Abstract
The human cerebellum has been implicated in the control of a wide variety of motor control parameters, such as force amplitude, movement extent, and movement velocity. These parameters often covary in both movement and isometric force production tasks, so it is difficult to resolve whether specific regions of the cerebellum relate to specific parameters. In order to address this issue, the current study used two experiments and SUIT normalization to determine whether BOLD activation in the cerebellum scales with the amplitude or rate of change of isometric force production or both. In the first experiment, subjects produced isometric pinch-grip force over a range of force amplitudes without any constraints on the rate of force development. In the second experiment, subjects varied the rate of force production, but the target force amplitude remained constant. The data demonstrate that BOLD activation in separate sub-areas of cerebellar regions lobule VI and Crus I/II scale with both force amplitude and force rate. In addition, BOLD activation in cerebellar lobule V and vermis VI was specific to force amplitude, whereas BOLD activation in lobule VIIb was specific to force rate. Overall, cerebellar activity related to force amplitude was located superior and medial, whereas activity related to force rate was inferior and lateral. These findings suggest that specific circuitry in the cerebellum may be dedicated to specific motor control parameters such as force amplitude and force rate.
Keywords: BOLD, Cerebellum, fMRI, Isometric
Introduction
A meta-analysis of human functional neuroimaging studies has found that the cerebellum is active during various motor tasks (Stoodley and Schmahmann, 2009). Furthermore, the observations of Stoodley and Schmahmann (2009) corroborate earlier work, which proposes localization of sensorimotor functions in the cerebellum to lobules IV, V, VI, and VIIIA/B (Nitschke et al., 1996; Schmahmann, 1991, 1996, 2004). Thus, the cerebellum plays a broad role in human neurological function, and it has long been known that there are segregated motor areas of the cerebellum. The role of the human cerebellum in sensorimotor control in general has yet to be fully defined. This is especially the case in terms of the control of force amplitude and rate of force development.
Smith and Bourbonnais (1981) found that some neurons in cerebellar lobules V and VI of monkeys scale in spike frequency with increasing grip force amplitude. Other electrophysiological studies found that the firing rate of cerebellar neurons of non-human primates increased during grasping and lifting movements (Espinoza and Smith, 1990). Additionally, cerebellar neural firing rate scales with increased grip force amplitude (Mason et al., 2006) and with EMG during precision grip force production (Townsend et al., 2006). Subsequent functional neuroimaging studies using isometric force production tasks have extended this finding to humans. For instance, one previous study using positron emission tomography (PET) and a finger flexion task found that regional cerebral blood flow in the cerebellar vermis was significantly correlated to isometric force amplitude (Dettmers et al., 1995). Similarly, an fMRI study found that the blood oxygenation level dependent (BOLD) signal in bilateral cerebellum scales with increasing isometric wrist force amplitude and increasing flexor carpi radialis EMG activity (i.e. the agonist muscle) (Sehm et al., 2010). Other human fMRI studies have also shown that the BOLD signal in the cerebellum increased in activation when individuals produce increased grip force amplitude (Dettmers et al., 1995; Keisker et al., 2009; Pope et al., 2005).
In addition to being involved in producing different levels of force, several neuroimaging and electrophysiological studies in human and non-human primates suggest that the cerebellum plays a role in modulating a number of movement parameters, such as movement extent or movement velocity. For instance, a number of electrophysiological studies in non-human primates found that Purkinje cell simple spike discharge is correlated with movement extent (Fu et al., 1997; Smith and Bourbonnais, 1981; Wetts et al., 1985). These findings have been replicated in a human study using PET, which shows that the ipsilateral cerebellum scales in activation with the extent of wrist movements (Turner et al., 2003). Other electrophysiological studies in non-human primates suggest that neural activation in the cerebellum plays a role in controlling movement velocity (Coltz et al., 1999; Mano et al., 1986; Mano and Yamamoto, 1980; van Kan et al., 1993; Wetts et al., 1985). This finding has also been demonstrated in humans, where regional cerebral blood flow (rCBF) in the ipsilateral anterior cerebellum correlated significantly with the velocity of joystick movements (Turner et al., 1998).
In summary, neural activation in the cerebellum has been implicated in the control of a number of parameters, such as force amplitude, movement extent, and movement velocity. However, in studies of both movement and isometric force, a number of these variables are correlated and it becomes difficult to identify whether specific areas of the cerebellum are involved in controlling a specific parameter. For instance, the rate of change of force covaries with force amplitude in isometric force production tasks (Spraker et al., 2007), and movement velocity can covary with movement extent, suggesting that force amplitude could be the main parameter regulated by the cerebellum. As such, the current study used two experiments to determine whether specific motor areas of the cerebellum scale in BOLD activity with amplitude or rate of change of isometric precision grip force production. This is the first study to use the spatially unbiased infratentorial template (SUIT) of the cerebellum (Diedrichsen, 2006; Diedrichsen et al., 2009) to examine these motor control parameters in relation to BOLD activity. In the first experiment, the amount of force was varied over a wide range without any constraints on the rate of force development. The second experiment required subjects to vary the rate at which they produced force keeping the amplitude of force constant. We tested two alternative hypotheses. The first hypothesis was that BOLD activity in the same regions of the cerebellum scale with both force amplitude and rate of change of force. The second hypothesis was that BOLD activity in distinct cerebellar areas scales with the force amplitude and rate of change of force.
Methods
The experiments reported in this paper are the same as those presented in prior studies (Force amplitude, Spraker et al., 2007; Rate of Force, Prodoehl et al., 2008). In the present paper, the focus is exclusively in the cerebellum.
Subjects
Twelve right-handed subjects participated in the force amplitude experiment (six male and six female, ages 20–35 yr). One male subject was not included in the analysis due to excessive head motion that correlated with the task. Eleven right-handed subjects (five males and six females, age 20–37 yr) participated in the rate experiment. The subjects were different in each experiment. Each of the subjects was naïve to the purpose of the experiment, had normal or corrected to normal vision, and was free of neurological disorders. All subjects provided informed consent to all procedures, which were approved by the local Institutional Review Board and were in accord with the Declaration of Helsinki.
Force Data Acquisition
Subjects used their right hand (thumb, first, and second fingers) to produce isometric force against the same custom grip device during the force amplitude experiment (Spraker et al., 2007) and the rate experiment (Prodoehl et al., 2008). At each sampling interval, the output from the custom grip device was displayed to the subject using a visual feedback system (Vaillancourt et al., 2003). The feedback was projected using a parallax biofeedback system (Thulborn, 1999) through a mirror located 35 cm from the subject's eyes. The force output was displayed at a refresh rate of 60 Hz and a resolution of 640 × 480 pixels.
Experimental Design
Before each scanning session in both the force amplitude and rate experiments, each subject participated in a 1h training session outside the scanner to minimize motor learning effects when inside the scanner. The individual's maximum voluntary contraction (MVC) was first calculated. The subject was asked to sustain a contraction of maximum force for three consecutive 5s trials. Each trial was separated by a period of rest. The MVC was calculated as the average force during the sustained maximum force contraction.
Force Amplitude Experiment
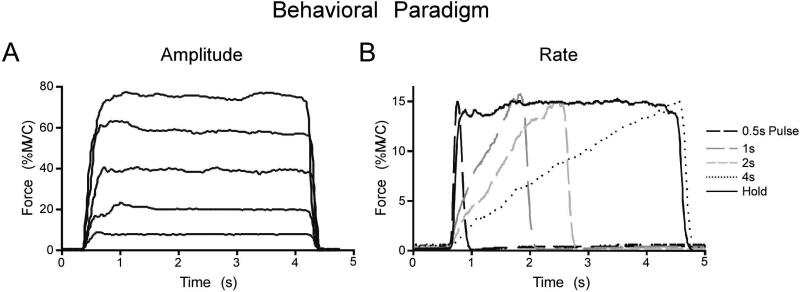
The force amplitude experiment required subjects to repeat the force task at five different amplitudes during five different scans, each lasting 4min 30sec. The force level requirement for each of the five functional scans was set to 5%, 20%, 40%, 60%, or 80% of each subject's collected MVC. Each functional scan started and ended with a 30s rest block alternating with four 30s force blocks. During rest blocks, subjects fixated on a stationary red target and white cursor but did not produce force. In each of the force blocks, subjects were cued to produce 4s force contractions separated by 2s of rest. During force blocks, the target would switch to green to cue the subjects to start producing force. When the target was green, the white cursor could be vertically displaced from its resting position with respect to the level of force generated by the subject and collected through the A/D board. Each force block required subjects to complete five isometric contractions. Figure 1A shows a single contraction event from the same subject at each of the five force levels that occurred in the five separate fMRI scans. In total, the subjects completed 20 isometric contractions at each of the 5%, 20%, 40%, 60%, and 80% MVC force levels.
Figure 1.
A. Recorded force trace from the force amplitude experiment showing one subject performing a single isometric contraction to the 5%, 20%, 40%, 60%, 80% MVC force levels. All contractions in the force amplitude experiment were approximately 4s long. B. A recorded force trace from the rate experiment showing one subject performing a single isometric contraction during the 0.5s Pulse, 1s, 2s, 4s, and Hold conditions. All contractions in the rate experiment had a peak force amplitude of 15% MVC.
Rate Experiment
The rate experiment consisted of five functional scans, each lasting 7min and 10s. Each functional scan started and ended with a 30s rest block, alternating with four 70s task blocks. Each 70s task block consisted of 30s force production with visual feedback, a 10s break, and 30s force production with auditory feedback. The current study focuses only on force production with visual feedback. During rest blocks, subjects fixated on a stationary red target and white cursor and did not produce force. During the force conditions subjects were required to generate force to 15% of MVC every 5s for a total of six force contractions per 30s block. For each functional scan, subjects were instructed to generate force in one of five contraction types: (1) 0.5s pulse, (2) 1s ramp, (3) 2s ramp, (4) 4s ramp, and (5) hold. Figure 1B shows a single contraction from a single subject for each of the five contraction types. The 0.5s, 1s, 2s, and 4s conditions change in both rate and duration of force production. The purpose of the hold condition is to distinguish between the control of force rate and the control of force duration since the hold condition is of a different rate but the same duration as that of the 4s condition (Vaillancourt et al., 2004). The force amplitude produced during each condition was carefully controlled using on-line visual feedback with the same cursor and target as in the force amplitude experiment. During rest blocks, subjects fixated on the stationary red target and white cursor and did not produce force. During the 0.5s pulse condition, the target bar turned green for 0.5s and returned to red for 4.5s. Subjects were cued to generate a force pulse to 15% MVC as quickly as possible within 0.5s and then release force for the remaining 4.5s (Figure 1B). During the 1s, 2s, and 4s ramp conditions, the target bar turned green for 1s, 2s, or 4s and then returned to red for the remainder of the 5s trial (4s, 2s, or 1s, respectively). Subjects were cued to gradually produce increasing force so that they achieved 15% MVC at the end of the ramp period (1s, 2s, or 4s) and then release force while the target bar turned red (Figure 1B). During the hold condition, the target bar turned green for 4s and returned to red for 1s. Subjects were cued to produce force as quickly as possible to 15% MVC and maintain the force level for the 4s period, then release force while the target bar turned red (Figure 1B).
Force Data Analysis
Force data were filtered at 20 Hz using a Butterworth filter (dual-pass; 4th order). After the force output was filtered off-line, visual inspection of the data was performed and 4 specific time points were marked for each contraction in the force amplitude experiment and the rate experiment. Point 1 was marked at the onset of force in all contractions. Points 2 and 3 were marked at the beginning and end of the sustained force period, respectively. In contractions where there was no sustained force period (i.e. 0.5s, 1s, 2s, 4s), point 2 was marked just before the peak force of the contraction and point 3 was marked just after the peak force of the contraction. Point 4 was marked at the offset of force in all contractions. Three main variables were calculated during force data analysis. First, mean force amplitude was calculated as the mean force output between points 2 and 3. Second, the rate of change of increasing force was obtained by averaging the first derivative of force between points 1 and 2. Third, the mean duration of each contraction was calculated as the time difference between the offset and onset of force (points 4 and 1).
Calculations were carried out for each individual contraction of each task. This resulted in 20 values per subject for each dependent measure for the force amplitude experiment (five contractions, four blocks) and 24 values per subject for each dependent measure for the rate experiment (six contractions, four blocks). These values were averaged to give three mean dependent measures per condition per subject for the force amplitude experiment and for the rate experiment. All calculations were performed using custom algorithms in MATLAB. Differences between the force amplitude conditions and differences between the rate conditions were analyzed using separate one-way ANOVAs for each dependent measure (Statistica, v6.1).
MRI Data Acquisition
Magnetic resonance images were collected using a quadrature, volume head coil inside a 3 Tesla MR Scanner (GE Healthcare, Waukesha, WI, 3T94 Excite 2.0). The functional images were obtained using a T2*-sensitive, single shot, gradient-echo echo-planar pulse sequence (echo-time 25 ms; repeat-time 2500 ms; flip angle 90°; field of view 200 mm2; imaging matrix 64 × 64; 42 axial slices at 3-mm thickness; 0-mm gap between slices). The EPIs covered the entire cerebellum for each subject. The high-resolution anatomical scans were obtained using a T1-weighted fast spoiled gradient echo pulse sequence (echo-time 1.98 ms; repeat-time 9 ms; flip angle 25°; field of view 240 mm2; imaging matrix 256 × 256; 120 axial slices at 1.5-mm thickness; 0-mm gap between slices).
MRI Data Analysis
The software packages Analysis of Functional NeuroImages (AFNI) and Statistical Parametric Mapping (SPM) were used to process and analyze the fMRI data sets. Within SPM, the spatially unbiased infratentorial template (SUIT) of the cerebellum was used to optimize normalization procedures specific to the cerebellum. Estimated within AFNI, head motion for all included subjects was less than 1mm in all directions. Voxel-wise analysis was the primary analysis used in this study, and the region of interest (ROI) analysis was performed in areas identified from the voxel-wise analysis to further confirm the finding.
Voxel-wise Analysis
Within AFNI, a voxel-wise analysis was first performed on the fMRI data from the force amplitude experiment and the rate experiment. Motion-corrected individual data sets were normalized by dividing the instantaneous signal in each voxel at each point in the time series by the mean signal in that voxel across each scan. After this, a Gaussian filter was applied to the resultant data sets (full-width half-maximum at 3mm). Then, the time-series data were regressed to a simulated hemodynamic response function for the task sequence (3Ddeconvolve, AFNI). The dependent variable at this level of analysis was the estimated β-coefficient of the regressed time series and its associated t-statistic. Before group analysis, each subject's anatomical and functional data set was transformed to MNI space using the SUIT procedure within SPM (Diedrichsen, 2006; Diedrichsen et al., 2009). The data were then transferred back to AFNI for group statistical analyses.
In AFNI, the output data from the force amplitude experiment and the rate experiment were analyzed using separate mixed-effect 2-way ANOVAs with condition as a fixed factor and subject as a random factor. The ANOVA for each experiment yielded the estimated group mean β-value for each condition and the estimated main effect of each condition (i.e. force amplitude, rate). All data sets were corrected for multiple comparisons using a Monte Carlo Simulation model (alphasim, http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim/). Group mean activation maps depicting force amplitude or rate scaling across conditions were thresholded to remove all voxels with F<4.5 (individual voxel p<0.005) with an activation cluster minimum of 17 voxels (136μl) (p<0.05, corrected). Voxels below the threshold were considered non-significant. A conjunction analysis was then performed to determine areas that only changed during the force experiment, areas that only changed during the rate experiment, and areas that changed during both experiments.
Analysis of Spatial Shift in Activation
To determine if the activation shifted in location for the force and rate experiments, we performed a statistical analysis comparing the 80% MVC condition with the 4s ramp condition. The unthresholded β-values for each voxel were compared within a combined ROI that included cerebellar lobules V and VI, and Crus I from the SUIT template. The center of mass was calculated using 3dclust (AFNI) for each subject. The x, y, and z center of mass coordinates for each subject were examined separately using three different 2-way ANOVAs comparing the force task vs rate task (force-80% vs. rate-4s) for each side. Force task was a between subject factor and hemispheric side was a within subject factor. For the x dimension the absolute value was used.
Regions of Interest Analysis
An ROI analysis of the BOLD percent signal change was used to further confirm the findings of the voxel-wise analysis. Percent signal change was determined by first calculating the mean signal within each voxel for rest and for task blocks across each individual motion-corrected functional time series. The mean percent signal change within each voxel was calculated using the following equation:
where μT is the mean signal during the task blocks and μR is the mean signal during the 30s rest blocks. Therefore, the output data represented the percent signal change in each voxel for each individual subject data set.
The ROIs used for statistical analysis were the areas that were detected by the voxel-wise ANOVA to have a significant main effect of condition (see above). We developed the ROIs by multiplying the individual ROIs from the SUIT mask by the conjunction analysis following the group level statistics (Diedrichsen et al., 2009). This provided region specific ROIs based on the SUIT normalization procedure that were specific to the force experiment and specific to the rate experiment. Group mean percent signal change within each ROI was examined to confirm the relation between force vs. percent signal change and rate vs. percent signal change in each experiment.
Results
Behavioral Performance
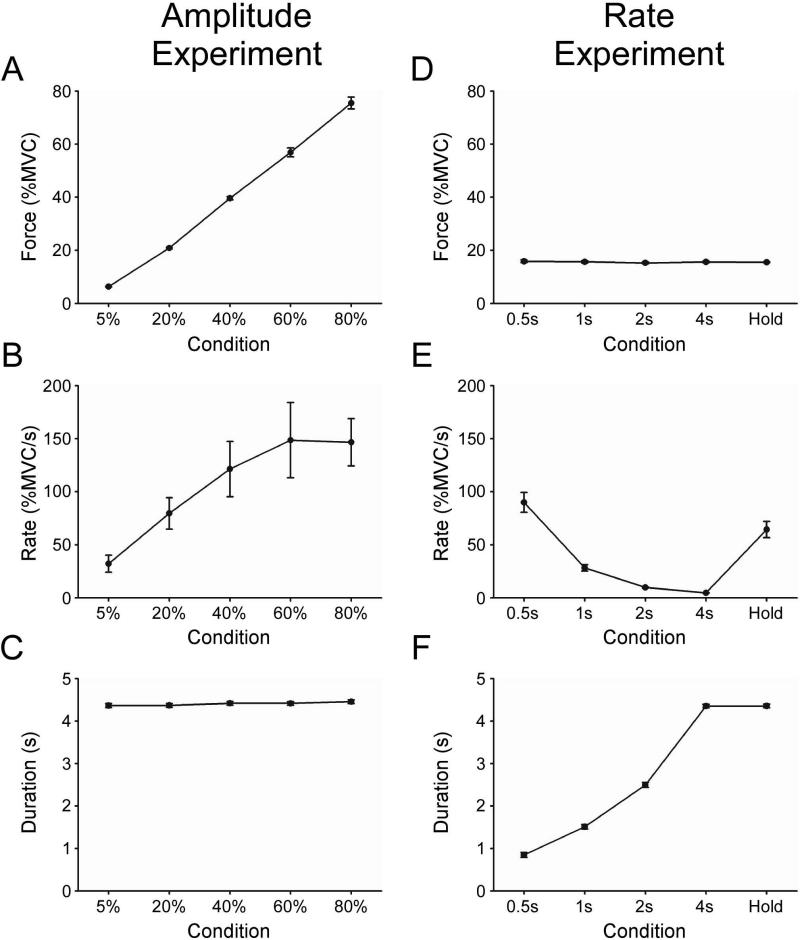
Figures 2A-C depict group mean force amplitude, rate of change of increasing force, and duration of force at the 5%, 20%, 40%, 60%, and 80% MVC target force levels. One-way ANOVA demonstrated that mean force amplitude (F(4,40)=640.91, p<0.01) and mean rate of change of force (F(4,40)=12.44, p<0.01) were significantly different across the target force levels. Duration of force was not different across increasing target force levels (F(4,40)=2.12, p=0.10).
Figure 2.
A-C and D-F depict behavioral data for the force amplitude experiment and the rate experiment, respectively. A plot of group mean force amplitude (A) depicts that subjects were able to produce force close to the target level for each of the five conditions. Rate of change of force increased with force amplitude (B), while a plot of duration of force shows that the contractions were of similar length during all five conditions (C). In the rate experiment, the group mean force was similar across all conditions (D). Subjects effectively produced contractions with decreasing rate of change of force across the 0.5s, 1s, 2s, and 4s conditions (E). Contractions increased in duration across the 0.5s, 1s, 2s, and 4s task conditions (F). Error is the standard error of the mean. In some instances, the error is not apparent because the error across subjects was very small and the y-axis scale is large.
Figures 2D-F depicts group mean force amplitude, rate of change of increasing force, and duration of force for the 0.5s, 1s, 2s, 4s, and Hold conditions. One-way ANOVA demonstrated that mean force amplitude was not different across conditions (F(4,40)=0.48, p=0.75). As expected, mean rate of change of force (F(4,40)= 45.95, p<0.01) and duration of force (F(4,40)=1359.69, p<0.01) were significantly different across conditions.
fMRI Results
Voxel-wise Analysis
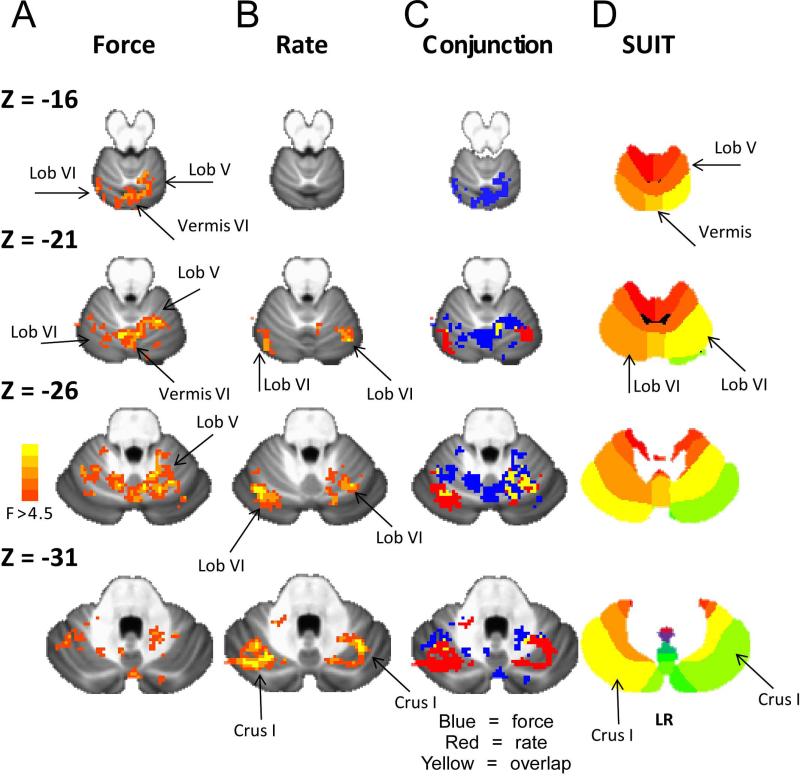
Figure 3 shows the F-test results for the force amplitude experiment and rate experiment. In addition, Figure 3 shows the conjunction analysis (Force alone = blue; Rate alone = red; overlap = yellow) indicating that specific areas of the cerebellum had a BOLD signal change across force amplitude and rate. In experiment 1, voxel-wise analysis revealed a significant main effect of force for BOLD activation in the cerebellum, including left lobule IV, left and right lobule V, left and right lobule VI, vermis VI, left and right Crus I, and left and right Crus II (Table 1; p<0.05, corrected). In experiment 2, voxel-wise analysis revealed that the rate experiment was significant for left and right lobule VI, left and right Crus I, left Crus II, and left and right lobule VIIb (Table 2; p<0.05, corrected). Finally, the overlap (yellow) shown in Figure 3C resulted in small clusters that did not reach the cluster size threshold for p<.05 corrected.
Figure 3.
Voxel-wise results of the ANOVA from the force experiment (A) and the rate (B) experiment overlaid on the SUIT T1 weighted average across 20 young healthy adults. C, shows the overlap following the conjunction analysis. Blue = force experiment. Red = rate experiment. Yellow = overlap between force and rate experiment. D, shows the SUIT template for each Z slice (Diedrichsen et al., 2009).
Table 1.
Force Regions of Interest
| ROI | SUIT MNI Coordinates | Volume | Max F-stat | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| L Lobule IV | -4.0 | -51.7 | -6.4 | 176 | 7.83 |
| Range | -8.0 – 0.0 | -48.0 – -56.0 | -9.0 – -3.0 | ||
| L Lobule V | -6.1 | -59.6 | -8.3 | 216 | 7.13 |
| Range | -10.2 – -2.0 | -52.0 – -64.0 | -11.0 – -5.0 | ||
| R Lobule V | 10.7 | -57.8 | -18.0 | 1288 | 11.76 |
| Range | 2.0 – 20.0 | -46.0 – -66.0 | -27.0 – -9.0 | ||
| L Lobule VI | -21.9 | -57.7 | -24.3 | 1984 | 10.92 |
| Range | -38.0 – -4.0 | -44.0 – -70.0 | -35.0 – -11.0 | ||
| Vermis VI | 1.0 | -68.2 | -21.9 | 1136 | 13.56 |
| Range | -4.0 – 6.0 | -60.0 – -76.0 | -29.0 – -13.0 | ||
| R Lobule VI | 10.8 | -64.9 | -18.9 | 216 | 9.83 |
| Range | 8.0 – 14.0 | -58.0 – -70.0 | -25.0 – -13.0 | ||
| L Crus I | -41.2 | -55.6 | -30.9 | 144 | 6.29 |
| Range | -46.0 – -38.0 | -50.0 – -60.0 | -33.0 – -29.0 | ||
| R Crus I | 20.5 | -80.5 | -30.5 | 176 | 6.74 |
| Range | 18.0 – 24.0 | -78.0 – -84.0 | -35.0 – -27.0 | ||
| L Crus II | -4.8 | -75.3 | -36.0 | 144 | 6.91 |
| Range | -10.0 – -2.0 | -72.0 – -76.0 | -41.0 – -33.0 | ||
| Vermis Crus II | 1.9 | -74.7 | -33.6 | 216 | 11.01 |
| Range | -2.0 – 4.0 | -72.0 – -78.0 | -37.0 – -31.0 | ||
| R Crus II | 8.5 | -79.5 | -38.0 | 576 | 8.95 |
| Range | 4.0 – 22.0 | -74.0 – -86.0 | -43.0 – -31.0 | ||
*Each center of mass coordinate and the range is shown.
Table 2.
Rate Regions of Interest
| ROI | SUIT MNI Coordinates | Volume | Max F-stat | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| L Lobule VI | -25.3 | -66.8 | -26.3 | 1448 | 20.71 |
| Range | -36.0 – -14.0 | -54.0 – -76.0 | -35.0 – -19.0 | ||
| R Lobule VI | 31.8 | -58.5 | -29.3 | 416 | 15.03 |
| Range | 26.0 – 36.0 | -54.0 – -66.0 | -33.0 – -25.0 | ||
| L Crus I | -37.1 | -67.5 | -32.3 | 2488 | 17.01 |
| Range | 52.0 – -20.0 | -56.0 – -78.0 | -43.0 – -23.0 | ||
| R Crus I | 31.4 | -67.4 | -30.2 | 408 | 11.35 |
| Range | 20.0 – 40.0 | -56.0 – -74.0 | -33.0 – -27.0 | ||
| L Crus II | -15.8 | -83.7 | -44.9 | 992 | 10.54 |
| Range | -26.0 – -6.0 | -78.0 – -88.0 | -53.0 – -37.0 | ||
| L Lobule VIIb | -22.4 | -72.9 | -51.5 | 624 | 10.28 |
| Range | -36.0 – -16.0 | -68.0 – -80.0 | -59.0 – -45.0 | ||
| R Lobule VIIb | 18.9 | -73.9 | -55.1 | 256 | 9.6 |
| Range | 12.0 – 26.0 | -70.0 – -78.0 | -59.0 – -51.0 | ||
*Each center of mass coordinate and the range is shown.
There was a topographic organization for where the force amplitude effects and rate effects were observed in the cerebellum. In the top slice of Figure 3 (Z = -16), only the force experiment resulted in changes in lobule V, lobule VI, and vermis VI, indicating that the force amplitude effects were more superior in the cerebellum. In slice Z = -21 of Figure 3, the activity in right and left lobule VI related to force amplitude was more medial to the activity in lobule VI that changed during the rate experiment. A similar observation is seen in Z = -26 and Z = -31, in which the activity that changed during the rate experiment was more lateral in lobule VI and Crus I. Also, Table 2 indicates that the rate experiment had activity in lobule VIIb whereas activity in lobule VIIb did not change during the force experiment. In summary, the general organization identified in Figure 3 is that the force amplitude effects were observed superior and medial whereas the rate effects were observed inferior and lateral in the cerebellum.
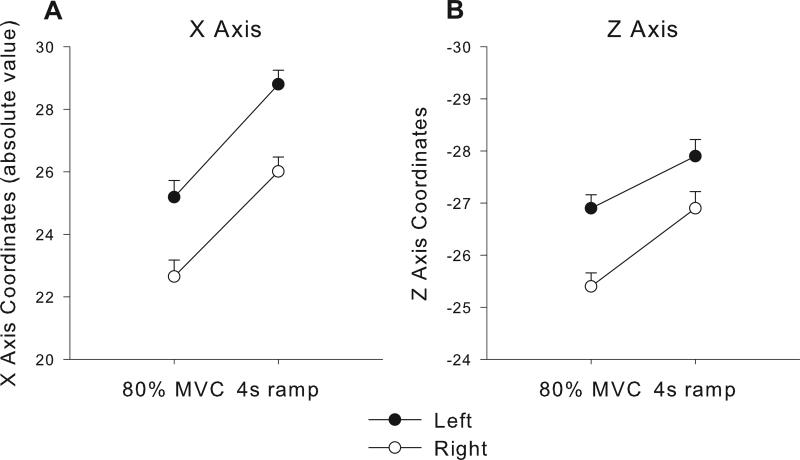
Analysis of Spatial Shift in Activation
Confirmation of the spatial shift in activation was performed for the x, y, and z center of mass coordinates for a combined ROI covering lobules V, VI, and Crus I. Figure 4 shows the results for the x and z dimensions since they both were significant. The 80% MVC condition from the force experiment was compared with the 4s ramp condition from the rate experiment. For the x dimension (Figure 4A), the rate experiment resulted in activation more lateral compared with the force experiment, which was supported by a main effect of force task (F(1,20)=34.17, p<0.001). The side effect was significantly more lateral for the right cerebellum compared to the left cerebellum (F(1,20)=49.96, p<0.001), but the force task by side interaction was not significant (F(1,20)=0.114, p=0.73). None of the effects were significant for the y dimension. For the z dimension (Figure 4B), the rate experiment resulted in activation more inferior compared with the force experiment, which was supported by a main effect of force task (F(1,20)=11.01, p<0.005). The left side was more inferior compared to the right side (F(1,20)=64.99, p<0.001), and the force task by side interaction was not significant (F(1,20)=2.82, p=0.11). These findings further confirm the lateral and inferior shift in activation during the rate experiment compared to the force experiment.
Figure 4.
Center of mass coordinates for the x (A) and z (B) dimensions are shown. The 80% MVC condition and the 4s ramp conditions were compared across left and right sides. The center of mass was quantified from each subject and condition separately using 3dclust (AFNI) in a combined ROI including cerebellar lobules V and VI and Crus I (SUIT). Error bars represent standard error.
Regions of Interest Analysis
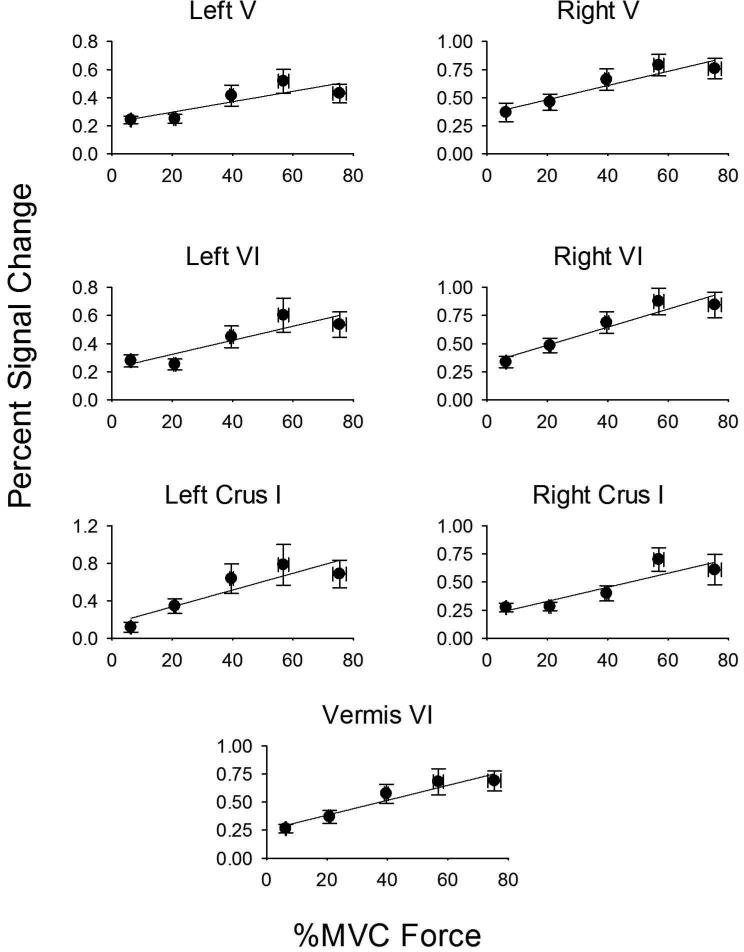
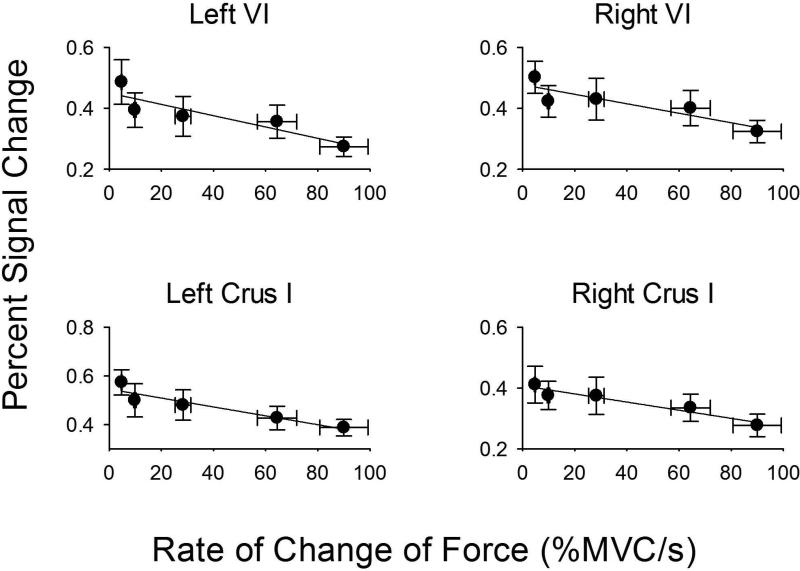
In order to determine the positive or negative slope for BOLD percent signal change across force and rate, we performed a region of interest analysis within the functionally-defined areas shown in Tables 1 and 2. Figure 5 shows percent signal change data for left and right lobule V, left and right lobule VI, left and right Crus I, and vermis VI. In each case, the percent signal change increased with the level of force as did all other areas in Table 1. The slope of the regression model between force level and percent signal change was significant for all areas shown Table 1 (all p's < 0.05). It is important to point out that these regions of interest were not significant in the voxel-wise analysis for the rate experiment, suggesting that the findings shown in Figure 5 are specific to the level of force. In Figure 6, the percent signal change is shown for left and right lobule VI, and left and right Crus I. In each ROI, there was a negative slope such that the faster the rate of change of force, the less percent signal change within the ROI. A similar negative relation was found for all other areas in Table 2. The slope in the regression between rate of force and percent signal change was significant for each ROI shown in Table 2 (all p's < 0.05). Since the duration of force also changed in the rate experiment (Figure 2), we performed regression between duration of force and percent signal change in the ROIs listed in Table 2. None of the regression analyses were significant (all p's > 0.20), suggesting that these findings were specific to the rate of force.
Figure 5.
Force level plotted against percent signal change in ROIs that include: left lobule V, right lobule V, left lobule VI, right lobule VI, left Crus I, right Crus I, and vermis VI. Force level is on the x-axis because this was the independent variable manipulated in the study. Each plot represents the average force/percent signal change across subjects, along with the standard error of the mean for the x and y axes. The error for force level (x axis) was small at the low force levels, and can only be visible at the high force levels. In each plot, the positive slope from the regression analysis was significant at p<.05.
Figure 6.
Rate of force plotted against percent signal change in ROIs that include: left lobule VI, right lobule VI, left Crus I, and right Crus I. Rate of force is on the x-axis because this was the independent variable manipulated in the study. Each plot represents the average rate/percent signal change across subjects, along with the standard error of the mean for the x and y axes. The error for rate (x axis) was small at the low rate levels, and can only be visible at the high rate levels. In each plot, the negative slope from the regression analysis was significant at p<.05.
Discussion
The human cerebellum has been implicated in controlling a number of parameters associated with force generation and movement. Many of these parameters have been correlated in previous studies, so it has proven difficult to identify those that are specifically related to neural activity in cerebellar sensorimotor areas. This study used two experiments to investigate how human cerebellar BOLD activity scales with force amplitude and rate of change of force during isometric, precision grip force control. The results demonstrate that specific areas of the cerebellum scale in activation with force amplitude and rate of change of force. The data demonstrate that BOLD activation in separate sub-areas of cerebellar regions lobule VI and Crus I/II scale with both force amplitude and force rate. In addition, BOLD activation in cerebellar lobule V and vermis VI was specific to force amplitude, whereas BOLD activation in lobule VIIb was specific to force rate. Overall, cerebellar activity related to force amplitude was located superior and medial, whereas activity related to force rate was inferior and lateral.
In the following, we compare our current findings using the BOLD fMRI technique with neurophysiological recordings in the cerebellum in which simple and complex firing patterns of Purkinje cells were recorded. It is important to note that the relation between the BOLD fMRI signal and the firing rate of Purkinje cells is not straightforward. While in the cerebral cortex the BOLD signal can be modeled as a nonlinear function of cerebral metabolic rate of oxygen consumption, cerebral blood flow, and blood volume (Buxton et al., 1998), the architecture and physiology in the cerebellar cortex is very different from the cerebral cortex (Diedrichsen et al., 2010; Thomsen et al., 2004). Most current evidence suggests that the BOLD fMRI signal in the cerebellum reflects mossy fiber input, rather than the firing of Purkinje cells (Diedrichsen et al., 2010). Despite this caveat, the anatomical location of our findings is consistent with the general locations reported in prior studies in animals.
Cerebellum and Force Amplitude
The current finding that BOLD activation in the cerebellum scaled with increasing force amplitude during isometric contractions is in agreement with previous studies in human and non-human primates. An electrophysiological study in monkeys found a significant positive correlation between neural firing rate in cerebellar lobules V and VI and the amplitude and rate of change of precision grip force (Smith and Bourbonnais, 1981). Another study found that the firing rate of neurons in the dentate and interpositus nuclei of monkeys was significantly correlated to the amplitude of prehensile force (Wetts et al., 1985). Several neuroimaging studies have extended these findings to humans. Studies of the somatotopy of digit representations related to producing force consistently identify lobules V and VI, and vermis lobules VI and VII during finger pressing tasks (Wiestler et al., 2011) and lobule V during hand movement tasks (Grodd et al., 2001). A previous PET study found that rCBF in the right anterior cerebellar vermis scaled significantly with index finger flexion force (Dettmers et al., 1995). While Dettmers and colleagues (1995) were only able to scan the superior portion of the cerebellum, this is in agreement with the current finding that BOLD activation in the vermis VI scaled with force amplitude. A subsequent fMRI experiment found that BOLD activation in right cerebellar lobules IV-V and left lobule VI increased as individuals produced greater levels of isometric wrist flexion force (Sehm et al., 2010).
Importantly, in previous experiments and the current study of force amplitude, subjects were required to produce different levels of isometric force amplitude without specific instructions concerning the rate of change of force. For instance, Dettmers and colleagues (1995) used a task that required subjects to perform an isometric key press with increasing levels of force amplitude once every second. The task used by Sehm and colleagues (2010) required subjects to move a cursor to a target box located at increasing distances on a screen that were related to increasing levels of isometric force amplitude. While these studies did not explicitly report rate of change of force, the instructions were similar to the task used in the current experiment where force amplitude and rate of change of force covaried (Figure 2B). Thus, using these tasks alone, one is unable to determine whether the activation in the cerebellum scales with isometric force amplitude or rate of change of force. Our study confirms prior literature by demonstrating that BOLD activation in the cerebellum does scale with force amplitude because the force experiment found cerebellar areas that were uniquely different to the rate experiment.
Cerebellum and Force Rate
We observed evidence that BOLD activity scales with the rate of force in sub-areas of the cerebellum that are distinct from those areas that scaled with the force amplitude. This finding could be related to the possibility that visual feedback had a greater effect on BOLD activity during the rate task, or that separate areas of the cerebellum regulate rate that are different from the areas that regulate force amplitude.
One interpretation of the findings in the rate experiment is that the dependence on visual feedback varied between the conditions. For instance, the 0.5s condition required subjects to generate a fast pulse to the target, which may have relied more on feedforward mechanisms and to a lesser degree on feedback mechanisms. In contrast, during the 4s condition subjects generated a slow ramp contraction that reached the target at approximately 4s, and this may have relied more on feedback mechanisms rather than feedforward mechanisms. Previously, Seidler and colleagues (2004) examined a Fitt's position control task that manipulated the index of difficulty. They found that the BOLD signal in right and left lobule V/VI decreased with an increase in the size of the target (decreased index of difficulty), suggesting that visual feedback from the target could influence cerebellar activity related to motor performance. Our finding in the left and right lobule VI during the rate experiment would be consistent with this interpretation. In addition, studies manipulating the frequency of visual feedback during a precision grip force task have found greater activity in lobule VI when frequency of feedback was at 25 Hz compared to 0.4 Hz (Vaillancourt et al., 2006). During reaching errors, it has also been shown that lobule VI is activated during both kinematic and dynamic execution errors (Diedrichsen et al., 2005). Future studies that compare the different rates of force development at different levels of visual feedback could further address this issue.
Another interpretation of the current findings is that the cerebellum regulates the rate of force in specific sub-areas of the cerebellum. Most prior studies examining rate have made use of a movement task, rather than a task involving force production. In movement tasks that require different velocities, this will involve a manipulation of both force amplitude and rate of force characteristics (Gottlieb et al., 1989). Thus, in movement tasks that vary in velocity, distinguishing the findings in relation to force amplitude or rate of force should be taken with caution. Below, we compare our findings during the isometric force rate experiment to prior studies that have manipulated movement velocity or frequency.
Coltz and colleagues (1999) recorded cerebellar Purkinje cells in cerebellar lobules IV, V, and VI in monkeys as they performed arm movements with increasing velocity (i.e. 2, 3, 4, and 5 cm/s) over a fixed distance. The results demonstrate that simple spike discharge rate increased significantly with movement velocity. Other studies recorded neurons in lobules IV-VI (Mano and Yamamoto, 1980) and in the nucleus interpositus (van Kan et al., 1993) of the cerebellum of monkeys and found that neural firing rate was correlated with wrist and elbow movement velocity. Indeed, one explanation for these findings is that the cerebellum plays a role in directly controlling movement velocity (Coltz et al., 1999). However, as velocity increases for movements over a fixed distance, muscle force and rate of change of force must also increase. This idea is in agreement with two other electrophysiological studies in non-human primates. The work of Wetts and colleagues (1985) demonstrates that the firing rate of neurons in the dentate and interposed nuclei is correlated to both the velocity of wrist movements and the torque produced by wrist flexor and extensor muscles. Our findings are consistent with those of Wetts and colleagues since specific areas of the cerebellum changed with force amplitude and rate of change of force (Figure 3). A subsequent study required monkeys to make elbow movements against resistive and assistive force fields to modulate muscle force without altering movement kinematics (Yamamoto et al., 2007). The data of Yamamoto and colleagues (2007) show that the simple spike activity of cerebellar neurons in lobules V and VI was much more closely related to changes in muscle force than movement velocity. Our findings are consistent with the work of Yamamoto and colleagues since we found that the BOLD signal scaled specifically with force in lobule V and in a sub-area of lobule VI.
Previous PET studies found that rCBF in the cerebellum had a significant positive relationship with the velocity of joystick movements about the wrist joint (Jenkins et al., 1997; Turner et al., 1998; van Mier et al., 1998; VanMeter et al., 1995). A study in humans using fMRI found that BOLD activation in bilateral lobule V, vermis VI, and vermis VIII scaled significantly with increasing frequency of bimanual, cyclical movements (Debaere et al., 2004). Another human fMRI study found that BOLD activation in bilateral cerebellar lobules IV/V, VI, and VIII increased significantly with increasing speech tempo (Riecker et al., 2006). These findings suggest that the cerebellum may play a role in controlling movement frequency. Importantly though, the tasks used in all of these studies required subjects to vary movement frequency within a fixed-duration task block, so there is a greater number of movements for high movement frequency conditions than low movement frequency conditions. A previous fMRI study has used an event-related finger-tapping task to independently investigate how BOLD activation in the human motor areas is related to movement frequency and movement quantity (Kim et al., 2005). Indeed, they found that increasing movement frequency failed to produce increased BOLD activation in the cerebellum, while BOLD activation in bilateral cerebellar lobule VI scaled with increasing movement quantity. A greater movement quantity would also be consistent with a greater overall level of muscle force on average, and is consistent with the current findings that specific areas of cerebellar lobule VI were related to force amplitude. This is in agreement with a human PET study that found increased rCBF in the cerebellum as subjects performed a 12-stroke finger movement task compared to a single finger movement (Catalan et al., 1998). It is important to note that in areas where we found significant changes with the rate of force, we did not find a significant relation with the duration of force. It remains possible that if duration were manipulated with the rate held constant, BOLD activity in specific areas of the cerebellum could change with the duration of force. In addition, in areas where the rate experiment yielded significant results, the relation between percent signal change and rate of force was negative. Prior studies that found a positive relation between BOLD activation or rCBF and movement velocity could have been influenced by the muscle force required during the movement, rate of change of force, or both.
Conclusion
This is the first study to independently manipulate the force amplitude and rate of change of force in separate experiments using the SUIT normalization procedure. The findings demonstrate that BOLD activation in specific sub-areas within the cerebellum of humans scales with increasing force amplitude and rate of change of force during isometric contractions. This sharpens the current understanding of the human motor system because previous experiments of isometric force production could not unambiguously determine whether increasing cerebellar activation is associated with amplitude of force or rate of change of force. Force amplitude and rate of change of force also covary with movement velocity and frequency in tasks where subjects are focused on completing movements under specific speed constraints. In this context, the current findings suggest an alternate interpretation of the current literature in which activation within the cerebellum is related to force production and rate of change of force, such that specific cerebellar areas are related to each motor parameter. The distinct role of the cerebellum during the rate of force could also be related to the use of visual feedback during slow ramp contractions. Finally, we identified a general pattern of cerebellar activity such that the BOLD signal related to force amplitude was superior and medial, whereas the BOLD signal related to force rate was inferior and lateral.
Highlights.
We study BOLD cerebellar activation during precision grip force production
We examine how cerebellar activation scales with amplitude and rate of force
Distinct sub-areas of lobule VI and Crus I/II scaling BOLD activation with force amplitude and rate
BOLD activation in lobule V was specific to force amplitude
BOLD activation in lobule VIIb was specific to force rate
Acknowledgments
Financial Support: This research was supported in part by grants from the National Institute of Health (R01-NS-58487, R01-NS-52318, R01-NS-28127, R01-NS-40902). DMC was supported by a faculty fellowship from Faber, K.U.Leuven.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121(Pt 2):253–264. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- Coltz JD, Johnson MT, Ebner TJ. Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci. 1999;19:1782–1803. doi: 10.1523/JNEUROSCI.19-05-01782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 2004;21:1416–1427. doi: 10.1016/j.neuroimage.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Schlerf J, Wiestler T. Advances in functional imaging of the human cerebellum. Curr Opin Neurol. 2010;23:382–387. doi: 10.1097/WCO.0b013e32833be837. [DOI] [PubMed] [Google Scholar]
- Espinoza E, Smith AM. Purkinje cell simple spike activity during grasping and lifting objects of different textures and weights. J Neurophysiol. 1990;64:698–714. doi: 10.1152/jn.1990.64.3.698. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol. 1997;78:478–491. doi: 10.1152/jn.1997.78.1.478. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single-joint movements. I. A speed-insensitive strategy. J Neurophysiol. 1989;62:342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH, Passingham RE, Brooks DJ. The effect of movement frequency on cerebral activation: a positron emission tomography study. J Neurol Sci. 1997;151:195–205. doi: 10.1016/s0022-510x(97)00145-7. [DOI] [PubMed] [Google Scholar]
- Keisker B, Hepp-Reymond MC, Blickenstorfer A, Meyer M, Kollias SS. Differential force scaling of fine-graded power grip force in the sensorimotor network. Hum Brain Mapp. 2009;30:2453–2465. doi: 10.1002/hbm.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Eliassen JC, Sanes JN. Movement quantity and frequency coding in human motor areas. J Neurophysiol. 2005;94:2504–2511. doi: 10.1152/jn.01047.2004. [DOI] [PubMed] [Google Scholar]
- Mano N, Kanazawa I, Yamamoto K. Complex-spike activity of cerebellar Purkinje cells related to wrist tracking movement in monkey. J Neurophysiol. 1986;56:137–158. doi: 10.1152/jn.1986.56.1.137. [DOI] [PubMed] [Google Scholar]
- Mano N, Yamamoto K. Simple-spike activity of cerebellar Purkinje cells related to visually guided wrist tracking movement in the monkey. J Neurophysiol. 1980;43:713–728. doi: 10.1152/jn.1980.43.3.713. [DOI] [PubMed] [Google Scholar]
- Mason CR, Hendrix CM, Ebner TJ. Purkinje cells signal hand shape and grasp force during reach-to-grasp in the monkey. J Neurophysiol. 2006;95:144–158. doi: 10.1152/jn.00492.2005. [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Kleinschmidt A, Wessel K, Frahm J. Somatotopic motor representation in the human anterior cerebellum. A high-resolution functional MRI study. Brain. 1996;119(Pt 3):1023–1029. doi: 10.1093/brain/119.3.1023. [DOI] [PubMed] [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC. Force related activations in rhythmic sequence production. Neuroimage. 2005;27:909–918. doi: 10.1016/j.neuroimage.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Wasson P, Corcos DM, Vaillancourt DE. Effects of visual and auditory feedback on sensorimotor circuits in the basal ganglia. J Neurophysiol. 2008;99:3042–3051. doi: 10.1152/jn.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Kassubek J, Groschel K, Grodd W, Ackermann H. The cerebral control of speech tempo: opposite relationship between speaking rate and BOLD signal changes at striatal and cerebellar structures. Neuroimage. 2006;29:46–53. doi: 10.1016/j.neuroimage.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex. 2010;20:34–45. doi: 10.1093/cercor/bhp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage. 2004;22:1775–1783. doi: 10.1016/j.neuroimage.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Smith AM, Bourbonnais D. Neuronal activity in cerebellar cortex related to control of prehensile force. J Neurophysiol. 1981;45:286–303. doi: 10.1152/jn.1981.45.2.286. [DOI] [PubMed] [Google Scholar]
- Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual Basal Ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98:821–834. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol. 2004;560:181–189. doi: 10.1113/jphysiol.2004.068072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR. Visual feedback to stabilize head position for fMRI. Magn Reson Med. 1999;41:1039–1043. doi: 10.1002/(sici)1522-2594(199905)41:5<1039::aid-mrm24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Townsend BR, Paninski L, Lemon RN. Linear encoding of muscle activity in primary motor cortex and cerebellum. J Neurophysiol. 2006;96:2578–2592. doi: 10.1152/jn.01086.2005. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM. Motor subcircuits mediating the control of movement velocity: a PET study. J Neurophysiol. 1998;80:2162–2176. doi: 10.1152/jn.1998.80.4.2162. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Corcos DM. Intermittent visuomotor processing in the human cerebellum, parietal cortex, and premotor cortex. J Neurophysiol. 2006;95:922–931. doi: 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. doi: 10.1016/j.neuroimage.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. J Neurophysiol. 2003;90:3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE. Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol. 1998;80:2177–2199. doi: 10.1152/jn.1998.80.4.2177. [DOI] [PubMed] [Google Scholar]
- VanMeter JW, Maisog JM, Zeffiro TA, Hallett M, Herscovitch P, Rapoport SI. Parametric analysis of functional neuroimages: application to a variable-rate motor task. Neuroimage. 1995;2:273–283. doi: 10.1006/nimg.1995.1035. [DOI] [PubMed] [Google Scholar]
- Wetts R, Kalaska JF, Smith AM. Cerebellar nuclear cell activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol. 1985;54:231–244. doi: 10.1152/jn.1985.54.2.231. [DOI] [PubMed] [Google Scholar]
- Wiestler T, McGonigle DJ, Diedrichsen J. Integration of sensory and motor representations of single fingers in the human cerebellum. J Neurophysiol. 2011;105:3042–3053. doi: 10.1152/jn.00106.2011. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kawato M, Kotosaka S, Kitazawa S. Encoding of movement dynamics by Purkinje cell simple spike activity during fast arm movements under resistive and assistive force fields. J Neurophysiol. 2007;97:1588–1599. doi: 10.1152/jn.00206.2006. [DOI] [PubMed] [Google Scholar]