Abstract
The cardiovascular system operates under demands ranging from conditions of rest to extreme stress. One mechanism of cardiac stress tolerance is action potential duration shortening driven by ATP-sensitive potassium (KATP) channels. KATP channel expression has a significant physiologic impact on action potential duration shortening and myocardial energy consumption in response to physiologic heart rate acceleration. However, the effect of reduced channel expression on action potential duration shortening in response to severe metabolic stress is yet to be established. Here, transgenic mice with myocardium-specific expression of a dominant negative KATP channel subunit were compared with littermate controls. Evaluation of KATP channel whole cell current and channel number/patch was assessed by patch clamp in isolated ventricular cardiomyocytes. Monophasic action potentials were monitored in retrogradely perfused, isolated hearts during the transition to hypoxic perfusate. An 80-85% reduction in cardiac KATP channel current density results in a similar magnitude, but significantly slower rate, of shortening of the ventricular action potential duration in response to severe hypoxia, despite no significant difference in coronary flow. Therefore, the number of functional cardiac sarcolemmal KATP channels is a critical determinant of the rate of adaptation of myocardial membrane excitability, with implications for optimization of cardiac energy consumption and consequent cardioprotection under conditions of severe metabolic stress.
Keywords: ATP-sensitive potassium channel, K-ATP, heart, glyburide, monophasic action potential
1. Introduction
The cardiovascular system operates under a wide span of demands, ranging from conditions of rest to extreme stress. Identification of mechanisms underlying cardiac stress resistance is an area of active investigation. One mechanism of cardiac stress tolerance is through action potential duration (APD) shortening driven by ATP-sensitive potassium (KATP) channels [1-14].
KATP channels are comprised of the pore-forming inwardly rectifying potassium channel, Kir6.x, with the regulatory sulfonylurea receptor, SUR [5,15-18]. The metabolic sensing of KATP channels occurs through modulation of the ATP sensitivity of Kir6.x by SUR which is closely integrated with cellular energetic networks [6,19-27]. When activated by a reduced ATP/ADP ratio, reflecting increased cellular metabolic demand, KATP channel-dependent potassium efflux shortens cardiac APD [1-3,5,28]. This potassium efflux limits sodium and calcium entry into the cell and thus reduces energy requirements for ion transport/exchange and contraction as well as prolongs the diastolic interval that supports myocardial relaxation and restoration of ion gradients and energetic resources [1-14].
Reflecting the importance of myocardial energy homeostasis, cardiac KATP channels are among the most densely expressed membrane protein complexes in cardiomyocytes [5,14,21,28]. Indeed, models based on data obtained in isolated cardiomyocytes, and indirect evidence in intact hearts, indicate that opening of <1% of sarcolemmal KATP channels is sufficient to dramatically shorten the APD [5,20,24,29-31]. By implication, KATP channel physiologic effects should thus be insensitive to KATP channel expression increases, and buffered against all but the most severe expression decreases. However, we have recently shown that changes in KATP channel expression have a significant physiologic impact with regard to both APD shortening and myocardial energy consumption in response to physiologic increases in heart rate [1]. Compared with control, a ~50% increase in KATP channel membrane expression results in greater and more rapid APD shortening associated with less myocardial oxygen consumption in response to heart rate acceleration within the physiologic range, while an ~80% reduction in functional KATP channels has the opposite effect [1]. These findings indicate the importance of the level of KATP channel expression in sensing slight changes in cardiomyocyte energetics coupled to physiologic increases in workload. Still, the effect of reduced channel expression on APD shortening in response to a severe metabolic stress that activates most or all available KATP channels, is yet to be established.
Here, we demonstrate that in hearts exposed to severe hypoxia, an 80-85% reduction in KATP channel current density results in a normal magnitude of APD shortening, but occurring at a significantly slower rate. Thus, this study provides direct evidence to support the previously proposed models linking dramatic shortening of the cardiac APD to activation of a small percentage of KATP channels. This study further indicates that the number of functional KATP channels is a critical determinant of the rate of adaptation of membrane excitability. These findings have implications for optimization of cardiac energy consumption and consequent cardioprotection, under conditions of severe metabolic stress.
2. Materials and Methods
2.1 Generation of genetically modified mice
Transgenic Tg[CX1-eGFP-Kir6.1AAA] mice (Fig. S1) were created on an FVB background using a non-conducting Kir6.1AAA pore mutant where a Gly-Phe-Gly motif critical for K+ permeation was replaced by Ala-Ala-Ala [8]. Expression of the Kir6.1AAA transgene has been shown to dramatically reduce KATP channel current in tissues with channels comprised of either Kir6.2 or Kir6.1 isoforms [1,3,8,32]. Kir6.1AAA was subcloned downstream of a loxP site-flanked eGFP coding region and a stop codon, in such a way that dominant negative suppression of Kir6.2/SUR2A potassium efflux could be initiated by expression of Cre recombinase. Accordingly, cardiac-specific reduction of KATP channel activity was achieved by crossing Tg[CX1-eGFP-Kir6.1AAA] and Tg[αMHC-Cre] mice [33]. The resulting offspring are notated Tg[αMHC-Kir6.1AAA]. Male WT, Tg[CX1-eGFP-Kir6.1AAA], and Tg[αMHC-Kir6.1AAA] littermate mice, aged 8-12 weeks, were used for experiments. Protocols conform to the Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Institutional Animal Care and Use Committee.
2.2 Isolated heart studies
Hearts were extracted from anesthetized mice and retrogradely perfused at 90 mmHg with Krebs-Henseleit buffer bubbled with 95% O2/5% CO2, at 37°C and pH 7.4 [1-3,12]. The AV node was mechanically dissociated and hearts paced at 130 ms cycle length (Bloom Electrophysiology, Fischer Imaging Corp., Denver, CO) using a platinum pacing catheter positioned in the right ventricle (NuMed; Hopkinton, NY). For hypoxia, a separate reservoir of Krebs-Henseleit buffer was bubbled with 95%N2/5%CO2, at 37°C and pH 7.4 [12]. A valve followed by a small bubble chamber and an oxygen sensor immediately above the heart was used to quickly switch the perfusing solutions. Oxygen partial tension was measured in the perfusate prior to heart passage (Model 210, Instech Laboratories, Plymouth Meeting, PA). Coronary flow was measured in series with the aortic cannula (T402, Transonic Systems, Ithica, NY).
A monophasic action potential (MAP) probe (EP Technologies; Sunnyvale, CA) was maintained at a single stable position on the LV epicardium, and amplified signals (IsoDam; World Precision Instruments; Sarasota, FL) were acquired at 2 kHz [1-3,12]. MAP recordings were analyzed (MATLAB, Mathworks, Natick, MA) for duration at 90% repolarization (APD90). Changes in APD90 (ΔAPD90) were calculated using the duration of the MAP just prior to the initiation of hypoxia as a reference. MAPs were only analyzed from tracings in which pacing capture was maintained without interruption. All calculations were manually reviewed.
2.3 Patch-clamp studies
Single ventricular cardiomyocytes were enzymatically isolated [25,26] and patch clamp studies performed as previously described [1].
2.4 Imaging
Bright field and fluorescence images were captured using an Olympus SZX12 stereoscope with 1x and 40X objectives and a fiberoptic white light or 100W mercury lamp. A GFP-specific filter was used for fluorescence images. An Olympus DP72 CCD camera and DP72 BSW software were used to acquire images. Fluorescence images were taken at the same light intensity, aperture setting and exposure time.
2.5 Statistical analysis
Results are expressed as mean±SEM. Comparisons between two groups are made using the 2-sided Student’s t-test and between multiple groups using analysis of variance (ANOVA) and appropriate post hoc tests. A p value <.05 is considered significant.
3. Results
3.1 Selective myocardial expression of the potassium impermeable Kir6.1AAA pore-forming mutant is tracked by elimination of green fluorescence
Tg[αMHC-Cre] mice are crossed with Tg[CX1-eGFP-Kir6.1AAA] mice to produce Tg[αMHC-Kir6.1AAA] with selective myocardial expression of Kir6.1AAA, tracked by elimination of eGFP expression (Fig. S1). Previous studies have confirmed myocardial-specific expression of the Kir6.1AAA protein in Tg[αMHCKir6.1AAA] mice [8]. Here, WT hearts (Fig. S1C) show faint autofluorescence of the great vessels (40X views). In contrast, hearts from Tg[CX1-eGFP-Kir6.1AAA] mice show bright green fluorescence of both myocardium and the great vessels (Fig. S1A, inset and rightmost column). Hearts from Tg[αMHC-Kir6.1AAA] mice show green fluorescence in the great vessels (40X views), but elimination of green fluorescence in the myocardium (40X and inset), consistent with the myocardial-specific expression of αMHC (Fig. S1B).
3.2 Transgenic expression of Kir6.1AAA significantly reduces the KATP channel current density
Cardiomyocytes isolated from hearts of Tg[αMHC-Kir6.1AAA] and control mice were exposed to the mitochondrial uncoupler, 2,4-dinitrophenol (DNP), and the specific KATP channel opener, pinacidil. Expression of the Kir6.1AAA transgene is associated with a reduction in whole cell KATP channel current, normalized to cell size, of ~85% (WT: 18.1±2.3 pA/pF, Tg[CX1-eGFPKir6.1AAA]: 17.4±1.7 pA/pF, Tg[αMHC-Kir6.1AAA]: 2.5±.6 pA/pF, Fig. S2A and B, *p<.05). Similarly, the number of KATP channels/patch is reduced by ~80% in the presence of the Kir6.1AAA transgene, compared with littermate controls (WT: 4.7±.7, Tg[CX1-eGFP-Kir6.1AAA]: 4.6±.5, Tg[αMHC-Kir6.1AAA]: .9±.2, Fig. S2C and E, p<.05), despite no differences in average pipette tip size (Fig. S2D, p=NS). These data are similar to our previously published findings [1].
3.3 Reduction in functional KATP channels results in the same magnitude, but a significantly slower time-course, of APD shortening in response to hypoxia
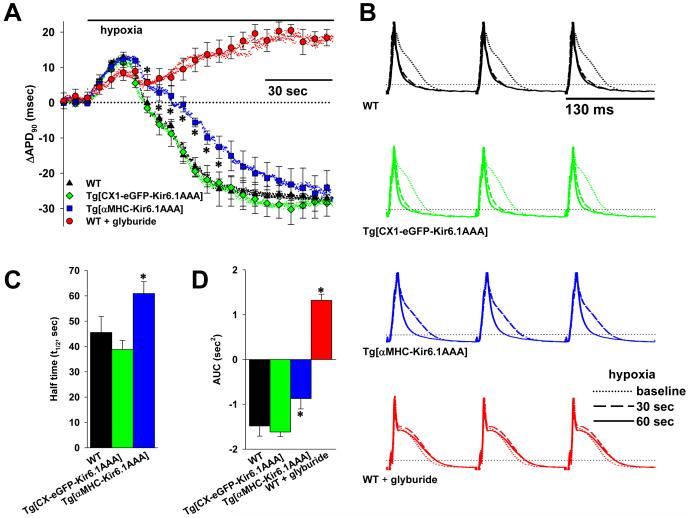
MAP duration at 90% repolarization (APD90) was measured from a single, stable position on the left ventricular epicardium of isolated hearts from Tg[αMHC-Kir6.1AAA] and control mice, while pacing at a fixed cycle length. The change in APD90 of each heart (ΔAPD90) compared to its own baseline duration is plotted as a function of time before and after a change in the heart perfusate to the hypoxic solution (Fig. 1A). In hearts from control mice, the ΔAPD90 follows a time-course in response to hypoxia characterized by an initial lengthening, followed by a continuous decrease until a new steady-state duration is achieved at about one minute. However, in hearts from Tg[αMHC-Kir6.1AAA] mice, the ΔAPD90 demonstrates a similar initial increase, but has a slower decreasing phase, although eventually achieving the same steady-state as in controls. As a result, the half-time of achieving the new steady state ΔAPD90 is significantly longer in hearts from Tg[αMHC-Kir6.1AAA] mice (WT: 45.5±6.3 sec, Tg[CX1-eGFP-Kir6.1AAA]: 38.8±3.6 sec, Tg[αMHC-Kir6.1AAA]: 60.9±4.7 sec, Fig. 1C, *p<.05). The area under the curve (AUC), which subtracts the effects of positive and negative APD90 deflections from baseline, is significantly smaller in hearts from Tg[αMHC-Kir6.1AAA] mice than controls (WT −1.48±.23 sec2, Tg[CX1-eGFP-Kir6.1AAA] −1.62±.10 sec2, Tg[αMHC-Kir6.1AAA] −.87±.23 sec2, Fig. 1D, *p<.05). Of note, there is no difference in coronary flow at baseline or during hypoxia in hearts from Tg[αMHC-Kir6.1AAA] mice compared with WT or Tg[CX1-eGFP-Kir6.1AAA] controls (2.95±.22, 3.15±.79, 3.2±.41 ml/min baseline and 2.55±.5, 2.4±.6, and 3.0±.8 ml/min during hypoxia, respectively, p=NS), consistent with limitation of dominant-negative Kir6.1AAA expression to the myocardium (Fig. S1), without effect on vascular KATP channels [32]. As an additional control, ΔAPD90 was also tracked in WT hearts perfused with the specific KATP channel blocker glyburide at a dose of 10μM, sufficient to completely block cardiac KATP channel current [34]. This also reveals an initial increase in ΔAPD90 in response to hypoxia, but is then followed by only a slight dip towards baseline, before continuing to lengthen until reaching a steady-state well above baseline (Fig. 1A). This is associated with a very positive AUC (1.32±.14 sec2, Fig. 1D). Consistent with the effect of glyburide on increasing vasoconstriction through blockade of Kir6.1-containing vascular smooth muscle KATP channels, the coronary flow is reduced both at baseline and during exposure to hypoxia (1.4±.12 ml/min baseline and 1.3±.09 ml/min during hypoxia, n=5, p<.05 compared to WT without glyburide treatment).
Figure 1. Reduction in functional KATP channels translates into slowed APD shortening in response to hypoxia.
A. Summary plots of beat-to-beat MAP duration shortening (ΔAPD90) following initiation of hypoxia in isolated, perfused hearts from WT (n=4), Tg[CX1-eGFPKir6.1AAA] (n=3), and Tg[αMHC-αMHC-Kir6.1AAA] vs. WT and Tg[CX1-eGFP-Kir6.1AAA] controls). Also graphed is the response in glyburide (10μM) treated hearts from WT mice (n=5, *p<.05 vs. WT hearts not treated with glyburide). Symbols with error bars mark the mean±S.E for each 40th APD. B. Representative examples of MAPs recorded from WT, Tg[CX1-eGFP-Kir6.1AAA], Tg[αMHC-Kir6.1AAA] and glyburide treated WT hearts paced at 130 ms cycle length. MAPs at baseline and after 30 and 60 seconds of hypoxia are superimposed for comparison. Horizontal dotted lines indicate 90% repolarization. C. Summary statistics showing the half time (t½) of the ΔAPD90 response (*p<.05 for Tg[αMHCKir6.1AAA] vs. combined WT and Tg[CX1-eGFP-Kir6.1AAA] controls). D. Summary statistics showing the area under the curve (AUC) of the ΔAPD90 time course (*p<.05 for Tg[αMHCKir6.1AAA] and glyburide-treated WT vs. combined WT and Tg[CX1-eGFP-Kir6.1AAA] controls).
4. Discussion
KATP channels are membrane protein complexes vital for preservation of myocardial energy balance under normal and disease conditions [1-3,5,6,12,14,15]. Such a basic physiologic role in cardiac adaptation is consistent with the high expression of these channels in cardiomyocytes [2,3,5,6,14,15,21,24]. Here we use myocardial-specific expression of a dominant-negative KATP channel subunit to reduce the number of functional KATP channels on the membrane of cardiomyocytes, with a consequent reduction in the inducible KATP channel current. This reduction is associated with a slowed time-course of APD shortening in isolated hearts in response to the metabolic stress imposed by hypoxia. This study is in agreement with previous studies that indicate a deficit in functional KATP channels results in both a limitation of APD shortening and increased myocardial oxygen consumption under heart rate acceleration, proarrhythmia and vulnerability to injury [1-3,8-10,12-14,35,36].
As such, the AUC (Fig. 1D), which quantifies the difference between positive and negative areas of APD90 deflection from baseline, can be taken as a measure of the efficiency of energy conservation in response to metabolic challenge, with more negative values representing overall greater responsiveness of APD shortening and thus improved dynamics of energy economy [1]. In the current study, we see that, despite the same steady-state degree of APD shortening in response to hypoxia, there is a smaller AUC in KATP channel deficient hearts. This reduction in dynamic control of energy efficiency could be expected to result in increased myocardial vulnerability to injury and dysfunction, likely contributing to the reduced exercise tolerance and increased mortality in these mice [8]. Furthermore, the achievement of the same degree of steady-state APD shortening in hearts with normal and ~80-85% reduced expression of functional KATP channels provides direct experimental support for models predicting that opening a small percentage of normally expressed KATP channels is sufficient to dramatically reduce APD [5,20,24,30]. In addition, the present data provide important detail about the time-course of shortening, indicating that the level of membrane KATP channel expression may impact cardioprotection through the rapidity of APD response, and thus efficiency of cardiac energetic conservation, under severe metabolic challenge.
The complex time-course of APD changes in response to hypoxia as measured here using MAPs in intact murine hearts (Fig. 1A), is similar to data obtained using sharp intracellular electrodes in isolated guinea-pig cardiomyocytes exposed to the mitochondrial uncoupler DNP [37]. In that study, the APD also initially increased following metabolic inhibition, before a phase of dramatic shortening. The initial APD increase was attributed to inhibition of the electrogenic sodium pump caused by ATP depletion, while the subsequent dramatic APD decrease was attributed to the simultaneous pronounced increase in outward current with a reversal potential consistent with a predominant potassium ion component. A similar phenomenon was more recently reported using the patch clamp technique, also in isolated guinea-pig ventricular myocytes in response to DNP [38], and in a study recording MAPs obtained through a suction electrode on the apex of intact ferret hearts exposed to cyanide [39]. The similarities in time-course of APD changes between these studies support that the phenomenon is unlikely to be a technical artifact, or to be species specific, although the magnitude of the effect would be difficult to compare between studies and could have a technical or species-related component. The effect may be more pronounced in the current study due to our beat-to-beat recording and higher metabolic rate (37°C and paced at 130ms C.L vs. cooler temperatures and/or slower pacing rates in the other studies). The differences demonstrated here in the time-course of APD adjustment in Tg[αMHC-Kir6.1AAA] hearts cannot be attributed to mitochondrial KATP channels as no expression of the Kir6.1AAA transgene has been found in the mitochondria [8].
Given the persistence of GFP fluorescence in the great vessels of Tg[αMHC-Kir6.1AAA] hearts (Fig. S1), and the preserved coronary flow, we also rule out disruption of KATP channel function in the vasculature as a cause for the altered APD response to hypoxia in Tg[αMHCKir6.1AAA] mice. In contrast, glyburide treated WT hearts, in which both myocardial and vascular KATP channels would be blocked [34], exhibit both reduced coronary flow and loss of APD shortening (Fig. 1A, B). Interestingly, not only does perfusion with glyburide result in an absence of APD shortening with hypoxia, but it is also associated with a slow increase in APD over time. This results in a markedly positive AUC, and would be expected to represent an energetically very unfavorable outcome. Indeed, we previously showed that oxygen consumption with heart rate acceleration is significantly increased in hearts perfused with glyburide, coincident with loss of APD shortening [1]. Those data, in addition to the data reported here with hypoxia, as well as numerous other studies [34], indicate a negative effect of glyburide on cardioprotective KATP channel current. This would suggest that this drug, used extensively for the treatment of diabetes due to its blockade of pancreatic β-cell KATP channel isoforms and related increase in insulin secretion, would have a detrimental and potentially dangerous effect on cardiac stress resistance. However, clinical studies have shown inconsistent results in this regard [40], likely due to the finding that the clinically desirable effect of glyburide inhibition of pancreatic KATP channel isoforms is irreversible, while the potentially harmful inhibition of cardiac channels is only transient [34].
The finding that cardiac KATP channel expression has an impact on the APD rate of response to metabolic challenge lends insight into heart diseases manifested by reduced KATP channel expression, such as some forms or stages of heart failure [11,12,41]. While reduced KATP channel expression may not impact the maximum KATP channel-dependent APD change, loss of prompt APD responsiveness may exacerbate metabolic deficits in the already energetically challenged myocardium. It is possible that disease states such as heart failure may also result in heterogenous loss of KATP expression such that parts of the myocardium respond more slowly to changes in heart rate or oxygen delivery resulting in a time-dependent dispersion of refractoriness contributing to an increased propensity to arrhythmias [42-44]. Thus, understanding mechanisms and effects of KATP channel expression regulation may provide novel strategies for combating the energetic and electrical derangements that contribute to progression of myocardial dysfunction.
Supplementary Material
Figure S1. Expression of Kir6.1AAA is tracked by elimination of GFP fluorescence. Brightfield and fluorescence images of hearts from A. Tg[CX1-eGFP-Kir6.1AAA], B. Tg[αMHCKir6.1AAA], and C. WT mice. Retained suture material from retroperfusion marks the aorta. RA: right atrium, LA: left atrium, RV: right ventricle, LV: left ventricle, PA: pulmonary artery, RVOT: right ventricular outflow tract, Ao: aorta.
Figure S2. Expression of Kir6.1AAA reduces the number of functional KATP channels. A. Summary statistics of KATP channel whole cell current stimulated by DNP (50 μM) and pinacidil (100 μM) in WT (n=8) and Tg[CX1-eGFP-Kir6.1AAA] control (n=6) ventricular myocytes (p=NS), vs. Tg[αMHC-Kir6.1AAA] (n=10) cardiomyocytes (*p<.05). Current is normalized to membrane capacitance (pA/pF). B. Representative whole cell current recordings at baseline and after stimulation of KATP channel opening by DNP and pinacidil. C. Summary statistics indicating the number of KATP channels in cell-attached patches of ventricular myocytes stimulated with DNP (200 μM) and pinacidil (100 μM), from WT (n=13) and Tg[CX1-eGFP-Kir6.1AAA] (n=16) hearts (p=NS), vs. Tg[αMHC-Kir6.1AAA] (n=17) hearts (*p<.05). D. There was no significant difference in the patch pipette tip size as estimated by tip resistance (WT: 4.6±.20 MΩ, Tg[CX1-eGFPKir6.1AAA]: 4.5±.2 MΩ, Tg[αMHC-Kir6.1AAA]: 4.4±.1 MΩ, p=NS). E. Representative cell-attached recordings before, during, and after wash-out of DNP and pinacidil. DNP: 2,4-dinitrophenol.
Highlights.
Sarcolemmal KATP channels are essential for cardiac resistance to stress
KATP channel activation shortens action potential duration (APD), conserving energy
Reduction in cardiac KATP channels does not alter magnitude of APD shortening
Cardiac KATP channel number defines rate of APD shortening under hypoxia
Sarcolemmal KATP channel expression defines cardiac energetic efficiency
Acknowledgments
This work was supported by the National Institutes of Health [HL092286 to D.H-Z., HL093368 to L.Z., HL085820 to W.A.C.]; The Carver Trust [01-224 to D.H-Z. and to L.Z.]; The Central Society for Clinical Research [to D.H-Z.]; and the Fraternal Order of Eagles [to D.H-Z. and L.Z.]
We are grateful to Dr. Michael D. Schneider for Tg[αMHC-Cre] mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- [1].Zingman LV, Zhu Z, Sierra A, et al. Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation. J. Mol. Cell. Cardiol. 2011;51:72–81. doi: 10.1016/j.yjmcc.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alekseev AE, Reyes S, Yamada S, et al. Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell. Metab. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- [5].Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol. Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J. Appl. Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- [7].Moore RL. Myocardial KATP channels are critical to Ca2+ homeostasis in the metabolically stressed heart in vivo. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1692–3. doi: 10.1152/ajpheart.00076.2007. [DOI] [PubMed] [Google Scholar]
- [8].Tong X, Porter LM, Liu G, et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H543–51. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamada S, Kane GC, Behfar A, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J. Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu XK, Yamada S, Kane GC, et al. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–8. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- [11].Bienengraeber M, Olson TM, Selivanov VA, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat. Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hodgson DM, Zingman LV, Kane GC, et al. Cellular remodeling in heart failure disrupts K(ATP) channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Suzuki M, Sasaki N, Miki T, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- [15].Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- [16].Inagaki N, Gonoi T, Clement JP, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- [17].Clement JP, 4th, Kunjilwar K, Gonzalez G, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- [18].Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for K(ATP) assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–167. doi: 10.1016/s0896-6273(00)81146-0. [DOI] [PubMed] [Google Scholar]
- [19].Bienengraeber M, Alekseev AE, Abraham MR, et al. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- [20].Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J. Physiol. 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am. J. Physiol. 1991;261:H1675–86. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- [22].O’Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- [23].Sasaki N, Sato T, Marban E, O’Rourke B. ATP consumption by uncoupled mitochondria activates sarcolemmal K(ATP) channels in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1882–8. doi: 10.1152/ajpheart.2001.280.4.H1882. [DOI] [PubMed] [Google Scholar]
- [24].Weiss JN, Venkatesh N. Metabolic regulation of cardiac ATP-sensitive K+ channels, Cardiovasc. Drugs Ther. 1993;7(Suppl 3):499–505. doi: 10.1007/BF00877614. [DOI] [PubMed] [Google Scholar]
- [25].Zingman LV, Alekseev AE, Bienengraeber M, et al. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- [26].Zingman LV, Hodgson DM, Bienengraeber M, et al. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J. Biol. Chem. 2002;277:14206–14210. doi: 10.1074/jbc.M109452200. [DOI] [PubMed] [Google Scholar]
- [27].Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- [29].Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J. Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferrero JM, Jr, Saiz J, Ferrero JM, Thakor NV. Simulation of action potentials from metabolically impaired cardiac myocytes. Role of ATP-sensitive K+ current. Circ. Res. 1996;79:208–221. doi: 10.1161/01.res.79.2.208. [DOI] [PubMed] [Google Scholar]
- [31].Findlay I, Faivre JF. ATP-sensitive K channels in heart muscle. Spare channels. FEBS Lett. 1991;279:95–97. doi: 10.1016/0014-5793(91)80259-6. [DOI] [PubMed] [Google Scholar]
- [32].Malester B, Tong X, Ghiu I, et al. Transgenic expression of a dominant negative K(ATP) channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- [33].Agah R, Frenkel PA, French BA, et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- [35].Lader JM, Vasquez C, Bao L, et al. Remodeling of atrial ATP-sensitive K+ channels in a model of salt-induced elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H964–74. doi: 10.1152/ajpheart.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rajashree R, Koster JC, Markova KP, et al. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal K(ATP) channels. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H584–90. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- [37].Isenberg G, Vereecke J, van der Heyden G, Carmeliet E. The shortening of the action potential by DNP in guinea-pig ventricular myocytes is mediated by an increase of a time-independent K conductance. Pflugers Arch. 1983;397:251–259. doi: 10.1007/BF00580257. [DOI] [PubMed] [Google Scholar]
- [38].Faivre JF, Findlay I. Action potential duration and activation of ATP-sensitive potassium current in isolated guinea-pig ventricular myocytes. Biochim. Biophys. Acta. 1990;1029:167–172. doi: 10.1016/0005-2736(90)90450-3. [DOI] [PubMed] [Google Scholar]
- [39].Elliott AC, Smith GL, Allen DG. Simultaneous measurements of action potential duration and intracellular ATP in isolated ferret hearts exposed to cyanide. Circ. Res. 1989;64:583–591. doi: 10.1161/01.res.64.3.583. [DOI] [PubMed] [Google Scholar]
- [40].Selvin E, Bolen S, Yeh HC, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch. Intern. Med. 2008;168:2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gopalakrishnan M, Triggle DJ, Rutledge A, et al. Regulation of K+ and Ca2+ channels in experimental cardiac failure. Am. J. Physiol. 1991;261:H1979–87. doi: 10.1152/ajpheart.1991.261.6.H1979. [DOI] [PubMed] [Google Scholar]
- [42].Michailova A, Lorentz W, McCulloch A. Modeling transmural heterogeneity of K(ATP) current in rabbit ventricular myocytes. Am. J. Physiol. Cell. Physiol. 2007;293:C542–57. doi: 10.1152/ajpcell.00148.2006. [DOI] [PubMed] [Google Scholar]
- [43].Kimura S, Bassett AL, Furukawa T, et al. Electrophysiological properties and responses to simulated ischemia in cat ventricular myocytes of endocardial and epicardial origin. Circ. Res. 1990;66:469–477. doi: 10.1161/01.res.66.2.469. [DOI] [PubMed] [Google Scholar]
- [44].Furukawa T, Kimura S, Furukawa N, et al. Role of cardiac ATP-regulated potassium channels in differential responses of endocardial and epicardial cells to ischemia. Circ. Res. 1991;68:1693–1702. doi: 10.1161/01.res.68.6.1693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of Kir6.1AAA is tracked by elimination of GFP fluorescence. Brightfield and fluorescence images of hearts from A. Tg[CX1-eGFP-Kir6.1AAA], B. Tg[αMHCKir6.1AAA], and C. WT mice. Retained suture material from retroperfusion marks the aorta. RA: right atrium, LA: left atrium, RV: right ventricle, LV: left ventricle, PA: pulmonary artery, RVOT: right ventricular outflow tract, Ao: aorta.
Figure S2. Expression of Kir6.1AAA reduces the number of functional KATP channels. A. Summary statistics of KATP channel whole cell current stimulated by DNP (50 μM) and pinacidil (100 μM) in WT (n=8) and Tg[CX1-eGFP-Kir6.1AAA] control (n=6) ventricular myocytes (p=NS), vs. Tg[αMHC-Kir6.1AAA] (n=10) cardiomyocytes (*p<.05). Current is normalized to membrane capacitance (pA/pF). B. Representative whole cell current recordings at baseline and after stimulation of KATP channel opening by DNP and pinacidil. C. Summary statistics indicating the number of KATP channels in cell-attached patches of ventricular myocytes stimulated with DNP (200 μM) and pinacidil (100 μM), from WT (n=13) and Tg[CX1-eGFP-Kir6.1AAA] (n=16) hearts (p=NS), vs. Tg[αMHC-Kir6.1AAA] (n=17) hearts (*p<.05). D. There was no significant difference in the patch pipette tip size as estimated by tip resistance (WT: 4.6±.20 MΩ, Tg[CX1-eGFPKir6.1AAA]: 4.5±.2 MΩ, Tg[αMHC-Kir6.1AAA]: 4.4±.1 MΩ, p=NS). E. Representative cell-attached recordings before, during, and after wash-out of DNP and pinacidil. DNP: 2,4-dinitrophenol.