Abstract
Brain morphometric studies often incorporate comparative hemispheric asymmetry analyses of segmented brain structures. In this work, we present evidence that common user guided structural segmentation techniques exhibit strong left-right asymmetric biases and thus fundamentally influence any left-right asymmetry analyses. In this study, MRI scans from ten pediatric subjects were employed for studying segmentations of amygdala, globus pallidus, putamen, caudate, and lateral ventricle. Additionally, two pediatric and three adult scans were used for studying hippocampus segmentation. Segmentations of the sub-cortical structures were performed by skilled raters using standard manual and semi-automated methods. The left-right mirrored versions of each image were included in the data and segmented in a random order to assess potential left-right asymmetric bias. Using shape analysis we further assessed whether the asymmetric bias is consistent across subjects and raters with the focus on the hippocampus.
The user guided segmentation techniques on the sub-cortical structures exhibited left-right asymmetric volume bias with the hippocampus displaying the most significant asymmetry values (p≪0.01). The hippocampal shape analysis revealed the bias to be strongest on the lateral side of the body and medial side of the head and tail.
The origin of this asymmetric bias is considered to be based in laterality of visual perception; therefore segmentations with any degree of user interaction contain an asymmetric bias. The aim of our study is to raise awareness in the neuroimaging community regarding the presence of the asymmetric bias and its influence on any left-right hemispheric analyses. We also recommend reexamining previous research results in the light of this new finding.
Keywords: Manual Segmentation, Subcortical Structures Neuroimaging, Asymmetry Analysis, Rater Bias
Introduction
Many morphometric brain studies use manual or semi-automatic segmentations of MR and CT images to make quantitative assessments of sub-cortical brain structures. Often such studies also analyze the asymmetry between structures in the left and right hemisphere. Such asymmetry has been studied in subcortical structures in many important neurological conditions. For example, in two separate studies Looi et al. have shown the caudate nucleus (2008) and putamen (2009) to display differential asymmetry in subjects with Alzheimer’s and Frontotemporal lobar degeneration as compared to normal controls. Schizophrenia patients have been found to exhibit a greater right than left volume asymmetry in the amygdala and hippocampus (Wang et al., 2001; Keshavan et al., 2002) in addition to a greater left than right lateral ventricle asymmetry (Buchsbaum et al., 1997; Puri et al., 1999). At the same time recent studies investigating hippocampal and amygdala volumes in adults with post-traumatic stress disorder have found smaller right relative to left hippocampal volumes in PTSD patients than in normal subjects (Pavic et al., 2006) while amygdala asymmetry has been shown to be preserved in PTSD patients (Woon, F.L., Hedges, D.W., 2008). Asymmetries in hippocampal (Geroldi et al., 2000; Chetelat, G., Baron, J.C., 2003; Shi et al., 2009) and lateral ventricle (Cherbuin et al., 2010) volumes have also been found to significantly correlate with mild cognitive disorders and Alzheimer’s disease. Hippocampal volumes have also been investigated in depression with studies suggesting that the left hippocampus shrinks to a greater extent than the right hippocampus, creating a significant hippocampal volume asymmetry in patients with depression (Kronmuller et al., 2009).
The study presented in this paper investigates bias in left-right asymmetry measures of user-guided segmentations that may significantly influence asymmetry analyses. We provide evidence that user interaction, whether through fully manual tracing or more limited user-interactions such as the placement of landmarks may introduce a systematic asymmetric bias.
Such an asymmetric bias may not be surprising as humans are known to naturally display a right-left perception bias. In a meta-analysis of 73 line-bisection studies, Jewell and McCourt (2000) found that normal controls commonly showed a leftward line bisection bias referred to as pseudoneglect. Perceptual bias is at least partially caused by hemispheric specialization such as right-hemispheric dominance in spatial and attention functions (Niemeier et al., 2007) with directional reading and writing systems also playing an important role in spatial-representational bias (Dobel et al., 2007). Further, right-left perception bias is not limited to humans, as a leftward gaze bias towards faces has also been shown in monkeys and dogs (Guo et al., 2009). Thus in user-guided segmentations of structures that display poor contrast and lack a clear boundary, raters may consistently trace left and right facing shapes differently. This may potentially lead to a systematic bias in asymmetry measures for user-guided segmentations of structures on the left and right sides of the brain.
There are currently no published studies available on perceptual bias in user-guided segmentation techniques. We only found a single neuroimaging study aiming to measure asymmetry (Thompson et al., 2008) that acknowledges a possible asymmetric bias. The goal of our study is to assess a potential left-right asymmetric bias in manual segmentations. This study has two-fold benefits. Firstly, it provides conclusive evidence for the presence of this bias. Secondly, it raises awareness in the neuroimaging community with regards to considering the asymmetric bias in future studies.
We studied the left-right asymmetric bias in fully manual segmentations of amygdala, putamen, and globus pallidus as well as semi-automated segmentations of the caudate, lateral ventricles, and hippocampus. We also performed a shape analysis for hippocampus segmentations, allowing us to pinpoint the degree of bias that the different hippocampal regions are experiencing. In order to assess the asymmetric bias segmentations are performed twice, both on an image in a standard view as well as on an image in a left-right mirrored radiologic view.
Materials and methods
1 Data Sets
For the amygdala, putamen, globus pallidus, caudate, and lateral ventricle segmentations we used 10 pediatric subjects’ ages 2.2 to 5.3 years from an autism study (Cascio et al., 2006; Hazlett et al., 2011). Five autistic cases and five controls were employed. A single expert rater performed all the segmentations bilaterally for a given structure.
For the hippocampus, two additional pediatric cases from the Autism study (a 2.2 year old control and a 2.9 year old autistic) were selected along with three adult cases (1 schizophrenic and 2 controls) from a separate schizophrenia study (Chakos et al., 2005). Five different raters bilaterally segmented the hippocampus for each of the five cases.
Participants from both data sets were scanned using a 1.5 T GE Signa Advantage scanner at Duke University. Images were acquired using a three-dimensional inversion recovery prepped axial spoiled gradient recalled protocol. The image matrices were 256×256×124 slices. The resolutions differed between the two datasets with the pediatric data at 0.78125×0.78125×1.5 mm resolution and the adult data set at 0.9375×0.9375×1.5 mm resolution.
2 Segmentations
Segmentations of amygdala, putamen, globus pallidus, caudate, hippocampus, and lateral ventricles were performed using standard manual and semi-automated methods. In order to study left-right asymmetric bias both original and left-right mirrored versions of each image were presented to the raters for segmentation. All images were randomized prior to segmentation. Five different raters performed the hippocampus segmentation, while each of the other structures was segmented by a single rater. All segmentations were performed according to well-defined segmentation protocols, which were defined as a part of clinical studies (detailed information in Chakos et al., 2005; Hazlett et al., 2011). All raters were right-handed.
2.1 Manual Segmentations
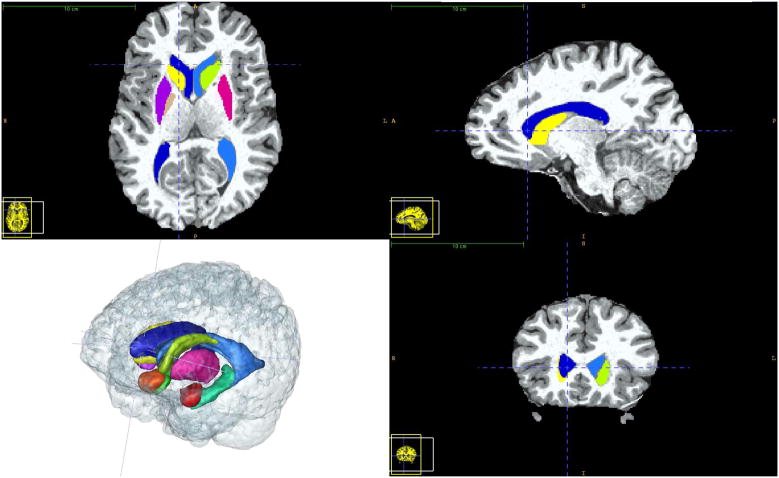
The amygdala, putamen, and globus pallidus were segmented via manual outlining in all three orthogonal slice directions using the ITK-SNAP (Yushkevich et al., 2006) segmentation tool (see Figure 1).
Figure 1.
Visualization of the sub-cortical structure segmentations using the ITK-SNAP segmentation tool. ITK-SNAP was used for the fully manual segmentations of the amygdala, putamen, and globus pallidus in addition to the semi-automatic caudate segmentation. All of the structures studied in this paper are shown in a healthy adult dataset.
2.2 Semi-Automatic Segmentations
The caudate, hippocampus, and lateral ventricles were segmented using different semi-automatic methods that are based on limited user interaction:
The caudate was initialized via the user initialized deformable contour tool in ITK-SNAP. The segmentation was then post-processed manually in regions abutting the nucleus accumbens.
The lateral ventricle segmentation is performed in a two-step process. First, a cerebro-spinal fluid (CSF) probability map is generated via an atlas based tissue classification tool (Prastawa et al., 2005). Second, the deformable contour tool in ITK-SNAP is employed to segment the ventricles on the probabilistic CSF map. The user needs to provide a seed point and stop the deformation upon convergence, as well as perform minimal manual post-processing. Of all structures, the lateral ventricles employed the least user input with the most constraints.
The hippocampus was segmented using a landmark based diffeomorphic template registration tool (Csernansky et al., 2008). The tool requires the raters to place landmarks at specific points around the perimeter of the hippocampus. The hippocampal surface is then determined automatically using a deformable registration of a prior template with the same landmark annotations. Next, the user iteratively adjusts the landmarks until the segmentation is satisfactory.
3 Volumetric Analysis
We tested for a volumetric asymmetric bias in two different ways. The first method compares the left-right asymmetry index between structures segmented on original images and on the mirrored images. The second method compares the segmentation between the original and mirrored image in each hemisphere separately.
Left-right volume asymmetry index was assessed as:
| (1) |
Where, VR and VL are the volumes of actual right and left brain structures, independent of presentation. The asymmetry index was compared between original and mirrored images. Assuming the absence of an asymmetric bias, we would expect the asymmetry index to be consistent, independent of presentation. Figure 2 visualizes the asymmetry indexes for all structures. We used a paired student t-test to test for the presence of a significant bias.
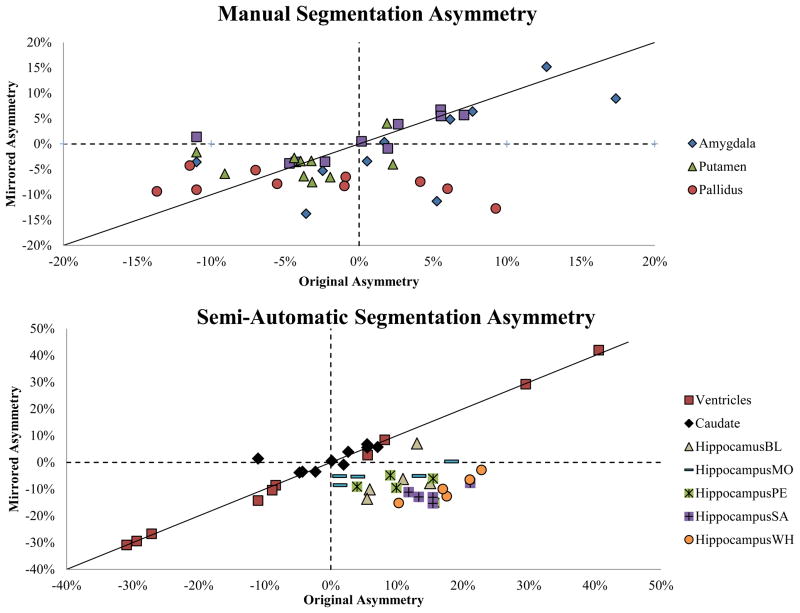
Figure 2.
Scatterplot of mirror vs. original volume asymmetry for manual and semi-automatic segmentations. Unbiased segmentations should fall along y = x. The amygdala and putamen appear to have slight rightward biases, while the pallidus has a strong rightward bias. The ventricles and caudate show very little bias, while the hippocampus asymmetry (shown for 5 raters) appears to be nearly inverted due to a powerful rightward bias.
We also investigated volumetric differences within each hemisphere. The hemispheric structure with original versus mirrored difference was assessed as:
| (2) |
Where, Vorig and Vmirr represent the volume of the structure within the hemisphere (right or left) on the original and mirrored image, respectively. Assuming the absence of an asymmetric bias, the volumetric differences between mirrored and original brain structures within each hemisphere should have an approximately Gaussian distribution with a mean of zero. The distribution is visually inspected via distribution histograms (see Figure 3). For significance testing, we employed a paired student t-test of the raw volumes for each hemisphere separately.
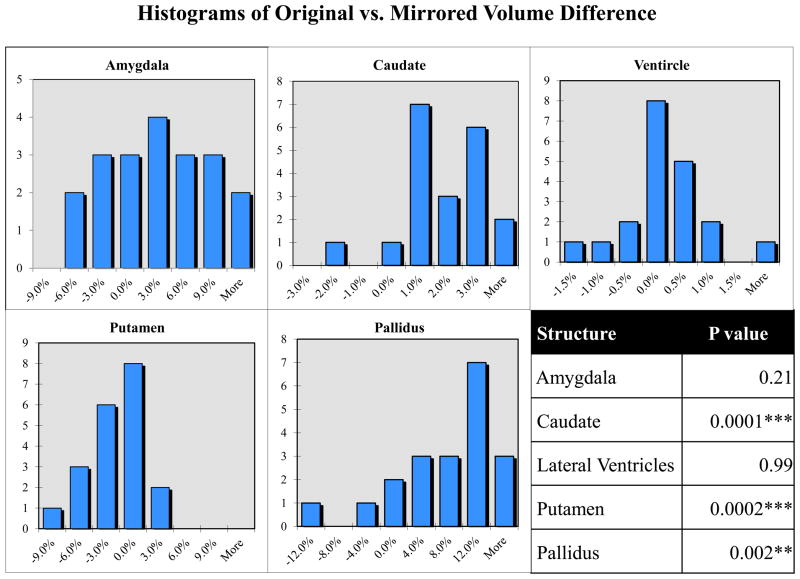
Figure 3.
Distribution of original vs. mirrored volume difference for right and left structures. The volume of each structure segmented on an original image is compared (via eq. 2) to the volume of the same structure segmented on the corresponding mirrored image. In the absence of bias, the histograms should consist of a normal distribution with a mean of zero. Significance values (student t-test) indicate a systematic hemispheric asymmetry bias.
4 Shape Analysis
While the volumetric analysis is enough to reveal the existence of a potential asymmetric bias, we also wanted to know if there are specific areas, which consistently show a large bias due to local shape or image contrast. For this purpose we chose to focus on one structure, the hippocampus. We performed a local shape analysis on the hippocampus segmentations via the UNC SPHARM-PDM (Spherical HARMonics Point Distribution Models) shape analysis toolbox. SPHARM-PDM allows us to discover the local hippocampal areas that were consistently affected by an asymmetric bias, across both raters and subjects.
The SPHARM-PDM toolbox presents a comprehensive set of tools for the computation of 3D structural statistical shape analysis. The SPHARM-PDM description is a sampled boundary description with object-inherent correspondence that can only represent objects of spherical topology (Brechbuhler et al., 1995). The input of SPHARM-PDM toolbox is our set of hippocampal binary segmentations. These segmentations are first processed to ensure spherical topology and then converted to surface meshes. Next, a spherical parameterization is computed from the surface meshes using an area-preserving, distortion minimizing spherical mapping. Further, the SPHARM description is computed from the mesh and its spherical parameterization (Styner et al., 2006). The correspondence is determined by aligning the principal curves of first order ellipsoid representation with the standard coordinate frame, so that the north pole of the first order ellipsoid aligns with the positive z axis, and its 0 meridian aligns with the x–z plane. This description is then sampled into triangulated surfaces via an icosahedron subdivision of the spherical parameterization. Hippocampal surfaces are well represented (local representation error is smaller than 0.1 mm on average) by a subdivision of level 10 resulting in 1002 surface points. Alignment of triangulated surfaces was finally performed using rigid body, Generalized Procrustes alignment which iteratively aligns the surfaces to the population mean.
5 Statistical Shape Analysis
After computation of the surface triangulation with SPHARM-PDM correspondence, local hippocampal difference vector maps were computed between the hippocampal surface models of segmentations from the original and mirrored images (see Figure 4). By projecting the local difference vectors to their normal surface, the vectors were converted to scalar signed difference maps (Styner et al., 2005).
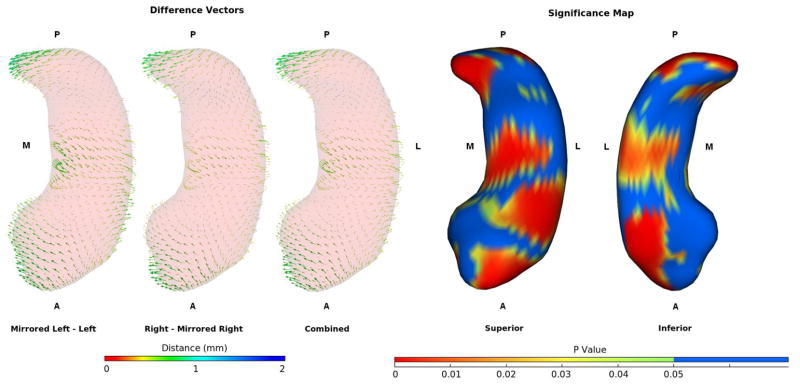
Figure 4.
Difference vectors between hippocampi segmented in mirrored and original images and the significance map for the combined data. Data is averaged across all subjects and raters. Vectors are lengthened by a factor of 2 for display clarity. The right and mirrored left segmentations are shifted medially at the head and tail while the bodies are shifted laterally, giving them a more curved appearance than the corresponding segmentations performed on the left side of the image.
Repeated measures ANOVA (R statistical package) was employed to calculate the statistical significance of the local signed difference at each location independently resulting in raw significance maps (Figure 4). All surfaces, i.e. from all subjects and all raters were included. RaterID, subjectID, and hemispheric side were modeled as covariates.
Results
As mentioned before, all results are discussed in their original assignment, independent of presentation.
Manual Segmentation Bias
The volumes of all the structures segmented fully manually (amygdala, globus pallidus, and putamen) showed different levels of evidence for the presence of a left-right asymmetric bias (Table 1). The putamen in particular showed significant leftward asymmetry (p < 0.05) in the original image segmentations and significant rightward asymmetry (p < 0.01) in the mirrored image segmentations. The globus pallidus showed highly significant rightward asymmetry (p < 0.001) in the mirrored image segmentations and insignificant leftward asymmetry in the original image segmentations. The asymmetry values for the amygdala and putamen showed insignificant trends (t-test p = 0.12 and p= 0.13) and the globus pallidus showed no difference in asymmetry values (p > 0.5). Figure 2 displays the original and mirrored segmentation volume asymmetries for each structure and for each subject. There are large discrepancies between original and mirrored asymmetries for the fully manually segmented structures, though the variability is high. The original versus mirrored differences within each hemisphere (Figure 3) provides further evidence of asymmetric bias and the student t-tests establish significance for the existence of bias. While the asymmetric bias in the amygdala was not significant (p = 0.21) the putamen and globus pallidus both displayed a highly significant (p < 0.01) asymmetric bias.
Table 1.
Average volumetric asymmetry for structural segmentations performed on mirrored and un-mirrored brain images and the asymmetry when the two are combined.
| Structure | Standard asymmetry | Radiologic (mirrored) asymmetry | Combined asymmetry |
|---|---|---|---|
| Amygdala | 3.44% | 0.18% | 1.82% |
| Caudate | 1.17% | −1.17% | 0.00% |
| Lateral Ventricles | −3.36% | 3.84% | 0.22% |
| Putamen | *−3.14% | **3.77% | 0.39% |
| Pallidus | −1.59% | ***7.96% | 2.99% |
| Hippocampus | **12.4% | **−8.30% | 1.97% |
Paired T-tests show significance for the asymmetry in the putamen, pallidus, and hippocampus when segmented in the radiologic view and significance in the opposite direction for the putamen and hippocampus in the standard view. No structures showed significant asymmetry when segmentations from the two views were combined. Only the Amygdala showed asymmetry in the same direction for both views.
p < 0.05,
p < 0.01,
p < 0.001
Semi-automatic Segmentation Bias
The segmentation variability for the more automated methods was far lower than for the manual segmentations.
Hippocampus segmentation
The volume bias in the landmark initialized hippocampus segmentation was the strongest for any structure, with nearly inverted asymmetry when presenting the images in a mirrored fashion (Figure 2). The paired t-test for the asymmetry values for the hippocampus was p = 0.00005 showing a prominent asymmetric bias. We did not perform the additional single structure difference test for the hippocampus because of the obvious bias.
We tested whether the template deformation methodology might be introducing the asymmetric bias into the segmentations. When the landmarks from the original dataset were computationally mirrored along with the images, rather than replaced by the rater, the segmentations were nearly identical with no detectable bias. We also computed the segmentations using a separate template with highly similar results with respect to the asymmetry measurements. These experiments suggest that there is no net asymmetric bias due to the particular template or the presentation of the image to the segmentation tool. Thus we confirmed that the user interaction is responsible for the asymmetric bias and not the deformable template methodology.
Caudate segmentation
The original versus mirrored difference within each hemisphere (Figure 3) reveals a highly significant asymmetric bias in the caudate segmentations (p = 0.0001), whereas the asymmetry analysis for the caudate was not found to be significant (p = 0.4).
Lateral ventricle segmentation
The lateral ventricle segmentations did not appear to be affected by any asymmetric bias. The charts in Figures 1 and 2 show that there is little difference between the original and the mirrored ventricle volumes. Further, neither the asymmetry value test nor the single structure test was significant (p > 0.9 for both).
Shape analysis results
Shape analysis of the hippocampus reveals that when segmented on the right hemisphere (right and mirrored left) the head and tail protrude further medially than when segmented on the left hemisphere (left and mirrored right). The bodies of the hippocampi segmented on the right hemisphere of the image are shifted laterally however, giving them an overall more curved appearance than those segmented on the left (Figure 4). Figure 4 also shows the consistency of the bias for hippocampi originating in each hemisphere. The two hemispheric models were combined for further analysis, though “hemisphere” was still used as a covariate in our repeated measures ANOVA. The raw p-value map (Figure 4) generated from a repeated measures ANOVA (rater, subject, and hemisphere were covariates) shows that the bias is highly significant in the areas where the bias is greatest as well as some areas where the bias appears small in magnitude. The bias in the tail is both strong and consistent and thus our p-value map indicates that almost every point on the tail is highly significant (p < 0.01). The bias in the body is not as high in magnitude but is very consistent across subjects and raters, and thus much of the bias there is significant even where its magnitude appears to be quite small. While the bias in the hippocampal head is quite high in magnitude, the bias is also extremely variable there (as can be seen in Figure 4). Thus the bias in the medial portion of the head does not reach significance.
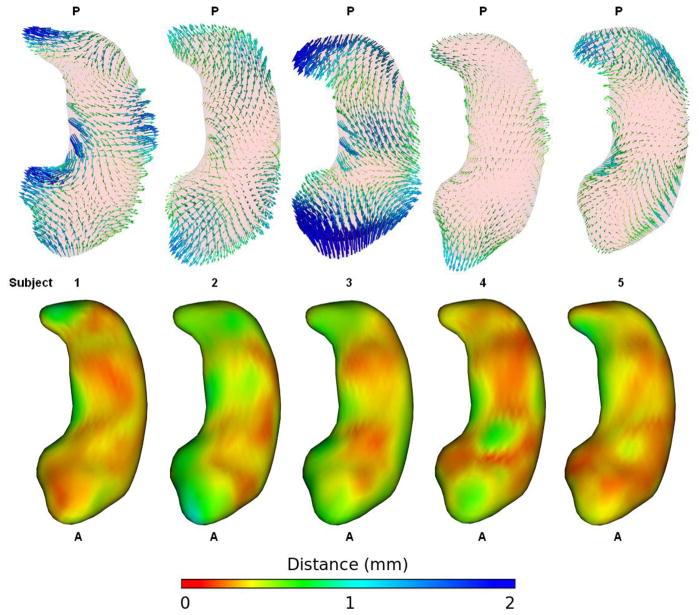
Figure 5 displays the bias vectors for each subject in order to assess consistency across subjects. Right and left structures are combined and the vectors are averaged across all 5 raters. The bias in each subject is quite large and generally follows the pattern of appearing more curved when segmented on the right side. However, there is a lot of variability evident between the subjects. Subjects 1 through 3 are adults and generally show a stronger bias than subjects 4 and 5, which come from the pediatric study. Subject 3 in particular stands out as having exceptionally strong bias. When segmented on the right side of the image the tail of Subject 3 is shifted medially and toward the anterior and the head is shifted very far posteriorly reducing the length of the structure. The body of subject 3 is also shifted laterally when it is segmented on the right, giving it a much more curved appearance. Pathology does not appear to account for the exaggerated bias in subject 3 as those segmentations were performed on a scan of a healthy adult while the segmentations for subject 1 (a scan of an adult with schizophrenia) and subject 4 (a scan of a child with autism) show patterns of bias much more consistent with the overall combined bias (presented in Figure 4).
Figure 5.
Difference vectors and standard deviation maps for each subject. Right and left hippocampi are combined and vectors are averaged across all raters. The vectors are lengthened by a factor of 2. These vectors are directly comparable to those in figures 3 and 6 sharing the same scale and color map. Subjects 1 through 3 are adults (subject 1 has schizophrenia), while subjects 4 and 5 are from the pediatric study (subject 4 has autism). The bias is very large among the adult subjects and all structures segmented on the right side of the image appear more curved. However, there is large variability between subjects in the direction of the difference vectors.
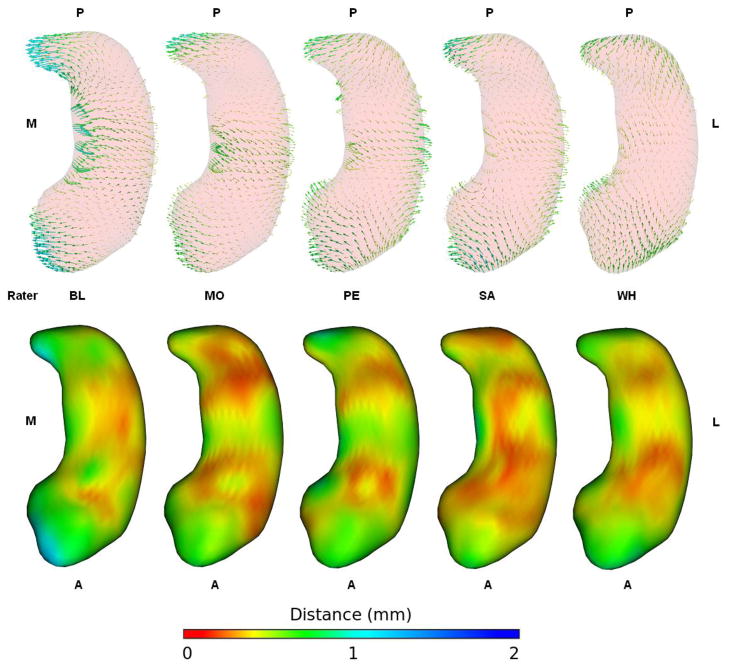
Figure 6 shows the bias vectors for each rater. Right and left structures are combined and the vectors are averaged across all 5 subjects. With the exception of Rater WH each rater’s vector map closely resembles the fully combined vector map in Figure 4. Rater BL stands out as a little more biased than the others but follows the same pattern as the others with segmentations done on the right side of the image having the head and tail shifted medially and the body shifted laterally resulting in a more curved appearance. Rater WH is the only one that deviates from this pattern, shifting the tail more anteriorly than medially and the head more posteriorly than medially, reducing the length of the structure but not making it more curved when segmenting on the right. Rater WH also managed to segment the body with very little bias and generally appears to have been a little less biased than the other raters when averaged across subjects. The volume asymmetry biases produced by Rater WH’s individual segmentations were still quite large (as shown in Figure 2).
Figure 6.
Difference vectors and standard deviation maps for each rater. Right and left hippocampi are combined and vectors are averaged across all subjects. The vectors are lengthened by a factor of 2. These vectors share the same color map and scale as those in figures 3 and 5. The bias appears to be very consistent between raters, with all raters except WH shifting the head and tail medially and the body laterally such that the structures appear more curved when segmented on the right side of the image.
Discussion
The main purpose of this study is to investigate a potential asymmetric bias in the user guided segmentation of sub-cortical brain structures using manual or semi-automated segmentation techniques. The only structural segmentation we found unaffected by such an asymmetric bias was the highly automated lateral ventricle segmentation. This is probably due in large part to the excellent contrast at the ventricle boundary eliminating any shape ambiguities and allowing for a very precise segmentation. The semi-automatic caudate segmentations and the fully manual segmentations of the amygdala, globus pallidus, and putamen provided evidence of left-right asymmetric volume bias. Unlike globus pallidus and putamen, we did not find significant evidence in the amygdala likely due to the large variability in our measurements.
The bias in our landmark based semi-automatic hippocampus segmentations was extremely strong. The asymmetry values were clearly different (p ≪ 0.01) and nearly inverted if presented in mirrored fashion. The resulting volume biases (as high as 11% in the hippocampus) are large enough to strongly influence most neuroimaging studies, which typically consider differences of 1 or 2% to be important. Shape analysis of the hippocampus revealed the bias to be greatest on the medial side of the head and tail and the lateral side of the body. This shape bias consistently approached 1 mm in many locations, enough to affect any clinical shape study. Repeated measures ANOVA revealed the bias located on the tail and body of the hippocampus to be highly significant. There was a lot of variability in the bias located on the medial portion of the head, preventing that area from reaching significance.
Laterality in visual perception is likely the main reason behind the asymmetric bias. While the presence of such an asymmetric bias may come as a surprise to some in the neuroimaging community, it is of “no great surprise to the perception community. Although I have not heard specifically about such a result, it seems immediately quite reasonable.” (Donald H. Mershon, NCSU). Keeping this in mind, it becomes essential to consider the factors, which may affect visual perception. Both hemispheric specialization (Neimeier et al., 2007) and cultural preferences have been hypothesized to be the origin of perceptual bias (Nachson et al., 1999; Chokron and Agostini, 2000; Health et al., 2005; Christian et al., 2007). Visual aesthetic preference has been shown to be affected by handedness, sex and age as well as related to directional scanning and reading/writing habits (De Agostini et al., 2010). An interesting finding demonstrates that directional preference is not limited to the visual modality but responds similarly to the auditory modality (Ouellet et al., 2010). Further, the direction of reading accounts for the preferred directionality of the mental time line as well (Ouellet et al., 2010). All of our raters were right handed and except for a single of the hippocampus raters (SA) all had a left to right primary reading direction. Interestingly, the bias in rater SA’s segmentations was quite similar to that of the other raters. The asymmetric bias seems to be thus independent of primary reading direction of the rater, though more study would be necessary to conclude this convincingly.
The role of our study is also to raise awareness in the neuroimaging community regarding the significance of the asymmetric bias. With its increasing recognition it has become essential to re-visit the results of the past research studies in the light of this new finding. The good news is that findings of neuroimaging studies analyzing group differences only of brain structures should not be affected in a major way by the asymmetric bias provided the groups do not significantly differ in shape or contrast. However, absolute asymmetry numbers must be questioned strongly. Meta-analysis of hippocampus asymmetry in normal controls (Pedraza et al., 2004) shows very large variability in asymmetry values between studies. Our findings suggest that studies which found a very large right greater than left hippocampus asymmetry were likely biased, while studies reporting a significant left greater than right asymmetry may have been segmented in a right-left mirrored radiologic view. Thus asymmetric bias may explain why there is so much variability in asymmetry measures of normal controls. A recent study (Li et al., 2007) investigating the characteristics of hippocampal volumes in healthy Chinese found hippocampi to be consistently asymmetrical with the right-side hippocampus being 3.2 – 6.8% larger than the left. McHugh et al (2007) calculated a comprehensive volumetric assessment of hippocampus on healthy older adults and reported 5.7 % larger right sided volume than left. Another study (Jeukens et al., 2009) carried out on two groups of 36 patients and 20 normal healthy controls performed a hippocampal volumetric analysis on their MR images. It reported the hippocampal volumes on the right side as larger compared to the left side in both the groups (6.9% in patients and 3.7% in controls). Some of the asymmetry in these studies may be due to rater bias.
As long as there is user-interaction involved there is likely to be some degree of left-right bias in segmentations. There are several image analysis techniques which can remove asymmetric bias from future research studies. (1) Since the direction of segmentation affects the final outcome, adopting a medial-to-lateral segmentation direction for both sides on sagittal slices only should remove the bias. However, this would not result in truly valid three dimensional segmentations due to the use of a single slice view; (2) Opting to do all segmentations on only one side, for instance doing all right side segmentations on an original image and all left side segmentations on a mirrored image, would also remove the bias; (3) Segmenting each structure on both the original and mirrored presentation and averaging the results would require twice as many segmentations but would eliminate rater asymmetric bias.
As a note, while free from bias caused by the rater, fully automated methods are not necessarily immune to asymmetric bias. Fennema-Notestine et al (2007) noticed a right greater than left volume asymmetric bias in their fully automatic atlas based FreeSurfer hippocampus segmentations. This was probably due to the use of an asymmetric atlas since fully automatic segmentations using a symmetric atlas should be free of asymmetric bias.
Supplementary Material
Highlights.
Expert raters segmented sub-cortical structures on mirrored and original MRI images
We compare left-right asymmetry for mirrored and original structures
We find significant asymmetric bias for manual and semi-automatic segmentations
We perform shape analysis tooutline areas with most significant bias
We propose potential sources and remedies for such a lateralized asymmetric bias
Acknowledgments
This research has been/is supported by the following grants: UNC Intellectual and Developmental Disabilities Research Center (IDDRC) HD 03110, the NIH Conte Center MH064065, and NIH RO1 MH61696 and NIMH MH64580. This work is also part of the National Alliance for Medical Image Computing (NAMIC), funded by the NIH through the NIH Roadmap for Medical Research, Grant U54 EB005149. The hippocampus data is from a study funded by the Stanley Foundation and UNC-MHNCRC (MH33127). We would like to thank Donald Mershon (NC State University) for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brechbuhler C, Gerig G, Kubler O. Parametrization of closed surfaces for 3-D shape description. Computer Vision, Graphics and Image Processing. 1995;61(1):154–170. [Google Scholar]
- Buchsbaum M, Yang S, Hazlett E, Siegel BV, Jr, Germans M, Haznedar M, O'Flaithbheartaigh S, Wei T, Silverman J, Siever LJ. Ventricular volume and asymmetry in schizotypal personality disorder and schizophrenia assessed with magnetic resonance imaging. Schizophrenia Research. 1997;27(1):45–53. doi: 10.1016/S0920-9964(97)00087-X. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Sachdev P, Anstey KJ. Mild Cognitive Disorders are Associated with Different Patterns of Brain asymmetry than Normal Aging: The PATH through Life Study. Front Psychiatry. 2010;11:1–11. doi: 10.3389/fpsyt.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Baron JC. Early diagnosis of alzheimer’s disease: contribution of structural neuroimaging. NeuroImage. 2002;18(2):525–541. doi: 10.1016/s1053-8119(02)00026-5. [DOI] [PubMed] [Google Scholar]
- Chokron, De Agostini M. Reading habits influence aesthetic preference. Cognitive Brain Research, Elsevier; 2000. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci USA. 1998 Sep;95(19):11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Agostini M, Kazandjian S, Cavezian C, Lellouch J, Chokron S. Visual aesthetic preference: Effects of handedness, sex, and age-related reading/writing directional scanning experience. Writing Systems Research; 2010. [Google Scholar]
- Dobel C, Diesendruck G, Bolte J. How writing system and age influence spatial representations of actions: a developmental, cross-linguistic study. Psychological Science. 2007;18:487–491. doi: 10.1111/j.1467-9280.2007.01926.x. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Buckner R, Killiany R, Blacker D, Dale AM. Feasibility of Multi-site Clinical Structural Neuroimaging Studies of Aging Using Legacy Data. Neuroinform. 2007;5(4):235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Gerig G, Styner M, Shenton ME, Lieberman JA. Shape versus Size: Improved understanding of the Morphology of Brain Structures. MICCAI; 2001. [Google Scholar]
- Geroldi C, Laasko MP, DeCarli C, Beltamello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. Apolipoprotein E genotype and hippocampal asymmetry in Alzheimer's disease: a volumetric MRI study. J Neurol Neurosurg Psychiatry. 2000;68:93–96. doi: 10.1136/jnnp.68.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Meints K, Hall C, Hall S, Mills D. Left gaze bias in humans, rhesus monkeys and domestic dogs. Animal Cognition. 2008;12:409–418. doi: 10.1007/s10071-008-0199-3. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011 May;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health RL, Mahmasanni O, Rouhana A, Nassif N. Comparison of aesthetic preferences among Roman and Arabic script readers. Psychology Press; 2005. [DOI] [PubMed] [Google Scholar]
- Jeukens CR, Vlooswijk MC, Majoie HJ, de Krom MC, Aldenkamp AP, Hofman PA, Jansen JF, Backes WH. Hippocampal MRI volumetry at 3 Tesla: reliability and practical guidance. Invest Radiol. 2009;44(9):509–17. doi: 10.1097/RLI.0b013e3181b4c180. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38(1):93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Keshevan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophrenia Research. 2002;58(2–3):173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Li YJ, Ga SN, Huo Y, Li SY, Gao XG. Characteristics of hippocampal volumes in healthy Chinese from MRI. Neurol Res. 2007;29(8):803–806. doi: 10.1179/016164107X223557. [DOI] [PubMed] [Google Scholar]
- Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, Botes L, Svensson L, Wahlund LO. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR Am J Neuroradiol. 2008;29(8):1537–1543. doi: 10.3174/ajnr.A1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi JC, Svensson L, Lindberg O, Zandbelt BB, Ostberg P, Orndahl E, Wahlund LO. Putaminal volume in frontotemporal lobar degeneration and Alzheimer disease: differential volumes in dementia subtypes and controls. AJNR Am J Neuroradiol. 2009;30(8):1552–1560. doi: 10.3174/ajnr.A1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TL, Saykin AJ, Wishart HA, Flashman LA, Cleavinger HB, Rabin LA, Mamourian AC, Shen L. Hippocampal volume and shape analysis in an older adult population. Clin Neuropsychol. 2007;21(1):130–145. doi: 10.1080/13854040601064534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeier M, Stojanoski B, Greco AL. Influences of time and spatial frequency on the perceptual bias: Evidence for competition between hemispheres. Neuropsychologia. 2007;45(5):1029–1040. doi: 10.1016/j.neuropsychologia.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Nachson I, Argaman E, Luria A. Effects of directional habits and handedness on aesthetic preference for left and right profiles. Journal of Cross-Cultural Psychology. 1999;30(1):106–114. [Google Scholar]
- Ouellet M, Santiago J, Israeli Z, Gabay S. Is the Future the Right Time? Experimental Psychology. 2010;57(4):308–314. doi: 10.1027/1618-3169/a000036. [DOI] [PubMed] [Google Scholar]
- Pavic L, Rudolf G, Rados M, Brklijacic B, Brajkovic L, Simentin-Pavic I, Ivanac G, Pavlisa G, Kalousek V. Smaller right hippocampus in war veterans with posttraumatic stress disorder. Psychiatry Research: Neuroimaging. 2007;154:191–198. doi: 10.1016/j.pscychresns.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. Journal of the International Neuropsychological Society. 2004;10(5):664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Med Image Anal. 2005 Oct;9(5):457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Puri BK, Richardson AJ, Oatridge A, Hajnal JV, Saeed N. Cerebral ventricular asymmetry in schizophrenia: a high resolution 3d magnetic resonance imaging study. International journal of psychophysiology. 1999;34:207–211. doi: 10.1016/s0167-8760(99)00078-1. [DOI] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;(19):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G. Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proc Natl Acad Sci USA. 2005;102(13):4872–4877. doi: 10.1073/pnas.0501117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Oguz I, Xu1 S, Brechbuhler C, Pantazis D, Levitt J, Shenton M, Gerig G. Framework for the statistical shape analysis of brain structures using spharm-pdm. Insight Journal. 2006 [PMC free article] [PubMed] [Google Scholar]
- Styner M, Smith RG, Graves MM, Mosconi MW, Peterson S, White S, Blocher J, El-Sayed M, Hazlett HC. Asymmetric bias in user guided segmentations of brain structures. SPIE Medical Imaging 2007: Image Processing; 2007. p. 65120K-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, Egan GF, Inder TE. Neonate Hippocampal Volumes: Prematurity, Perinatal Predictors, and 2-Year Outcome. Ann Neurol. 2008;63:642–651. doi: 10.1002/ana.21367. [DOI] [PubMed] [Google Scholar]
- Wang L, Joshi SC, Miller MI, Csernansky JG. Statistical analysis of hippocampal asymmetry in schizophrenia. NeuroImage. 2001;14(3):531–545. doi: 10.1006/nimg.2001.0830. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Amygdala Volume in Adults with Posttraumatic Stress Disorder: A Meta-Analysis. The Journal of Neuropsychiatry and Clinical Neurosciences. 2008;21:5–12. doi: 10.1176/jnp.2009.21.1.5. [DOI] [PubMed] [Google Scholar]
- Yushkevich P, Piven J, Hazlett H, Smith R, Ho S, Gee J. User-guided 3d active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;1(31):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.