Abstract
Phorbol esters bind to and activate a calcium phospholipid-dependent protein kinase (C kinase). Some researchers believe that activation of C kinase is necessary for the induction of phorbol ester biologic effects. Our research indicates that bryostatin, a macrocyclic lactone that binds to the phorbol ester receptor in human polymorphonuclear leukocytes, also binds to this receptor in the human promyelocytic leukemia cell line, HL-60. Bryostatin activates partially purified C kinase from HL-60 cells in vitro, and when applied to HL-60 cells in vivo, it decreases measurable cytoplasmic C kinase activity. Unlike the phorbol esters, bryostatin is unable to induce a macrophage-like differentiation of HL-60 cells; however, bryostatin, in a dose-dependent fashion, blocks phorbol ester-induced differentiation of HL-60 cells and, if applied within 48 hr of phorbol esters, halts further differentiation. These results suggest that activation of the C kinase by some agents is not sufficient for induction of HL-60 cell differentiation and imply that some of the biologic effects of phorbol esters may occur through a more complex mechanism than previously thought.
Full text
PDF
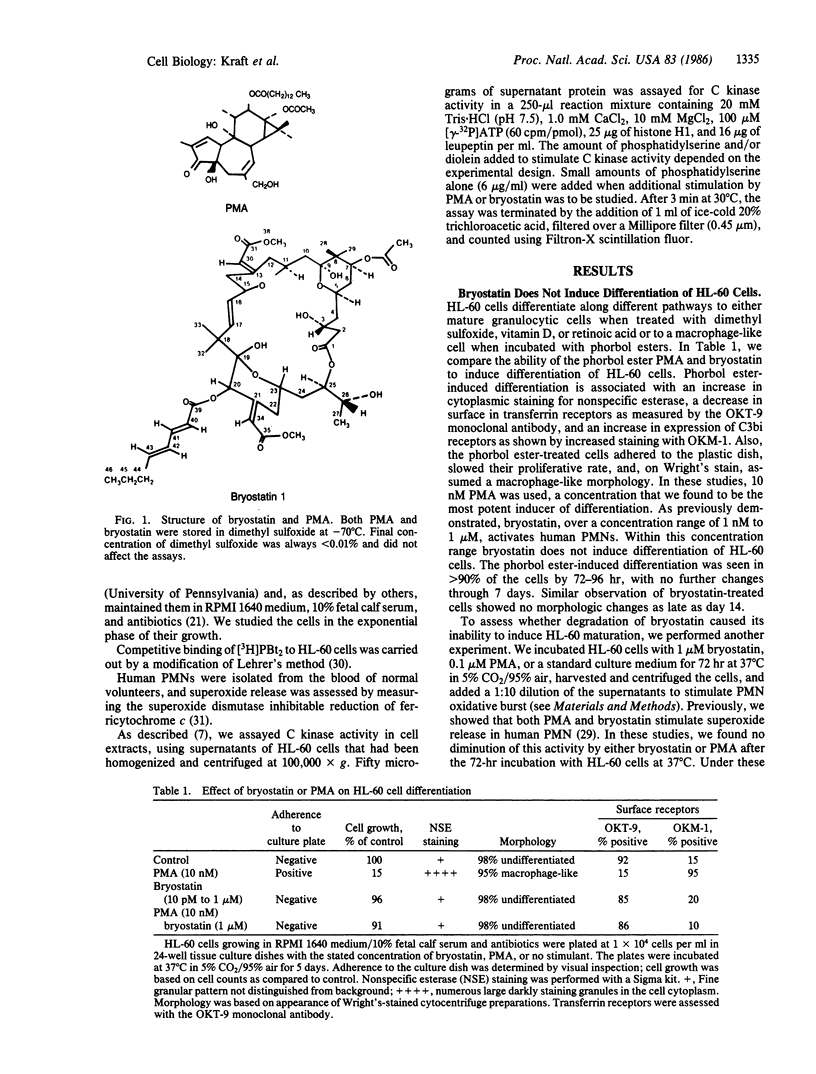
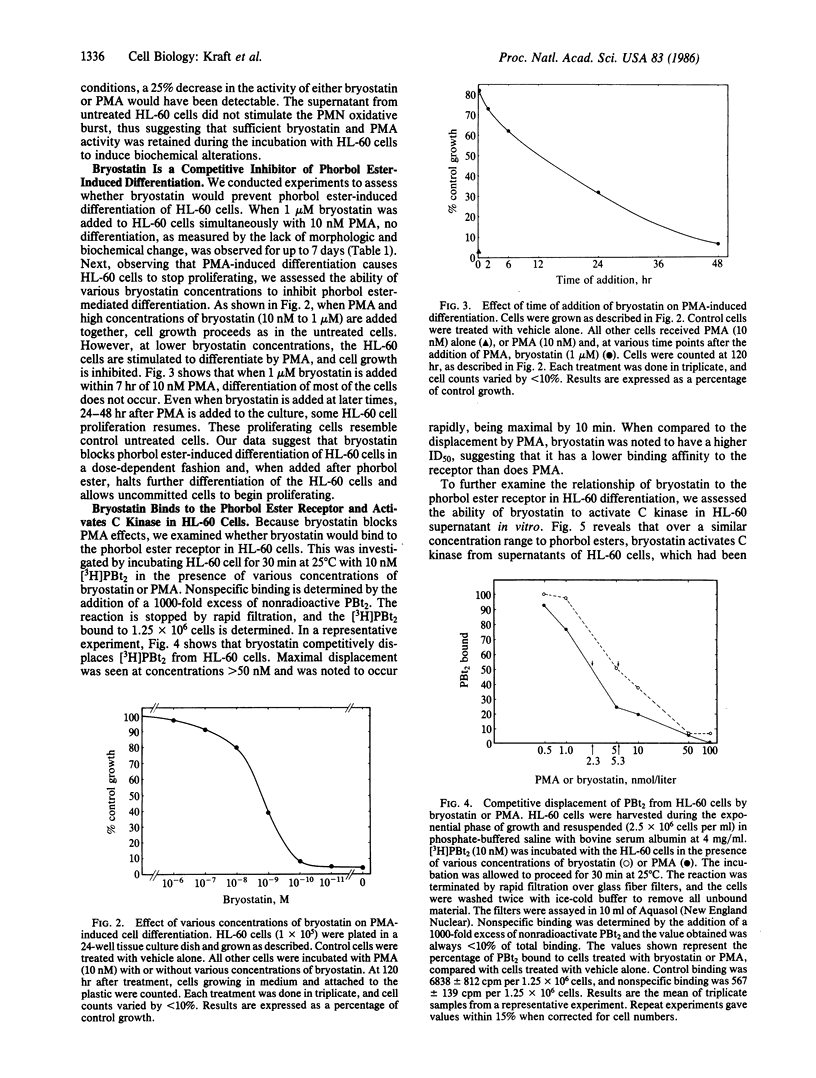
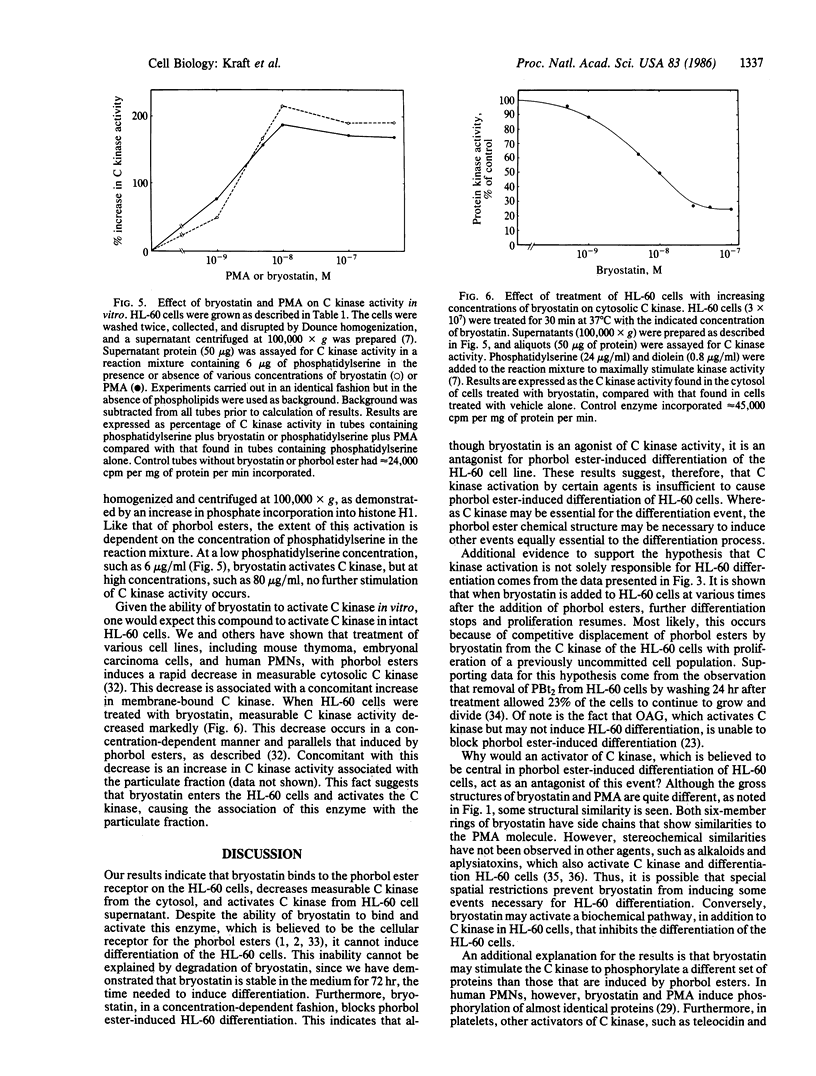

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcoleo J. P., Weinstein I. B. Activation of protein kinase C by tumor promoting phorbol esters, teleocidin and aplysiatoxin in the absence of added calcium. Carcinogenesis. 1985 Feb;6(2):213–217. doi: 10.1093/carcin/6.2.213. [DOI] [PubMed] [Google Scholar]
- Ashendel C. L., Staller J. M., Boutwell R. K. Protein kinase activity associated with a phorbol ester receptor purified from mouse brain. Cancer Res. 1983 Sep;43(9):4333–4337. [PubMed] [Google Scholar]
- Ashendel C. L., Staller J. M., Boutwell R. K. Solubilization, purification, and reconstitution of a phorbol ester receptor from the particulate protein fraction of mouse brain. Cancer Res. 1983 Sep;43(9):4327–4332. [PubMed] [Google Scholar]
- Berkow R. L., Kraft A. S. Bryostatin, a non-phorbol macrocyclic lactone, activates intact human polymorphonuclear leukocytes and binds to the phorbol ester receptor. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1109–1116. doi: 10.1016/0006-291x(85)90205-0. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Tzeng D. Y., Williams L. V., Baehner R. L. The comparative responses of human polymorphonuclear leukocytes obtained by counterflow centrifugal elutriation and Ficoll-Hypaque density centrifugation. I. Resting volume, stimulus-induced superoxide production, and primary and specific granule release. J Lab Clin Med. 1983 Nov;102(5):732–742. [PubMed] [Google Scholar]
- Cassileth P. A., Suholet D., Cooper R. A. Early changes in phosphatidylcholine metabolism in human acute promyelocytic leukemia cells stimulated to differentiate by phorbol ester. Blood. 1981 Aug;58(2):237–243. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper R. A., Braunwald A. D., Kuo A. L. Phorbol ester induction of leukemic cell differentiation is a membrane-mediated process. Proc Natl Acad Sci U S A. 1982 May;79(9):2865–2869. doi: 10.1073/pnas.79.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H., Tanaka Y., Miyake R., Kikkawa U., Nishizuka Y., Sugimura T. Activation of calcium-activated, phospholipid-dependent protein kinase (protein kinase C) by new classes of tumor promoters: teleocidin and debromoaplysiatoxin. Biochem Biophys Res Commun. 1984 Apr 30;120(2):339–343. doi: 10.1016/0006-291x(84)91259-2. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Protein kinase C and calcium ion in mitogenic response of macrophage-depleted human peripheral lymphocytes. J Biol Chem. 1985 Feb 10;260(3):1366–1369. [PubMed] [Google Scholar]
- Kajikawa N., Kaibuchi K., Matsubara T., Kikkawa U., Takai Y., Nishizuka Y., Itoh K., Tomioka C. A possible role of protein kinase C in signal-induced lysosomal enzyme release. Biochem Biophys Res Commun. 1983 Oct 31;116(2):743–750. doi: 10.1016/0006-291x(83)90587-9. [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Takai Y., Minakuchi R., Sano K., Nishizuka Y. Phospholipid turnover as a possible transmembrane signal for protein phosphorylation during human platelet activation by thrombin. Biochem Biophys Res Commun. 1980 Nov 17;97(1):309–317. doi: 10.1016/s0006-291x(80)80169-0. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kaibuchi K., Castagna M., Yamanishi J., Sano K., Tanaka Y., Miyake R., Takai Y., Nishizuka Y. Protein phosphorylation and mechanism of action of tumor-promoting phorbol esters. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:437–442. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Kreutter D., Caldwell A. B., Morin M. J. Dissociation of protein kinase C activation from phorbol ester-induced maturation of HL-60 leukemia cells. J Biol Chem. 1985 May 25;260(10):5979–5984. [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Ganong B. R., Bell R. M. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985 Feb 10;260(3):1358–1361. [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. E., Koeffler H. P. Specific binding of [3H]phorbol dibutyrate to phorbol diester-responsive and -resistant clones of a human myeloid leukemia (KG-1) line 1. Cancer Res. 1983 Aug;43(8):3563–3566. [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., White J. R., Sha'afi R. I. Phorbol esters inhibit the fMet-Leu-Phe- and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J Biol Chem. 1985 Feb 25;260(4):2125–2131. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Smith L., Pettit G. R. Bryostatins: potent, new mitogens that mimic phorbol ester tumor promoters. Biochem Biophys Res Commun. 1985 Nov 15;132(3):939–945. doi: 10.1016/0006-291x(85)91898-4. [DOI] [PubMed] [Google Scholar]
- Sugden D., Vanecek J., Klein D. C., Thomas T. P., Anderson W. B. Activation of protein kinase C potentiates isoprenaline-induced cyclic AMP accumulation in rat pinealocytes. 1985 Mar 28-Apr 3Nature. 314(6009):359–361. doi: 10.1038/314359a0. [DOI] [PubMed] [Google Scholar]
- Tapley P. M., Murray A. W. Modulation of Ca2+-activated, phospholipid-dependent protein kinase in platelets treated with a tumor-promoting phorbol ester. Biochem Biophys Res Commun. 1984 Jul 18;122(1):158–164. doi: 10.1016/0006-291x(84)90453-4. [DOI] [PubMed] [Google Scholar]
- Vandenbark G. R., Kuhn L. J., Niedel J. E. Possible mechanism of phorbol diester-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1984 Feb;73(2):448–457. doi: 10.1172/JCI111231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz P. W., Mueller G. C. Rapid stimulation of phospholipid metabolism in bovine lymphocytes by tumor-promoting phorbol esters. Cancer Res. 1978 Sep;38(9):2900–2904. [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Yamamoto S., Gotoh H., Aizu E., Kato R. Failure of 1-oleoyl-2-acetylglycerol to mimic the cell-differentiating action of 12-O-tetradecanoylphorbol 13-acetate in HL-60 cells. J Biol Chem. 1985 Nov 15;260(26):14230–14234. [PubMed] [Google Scholar]



