Abstract
Accumulating evidence indicates that IL-27, a member of the IL-12 family of cytokines, alleviates the severity of autoimmune diseases in both mice and men. The IL-27-induced activation of Signal transducer and activator of transcription (Stat)1 and Stat3 promotes the generation of IL-10-producing type 1 regulatory T (Tr1) cells that inhibit effector T cells. In addition, IL-27 also suppresses the development of pathogenic IL-17-producing CD4+ T cells (TH17) cells, suggesting that pharmacological manipulations of IL-27 signaling pathway could be exploited therapeutically in regulating tissue inflammation. Here, we review how IL-27 controls inflammation through the regulation of Tr1 and TH17 responses.
Keywords: IL-27, TH17 cells, Tr1 cells, FoxP3+ regulatory T cells, Stat, Maf
1. Introduction
Since the original classification by Mosmann and Coffman of CD4+ helper T (TH) lymphocytes into TH1 and TH2 subsets[1], the repertoire of TH subsets has expanded to include additional effector and regulatory T cell subsets such as TH17 cells and regulatory T cells (Foxp3+ Tregs and Tr1 cells). TH1 cells, which predominantly produce interferon (IFN)-γ and lymphotoxin, are essential for eliminating intracellular pathogens, but were also regarded as the major effector T cells in inducing tissue inflammation in organ-specific autoimmunity. However, mice lacking the component of TH1-IFN-γ pathway (Il12−/−, Ifng−/−, Ifngr1−/−, Il12rb2−/−) were not protected but overly susceptible to autoimmune diseases including Experimental Autoimmune Encephalomyelitis (EAE)[2], Experimental Autoimmune Uveitis (EAU)[3] and collagen-induced arthritis (CIA)[4]. Subsequent studies revealed that TH17 cells, instead of TH1 cells, induce tissue inflammation in autoimmune diseases. Although TH17 cells are essential for eliminating extracellular pathogens [5, 6], exaggerated TH17 response promotes autoimmunity. Elevated amounts of IL-17A and IL-17F are detected in several autoimmune diseases including multiple sclerosis (MS) [7], rheumatoid arthritis (RA) [8] and psoriasis[9]. The involvement of TH17 cells in tissue inflammation was confirmed in mouse models such as EAE where IL-17-neutralizing antibodies ameliorate clinical scores [10] or CIA where IL-17-deficient animals develop attenuated disease[11]. The differentiation factors for both mouse and human TH17 cells were found to be a combination of TGF-β1 and IL-6 or TGF-β1 and IL-21[12]. The activation of Signal transducer and activator of transcription (Stat)3 by IL-6 or IL-21 is critical for inducing the expression of the TH17 cell master transcription factors retinoid-related orphan receptor (ROR)γt, encoded by the gene Rorc, and RORα (Rora) [13] [14, 15]. Rorc−/− and Rora−/− mice show defective TH17 cell generation [15]. In addition, Chip-Sequencing analysis revealed Stat3 binding sites in the promoters regions of il17a and il17f gene[12]. Furthermore RORγt drives the expression of GM-CSF that is essential for inducing pathogenic TH17 cells, and mice deficient in making GM-CSF are resistant to develop EAE[16]. These observations indicate that RORγt is essential for the development of TH17 cells. Indeed TH17 cell generation can be inhibited by directly targeting RORγt using small chemical compounds such as digoxin and SR1001[17]. While IL-23 is not required for the induction of TH17 cell differentiation, IL-23 has a prominent role in expansion and stabilization of pathogenic TH17 cells [18–20]. Both IL-12p19−/− and IL-23R−/− mice are resistant to EAE, and few TH17 cells are found in the central nervous system (CNS) of those mice[21–23]. The IL-23-TH17 pathway has been shown to be critical in many autoimmune diseases, which is consistent with the fact that IL-23R polymorphisms has been genetically associated with a number of human autoimmune diseases including psoriasis, inflammatory bowel diseases (IBD) and ankylosing spondylitis[24]. More recent studies suggested that TH17 cells could also be induced with the combination of IL-1β, IL-6 and IL-23 in the absence of TGF-β1, suggesting that TH17 cells might actually represent a heterogeneous population of proinflammatory cells that are highly pathogenic and can be induced by multiple different ways.
Exaggerated inflammatory responses are prevented by regulatory T cell subsets that suppress activation of effector T cells. CD4+ regulatory T cells comprise Foxp3+ regulatory T-cells (Tregs) and IL-10-producing regulatory type I cells (Tr1) cells [25]. Foxp3+Tregs are important to maintain self-tolerance as illustrated by the severe autoimmune inflammation observed in mice deficient in Foxp3[26] or in patients with dysfunctional FOXP3 protein[27]. Although Foxp3+ Tregs inhibit effector T cell responses, they lose their suppressive functions in inflammatory conditions[28]. Therefore, IL-10-producing Tr1 cells might be crucial in controlling tissue inflammation. In humans, Tr1 cells were first described in severe combined immunodeficient (SCID) patients who had developed long-term tolerance to stem cell allografts, supporting the existence of these cells in humans and suggesting that they may play a role in mediating T cell tolerance [29]. Tr1 cells mediate immune suppression by secreting the suppressive cytokine IL-10 and by killing effector cells via Granzyme-B and Perforin [30, 31]. While IL-10 was initially described to be the differentiation factor for Tr1 cells, these T cells could not expand in the presence of IL-10. Therefore there was an emphasis on identifying growth/differentiation factors for Tr1 cells. Recent identification of IL-27 as a differentiation/growth factor for Tr1 cells has revived the interest in examining their role in tissue inflammation [32–34].
2. IL-27 dampens autoimmune inflammation
IL-27, an heterodimeric cytokine composed by the subunit p28 (IL-27p28) and the Epstein-Barr virus-induced gene 3 (EBI3), is mainly produced by activated antigen-presenting cells APCs[35]. IL-27 signals through a receptor complex consisting of the common IL-6 receptor chain, gp130, and the unique IL-27 receptor alpha chain (IL-27Ra or WSX-1) that is homologous to IL-12Rβ2 of IL-12 receptor[35, 36]. Based on the structural homology between IL-12 and IL-27 and their receptors, IL-27 was initially described as a proinflammatory cytokine that could induce TH1 differentiation, which was consistent with the ability of IL-27 to induce T-bet (Tbx21), the master transcription factor for the generation of TH1 cells. Subsequent work, using both TH1 and TH2 associated pathogens, established that IL-27 suppresses TH cells (TH1, TH2 and TH17 cells) functions in vivo, as Il27ra−/− mice showed enhanced T cell functions (reviewed in [37]). However, the mechanism by which IL-27-induced inhibition of T cell functions was not understood until the discovery that IL-27 can induce IL-10 production from CD4+ T cells.
3. IL-27 controls T cell responses
3.1 Regulation of TH1 and TH2 differentiation
While IL-27 induces T-bet and expression of IL-12Rβ2 in naïve CD4+ T cells, IL-27 signaling is not mandatory for TH1 differentiation as illustrated by mice lacking the IL-27R subunit (Il27ra−/−) that can mount adequate TH1 responses to eliminate intracellular pathogens [38–40]. Moreover, Il27ra−/− mice die due to uncontrolled immunopathology and severe tissue inflammation associated with exaggerated T cell responses and enhanced production of IFN-γ and TNF-α [38–40]. IL-27 was also reported to control the generation of TH2 cells. IL-27 treatment during Strongyloides venezuelensis infection decreases TH2 responses against the parasite and treated mice failed to develop intestinal mastocytosis and exhibited a marked delay in parasite expulsion [41]. Furthermore, intranasal administration of IL-27 inhibits OVA-induced airway hyperresponsiveness and inflammation in OVA-sensitized animals[41]. At the transcriptional level, IL-27 has been shown to suppress the master TH2 transcription factor GATA-3[41]. Recently, genome-wide association study (GWAS) has shown that a single nucleotide polymorphism (SNP) in the IL-27p28 gene was associated with an increased susceptibility to asthma[42] or COPD[43] and IL-27 has been proposed as a potential treatment for bronchial asthma.
3.2 Inhibition of TH17 cell differentiation
In addition to inhibiting both TH1 and TH2 development, IL-27 prevents the development of TH17 cells in vitro and in vivo. Il27ra−/− mice are overly susceptible to EAE compared to wild-type mice and present an increased accumulation of TH17 cells in the draining lymph nodes and in the CNS [44]. In this model, neutralization of IL-17 in Il27ra−/− mice during EAE disease course attenuated their disease phenotype [44]. Accordingly, recombinant IL-27 treatment decreases the disease incidence and severity in EAE with the inhibition of development of TH17 cells [45]. Similarly, Il27ra−/− mice chronically infected with T. gondii developed severe neuropathology mediated by CD4+ T cells, associated with increased TH17 cell development. IL-27 inhibits the production of IL-17 by BMNCs from chronically infected mice stimulated with IL-23[46]. Finally in the absence of IL-27 during murine flu infection, flu-specific T cell responses are skewed towards TH17[47].
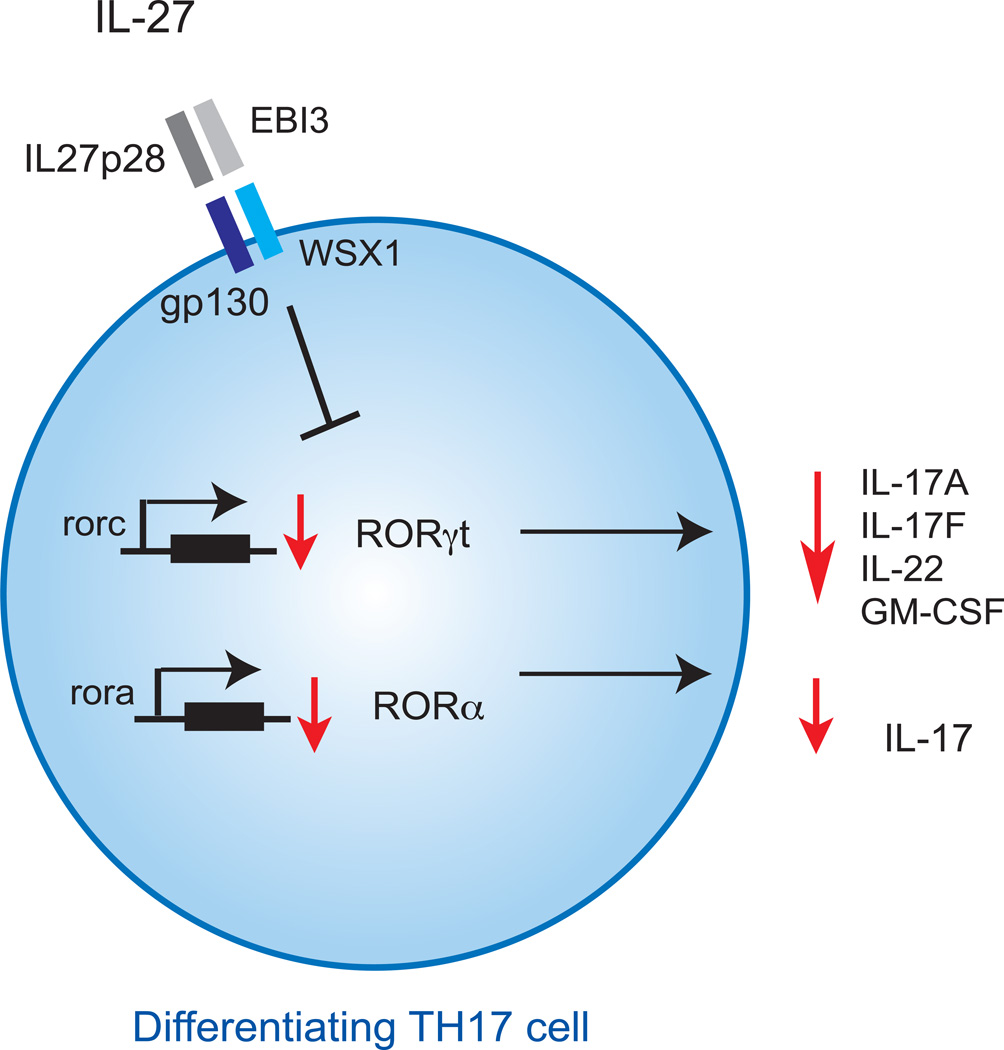
Above observations clearly indicated that IL-27 is negative regulator of development of TH17 cells. However, the mechanism by which IL-27 inhibits the development of TH17 cells is not clearly understood. Accumulating data suggest that IL-27 utilizes multiple mechanisms to inhibit the development of TH17 cells (Fig. 1 and 2). During TH17 cell differentiation, IL-27 directly suppresses the expression of both RORγt, the master transcription factor of TH17 cells [48] and RORα[49] (Fig. 1). IL-27 inhibits expression of RORγt in TH17 cells both in mouse and man [48]. Interestingly, IL-27 decreases the expression of GM-CSF and thereby dampens the pathogenicity of TH17 cells[16]. By blocking GM-CSF secretion and by inhibiting both RORα and RORγt expression, IL-27 interferes with TH17 cell differentiation at several levels, explaining its potent ability to suppress the induction of TH17 cells.
Figure 1. IL-27 inhibition of differentiating TH17 cells.
On differentiating TH17 cells, IL-27 inhibits the expression of transcription factors Rorγt and Rorα, thereby impairing the secretion the TH17-related cytokines, IL-17A, IL-17F, IL-22 and GM-CSF.
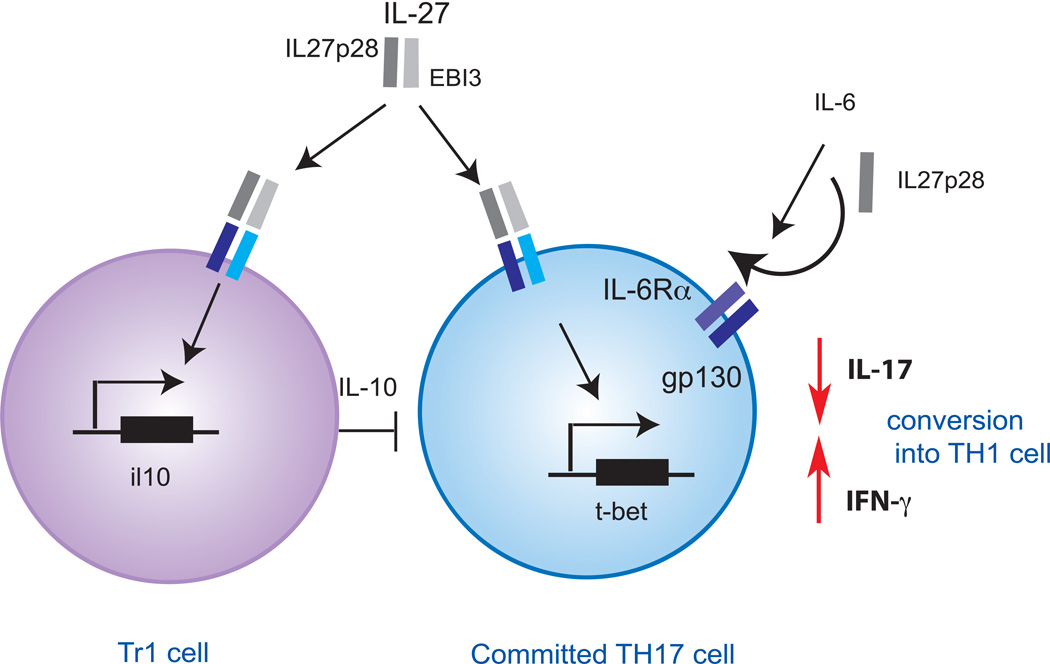
Figure 2. IL-27 inhibition of committed TH17 cells.
IL-27 induces the differentiation of Tr1 cells that inhibit TH17 cells in an IL-10-dependant manner. IL27p28 monomers interfere with IL-6 cytokine signaling through gp130 and thereby inhibit the maintenance of TH17 cells and their IL-17 secretion. IL-27 further induces t-bet expression that drives IFN-γ production and promotes the conversion of TH17 cells into TH1 cells.
Whether IL-27 can directly suppress effector/memory TH17 cells or fully differentiated TH17 cells is still debated. Indeed, TH17 maintained in culture for at least two rounds become unresponsive to IL-27 as IL-27 fails to inhibit the expression of RORα and RORγt in these cells[49]. However, IL-27 could modulate effector/memory TH17 cells using different strategies. Among the two IL-27 cytokine subunits, EBI3 is constitutively expressed but IL-27p28 secretion is transcriptionally regulated. IL-27p28 monomers can interfere with the IL-6-mediated production of IL-17 by preventing IL-6 signaling through gp130, suggesting that IL-27p28 monomers could also be exploited in regulating T cell responses [50]. IL-27p28 thus limits the generation and maintenance of TH17 cells in vivo without directly interfering with TH17 transcriptional program (Fig. 2). Furthermore, it has been proposed that TH17 could be converted into TH1 cells that are presumably less pathogenic [51, 52]. One putative mechanism by which IL-27 could converts TH17 into TH1 cells may be by inducing the expression of T-bet that drives IFN-γ expression and reduces the expression of IL-17 (Fig. 2). However, this hypothesis by which IL-27 may increase TH17 plasticity has not been proven experimentally.
3.3 Induction of Tr1 cells
IL-27, while inhibiting TGF-β-induced Foxp-3+ Tregs, induces IL-10+, IFNγ+ T cells that are immunosuppressive, a phenotype in line with the previously described Tr1 cells [32–34, 53, 54]. The role of IL-27 in generation of IL-10-producing Tr1 cells was further emphasized in vivo. IL-27 treated MOG-specific splenocytes lose their ability to transfer EAE in an IL-10 dependent manner [33]. Furthermore, during flu infection, IL-27 generates regulatory T cells that inhibit TH17 cells by secreting IL-10 and IFN-γ. In the absence of IL-10, flu-specific T cell responses developed a stronger TH17 component [47]. Furthermore, it has been shown that Tr1 cells can inhibit TH17 cells in vivo in an IL-10 dependent manner during murine colitis [55] (Fig. 2). Akin to what has been observed in murine T cells, activation of naïve human T cells in the presence of IL-27 similarly induces Tr1 cells that produce both IFN-γ and IL-10 [56].
4. Molecular pathways involved in IL-27 biology
Similar to other type 1 cytokine receptors, IL-27 also induces the activation of Janus kinase/Stat pathway. IL-27 predominantly induces the phosphorylation of Stat1 and Stat3. Here we will discuss the IL-27-induced signaling events following the activation of the Stats and analyze their roles in inhibiting TH17 cell and in inducing Tr1 cell differentiation.
4.1 IL-27 and Stat1 activation
4.1.1 Stat1 activation by IL-27 represses TH17 differentiation and induces Tr1 cells
The activation of the IL-27 specific subunit WSX-1 drives the tyrosine phosphorylation of JAK1 that further activates Stat1. Indeed, JAK1, but not other JAKs, coprecipitates with the WSX1 subunit[57].
The Stat1 signaling pathway is necessary for IL-27-induced T-bet expression [58]. T-bet not only drives the expression of IFN-γ but also plays an important role in the inhibition of TH17 cytokines, independently of IFN-γ. T-bet can reprogram committed TH17 cells by repressing TH17 gene program, which results in fewer transcripts of Rorc, il17a, il17f, il23r [59]. These finding were supported by studies showing that T-bet utilizes Runt-related transcription factor 1 (Runx1), a transcriptional activator that sequesters Rorc away from the regulatory regions on Rorc promoter[59]. Indeed Runx1 binding site is located upstream of T-bet binding site on Rorc promoter. By sequestering Runx1, T-bet inhibits the expression of RORγt, resulting impaired development of TH17 cell[59] (Fig. 3).
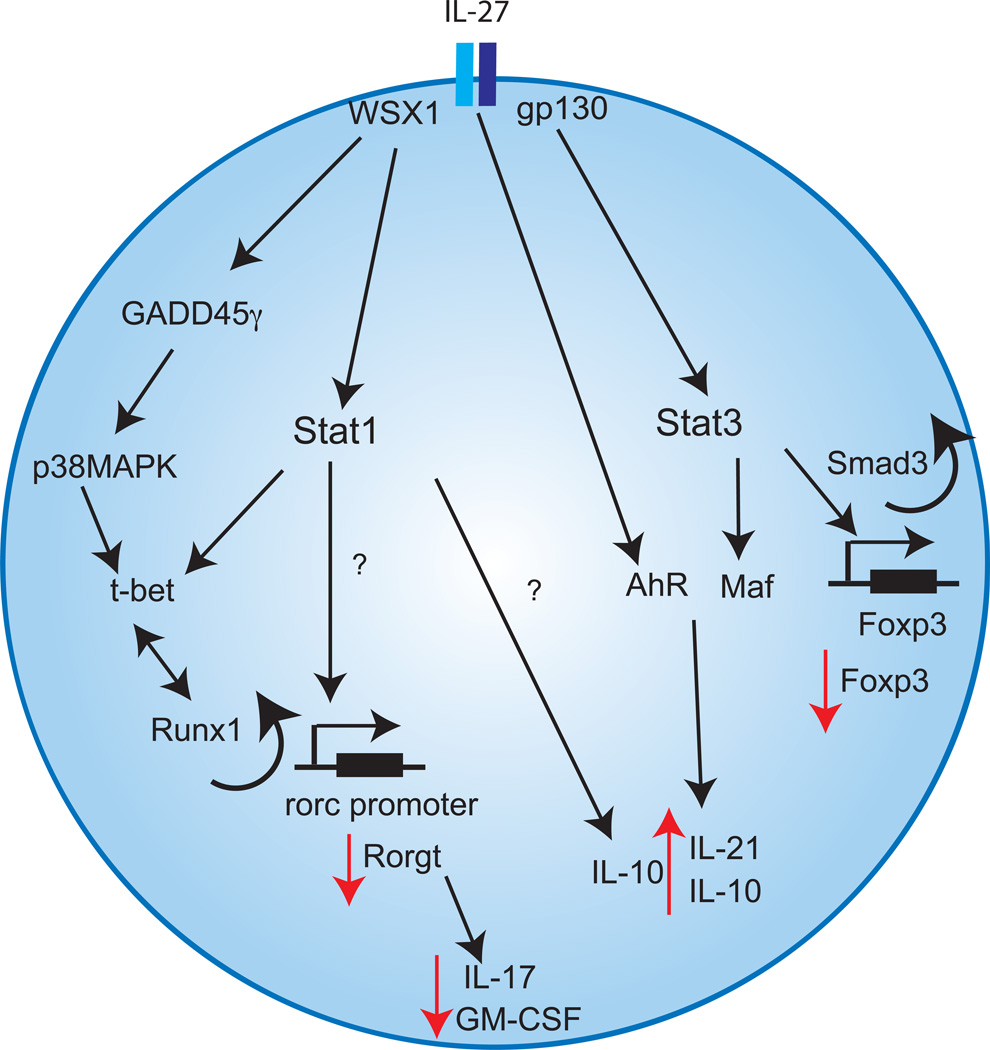
Figure 3. Reciprocal regulation of TH17 and Tr1 cells by IL-27.
The molecular mechanisms by which IL-27 promotes Foxp3−IL-10+Tr1 cell differentiation and represses TH17 cell development through activation of Stat1 and Stat3 activation are shown. IL-27 activates Stat1 through the subunit WSX1 that inhibits RoRγt expression through t-bet-dependent as well as t-bet-independent pathways. Alternatively, IL-27 can promote t-bet expression in a Stat1 independent pathway via GADD45γ. In addition, IL-27 activates Stat3 signaling through gp130. Stat3 induction then drives Maf transcription. Maf together with Ahr transactivates il21 and il10 promoters. On the other hand, IL-27 inhibits Foxp3 transcription in a Stat3/Smad3 dependent manner.
Stat1−/− and T-bet−/− mice exhibit an increased number of TH17 cells both during systemic inflammation in vivo or during TH17 cells differentiation in vitro. IL-17 production is greater in the absence of T-bet compared to the absence of Stat1[60]. This may be related to the fact that T-bet might also be induced in a Stat1 independent manner. In this vein, Owaki et al have shown that IL-27 induces a Stat1 independent T-bet expression[61]. Indeed IL-27 induces the expression of GADD45γ that further drives the phosphorylation of p38 MAPK leading to T-bet expression (Fig. 3).
It has been further proposed that Stat1 could inhibit RORα and RORγt expression in differentiating TH17 cells in a T-bet independent manner (Fig. 3). While a direct inhibitory effect of Stat1 on RORα and RORγt expression has not been ruled out, Stats could also indirectly affect TH17 responses by promoting the function of auxiliary inhibitory TH17 factors. Different repressors of TH17 cells differentiation have been identified, including Ets-1, which negatively regulates TH17 cell differentiation[62]. Stat1 and Ets-1 have been shown to bind together[63] and might cooperate to inhibit TH17 cell differentiation by directly or indirectly interfering with RORγt function in TH17 cells.
IL-27 has been shown to induce IL-10 expression from CD4+ T cells using both Stat1 and Stat3 pathways (Fig. 3). Indeed, in the absence of Stat1 signaling, IL-27 driven IL-10 production is decreased. While it is clear that the Stat1 driven IL-10 secretion is independent of t-bet signaling, the underlying mechanisms still remain unclear [34].
4.2 IL-27 and Stat3 activation
4.2.1 Stat3 activation by IL-27 does not enhance TH17 cell differentiation
IL-27 utilizes gp130 subunit of IL-6 receptor complex, which results in activation of Stat3 signaling. A genetic defect in Stat3 signaling in humans, in hyperIgE syndrome, results in defective TH17 cells and in unrelenting fungal infections, supporting the critical role of Stat3 in the generation of TH17 cells[64]. At the first glance, it is puzzling that IL-6 and IL-27, which both activate Stat3 pathways, have antagonistic properties. It has been proposed that IL-6 leads to a faster and more persistent pattern of Stat3 phosphorylation that is crucial to drive pro-inflammatory signals downstream Stat3. pStat-3 directly binds to il17a and il17f promoters and transactivate these genes by collaborating with other trancription factors like IRF-4 and RORγt. Furthermore, the formation of Stat1-Stat3 heterodimers in response to IL-27 rather than the formation of mainly Stat3 homodimers in response to IL-6 or IL-21 may play a role in the difference between IL-6 and IL-27 signaling. Indeed preliminary data from our laboratory supports this hypothesis. In addition, IL-6 activation rapidly induces Stat3 repressor SOCS3[65]. SOCS3 is an essential negative regulator of Stat3 phosphorylation and constrains TH17 cell differentiation[66, 67]. While IL-27 induces expression of SOCS3, IL-27-mediated inhibition of IL-17 production is independent of SOCS3[46]. It therefore seems unlikely that IL-27-induced SOCS3 contributes to the inhibition of TH17 cells. Instead, the inhibition of TH17 differentiation might mainly be mediated through Stat1 and T-bet as discussed above.
4.2.2 Stat3 activation by IL-27 promotes Tr1 cell differentiation
IL-27-induced Stat3 phosphorylation is essential for the anti-inflammatory role of IL-27, as it triggers IL-10 secretion from CD4+ T cells[34] (Fig. 3). Sustained activation of Stat3 leads to the induction of the transcription factor Maf[68]. We and others have recently shown that Maf is essential for IL-10 production induced by IL-27[53]. Similarly to Stat3 deficient CD4+ T cells, Maf deficient CD4+ T cells cannot produce IL-10 in response to IL-27. It has been further shown that Maf directly transactivates il10 and il21 promoters[53]. In addition to Maf, IL-10 production by IL-27 is regulated by the ligand activated transcription factor Aryl hydrocarbon receptor (AhR) that binds to Maf resulting in a complex that induces both il10 and il21 transcription[69]. The finding of AhR involvement in IL-10 production is significant as it provides impetus to design AhR ligands that can modulate the anti-inflammatory properties of Tr1 cells both in vitro and in vivo (reviewed in [31]). The expression of the cytokine IL-21 is further essential for IL-27-induced-IL-10 production[53] (reviewed in [37]). In the absence of IL-21, IL-10 production is reduced in Tr1 cells. IL-21 secretion can be further amplified by AhR activation[69].
4.2.3 Stat3 activation by IL-27 and inhibition of Foxp3
IL-27 inhibits the generation of Foxp3+Tregs[70]. The fact that Foxp3+Tregs express IL-27R strongly suggested that IL-27 might block the development of those regulatory cells in vitro[71]. IL-27 indeed leads to a decreased expression of Foxp3 through a mechanism that is at least partially dependent on Stat3[70]. Smad3 binding to Foxp3 promoter is implicated in Foxp3 transcription. It has been proposed that IL-27-induced pStat3 binds to a gene silencer region (enhancer II) in a conserved region of Foxp3 gene that reduces the acetylation in the region of Smad3 binding site and decreases the binding of pSmad 3 to Foxp3 promoter [72]. This results in a decreased accessibility and binding of Smad3 to Foxp3 promoter and thereby decreases Foxp3 transcription (Fig. 3). IL-27 impacts Foxp3+Treg development and function in vivo. Indeed mice that overexpress both IL-27 subunits, IL-27p28 and EBI3, have decreased number of Foxp3+Tregs and developed spontaneous inflammation similar to mice that lack Foxp3+Tregs such as the scurfy Foxp3 mutant mice or IL-2−/− mice[73]. Interestingly, IL-27 transgenic mice are deficient in IL-2. Those results are in accordance with another recent study showing that IL-27 inhibits Foxp3+Treg in vivo in a murine T cell transfer colitis model. Il27ra−/− deficient T cells transferred an attenuated disease due to a larger percentage of transferred cells expressing Foxp3 compared to wild-type T cells[74].
5. Therapeutic implications
5. 1. IL-27 confers protection against Multiple sclerosis
Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system resulting in inflammation, demyelization and axonal loss. It is a common neurological disorder, which attacks young adults. TH17 cells were shown to contribute to MS development[75]. By contrast, IL-27 protects against autoimmune inflammation in the mouse model EAE as exemplified by Il27ra−/− mice which develop an accelerated EAE disease course compared to WT controls and show increased levels of TH17 cells in the CNS [44]. Furthermore, daily intrathecal treatment with IL-27 during EAE alleviates the disease and decreases both the inflammation in the brain and the number of infiltrating TH17 cells[45]. Similarly in a T cell adoptive transfer model, pre-treatment of autoreactive CD4+ T cells with IL-27 leads to a reduction of their pathogenicity in an IL-10 dependent manner[33]. Interestingly, IL-27 was also shown to mediate the protective effect of Bone marrow stromal cells (BMSCs) that prevent EAE in mice and suppress IL-17 production[76].
Support for IL-27 in regulating autoimmune tissue inflammation has also been provided in humans. The immunomodulatory drug IFN-β, used in the first line of treatment for MS, has been shown to induce IL-27 production from dendritic cells (DCs). Interferon (IFN)-β, a member of the type I interferon family, is an approved treatment for relapsing remitting MS (RRMS) that reduces the rate of relapses by 30%. While the therapeutic mechanisms of IFN-β remain poorly understood, recent studies indicate that IL-27 contributes to its regulatory properties both in mouse[77] and human[78] [79]. One limitation of IFN-β treatment is that 20–50% of patients fail to respond to therapy thus delaying a change in the treatment strategy of those patients. While the presence of neutralizing antibodies (Nabs) against IFN-β in the blood has been proposed to correlate with treatment failure[80], a proportion of non-responder patients do not develop Nabs, limiting the use of Nabs to predict the response to IFN-β therapy [81]. IL-27 secretion from PBMC from RRMS patients has been proposed as a predictive factor of clinical response to IFN-β treatment. Indeed, PBMC isolated from RRMS patients that respond to IFN-β treatment secrete more IL-27 when exposed in vitro to IFN-β than PBMC isolated from “non-responder” patients[78]. Finally, other therapies proposed for treating MS, such as Statins, which in addition to their cholesterol-lowering activity have anti-inflammatory properties, were shown to increase in vitro IL-27 secretion from human monocytes of MS patients [82]
5.2 IL-27 protects against rheumatoid arthritis
Rheumatoid arthritis (RA) is a systemic inflammatory disorder that principally attacks synovial joints. TH17 cells and IL-17 expression is elevated in RA synovial tissue and fluid macrophages compared to controls[83, 84]. Elevated levels of IL-17 have been reported in the animal model of RA, collagen-induced arthritis (CIA), and IL-17 neutralization prevents bone destruction suggesting a pathological role of TH17 cells in the development of RA [85]. Administration of IL-27 in mice suffering from CIA reduces the severity of the disease, as shown by reduced cellular infiltration in the joints, synovial hyperplasia, and joint erosion[84]. IL-27 treatment further decreases serum levels of IL-6. In addition, lymphocytes isolated from spleen and lymph node of IL-27-treated mice produce significantly reduced amounts of IFN-γ and IL-17 when cultured with type II collagen in vitro compared with lymphocytes from control mice. Similar results were obtained when IL-27 was ectopically expressed in the joints [86]. These studies highlight in the therapeutic potential of IL-27 in RA, especially with the feasibility of local, intra-articular, administration of recombinant IL-27.
5.3 Controversial role of IL-27 in inflammatory Bowel Disease
IL-27 is implicated in the pathogenesis of IBD, Crohn’s disease and ulcerative colitis. Genome wide studies have identified SNPs in the gene encoding p28 subunit associated with a lower expression of IL-27 and early onset inflammatory bowel disease, which would be consistent with a protective role of IL-27 in IBD[87]. Two other studies have found transcripts for IL-27p28[88] and Ebi3[89] to be overexpressed in biopsy samples from IBD patients. The function of IL-27 has been assessed using different murine models of IBD. In the mouse IBD model of acute inflammation, which relies on the presence of dextran Sulfate Sodium (DSS) to induce inflammation, Il27ra−/− mice receiving 5–10% DSS in drinking water were more susceptible to disease[90]. Il27ra−/− deficient mice showed a reduction in TH1 IFNγ-producing cells and an increase in TH17 cells in gut-associated lymphoid tissue pointing toward an important regulatory role of IL-27 in dampening TH17 cell function [90]. In the 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced mouse acute colitis model, subcutaneous scIL-27 (EBI3 and p28 subunits generated as a single-chain human IL-27) treatment significantly improved in a dose-dependent manner the extent of the lesions as well as necrosis, ulceration and thickening of mucosal epithelium. scIL-27 suppressed several inflammatory cytokines in inflamed colon, including IL-17 [91]. However, in a T cell transfer colitis model, IL-27 was shown to exert proinflammatory effects as it suppressed induced Treg development in vivo[74]. In contrast, in the DSS model, Il27ra−/− mice treated with lower doses of DSS (0.5% in drinking water), were protected compared to WT controls[92]. The implication of different pathogenic or regulatory subsets and the heterogenicity of the models may explain the different responses to IL-27 treatment in murine models of colitis. However, in models where TH17 cells are implicated in the development of the disease, the anti-inflammatory role of IL-27 appears to be dominant. Indeed, TH17 cells have been shown to be crucial for the development of TNBS-induced colitis as IL-17 receptor A (IL-17RA) knockout mice do not develop TNBS colitis[93] and IL-17F-deficient mice develop more severe DSS colitis than controls[94]. A better understanding of the pathogenesis of IBD should provide additional insight into the role of IL-27 in colitis.
6. Open questions and concluding remarks
While IL-27 promotes Tr1 cells, it inhibits CD4+Foxp3+Tregs induced by TGF-β. These observation are reminiscent of the action of AhR ligands such as FICZ that promotes Tr1 cells but inhibits Foxp3+Tregs. This paradoxical effect on regulatory T cells might stem from different and/or complementary roles of regulatory T cells. Tr1 cells but not Foxp3+Tregs may develop in situ in the inflamed tissue as IL-27 can be secreted be resident cells in the target organ, such as in the brain during EAE and MS. Foxp3+ Tregs can not inhibit highly pathogenic effector T cells in the target organ[95] but they induce tolerogenic plasmacytoid dendritic cell (DC) that secrete IL-27 thus promoting Tr1 cell generation [32]. Under inflammatory settings, Foxp3+Tregs can produce cytokines that belong to other lineages [96, 97] and we propose that Tr1 cells could be more stable and thereby regulate tissue inflammation at the target site.
IL-27 controls inflammation by inhibiting TH17 cells and by promoting the development of IL-10-producing regulatory Tr1 cells. Despite their opposite in vivo functions, Tr1 and TH17 cells harbor striking similarities. First, they rely on the transcription factors Maf and AhR for their generation. Second, they require IL-21 for their growth. Third, they produce IL-10. In this regard, Ghoreschi et al showed that TH17 differentiated with TGF-β and IL-6 (TH17(β)) produced IL-10 and were poorly pathogenic in vivo in contrast to TH17 cells induced by IL-6, IL-1β and IL-23 (TH17)(23)) that did not produce IL-10 and were highly pathogenic. In addition, TGF-β induced TH17 expressed higher levels of Maf and AhR compared to TH17 induced with IL-1, IL-6 and IL-23 (23). This observation would thus be in line with a previous work suggesting that the Maf-driven induction of IL-10 in TH17 cells reduced their pathogenicity[98]. Since we have shown that the expression of Maf and AhR is required for the production of IL-10 and IL-21 in Tr1 cells, it might be interesting to explore whether IL-27 could actually be converting TH17 to Tr1 cells. We are currently conducting a functional transcriptional analysis of Tr1 (differentiated with IL-27) and TH17 (IL-6 and TGF-β) cells using a computational approach and a whole genome microarray analysis to address this question.
In the same line, IL-21 has been ascribed a functional role in promoting both TH17[99, 100] and Tr1 cells[53]. The role of IL-21 during autoimmune disease such as EAE is controversial. While initial studies have proposed that IL-21R−/− mice presented a less severe EAE disease [100], longer observation of EAE disease course showed that IL-21R−/− mice developed a more severe disease [101, 102]. Besides being a growth factor for TH17 cells [103], IL-21 may behave as an anti-inflammatory effect by promoting IL-10 secretion from different T cell subtypes. It remains to be seen whether IL-27 and its downstream cytokine IL-21 can modulate the pathogenicity and stability of different subtypes of TH17 cells that have been further treated with IL-23. In conclusion, IL-27 not only induces the generation of anti-inflammatory Tr1 cells but broadly controls autoimmune responses by inhibiting effector T cells in various target organs.
Highlights.
IL-27 controls autoimmune responses by promoting Tr1 cells and inhibiting TH17 cells.
IL-27 triggers Stat1 and Stat3 signaling
IL-27 induces Maf and AhR that control IL-10 secretion from Tr1 cells
Stat1 activation represses TH17 cells and induces Tr1 cells
Stat3 activation enhances Tr1 cells
IL-27 alleviates human autoimmune diseases.
Acknowledgements
The authors are supported by grants from Swiss National Science Foundation (SFGBM) and the Novartis Foundation (C.P.) and the Agence Nationale de la Recherche [ANR-10-PDOC-014-01] (L.A.). AA is supported by research grant from Crohn’s and Colitis Foundation of America, New York. Studies in our laboratory were funded by the National Institutes of Health [NS030843, AI039671, AI056299] (V.K.K.).
Abbreviation
- Tr1 cells
type 1 regulatory T cells
- TH17
T helper 17
- Stat
Signal Transducer and Activator of Transcription
- Maf
Transcription factor Maf
- Ahr
Aryl hydrocarbon receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 3.Jones LS, Rizzo LV, Agarwal RK, Tarrant TK, Chan CC, Wiggert B, et al. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 1997;158:5997–6005. [PubMed] [Google Scholar]
- 4.Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Billiau A. Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol. 1998;28:2143–2151. doi: 10.1002/(SICI)1521-4141(199807)28:07<2143::AID-IMMU2143>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 6.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 8.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- 9.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 10.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 12.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 15.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 17.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 19.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 21.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 22.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XJ, Huang W, Yang S, Sun LD, Zhang FY, Zhu QX, et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet. 2009;41:205–210. doi: 10.1038/ng.310. [DOI] [PubMed] [Google Scholar]
- 25.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 26.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 27.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 28.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 29.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnani CF, Alberigo G, Bacchetta R, Serafini G, Andreani M, Roncarolo MG, et al. Killing of myeloid APC via HLA Class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol. 2011;41:1652–1662. doi: 10.1002/eji.201041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Seminars in Immunology. 2011 doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 34.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 35.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 36.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 37.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J Interferon Cytokine Res. 2010;30:381–388. doi: 10.1089/jir.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 39.Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, et al. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 40.Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, et al. Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol. 2006;168:158–169. doi: 10.2353/ajpath.2006.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 42.Chae SC, Li CS, Kim KM, Yang JY, Zhang Q, Lee YC, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–361. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]
- 43.Huang N, Liu L, Wang XZ, Liu D, Yin SY, Yang XD. Association of interleukin (IL)-12 and IL-27 gene polymorphisms with chronic obstructive pulmonary disease in a Chinese population. DNA Cell Biol. 2008;27:527–531. doi: 10.1089/dna.2007.0715. [DOI] [PubMed] [Google Scholar]
- 44.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 45.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 46.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 47.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 49.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stumhofer JS, Tait ED, Quinn WJ, 3rd, Hosken N, Spudy B, Goenka R, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 55.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F, et al. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol Lett. 2011;136:21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 58.Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells. J Immunol. 2004;173:3871–3877. doi: 10.4049/jimmunol.173.6.3871. [DOI] [PubMed] [Google Scholar]
- 59.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol. 2011;12:96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villarino AV, Gallo E, Abbas AK. STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J Immunol. 2010;185:6461–6471. doi: 10.4049/jimmunol.1001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owaki T, Asakawa M, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 induces Th1 differentiation via p38 MAPK/T-bet- and intercellular adhesion molecule-1/LFA-1/ERK1/2-dependent pathways. J Immunol. 2006;177:7579–7587. doi: 10.4049/jimmunol.177.11.7579. [DOI] [PubMed] [Google Scholar]
- 62.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yockell-Lelievre J, Spriet C, Cantin P, Malenfant P, Heliot L, de Launoit Y, et al. Functional cooperation between Stat-1 and ets-1 to optimize icam-1 gene transcription. Biochem Cell Biol. 2009;87:905–918. doi: 10.1139/o09-055. [DOI] [PubMed] [Google Scholar]
- 64.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, Lhocine N, et al. General nature of the STAT3-activated anti-inflammatory response. J Immunol. 2006;177:7880–7888. doi: 10.4049/jimmunol.177.11.7880. [DOI] [PubMed] [Google Scholar]
- 66.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 67.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J Immunol. 2005;174:2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- 69.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 71.Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, et al. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- 72.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–325. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tait Wojno ED, Hosken N, Stumhofer JS, O'Hara AC, Mauldin E, Fang Q, et al. A Role for IL-27 in Limiting T Regulatory Cell Populations. J Immunol. 2011 doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 208;115:123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Wang G, Sun B, Li H, Mu L, Wang Q, et al. Interleukin-27 suppresses experimental autoimmune encephalomyelitis during bone marrow stromal cell treatment. J Autoimmun. 2008;30:222–229. doi: 10.1016/j.jaut.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sweeney CM, Lonergan R, Basdeo SA, Kinsella K, Dungan LS, Higgins SC, et al. IL-27 mediates the response to IFN-beta therapy in multiple sclerosis patients by inhibiting Th17 cells. Brain Behav Immun. 2011;25:1170–1181. doi: 10.1016/j.bbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Zhang X, Jin J, Tang Y, Speer D, Sujkowska D, Markovic-Plese S. IFN-beta1a inhibits the secretion of Th17-polarizing cytokines in human dendritic cells via TLR7 up-regulation. J Immunol. 2009;182:3928–3936. doi: 10.4049/jimmunol.0802226. [DOI] [PubMed] [Google Scholar]
- 80.Sorensen PS, Ross C, Clemmesen KM, Bendtzen K, Frederiksen JL, Jensen K, et al. Clinical importance of neutralising antibodies against interferon beta in patients with relapsing-remitting multiple sclerosis. Lancet. 2003;362:1184–1191. doi: 10.1016/S0140-6736(03)14541-2. [DOI] [PubMed] [Google Scholar]
- 81.van der Voort LF, Kok A, Visser A, Oudejans CB, Caldano M, Gilli F, et al. Interferon-beta bioactivity measurement in multiple sclerosis: feasibility for routine clinical practice. Mult Scler. 2009;15:212–218. doi: 10.1177/1352458508096877. [DOI] [PubMed] [Google Scholar]
- 82.Zhang X, Jin J, Peng X, Ramgolam VS, Markovic-Plese S. Simvastatin inhibits IL-17 secretion by targeting multiple IL-17-regulatory cytokines and by inhibiting the expression of IL-17 transcription factor RORC in CD4+ lymphocytes. J Immunol. 2008;180:6988–6996. doi: 10.4049/jimmunol.180.10.6988. [DOI] [PubMed] [Google Scholar]
- 83.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10:R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, et al. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67:1474–1479. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 85.Kelchtermans H, Schurgers E, Geboes L, Mitera T, Van Damme J, Van Snick J, et al. Effector mechanisms of interleukin-17 in collagen-induced arthritis in the absence of interferon-gamma and counteraction by interferon-gamma. Arthritis Res Ther. 2009;11:R122. doi: 10.1186/ar2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, et al. Local expression of IL-27 ameliorates collagen induced arthritis. Arthritis Rheum. 2011;63:2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, Zeuzem S, et al. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis. 2005;11:16–23. doi: 10.1097/00054725-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Omata F, Birkenbach M, Matsuzaki S, Christ AD, Blumberg RS. The expression of IL-12 p40 and its homologue, Epstein-Barr virus-induced gene 3, in inflammatory bowel disease. Inflamm Bowel Dis. 2001;7:215–220. doi: 10.1097/00054725-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Troy AE, Zaph C, Du Y, Taylor BC, Guild KJ, Hunter CA, et al. IL-27 regulates homeostasis of the intestinal CD4+ effector T cell pool and limits intestinal inflammation in a murine model of colitis. J Immunol. 2009;183:2037–2044. doi: 10.4049/jimmunol.0802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol. 300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 92.Honda K, Nakamura K, Matsui N, Takahashi M, Kitamura Y, Mizutani T, et al. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm Bowel Dis. 2005;11:1044–1052. doi: 10.1097/01.mib.0000191611.05466.1f. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 94.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 98.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 101.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 102.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 103.Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]