Abstract
Recent clinical reports found a high incidence of recurrent otitis media in children suffering hyperacusis, a marked intolerance to an otherwise ordinary environmental sound. However, it is unclear whether the conductive hearing loss caused by otitis media in early age will affect sound tolerance later in life. Thus, we have tested the effects of tympanic membrane (TM) damage at an early age on sound perception development in rats. Two weeks after the TM perforation, more than 80% of the rats showed audiogenic seizure (AGS) when exposed to loud sound (120 dB SPL white noise, < 1 minute). The susceptibility of AGS lasted at least sixteen weeks after the TM damage, even the hearing loss recovered. The TM damaged rats also showed significantly enhanced acoustic startle responses compared to the rats without TM damage. These results suggest that early age conductive hearing loss may cause an impaired sound tolerance during development. In addition, the AGS can be suppressed by the treatment of vigabatrin, acute injections (250 mg/kg) or oral intakes (60 mg/kg/day for 7 days), an antiepileptic drug that inhibits the catabolism of GABA. c-Fos staining showed a strong staining in the inferior colliculus (IC) in the TM damaged rats, not in the control rats, after exposed to loud sound, indicating a hyper-excitability in the IC during AGS. These results indicate that early age conductive hearing loss can impair sound tolerance by reducing GABA inhibition in the IC, which may be related to hyperacusis seen in children with otitis media.
Keywords: Otitis media, Audiogenic seizure, Acoustic startle reflex, Hyperacusis, Inferior colliculus, GABA
INTRODUCTION
Otitis media, an inflammation of the middle ear, is the most commonly diagnosed illness among preschool children in the United States (Lanphear et al., 1997). The incidence of recurrent otitis media has increased significantly in children, especially infants, in the last decade, most likely due to the increased enrollment in child care services and a higher prevalence of allergic conditions (Kristjansson et al., 2010; Martines et al., 2010). Chronic otitis media in early childhood is a serious concern as it induces recurrent conductive hearing loss, which adversely affects language acquisition, learning and social interactions (O'Leary et al., 2009). Recent clinical reports suggest that early age hearing loss may be related with hyperacusis, a disorder characterized by a marked intolerance to ordinary environmental sounds. Coelho et al. found that hearing loss was often reported in children who experienced hyperacusis and tinnitus (~37%) and suggested that the mild hearing loss might be an associated risk factor for hyperacusis and tinnitus (Coelho et al., 2007). The vast majority of children with Williams syndrome, a genetic neural developmental disorder, suffer from hyperacusis. Although the cause of hyperacusis in Williams syndrome may be related with the complex cognitive deficits caused by this neurodevelopmental disorder, interestingly, children with Williams syndrome often show a high frequency hearing loss which resembles the configuration of noise-induced hearing loss (Gothelf et al., 2006) or recurrent otitis media (Klein et al., 1990; Miani et al., 2001). These studies suggest that early age hearing loss (conductive or sensorineural) may affect the development of the central auditory system (Popescu et al., 2010; Xu et al., 2007) and consequently, impairs the sound perception.
Previous animal studies have shown that early age conductive hearing loss can impair sound tolerance (Chen et al., 1973; Gates et al., 1973; McGinn et al., 1973). Chen et al reported that a high incidence of audiogenic seizure (AGS) behavior was induced in mice by rupturing their tympanic membranes (TM) at an early age (postnatal 14 or 21 days), but not in adult (Chen et al., 1973). During exposure to loud sound, i.e., ringing bells for 120 seconds (~125 dB SPL), the TM damaged mice exhibited wild running followed by erratic leaping, clonic convulsion and even death. This study suggests that sound deprivation at early age caused by conductive hearing loss can impair sound tolerance. However, in most of those studies, AGS has only been tested for 2-3 weeks after TM damage. The long term effect of the TM damage on sound tolerance has never been tested. It was also unclear whether the TM damage will affect the sound loudness perception. People with hyperacusis often report increased sensitivity to sound, regardless the hearing loss they may have. Therefore, we developed a conductive hearing loss animal model in young Sprague-Dawley rats by surgically perforating their TMs (bilateral and monaural), a common occurrence in children with chronic suppurative otitis media (Johnston et al., 2004; Lasisi et al., 2008; Martines et al., 2010), and assess their behavioral response to loud sound later in life. The susceptibility to AGS and the acoustic startle reflex have been tested after the TM damage up to 16 weeks post-surgery. We also used the staining of c-Fos, an immediate early gene which can be utilized as a marker of repetitive neuronal activation (Friauf, 1992), to identify the source of AGS in the central auditory system.
Materials and Methods
Animal models for conductive hearing loss
22 Sprague-Dawley rat pups were randomly assigned to either the TM damaged group (TM Group, n = 14) or the Control Group (n = 8). For the TM Group, bilateral TMs were surgically destroyed at postnatal 16 days (P-16d), shortly after their ear canals fully opened which has been referred as the critical period of the central auditory system development (de Villers-Sidani et al., 2008). The TM damage surgery was performed under a surgical microscope and the rats were under light anesthesia with isoflurane (1.5-2%). The TM was visualized under the surgical microscope. The entire TM was ruptured using a sterile 22 gauges needle. The middle ear ossicles, except the manubrium of the malleus, were avoided to be touched during the surgery. The surgery lasted for less than 5 minutes for each rat. Rats in the Control Group were also anesthetized with isoflurane (1.5-2%) for 5 minutes without surgery. All animals were then returned to their cages. The hearing loss induced by TM damage was evaluated using auditory brainstem (ABR) tests at 2 and 6 weeks after the surgery.
AGS test and vigabatrin treatment
The susceptibility to AGS was tested following the procedure described in a previous literature (Chakravarty et al., 1999). In the test, rats were individually placed in a round training pan (diameter 40 cm, height 50 cm) with a loud speaker installed at its top (D-59, GMI Sound Corp., USA) in a sound proof room. The movement of the rats was captured by a video camera and monitored by the testers outside the testing room. The rats were allowed to habituate to the testing environment for 2 minutes. Then, they were exposed to a loud white noise (120 dB SPL) for up to 60 seconds if no seizure was induced. Wild running and erratic leaping were used as a sign to identify the AGS (Chakravarty et al., 1999). Once AGS was observed, the sound exposure will be terminated.
The effect of vigabatrin on AGS was tested on a group of rats in the TM Group (2-4 months old) through acute injection or chronically oral administration. For acute test, vigabatrin (Sabril, Anaofi Aventis, UK) was dissolved in saline solution (25 mg/ml) right before the treatment. The rats were tested for AGS 2 hours after the injection of vigabatrin (250 mg/kg, intraperitoneal injection, i.p.). The effect of saline (4-5 ml, i.p.) on AGS was also tested on these rats 3-5 days later as control. For the chronic effect of vigabatrin, vigabatrin was dissolved in water at 1 mg/ml concentration and treated for seven days. The averaged water taken was 20±0.7 ml (n = 7) and the dose of vigabatrin was about 60 mg/kg/day.
Acoustic startle reflex test
The acoustic startle reflex was recorded from rats in the TM Group and the Control Group (four for each group, only male rats were used) (Sun et al., 2009). During the test, rats were placed in custom-fabricated wire mesh cages resting on the Plexiglas floor within sound isolated testing chambers. Sounds were delivered by a loudspeaker located above the cage. A piezoelectric transducer attached to the bottom of the testing platform generated a voltage proportional to the magnitude of the startle response. Sound stimuli consisting of narrow-band noise (centered at 4 and 8 kHz with a 2 kHz bandwidth) were randomly presented at various intensities (60 to 110 dB SPL with 10 dB step). Ten trials have been used at each intensity with 18-22 second inter-trial intervals.
Expression of c-Fos in the IC
The expression of c-Fos in the IC induced by noise exposure was examined in rats from both the TM Group (n = 3) and the Control Group (n = 3). Rats were exposed to 120 dB SPL white noise for one minute or less if AGS was induced. Two hours after noise exposure, the rats were sacrificed with an overdose of pentobarbital and then intracardially perfused with phosphate-buffered saline followed by formalin. Our decision to sacrifice rats 2 h after the noise exposure was based on a previous study that found the immunoreactivity of c-Fos peaked at 2 hours after sound exposure (Klein et al., 2004). Following the intracardial perfusion, the brain was post-fixed and sliced into 40 μm coronal sections using a cryostat microtome. The c-Fos immunohistochemistry was performed on free-floating brain slices by exposing them for 2 h to the primary rabbit polyclonal antibody (sc-52, Santa Cruz), followed by 1 h exposure to the secondary antibody (biotinylated goat anti-rabbit IgG, BA-1000, Vector Lab) in room temperature. The immunoreactivity was detected following the avidin-biotin complex immunoperoxidase reaction (ABC; Vector Lab) and visualized by 3'-diaminobenzidine (DAB) staining. c-Fos staining was then examined under a microscope.
GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA) was used to perform the statistical analyses and generate the graphs. Results are presented as the mean ± standard error (SEM) of the mean.
Results
Hearing loss caused by the TM damage
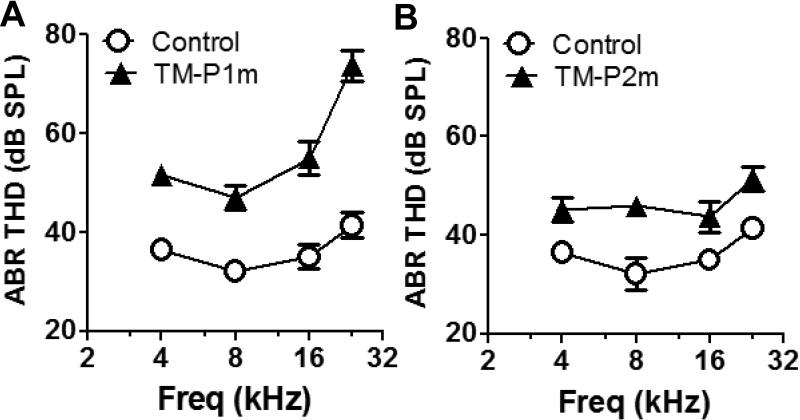
ABR was used to evaluate the hearing loss caused by the TM damage. Two weeks after the surgery, the averaged ABR threshold in the TM Group (n = 12) was 15, 15, 20 and 32 dB higher than the Control Group (n = 7) at 4, 8, 16 and 24 kHz, respectively (Two-way ANOVA, F(1, 68) = 106, P < 0.0001, Figure 1A). Six weeks after the TM damage, the difference of the averaged ABR threshold in the TM Group (n = 4) compared to the Control Group (n = 4) reduced to 9, 13, 9 and 10 dB at 4, 8, 16 and 24 kHz (Two-way ANOVA, F(1, 24) = 39, P < 0.0001, Figure 1B). These results are consistent with previous reports that most acute TM perforations healed spontaneously in several weeks (Amoils et al., 1992; Bigelow et al., 1998).
Figure 1.
Early age tympanic membrane damage induced hearing loss and audiogenic seizure (AGS). A. Tympanic membrane (TM) damage caused a 10 to 30 dB hearing threshold increase compared to the Control Group two weeks after the surgery. B. The differences of the hearing threshold between the TM Group and the Control Group reduced to 10-15 dB six weeks after the TM damage (Results are presented as mean ± SEM)
The Susceptibility of AGS induced by TM damage
The susceptibility to AGS in the TM Group and the Control Group was repeatedly tested at multiple time points after the surgery (1 week to 16 weeks) (Table 1). One week after the TM damage, none of the rats in the TM Group showed AGS. However, two weeks after surgery, 85% of the rats in the TM Group (12 out of 14) presented with AGS when they were exposed to 120 dB SPL white noise (< 60 seconds). The AGS behavior was characterized by wild running, erratic leaping and clonic convulsions during exposure to loud sound (Chen, 1978). Retesting at post-surgery 4, 6, 8 and 16 weeks, confirmed that the increased susceptibility to AGS was persistent in the TM group. The ratios of AGS susceptible rats at post-surgery 4, 6, 8 and 16 weeks are 100% (13 out of 13), 91% (10 out of 11), 78% (7 out of 9) and 100% (7 out of 7), respectively. The susceptibility of AGS of these rats maintained the same during the development. In contrast, none of the rats in the Control Group (n = 8) showed AGS during the tests at postnatal 3 to 18 weeks, which result is consistent with the literature that Sprague-Dawley rats are a genetically AGS-resistant strain (Pierson et al., 1992).
Table 1.
Effects of tympanic membrane (TM) damage on audiogenic seizure (AGS) susceptibility
| Post TM Weeks | Number of rats | No AGS | AGS |
|---|---|---|---|
| Bilateral TM damage at P16 | |||
| 1 | 14 | 14 | 0 |
| 2 | 14 | 2 | 12 |
| 4 | 13 | 0 | 3 |
| 6 | 11 | 1 | 10 |
| 8 | 9 | 2 | 7 |
| 16 | 7 | 0 | 7 |
| Monaural TM damage at P16 | |||
| 2 | 8 | 2 | 6 |
| Bilateral TM damage at P45 | |||
| 2 | 4 | 4 | 0 |
In addition, we tested the AGS in a group of rats with monaural TM damage at P-16d (n=8). Two weeks after the surgery, 75% of rats (6 out of 8) also exhibited AGS when exposed to 120 dB SPL white noise (< 60 seconds). Four adult rats had TM damage at P45. Four weeks later, none of these rats developed AGS.
The TM damage enhanced the startle amplitude
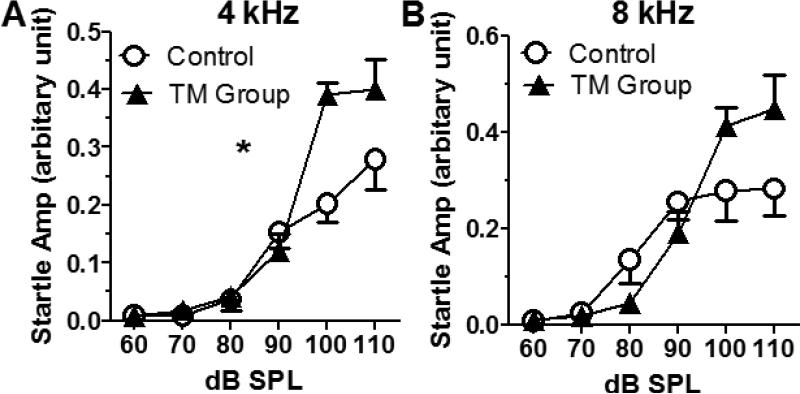
To test if early age conductive hearing loss also affects the sensitivity of sound stimuli, the acoustic startle response of the rats in the TM Group (bilateral TMs were damaged at P16d) and the age-matched rats in the Control Group were compared. The acoustic startle reflex is characterized by a contraction of the skeletal and facial muscles in response to sudden, relatively intense sounds, and its amplitude is related exponentially to sound intensity – similar to sound loudness response curve to the sound intensity (Stevens, 1955). Four rats for each group (3-4 weeks after the TM damage) were tested. Compared to the Control Group, there was a significant increase of the acoustic startle response at 4 kHz in the TM Group (two-way ANOVA, F(1,36)=9.38, p=0.004, n = 4, Figure 2A). At 8 kHz, the startle response of the TM group was enhanced at high intensities (100 and 110 dB SPL), despite a slightly reduced response at lower intensities due to the hearing loss (two-way ANOVA, P>0.05, n = 4, Figure 2B). These results suggest that the early age TM damage may increase the sound reaction (sensitivity).
Figure 2.
Tympanic membrane (TM) damage caused increased acoustic startle response. A. Rats with TM damage showed higher acoustic startle response at 4 kHz (Two-way ANOVA, p = 0.004, F(1,36)= 9.38, n = 4) compared to the Control Group. B. At 8 kHz, the startle responses of the TM Group were higher at 100 and 110 dB SPL, but slightly lower from 70 to 90 dB SPL compared to the Control Group (Results are presented as mean ± SEM, * P < 0.05).
C-Fos Staining
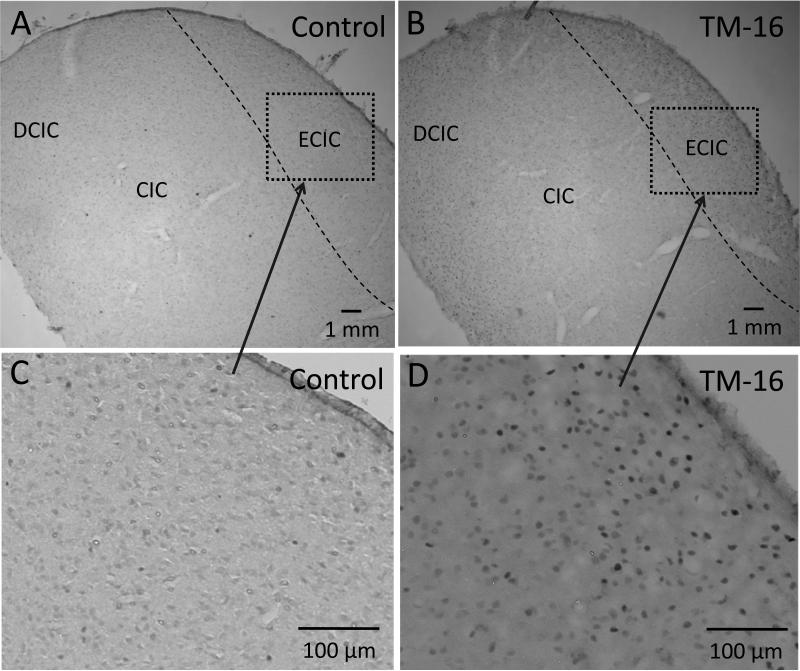
c-Fos staining was performed on brain tissue harvested from rats in the TM Group and the Control Group (6-8 weeks old; 3 from each group) 2 hours after loud sound exposure (120 dB, 1 minute). During the loud sound exposure, the rats in the TM Group showed AGS, whereas the rats in the Control Group did not. Figure 3 shows examples of c-Fos staining in a control rat (A and C) and a TM damaged rat (B and D). Strong c-Fos staining can be seen in the IC of the TM damaged rats; whereas only scattered c-Fos positive nuclei can be found in the control rats. To quantify the c-Fos staining in the IC, we counted the c-Fos positive nuclei in a serial of sections of the IC (determined by eye). The averaged number of the c-Fos positive nuclei in the central and dorsal nuclei of the IC was 42 ± 8.4 / mm2 (counted from 8 sections) and 11.7 ± 2.6 / mm2 (6 sections) in the TM damaged rats and the control rats respectively. The difference was significant (the Student's t-test, p=0.017). In the external nucleus of the IC (ECIC), the averaged number of c-Fos positive nuclei was 42 ± 8.4 / mm2 (n = 8) in the TM damaged rats which was significantly higher than 4.6 ± 1.4 / mm2 (n = 6) in the control rats (the Student's t-test, p=0.001). The c-Fos staining in the cochlear nucleus and the auditory cortex were either absent or very light in both groups. These results are similar to the results seen in mice genetically prone to AGS (Klein et al., 2004; Kwon et al., 1997), suggesting that early age TM damage can cause sound-evoked hyperexcitability in the IC, which may initiate the AGS.
Figure 3.
The c-Fos staining in the inferior colliculus in a control rat (A and C) and a tympanic membrane (TM) damaged rat (B and D). The inferior colliculus tissue was harvested 2 hours after exposure to 120 dB SPL white noise (1 minute). A strong c-Fos staining can be seen in the TM damaged rats primarily in the external nuclei (ECIC), the dorsal nuclei (DCIC) and the central nuclei of the inferior colliculus (CIC) (B and D), not in the rat in the Control Group (A and C).
Effects of vigabatrin on AGS
To test if GABA inhibition was involved in the AGS caused by the TM damage, effect of vigabatrin (250 mg/kg, i.p.), an antiepileptic drug which can increase the ambient GABA concentration in the brain (Willmore et al., 2009), has been tested in eleven rats in the TM Group. Saline (4-5 ml, i.p.) was used as the control. The AGS was tested (white noise exposure, 120 dB SPL, 1 minute or less) 2-3 hours after vigabatrin or saline treatment. After saline injection, 10 out of 11 rats showed AGS; whereas only one of the eleven rats showed AGS after the injection of vigabatrin (Table 2, Chi-square test, P<0.0001). Two-weeks after the acute vigabatrin treatment, AGS was retested in these rats. 10 out of 11 rats showed AGS. Vigabatrin treatment through oral intake (60 mg/kg/day, seven days) can also suppress AGS in 86% of the tested rats (6 out of 7 rats, table 3, Chi-square test, P<0.0001). Two weeks after stopping vigabatrin treatment, AGS can be induced in all of the rats (7 out of 7 rats) using the same procedure. Therefore, these data suggest the AGS caused by the TM damage may be related to lack of GABAergic inhibition.
Table 2.
Effects of acute vigabatrin or saline treatment (250 mg/kg, i.p.) on audiogenic seizure (AGS)
| Testing time | Number of rats | No AGS | AGS |
|---|---|---|---|
| Vigabatrin (2-3 hrs post treatment) | 11 | 10 | 1 |
| Saline (2-3 hrs post treatment) | 11 | 1 | 10 |
| Vigabatrin (2 weeks post treatment) | 11 | 1 | 10 |
Table 3.
Effects of chronic vigabatrin treatment (60 mg/kg/day, 7 days) on audiogenic seizure (AGS)
| Testing time | Number of rats | No AGS | AGS |
|---|---|---|---|
| Post 0 day | 7 | 6 | 1 |
| Post 2 weeks | 7 | 0 | 7 |
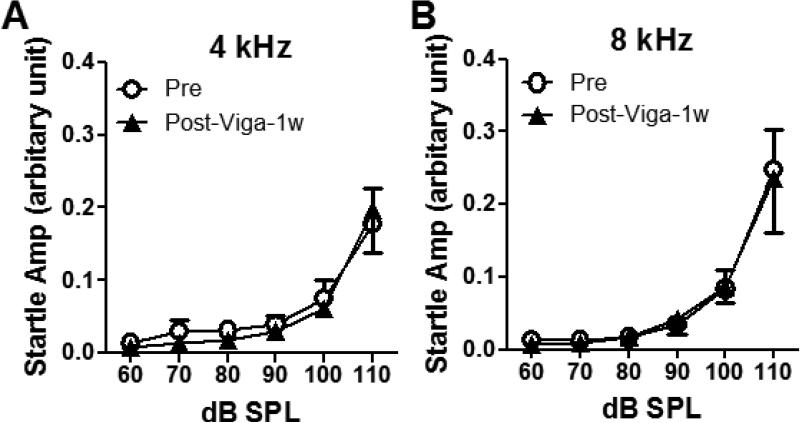
To confirm the suppressive effect of vigabatrin on AGS was not due to the impairment of rats’ motor control systems, the acoustic startle response before and after the vigabatrin treatment was tested in the rats in the TM Group (3-4 months after TM damage). There was no significant difference between the startle amplitude before and after seven days treatment of vigabatrin through the drinking water (60 mg/kg/day for seven days, Figure 4A-B). Therefore, the suppression of AGS was not due to the impairment on their motor controls.
Figure 4.
Oral treatment of vigabatrin (60 mg/kg/day, seven days) did not affect the acoustic startle response. Acoustic startle responses were tested in adult rats with TM damage at early age before and after vigabatrin treatment at (A) 4 kHz and (B) 8 kHz. There was no significant different before and after the treatment (n = 4, two-way ANOVA, P>0.05) (Results are presented as mean ± SEM).
DISCUSSION
One of the most interestingly findings of this study is that early age TM perforation (bilateral or monaural) can cause AGS in rats. This result suggests that hearing loss during the developmental period (not in adult) can cause severe long-term disorders in later life. Although most of the rats with TM damage at P16 developed AGS, about 1-2 rats did not develop AGS. Since during the TM surgery, the damage of the middle ear structure (ossicles and middle ear muscles) was avoided in order to prevent a permanent hearing loss, we think the susceptibility of AGS may be affected the recovery time from the TM damage. Interestingly, the monaural TM damage caused AGS in 75% of the rats (6 out of 8). The ratio was only slightly lower than the bilateral TM damage group (86%, 12 out of 14). These results suggest that even the monaural hearing loss is sufficient to affect the development of the central auditory system and impair sound processing.
Interestingly, the TM damaged rats also exhibited enhanced acoustic startle response even with an elevated hearing threshold, suggesting an increased sensitivity to acoustic stimulation. As increased sound sensitivity and reduced sound tolerance are typical signs for hyperacusis, our results suggest the TM damage at early age may cause hyperacusis behavior in rats (Ison et al., 2007; Sun et al., 2009; Turner et al., 2008). The cause of hyperacusis in human is still largely unknown. A recent studies on patients with hyperacusis have shown elevated activations in the auditory midbrain, thalamus, and primary auditory cortex, which may be related to the reduced central inhibition (Gu et al., 2010).
We also identified a strong c-Fos staining in the IC in the TM damaged rats after AGS, suggesting the AGS may be related to the hyperactivity in the IC responding to sound stimuli. These results are consistent with previous studies on AGS using mice genetically prone to AGS (Klein et al., 2004; Kwon et al., 1997). In addition, we found the AGS can be reversibly suppressed by vigabatrin treatments (i.p. injection or oral take), suggesting that the AGS caused by the TM damage may be related to a deficiency of GABA inhibition. Interestingly, the vigabatrin treatment (oral taking) can block audiogenic seizure without affecting the amplitude of acoustic startle reflex. This suggests that different change in the central nervous systems may be involved in causing audiogenic seizure and enhanced acoustic startle reflex.
How early age hearing loss affects the central auditory system development is not yet clear. Previous studies have shown that normal sound stimuli are crucial for the functional development of the central auditory system (Chang et al., 2003; Kral et al., 2002). Failure to receive proper excitatory input from the cochlea is known to cause the functional impairment in the central auditory system and various sound processing deficits. For instance, moderate hearing loss during development can alter the temporal properties of synapses and spikes (Xu et al., 2007), and prevent the synaptic inhibitory plasticity development which was regulated by the GABA-B receptors in the auditory cortex (Takesian et al., 2010). The conductive hearing loss at early age can also disrupt the binaural integration of interaural level differences in the IC (Popescu et al., 2010). However, the specific damage on the central inhibitory system caused by early age hearing loss still need more experiments to be revealed.
In summary, this study examined the behavioral consequences of early age conductive hearing loss on sound perception development. Our results suggest that early age sound deprivation caused by conductive hearing loss may cause an irreversible impairment in the central auditory system and increases the risk of developing hyperacusis.
Highlights.
Tympanic membrane damage at early age causes audiogenic seizure in rats.
Tympanic membrane damage at early age causes enhanced acoustic startle response
Extensively increased c-Fos staining in the inferior colliculus after audiogenic seizure
Vigabatrin treatment can suppress audiogenic seizure
Acknowledgements
This project was supported by Action on Hearing Loss (G42)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amoils CP, Jackler RK, Milczuk H, Kelly KE, Cao K. An animal model of chronic tympanic membrane perforation. Otolaryngol Head Neck Surg. 1992;106:47–55. doi: 10.1177/019459989210600127. [DOI] [PubMed] [Google Scholar]
- Bigelow DC, Kay D, Saunders JC. Effect of healed tympanic membrane perforations on umbo velocity in the rat. Ann Otol Rhinol Laryngol. 1998;107:928–34. doi: 10.1177/000348949810701105. [DOI] [PubMed] [Google Scholar]
- Chakravarty DN, Faingold CL. Differential roles in the neuronal network for audiogenic seizures are observed among the inferior colliculus subnuclei and the amygdala. Exp Neurol. 1999;157:135–41. doi: 10.1006/exnr.1999.7047. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chen CS. Acoustic trauma-induced developmental change in the acoustic startle response and audiogenic seizures in mice. Exp Neurol. 1978;60:400–3. doi: 10.1016/0014-4886(78)90094-8. [DOI] [PubMed] [Google Scholar]
- Chen CS, Gates R, Bock GR. Effect of priming and tympanic membrane destruction on development of audiogenic seizure susceptibility in BALB-c mice. Exp Neurol. 1973;39:277–84. doi: 10.1016/0014-4886(73)90230-6. [DOI] [PubMed] [Google Scholar]
- Coelho CB, Sanchez TG, Tyler RS. Hyperacusis, sound annoyance, and loudness hypersensitivity in children. Prog Brain Res. 2007;166:169–78. doi: 10.1016/S0079-6123(07)66015-4. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RC, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci. 2008;11:957–65. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E. Tonotopic Order in the Adult and Developing Auditory System of the Rat as Shown by c-fos Immunocytochemistry. Eur J Neurosci. 1992;4:798–812. doi: 10.1111/j.1460-9568.1992.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Gates GR, Chen CS, Bock GR. Effects of monaural and binaural auditory deprivation on audiogenic seizure susceptibility in BALB-c mice. Exp Neurol. 1973;38:488–93. doi: 10.1016/0014-4886(73)90170-2. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Farber N, Raveh E, Apter A, Attias J. Hyperacusis in Williams syndrome: characteristics and associated neuroaudiologic abnormalities. Neurology. 2006;66:390–5. doi: 10.1212/01.wnl.0000196643.35395.5f. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104:3361–70. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE. Age-Related Hearing Loss in C57BL/6J Mice has both Frequency-Specific and Non-Frequency-Specific Components that Produce a Hyperacusis-Like Exaggeration of the Acoustic Startle Reflex. J Assoc Res Otolaryngol. 2007;8:539–50. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LC, Feldman HM, Paradise JL, Bernard BS, Colborn DK, Casselbrant ML, Janosky JE. Tympanic membrane abnormalities and hearing levels at the ages of 5 and 6 years in relation to persistent otitis media and tympanostomy tube insertion in the first 3 years of life: a prospective study incorporating a randomized clinical trial. Pediatrics. 2004;114:e58–67. doi: 10.1542/peds.114.1.e58. [DOI] [PubMed] [Google Scholar]
- Klein AJ, Armstrong BL, Greer MK, Brown FR., 3rd Hyperacusis and otitis media in individuals with Williams syndrome. J Speech Hear Disord. 1990;55:339–44. doi: 10.1044/jshd.5502.339. [DOI] [PubMed] [Google Scholar]
- Klein BD, Fu YH, Ptacek LJ, White HS. c-Fos immunohistochemical mapping of the audiogenic seizure network and tonotopic neuronal hyperexcitability in the inferior colliculus of the Frings mouse. Epilepsy Res. 2004;62:13–25. doi: 10.1016/j.eplepsyres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Kristjansson S, Skuladottir HE, Sturludottir M, Wennergren G. Increased prevalence of otitis media following respiratory syncytial virus infection. Acta Paediatr. 2010;99:867–70. doi: 10.1111/j.1651-2227.2009.01637.x. [DOI] [PubMed] [Google Scholar]
- Kwon J, Pierson M. Fos-immunoreactive responses in inferior colliculi of rats with experimental audiogenic seizure susceptibility. Epilepsy Res. 1997;27:89–99. doi: 10.1016/s0920-1211(97)01024-3. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.3.e1. [DOI] [PubMed] [Google Scholar]
- Lasisi AO, Olayemi O, Irabor AE. Early onset otitis media: risk factors and effects on the outcome of chronic suppurative otitis media. Eur Arch Otorhinolaryngol. 2008;265:765–8. doi: 10.1007/s00405-007-0544-1. [DOI] [PubMed] [Google Scholar]
- Martines F, Bentivegna D, Di Piazza F, Martinciglio G, Sciacca V, Martines E. The point prevalence of otitis media with effusion among primary school children in Western Sicily. Eur Arch Otorhinolaryngol. 2010;267:709–14. doi: 10.1007/s00405-009-1131-4. [DOI] [PubMed] [Google Scholar]
- McGinn MD, Willott JF, Henry KR. Effects of conductive hearing loss on auditory evoked potentials and audiogenic seizures in mice. Nature - New Biology. 1973;244:255–6. doi: 10.1038/newbio244255a0. [DOI] [PubMed] [Google Scholar]
- Miani C, Passon P, Bracale AM, Barotti A, Panzolli N. Treatment of hyperacusis in Williams syndrome with bilateral conductive hearing loss. Eur Arch Otorhinolaryngol. 2001;258:341–4. doi: 10.1007/s004050100364. [DOI] [PubMed] [Google Scholar]
- O'Leary SJ, Triolo RD. Surgery for otitis media among Indigenous Australians. Med J Aust. 2009;191:S65–8. doi: 10.5694/j.1326-5377.2009.tb02930.x. [DOI] [PubMed] [Google Scholar]
- Pierson M, Liebmann SL. Noise exposure-induced audiogenic seizure susceptibility in Sprague-Dawley rats. Epilepsy Res. 1992;13:35–42. doi: 10.1016/0920-1211(92)90005-e. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–31. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS. The measurement of loudness. Journal of the Acoustical Society of America. 1955;27:815–829. [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci. 2010;30:2716–27. doi: 10.1523/JNEUROSCI.3903-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–92. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Abelson MB, Ben-Menachem E, Pellock JM, Shields WD. Vigabatrin: 2008 update. Epilepsia. 2009;50:163–73. doi: 10.1111/j.1528-1167.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–26. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]