Abstract
Despite advances in developing novel therapeutic strategies, a major factor underlying cancer related death remains resistance to therapy. In addition to biochemical resistance, mediated by xenobiotic transporters or binding site mutations, resistance can be physiological; emerging as a consequence of the tumor’s physical microenvironment. This review focuses on extracellular acidosis, an end result of high glycolytic flux and poor vascular perfusion. Low extracellular pH, pHe, forms a physiological drug barrier described by an “ion trapping” phenomenon. We describe how the acid-outside plasmalemmal pH gradient negatively impacts drug efficacy of weak base chemotherapies but is better suited for weakly acidic therapeutics. We will also explore the physiologic changes tumor cells undergo in response to extracellular acidosis which contribute to drug resistance including reduced apoptotic potential, genetic alterations, and elevated activity of a multidrug transporter, p-glycoprotein, pGP. Since low pHe is a hallmark of solid tumors, therapeutic strategies designed to overcome or exploit this condition can be developed.
Keywords: Microenvironment, Acidosis, Ion Trapping, Drug resistance
Introduction
A major obstacle to overcome during the treatment of solid tumors is resistance to therapy 1, 2. One factor contributing to this problem is the physical tumor microenvironment (pO2 and pH) and its impact on therapeutic efficacy 3–5. Hypoxia (Figure 1) and high glycolytic activity are common characteristics of solid tumors leading to increased production and secretion of lactate and H+ to the extracellular space. The culmination of elevated glycolysis coupled with poor vascular perfusion is an acidic extracellular space 6–8. Non-invasive measurements have shown that pHe ranges from 6.5 to 6.9 while intracellular pH, pHi, remains neutral to alkaline 7, 9 creating an acid-outside pH gradient typically not observed in normal tissue 10.
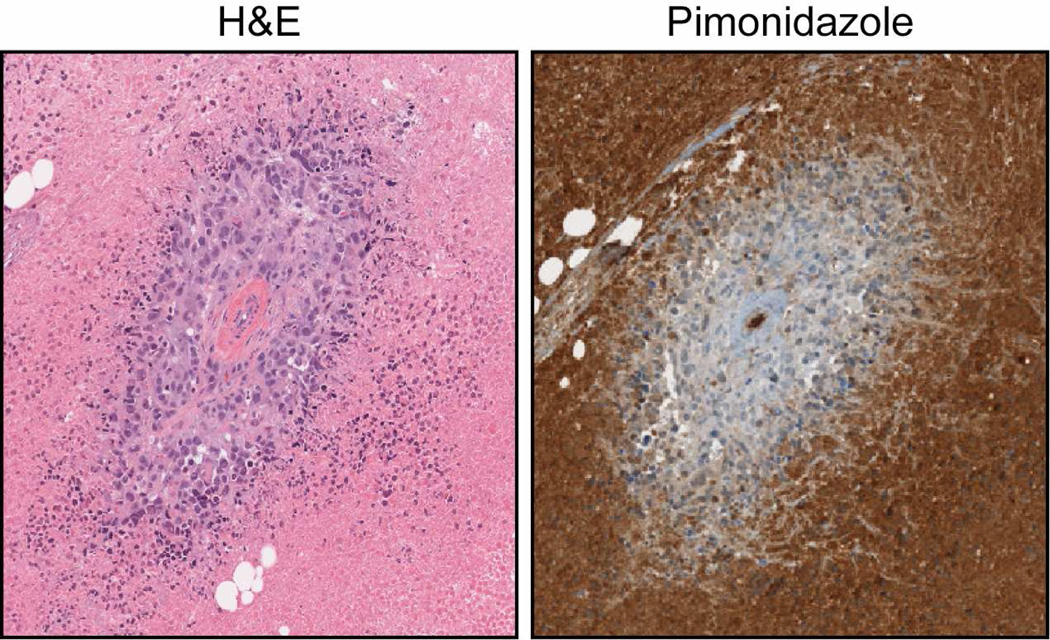
Figure 1. Tumor Microenvironment.
This is an immunohistochemical example of intratumoral diffusion limited hypoxia of MDA-MB-231 mammary fat pad tumors using pimonidazole to detect hypoxic tissue. Pimonidazole is a nitromidazole that binds to thiol groups at oxygen levels below 1%. The H&E stain identifies a vascular cross section surrounded by a population of well-oxygenated cells. Diffusion limited hypoxia (pimonidazole stain) surrounding patent vasculature is common in solid tumors where tumor growth extends beyond the oxygen diffusion limit (~200 µM). Due to significant changes in metabolism, hypoxic regions (pimonidazole positive) are most likely acidic generating an acid-outside pH gradient.
Tumor cells exposed to these harsh intratumoral physical conditions undergo many changes and it is becoming increasingly evident that acidosis plays an important role in the somatic evolution and progression of cancer from pre-invasive to malignant disease 6, 11–13. Early studies by Morita et al. described the clastogenic properties of low pHe on mammalian cell lines in-vitro 14–17. Other early studies by LeBoeuf observed that low pHe inhibits gap junctions, which are classified as tumor suppressors 18. These alterations may contribute to the observation that low pHe can promote the transformation of normal cells to a neoplastic phenotype 19. Additional studies show that a low extracellular pH increases the expression of vascular endothelial growth factor (VEGF), carbonic anhydrase, interlukin-8, cathepsin B, and matrix metalloproteinases- 2 and -9, all of which are associated with increased tumor cell survival, migration and invasion 20–23.
A low extracellular pH also contributes to drug resistance both in-vitro and in-vivo. The acid-outside pH gradient generated between intra- and extracellular space affects the distribution and uptake of select weak base chemotherapeutic drugs resulting in physiological drug resistance 24–27. Tumors cells adapted to low pHe in-vitro harbor p53 mutations and have elevated activity of p-glycoprotein both of which can contribute to drug resistance 28–30. In addition, chronically adapted low pHe cells are radio-insensitive in-vitro 31.
This review will focus on drug resistance and the extracellular acidic microenvironment. It will begin by discussing “ion trapping”, a phenomenon that describes how low pHe negatively impacts the uptake of weak base chemotherapeutics followed by the use of strategies to alkalinize tumor pH in order to increase therapeutic efficacy. We will conclude this review with a section on cellular adaptation and responses to acidosis that may contribute to drug resistance.
Low pH and physiological drug resistance
The cell membrane functions as a semi-permeable structure between the intra- and extracellular microenvironment. Small-uncharged molecules readily diffuse across the phospholipid portions of membranes while charged species tend to remain impermeable. Because of this characteristic, the acidic extracellular space of solid tumors creates a physiological barrier for the cellular uptake of weak bases 3. This phenomenon is termed “ion trapping” (Figure 2). Ion trapping occurs when there is a large permeability difference between ionized (impermeant) and non-ionized (permeant) species of a drug. On each side of the membrane, an equilibrium between ionized and non-ionized forms of the drug are established according to a Henderson-Hasselbach relationship. For a weak base, the ratio of ionized BH+ to non-ionized B is 10−(pH-pK). Thus, if the pKa is 8.3, the ratio will be ~10:1 at pH 7.3 (typical for pHi) and ~100:1 at a pH of 6.3 (lower range of pHe). As the non-ionized form of the drug equi-distributes on both sides of the membrane, more drug is sequestered in the lower pH of the extracellular environment, reducing therapeutic efficacy 32.
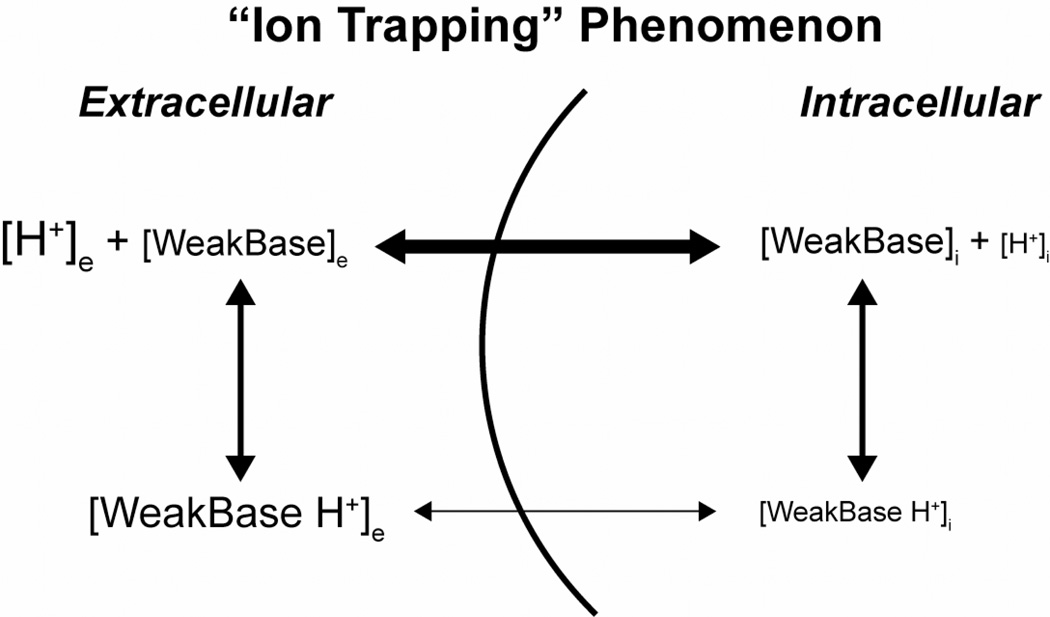
Figure 2. “Ion Trapping” phenomenon.
This example assumes the extracellular H+ concentration is greater than the intracellular H+ concentration (i.e. pHe < pHi). Uncharged ionizable weak bases such as doxorubicin freely permeate membranes [WeakBase]. However, in acidic solutions, weak bases are ionized becoming positively charged protonated species [WeakBase H+] reducing cell permeability. Therefore, positively charged weak bases become trapped in extracellular compartments reducing cellular uptake and efficacy. Weak acids tend to concentrate in more alkaline environments such as intracellular compartments. (Adapted from Raghunand and Gillies, Drug. Resist Update 3)
Most chemotherapeutic drugs have ionizable species under physiological conditions that may enhance or hinder their ability to cross membranes. Uptake and efficacy of weak base chemotherapeutics with a dissociation constant of 7.5–9.5 such as anthracyclines, anthraquinones, and vinca alkaloids are reduced by the acid-outside pH gradient of solid tumors, as shown by in-vitro and in-vivo studies 10, 24–27, 33.
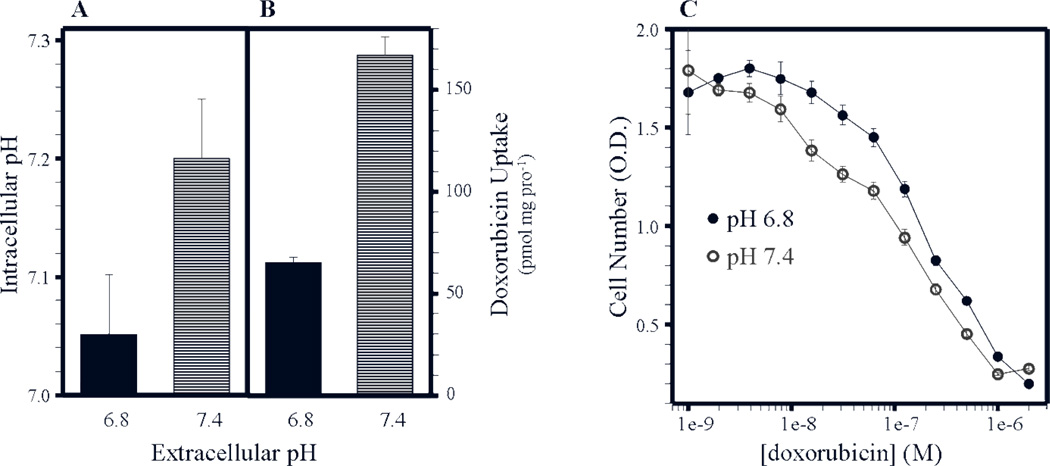
Figure 3A illustrates in-vitro plasmalemmal pH gradients in MCF-7 cells as a function of the extracellular pH. MCF-7 cells cultured at a pHe of 6.8 and 7.4 had a pHi of 7.05 and 7.2 respectively generating both acid-outside and alkaline-outside plasmalemmal pH gradients. Doxorubicin is an anthracycline consisting of an ionizable primary amine with a basic pKa of 8.3. Doxorubicin has been previously shown to undergo ion trapping3 in acid conditions and is a substrate for p-glycoprotein, a drug exporter with enhanced activity in acidic environments34. Intracellular accumulation of 14C-labeled doxorubicin was greater in MCF-7 cells cultured at a pHe of 7.4 (~168 pmol/mg/protein−1) than that of cells cultured at a pHe 6.8 (65 pmol/mg/protein−1) increasing in-vitro toxicity (Figure 3B and 3C). Table 1 is a list of additional weak base and weak acid chemotherapeutics and their respective pKas plus their LD50 against MCF-7 cells cultured at a pHe of 6.8 or 7.4 25.
Figure 3. Increased doxorubicin uptake and efficacy under alkaline conditions.
(A) Intracellular pH measurements and (B) doxorubicin uptake were determined as a function of extracellular medium pH in MCF-7 cells. (C) The effect of doxorubicin toxicity on MCF-7 cells in-vitro as a function of extracellular medium pH. (Adapted from Mahoney et al., Biochemical Pharmacology 25)
Table 1.
Summary of weak base and weak acid chemotherapeutic pKa values 25 and LD50 against MCF-7 cells cultured at a pHe of 6.8 and 7.4 25, 26.
| pKa | LD50 pHe 6.8 | LD50 pHe 7.4 | |
|---|---|---|---|
| Weak Bases | |||
| Doxorubicin | 8.30 | 312 ± 29 (nM) | 176 ± 33 (nM) |
| Daunorubicin | 8.30 | 384 ± 61 (nM) | 158 ± 37 (nM) |
| Mitoxantrone | 7.6 – 8.2 | 703 ± 62 (nM) | 262 ± 46 (nM) |
| Weak Acids | |||
| Chlorambucil | 5.8 | 14.3 ± 3 (µM) | 22 ± 4 (µM) |
| 5-Fluorouracil | 7.6 | 29 ± 13 (µM) | 27 ± 8 (µM) |
Conversely, if weak bases are protonated and trapped extracellularly in acidic environments, then uptake of weak acidic chemotherapeutics such as chlorambucil should be enhanced under similar acid-outside pH conditions. Chlorambucil, with a dissociation constant of 5.78, readily crosses the plasma membrane of cells cultured at a low pHe. In-vivo experimental acidosis following a bolus injection of glucose resulted in a 2.3 fold increase in the efficacy of chlorambucil compared to weak base doxorubicin 24. Intratumoral alkalization with sodium bicarbonate (NaHCO3) greatly reduced chlorambucil efficacy both in in-vitro and in-vivo studies (to be discussed in the next section). Friberg and Moan showed similar effects with the photosensitizing agent Hematoporphyrin IX (HpIX). Uptake of HpIX was increased in T-47D cells cultured under acidic conditions compared to neutral conditions 35 implying that the “ion trapping” phenomenon must be taken into consideration while designing and implementing all therapeutic strategies in addition to chemotherapy.
Melphalan is a weak acid chemotherapeutic compound with pKa values of 1.83 and 9.13 at pH 7.4 36 and is approved clinically for treatment of multiple myeloma and ovarian cancer 37. Conforming with the “ion trapping” hypothesis, increased cellular uptake of melphalan is observed in cells cultured at low pHe 38, 39 and the anti-tumoral effect of melphalan is enhanced by low pHe across many tumor xenograft models 40–42. Melphalan is one such compound that may benefit from a therapeutic approach that takes the “ion trapping” hypothesis into consideration. Melphalan is used in isolated limb perfusion and infusion models both pre-clincally and clinically for the treatment of melanoma 43, 44. Isolation of the limb temporarily halts blood circulation to the extremity resulting in local hypoxia and acidosis. Delivery of melphalan directly into the isolated limbs dramatically increases the compounds efficacy prolonging patient survival and reducing the number of limb amputees 45–51. These results suggest that inclusion of “ion trapping” in further studies may prove to be a viable therapeutic strategy.
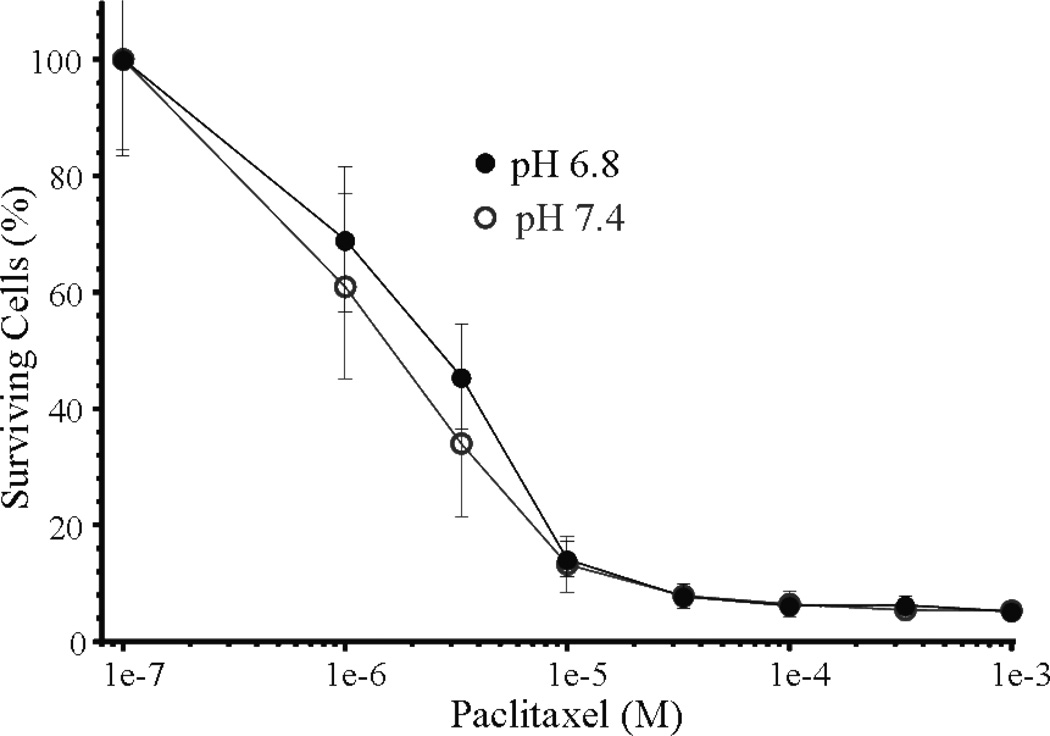
Paclitaxel is commonly used in the clinic to treat early stage breast cancer and has been used in-vitro to induce cell death in MCF-7 cells 52, 53. Paclitaxel is not ionizable and drug distribution should not be affected by extracellular pH. The effect of pH on paclitaxel efficacy determined in-vitro (Figure 4) showed no significant differences in toxicity in MCF-7 cells cultured at a pHe of 6.8 or 7.4 26. In addition, paclitaxel treatment in combination with sodium bicarbonate did not alter tumor growth rates suggesting the increased therapeutic benefit stemming from extracellular alkalinization by sodium bicarbonate may be drug selective. These results confirm that not all chemotherapeutics are ionizable under physiological conditions and are therefore not candidates for “ion trapping” 26.
Figure 4. Extracellular pH has no effect on paclitaxel cytotoxicity.
The effect of taxol cytotoxicity as a function of extracellular medium pH (Adapted from Raghunand et al. Br. J Cancer 26)
Experimental Alkalization of pHe
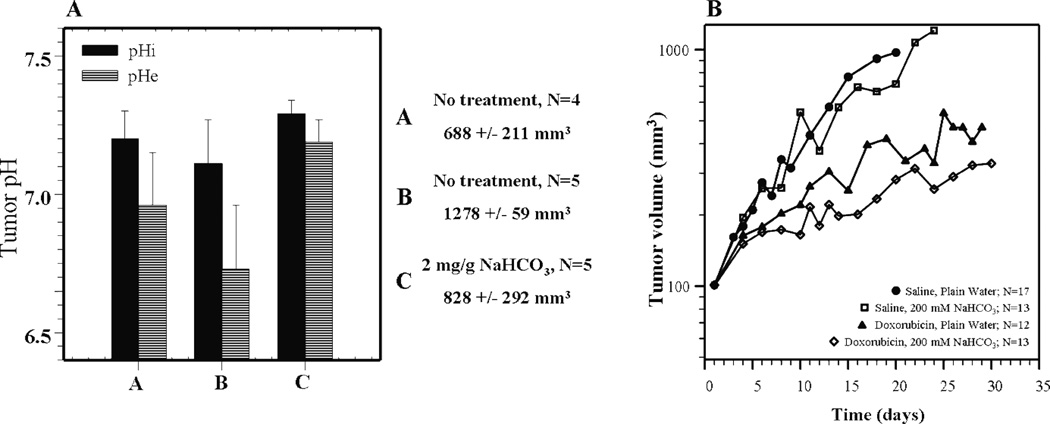
Experimental and mathematical models demonstrate that it is possible to raise extracellular pH of tumors using systemic buffers 54–57. An in-silico tumor model developed by Silva et al. determined that the buffer best suited to raise intratumoral pH should have a pKa of ~7.0 57. As stated by Silva, candidate buffers cholamine chloride (pKa, 7.1), BES (pKa, 7.15), TES (pKa, 7.5), and HEPES (pKa, 7.55) are available, but the effects of these buffers in-vivo need additional testing 58. Sodium bicarbonate is a physiological buffer with a pKa of 6.1 that regulates the pH in blood and tissue 59. Chronic administration of sodium bicarbonate increased the pHe of MCF-7 mammary fat pad tumors with little detectable effect on pHi (Figure 5A). These values were determined using 31P MR spectra to measure the chemical shift of exogenously added 3-APP (pHe) and endogenous inorganic phosphates (pHi). Notice that the pHe and pHi differed between two sets of control tumors grouped by size, but an acid-outside membrane gradient was present in both sets 33.
Figure 5. Sodium bicarbonate significantly increases extracellular pH of tumors in-vivo.
(A) Intracellular pH (pHi) and extracellular pH (pHe) measurements of untreated MCF-7 tumors (A and B) of varying sizes. Notice that pHe is acidic irrespective of tumor size. Administration of sodium bicarbonate significantly alkalinizes the extracellular pH. Tumor pHe and pHi were measured by 31P MRS. (C) In-vivo MCF-7 tumor volume measurements from mice either treated with 200 mM sodium bicarbonate (cyan), 2.0 mg/kg doxorubicin (red), or co-administration of 200 mM sodium bicarbonate and 2.0 mg/kg doxorubicin (purple). Administration of doxorubicin alone reduced tumor volume, but a greater reduction of tumor size was observed with co-administration of sodium bicarbonate. (Adapted from Raghunand et al. Br. J Cancer 33)
Although it affected the pHe, treatment with sodium bicarbonate alone had no effect on growth of primary tumors. However, combining sodium bicarbonate with doxorubicin reduced tumor volume and delayed growth compared to doxorubicin alone suggesting that alkalinization by sodium bicarbonate may enhance doxorubicin uptake (Figure 5B). These data support the in-vitro data of MCF-7 cells cultured at a pHe of 7.4 have increased doxorubicin uptake and sensitivity to treatment (Figure 3B and 3C). Even more striking results have been observed using mitoxantrone 60, 61, and a generalized model has been developed that uses the pH-dependent partition coefficients to predict the severity of ion trapping in drug distribution 25, 26.
Epirubicin, also a weak base with a pKa of 8.1 62, is an anthracycline that inhibits DNA and RNA synthesis. Epirubicin is used clinically to treat breast cancer and has been investigated as a treatment for superficial bladder cancer via intravesical delivery 63, 64. In-vitro studies show that epirubicin exhibits increased efficacy against human bladder cancer cells 65, 66 and chineese hamster ovary cells cultured under alkaline conditions 67. Clinically, issues may arise during intravesical delivery of epirubicin directly into the bladder since the patient urine may be acidic potentially decreasing cellular uptake of epirubicin. Buffering the pH of the bladder or alkalinizing the pH of epirubicin prior to delivery may have a beneficial impact on the therapeutic efficacy 65, 66, however, this has yet to be investigated.
Maintaining an alkaline intracellular environment is critical for cell survival. Cells maintain an intracellular alkaline environment by transporting intracellular H+ to the extracellular space via a number of mechanisms, including vacuolar-ATPase, Na+/H+ exchanger (NHE-1), carbonic anhydrases (CA-IX) and anion exchangers 68–72. Due to elevated glycolytic activity of tumor cells, dependence on these mechanisms for survival is critical. Vacuolar-ATPase located at the plasma membrane through membrane recycling has elevated expression and activity in metastatic tumors 73. Na+/H+ exchange expression correlates with hypoxic/necrotic regions of an in-vitro tumor spheroid 12. Carbonic anhydrases reversibly convert carbon dioxide and water to bicarbonate and a proton. Inhibition of CA-IX reduces tumor acidity and pH heterogeneity 74, 75. The end result is acidification of the extracellular space. Proton pump inhibitors (PPIs) are a selective class of vacuolar-ATPase inhibitors that are commonly used to treat patients with gastric disease 76. PPIs reduce the outward flux of H+ raising the pH of the extracellular environment 76. Some efficacy of PPIs has been observed in solid tumor models and in-vitro against melanoma cells. Luciani et al. utilized PPI omeprazole to reduce v-H+ -ATPase activity and to breakdown the acid-outside physiological barrier 77. The result was alkalization of both extracellular pH and intracellular vacuoles. They showed that pre-treatment with PPIs increased the uptake and efficacy of compounds that were under normal tumor conditions excluded from intracellular compartments 77.
Cellular adaptations to low pHe
The tumor physical microenvironment is composed of low oxygen tension and high acidity. These conditions lead exposed cells to physiological changes as well as to selective pressures. Physiological changes include changes in gene expression 78, apoptotic potential 31, autophagy 79, as well as drug resistance 3. Because acidity may cause p53-dependent apoptosis, selection of p53-mutant cells may occur 30. This loss of apoptotic potential and other adaptive changes are likely driven by microenvironment-induced genomic instability and inhibition of DNA repair 15, 80, 81.
Drug resistance is a major adaptive change in aggressive cancers and is a confounding factor during treatment. This may arise due to the chronic exposure to an acidic microenvironment. A major mechanism of drug resistance involves the activity or expression of the multidrug transporter, p-glycoprotein (pGP) 28, 29. pGP, encoded by the MDR1 gene, actively pumps cytotoxins, such as doxorubicin and paclitaxel, out of the cell 82. Although mRNA levels are not changed during acidosis, the activity of pGP is increased, and this effect is amplified by hypoxia 28. The localization of pGP is also crucial, and has been reported to change after induction of selective pressures 83. The changes in pGP activity during acidosis is accompanied with changes in intracellular pH, which may decrease the effectiveness of chemotherapeutics 84, 85, or the capacity of drugs to be pumped out of the cell 86.
Conclusions
We described mechanisms by which low pHe contributes to chemotherapy resistance. Since maintained acidification of the extracellular space is a hallmark of solid tumors, novel methods are needed to overcome low pHe drug resistance in order to improve therapeutic efficacy of current and future compounds. One approach is to alkalinize the microenvironment through the use of systemic buffers. While sodium bicarbonate successfully increased the efficacy of weak base chemotherapies in-vivo, a systemic buffer with a pKa of ~7.0 is predicted to be more effective. The opposite approach is to take advantage of low pHe through increased use and design of weak acid compounds. Many groups have developed low pH activated micelle systems that are designed to enter the core of solid tumors followed by the release of toxins within the acidic microenvironment, however additional in-vivo studies are required to determine their effectiveness 87. Although periods of hypoxia can be transient 88, 89, acidification of the extracellular microenvironment likely remains constant due to aerobic glycolysis. Because acidosis provides a modality for selection and for drug resistance, new techniques and pharmacological agents must be developed to adress tumor acidification.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for the financial support from the National Cancer Institute and the Physical Science of Oncology Center program (U54 CA143970).
ABBREVIATIONS
- pHi
intracellular pH
- pHe
extracellular pH
- VEGF
vascular endothelial growth factor
- pGP
p-glycoprotein
- NaHCO3
sodium bicarbonate
- 3-APP
3-aminopropyl phosphonate
- NHE1
Na+/H+ exchanger
- CA-IX
carbonic anhydrase 9
- PPI
proton pump inhibitor
Footnotes
SUPPORTING INFORMATION
Further information on tumor development and pimonidazole immunohistochemistry methods is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 2.Liu FS. Mechanisms of chemotherapeutic drug resistance in cancer therapy--a quick review. Taiwan J Obstet Gynecol. 2009;48(3):239–244. doi: 10.1016/S1028-4559(09)60296-5. [DOI] [PubMed] [Google Scholar]
- 3.Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3(1):39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- 4.Shekhar MP. Drug resistance: challenges to effective therapy. Curr Cancer Drug Targets. 2011;11(5):613–623. doi: 10.2174/156800911795655921. [DOI] [PubMed] [Google Scholar]
- 5.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 6.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8(1):56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 7.Hashim AI, Zhang X, Wojtkowiak JW, Martinez GV, Gillies RJ. Imaging pH and metastasis. NMR Biomed. 2011 doi: 10.1002/nbm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(23):6449–6465. [PubMed] [Google Scholar]
- 9.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64(3):425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56(6):1194–1198. [PubMed] [Google Scholar]
- 11.Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol. 2008;18(5):330–337. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97(5):646–653. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr. 2007;39(3):251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 14.Morita T. Low pH leads to sister-chromatid exchanges and chromosomal aberrations, and its clastogenicity is S-dependent. Mutat Res. 1995;334(3):301–308. doi: 10.1016/0165-1161(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 15.Morita T, Nagaki T, Fukuda I, Okumura K. Clastogenicity of low pH to various cultured mammalian cells. Mutat Res. 1992;268(2):297–305. doi: 10.1016/0027-5107(92)90235-t. [DOI] [PubMed] [Google Scholar]
- 16.Morita T, Watanabe Y, Takeda K, Okumura K. Effects of pH in the in vitro chromosomal aberration test. Mutat Res. 1989;225(1–2):55–60. doi: 10.1016/0165-7992(89)90033-x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56(24):5754–5757. [PubMed] [Google Scholar]
- 18.Ruch RJ, Klaunig JE, Kerckaert GA, LeBoeuf RA. Modification of gap junctional intercellular communication by changes in extracellular pH in Syrian hamster embryo cells. Carcinogenesis. 1990;11(6):909–913. doi: 10.1093/carcin/11.6.909. [DOI] [PubMed] [Google Scholar]
- 19.LeBoeuf RA, Lin PY, Kerckaert G, Gruenstein E. Intracellular acidification is associated with enhanced morphological transformation in Syrian hamster embryo cells. Cancer Res. 1992;52(1):144–148. [PubMed] [Google Scholar]
- 20.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54(24):6517–6525. [PubMed] [Google Scholar]
- 21.Shi Q, Le X, Wang B, Abbruzzese JL, Xiong Q, He Y, Xie K. Regulation of vascular endothelial growth factor expression by acidosis in human cancer cells. Oncogene. 2001;20(28):3751–3756. doi: 10.1038/sj.onc.1204500. [DOI] [PubMed] [Google Scholar]
- 22.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res. 2000;60(16):4610–4616. [PubMed] [Google Scholar]
- 24.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5(5):1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66(7):1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 26.Raghunand N, Mahoney BP, Gillies RJ. umor acidity, ion trapping and chemotherapeutics. II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. T. 2003;66(7):1219–1229. doi: 10.1016/s0006-2952(03)00468-4. [DOI] [PubMed] [Google Scholar]
- 27.Vukovic V, Tannock IF. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75(8):1167–1172. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007;17(1):239–244. [PubMed] [Google Scholar]
- 29.Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. mpact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8(2):143–152. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene. 1999;18(21):3199–3204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsubo T, Igawa H, Saito T, Matsumoto H, Park HJ, Song CW, Kano E, Saito H. Acidic environment modifies heat- or radiation-induced apoptosis in human maxillary cancer cells. Int J Radiat Oncol Biol Phys. 2001;49(5):1391–1398. doi: 10.1016/s0360-3016(00)01590-x. [DOI] [PubMed] [Google Scholar]
- 32.Roos A. Weak acids, weak bases and intracellular pH. Respir Physiol. 1978;33(1):27–30. doi: 10.1016/0034-5687(78)90080-4. [DOI] [PubMed] [Google Scholar]
- 33.Raghunand N, He X, van Sluis R, Mahoney B, Baggett B, Taylor CW, Paine-Murrieta G, Roe D, Bhujwalla ZM, Gillies RJ. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999;80(7):1005–1011. doi: 10.1038/sj.bjc.6690455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goda K, Balkay L, Marian T, Tron L, Aszalos A, Szabo G., Jr Intracellular pH does not affect drug extrusion by P-glycoprotein. J Photochem Photobiol B. 1996;34(2–3):177–182. doi: 10.1016/1011-1344(95)07282-9. [DOI] [PubMed] [Google Scholar]
- 35.Friberg EG, Cunderlikova B, Pettersen EO, Moan J. pH effects on the cellular uptake of four photosensitizing drugs evaluated for use in photodynamic therapy of cancer. Cancer Lett. 2003;195(1):73–80. doi: 10.1016/s0304-3835(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 36.Wu ZY, Smithers BM, Roberts MS. Tissue and perfusate pharmacokinetics of melphalan in isolated perfused rat hindlimb. J Pharmacol Exp Ther. 1997;282(3):1131–1138. [PubMed] [Google Scholar]
- 37.Falco P, Bringhen S, Avonto I, Gay F, Morabito F, Boccadoro M, Palumbo A. Melphalan and its role in the management of patients with multiple myeloma. Expert Rev Anticancer Ther. 2007;7(7):945–957. doi: 10.1586/14737140.7.7.945. [DOI] [PubMed] [Google Scholar]
- 38.Skarsgard LD, Skwarchuk MW, Vinczan A, Kristl J, Chaplin DJ. The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res. 1995;15(1):219–223. [PubMed] [Google Scholar]
- 39.Miller L, Deffie AM, Bose R, Goldenberg GJ. Modulation of melphalan uptake in murine L5178Y lymphoblasts in vitro by changes in ionic environment. Biochem Pharmacol. 1992;43(5):1154–1158. doi: 10.1016/0006-2952(92)90627-u. [DOI] [PubMed] [Google Scholar]
- 40.Siemann DW, Chapman M, Beikirch A. Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys. 1991;20(2):287–289. doi: 10.1016/0360-3016(91)90106-e. [DOI] [PubMed] [Google Scholar]
- 41.Wood PJ, Sansom JM, Newell K, Tannock IF, Stratford IJ. Reduction of tumour intracellular pH and enhancement of melphalan cytotoxicity by the ionophore Nigericin. Int J Cancer. 1995;60(2):264–268. doi: 10.1002/ijc.2910600222. [DOI] [PubMed] [Google Scholar]
- 42.Kuin A, Aalders M, Lamfers M, van Zuidam DJ, Essers M, Beijnen JH, Smets LA. Potentiation of anti-cancer drug activity at low intratumoral pH induced by the mitochondrial inhibitor m-iodobenzylguanidine (MIBG) and its analogue benzylguanidine (BG) Br J Cancer. 1999;79(5–6):793–801. doi: 10.1038/sj.bjc.6690127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kroon HM. Treatment of locally advanced melanoma by isolated limb infusion with cytotoxic drugs. J Skin Cancer. 2011:106573. doi: 10.1155/2011/106573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kroon HM, Moncrieff M, Kam PC, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15(11):3003–3013. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 45.Beasley GM, Ross MI, Tyler DS. Future directions in regional treatment strategies for melanoma and sarcoma. Int J Hyperthermia. 2008;24(3):301–309. doi: 10.1080/02656730701827573. [DOI] [PubMed] [Google Scholar]
- 46.Grunhagen DJ, de Wilt JH, van Geel AN, Eggermont AM. Isolated limb perfusion for melanoma patients--a review of its indications and the role of tumour necrosis factor-alpha. Eur J Surg Oncol. 2006;32(4):371–380. doi: 10.1016/j.ejso.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Hoekstra HJ. The European approach to in-transit melanoma lesions. Int J Hyperthermia. 2008;24(3):227–237. doi: 10.1080/02656730701816402. [DOI] [PubMed] [Google Scholar]
- 48.Kelley ST, Menon C, Buerk DG, Bauer TW, Fraker DL. Acidosis plus melphalan induces nitric oxide-mediated tumor regression in an isolated limb perfusion human melanoma xenograft model. Surgery. 2002;132(2):252–258. doi: 10.1067/msy.2002.125713. [DOI] [PubMed] [Google Scholar]
- 49.Lindner P, Doubrovsky A, Kam PC, Thompson JF. Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol. 2002;9(2):127–136. doi: 10.1007/BF02557363. [DOI] [PubMed] [Google Scholar]
- 50.Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L, Villegas-Portero R, Nieto-Garcia A. Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist. 15(4):416–427. doi: 10.1634/theoncologist.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noorda EM, Vrouenraets BC, Nieweg OE, van Geel AN, Eggermont AM, Kroon BB. Safety and efficacy of isolated limb perfusion in elderly melanoma patients. Ann Surg Oncol. 2002;9(10):968–974. doi: 10.1007/BF02574514. [DOI] [PubMed] [Google Scholar]
- 52.Morse DL, Gray H, Payne CM, Gillies RJ. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther. 2005;4(10):1495–1504. doi: 10.1158/1535-7163.MCT-05-0130. [DOI] [PubMed] [Google Scholar]
- 53.Saunders DE, Lawrence WD, Christensen C, Wappler NL, Ruan H, Deppe G. Paclitaxel-induced apoptosis in MCF-7 breast-cancer cells. Int J Cancer. 1997;70(2):214–220. doi: 10.1002/(sici)1097-0215(19970117)70:2<214::aid-ijc13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 54.Gillies RJ, Martinez-Zaguilan R. Regulation of intracellular pH in BALB/c 3T3 cells. Bicarbonate raises pH via NaHCO3/HCl exchange and attenuates the activation of Na+/H+ exchange by serum. J Biol Chem. 1991;266(3):1551–1566. [PubMed] [Google Scholar]
- 55.Martin NK, Gaffney EA, Gatenby RA, Gillies RJ, Robey IF, Maini PK. A mathematical model of tumour and blood pHe regulation: The HCO3-/CO2 buffering system. Math Biosci. 2011;230(1):1–11. doi: 10.1016/j.mbs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009;69(6):2260–2268. doi: 10.1158/0008-5472.CAN-07-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva AS, Yunes JA, Gillies RJ, Gatenby RA. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 2009;69(6):2677–2684. doi: 10.1158/0008-5472.CAN-08-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RM. Hydrogen ion buffers for biological research. Biochemistry. 1966;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 59.Putnam RW, Roos A. Which value for the first dissociation constant of carbonic acid should be used in biological work? Am J Physiol. 1991;260(5 Pt 1):C1113–C1116. doi: 10.1152/ajpcell.1991.260.5.C1113. [DOI] [PubMed] [Google Scholar]
- 60.Jahde E, Glusenkamp KH, Rajewsky MF. Protection of cultured malignant cells from mitoxantrone cytotoxicity by low extracellular pH: a possible mechanism for chemoresistance in vivo. Eur J Cancer. 1990;26(2):101–106. doi: 10.1016/0277-5379(90)90290-a. [DOI] [PubMed] [Google Scholar]
- 61.Raghunand N, Mahoney B, van Sluis R, Baggett B, Gillies RJ. Acute metabolic alkalosis enhances response of C3H mouse mammary tumors to the weak base mitoxantrone. Neoplasia. 2001;3(3):227–235. doi: 10.1038/sj.neo.7900151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li R, Huang J. Chromatographic behavior of epirubicin and its analogues on high-purity silica in hydrophilic interaction chromatography. J Chromatogr A. 2004;1041(1–2):163–169. doi: 10.1016/j.chroma.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 63.Onrust SV, Wiseman LR, Goa KL. Epirubicin: a review of its intravesical use in superficial bladder cancer. Drugs Aging. 1999;15(4):307–333. doi: 10.2165/00002512-199915040-00006. [DOI] [PubMed] [Google Scholar]
- 64.Bassi P, Spinadin R, Longo F, Saraeb S, Pappagallo GL, Zattoni F, Pagano F. Delayed high-dose intravesical epirubicin therapy of superficial bladder cancer. A way to reduce the side effects and increase the efficacy--a phase 2 trial. Urol Int. 2002;68(4):216–219. doi: 10.1159/000058438. [DOI] [PubMed] [Google Scholar]
- 65.Groos E, Walker L, Masters JR. Intravesical chemotherapy. Studies on the relationship between pH and cytotoxicity. Cancer. 1986;58(6):1199–1203. doi: 10.1002/1097-0142(19860915)58:6<1199::aid-cncr2820580604>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 66.Harris NM, Duffy PM, Crook TJ, Anderson WR, Sharpe P, Hayes MC, Cooper AJ, Solomon LZ. Intravesical pH: a potentially important variable affecting efficacy and the further development of anthracycline chemotherapy for superficial bladder cancer. BJU Int. 2002;90(9):957–964. doi: 10.1046/j.1464-410x.2002.02999.x. [DOI] [PubMed] [Google Scholar]
- 67.Kleeberger L, Rottinger EM. Effect of pH and moderate hyperthermia on doxorubicin, epirubicin and aclacinomycin A cytotoxicity for Chinese hamster ovary cells. Cancer Chemother Pharmacol. 1993;33(2):144–148. doi: 10.1007/BF00685332. [DOI] [PubMed] [Google Scholar]
- 68.Chiche J, Ilc K, Laferriere J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 69.Hinton A, Sennoune SR, Bond S, Fang M, Reuveni M, Sahagian GG, Jay D, Martinez-Zaguilan R, Forgac M. Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem. 2009;284(24):16400–16408. doi: 10.1074/jbc.M901201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin C, Pedersen SF, Schwab A, Stock C. Intracellular pH gradients in migrating cells. Am J Physiol Cell Physiol. 2011;300(3):C490–C495. doi: 10.1152/ajpcell.00280.2010. [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265(4 Pt 1):C1015–C1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 72.Miraglia E, Viarisio D, Riganti C, Costamagna C, Ghigo D, Bosia A. Na+/H+ exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int J Cancer. 2005;115(6):924–929. doi: 10.1002/ijc.20959. [DOI] [PubMed] [Google Scholar]
- 73.Sennoune SR, Bakunts K, Martinez GM, Chua-Tuan JL, Kebir Y, Attaya MN, Martinez-Zaguilan R. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol. 2004;286(6):C1443–C1452. doi: 10.1152/ajpcell.00407.2003. [DOI] [PubMed] [Google Scholar]
- 74.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29(50):6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 75.Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem. 2009;284(30):20299–20310. doi: 10.1074/jbc.M109.006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horn J. The proton-pump inhibitors: similarities and differences. Clin Ther. 2000;22(3):266–280. doi: 10.1016/S0149-2918(00)80032-6. discussion 265. [DOI] [PubMed] [Google Scholar]
- 77.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, Federici C, Iessi E, Parmiani G, Arancia G, Belardelli F, Fais S. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96(22):1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 78.Chen JL, Lucas JE, Schroeder T, Mori S, Wu J, Nevins J, Dewhirst M, West M, Chi JT. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 2008;4(12):e1000293. doi: 10.1371/journal.pgen.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delikatny EJ, Cooper WA, Brammah S, Sathasivam N, Rideout DC. Nuclear magnetic resonance-visible lipids induced by cationic lipophilic chemotherapeutic agents are accompanied by increased lipid droplet formation and damaged mitochondria. Cancer Res. 2002;62(5):1394–1400. [PubMed] [Google Scholar]
- 80.Papp-Szabo E, Josephy PD, Coomber BL. Microenvironmental influences on mutagenesis in mammary epithelial cells. Int J Cancer. 2005;116(5):679–685. doi: 10.1002/ijc.21088. [DOI] [PubMed] [Google Scholar]
- 81.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60(16):4372–4376. [PubMed] [Google Scholar]
- 82.Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42(3):155–199. [PubMed] [Google Scholar]
- 83.Petriz J, Gottesman MM, Aran JM. An MDR-EGFP gene fusion allows for direct cellular localization, function and stability assessment of P-glycoprotein. Curr Drug Deliv. 2004;1(1):43–56. doi: 10.2174/1567201043480072. [DOI] [PubMed] [Google Scholar]
- 84.Belhoussine R, Morjani H, Sharonov S, Ploton D, Manfait M. Characterization of intracellular pH gradients in human multidrug-resistant tumor cells by means of scanning microspectrofluorometry and dual-emission-ratio probes. Int J Cancer. 1999;81(1):81–89. doi: 10.1002/(sici)1097-0215(19990331)81:1<81::aid-ijc15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 85.Weylandt KH, Nebrig M, Jansen-Rosseck N, Amey JS, Carmena D, Wiedenmann B, Higgins CF, Sardini A. ClC-3 expression enhances etoposide resistance by increasing acidification of the late endocytic compartment. Mol Cancer Ther. 2007;6(3):979–986. doi: 10.1158/1535-7163.MCT-06-0475. [DOI] [PubMed] [Google Scholar]
- 86.Frezard F, Pereira-Maia E, Quidu P, Priebe W, Garnier-Suillerot A. P-glycoprotein preferentially effluxes anthracyclines containing free basic versus charged amine. Eur J Biochem. 2001;268(6):1561–1567. [PubMed] [Google Scholar]
- 87.Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol Pharm. 2009;6(4):1041–1051. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 89.Bennewith KL, Durand RE. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res. 2004;64(17):6183–6189. doi: 10.1158/0008-5472.CAN-04-0289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.