Abstract
Successful long-term memory (LTM) depends upon effective control of information in working memory (WM), and there is evidence that both WM and LTM are impaired by schizophrenia. This study tests the hypothesis that LTM deficits in schizophrenia may result from impaired control of relational processing in WM due to dorsolateral prefrontal cortex (DLPFC) dysfunction. fMRI was performed on 19 healthy controls and 20 patients with schizophrenia during WM tasks emphasizing relational (reorder trials) versus item-specific (rehearse trials) processing. WM activity was also examined with respect to LTM recognition on a task administered outside the scanner. Receiver operator characteristics analysis assessed familiarity and recollection components of LTM. Patients showed a disproportionate familiarity deficit for reorder versus rehearse trials against a background of generalized LTM impairments. Relational processing during WM led to DLPFC activation in both groups. However, this activation was less focal in patients than in controls, and patients with more severe negative symptoms showed less of a DLPFC increase. fMRI analysis of subsequent recognition performance revealed a group by condition interaction. High LTM for reorder versus rehearse trials was associated with bilateral DLPFC activation in controls, but not in patients who activated the left middle temporal and inferior occipital gyrus. Results indicate that although patients can activate the DLPFC on a structured relational WM task, this activation is less focal and does not translate to high retrieval success, suggesting a disruption in the interaction between WM and LTM processes in schizophrenia.
Keywords: episodic memory, functional imaging, neurocognition, familiarity, recollection
1. INTRODUCTION
Working memory (WM) and long-term memory (LTM) are core cognitive deficits in schizophrenia (Clare et al., 1993) that limit patients’ functional outcome (Green, 1996) and are not ameliorated by available medications (Goldberg et al., 2007; Keefe et al., 2007). Accordingly, there is interest in identifying neural mechanisms to guide development of new treatments to improve these memory processes (Carter & Barch, 2007). Although WM and LTM are typically studied in isolation, there is evidence that successful LTM encoding and retrieval depend upon efficient control of information processing during WM (Ranganath, 2010). The current study tests the hypothesis that LTM deficits in schizophrenia may be related to impaired control of information in WM due to dysfunction of the lateral prefrontal cortex (PFC).
The PFC is critical for controlling active maintenance and manipulation of information in WM (D'Esposito et al., 1999). Several findings suggest that the PFC may also contribute to LTM encoding through its control of information processing in WM (Blumenfeld & Ranganath, 2007). Recent functional magnetic resonance imaging (fMRI) studies demonstrated that different PFC subregions might contribute to memory in different ways (Blumenfeld & Ranganath, 2006; Hunt & Einstein, 1981). Whereas ventrolateral prefrontal cortex (VLPFC) is linked to controlled processing of item-specific information (Badre & Wagner, 2007; Blumenfeld & Ranganath, 2007), DLPFC activity is associated with processing of relations amongst items (D'Esposito et al., 1999; Wagner et al., 2001). A recent meta-analysis suggests that individuals with schizophrenia show DLPFC dysfunction during LTM encoding and retrieval (Ragland et al., 2009), but it is unclear whether these deficits are related to processing of information in WM. Here, we tested the hypothesis that LTM deficits in schizophrenia may result from impaired relational processing of information in WM due to DLPFC dysfunction.
To test this prediction, we adapted a paradigm developed by Blumenfeld and Ranganath (Blumenfeld & Ranganath, 2006). Participants were scanned while performing two WM tasks: On “rehearse” trials, participants maintained sequences of 3 objects across a delay period, whereas on “reorder” trials participants mentally rearranged the 3 objects according their physical weight and maintained that new order across the delay. Following scanning, subjects performed a LTM recognition test on items encountered in the scanner. In healthy individuals, DLPFC activity was increased during reorder trials, and this enhancement was associated with successful LTM encoding. Given evidence of DLPFC dysfunction and disproportionate relational memory deficits in schizophrenia (Clare et al., 1993; Lepage et al., 2006; Ranganath, Minzenberg & Ragland, 2008; Titone et al., 2004), we predicted that DLPFC activation during relational processing would be less strongly linked to successful LTM encoding in patients.
A second objective was to assess the nature of retrieval impairments in individuals with schizophrenia. Evidence from recent studies has supported the idea that patients with schizophrenia have selective deficits in recollection of past events, whereas familiarity-based recognition may be largely intact (Bonner-Jackson et al., 2008; Huron et al., 1995; Thoma et al., 2006; van Erp et al., 2008). However, much of the data supporting this view has been obtained from tests using the remember-know paradigm, which creates significant meta-cognitive demands and can depend heavily upon how subjects are instructed (Rotello et al., 2005). To circumvent these issues, we used receiver operator characteristic (ROC) analyses to assess effects of relational and item-specific encoding on familiarity and recollection in schizophrenia patients.
2. MATERIAL AND METHODS
2.1. Participants
Data are presented on 20 patients with schizophrenia and 19 healthy control participants. Individuals were matched at the group level for age, gender, handedness, parental education, and premorbid intellectual ability as assessed by the reading subtest of the Wide Range Achievement Test (see Table 1). Not included were 5 patients and 7 controls that performed below chance, had excessive movement (more than 3 mm), or missing data due to technical problems (faulty response box). The Structured Clinical Interview for DSM-IV-TR confirmed the diagnosis of schizophrenia in patients, and confirmed that controls were free of lifetime history of Axis I disorder. Master’s or Doctoral-level clinicians completed the SCID interview, and diagnoses were confirmed by consensus conference. Symptoms were rated with the Scale for Assessment of Negative Symptoms (SANS; (N.C. Andreasen, 1983)), Scale for Assessment of Positive Symptoms (SAPS; (N. C. Andreasen, 1984)), and Brief Psychiatric Rating Scale (BPRS; (Overall & Gorham, 1980)). Selected items from the BPRS, SANS and SAPS were used to compute positive, disorganization and negative symptom scores (Barch et al., 2003). All but two patients were receiving antipsychotics (1 typical, 17 atypical), with an average daily dose of 195 mg/day (SD=250, range=.5–700 mg) in chlorpromazine equivalents (Minzenberg et al., 2004). Exclusion criteria were: IQ < 70, drug or alcohol abuse or dependence in the previous three months, major medical or neurological illness, significant head trauma, or any known MRI contraindication.
Table 1.
Demographic characteristics of study sample
| Controls (n-19) | Patients (n=20) | ||||
|---|---|---|---|---|---|
| Demographic Variable | Mean | SD | Mean | SD | p-value |
| Age (years) | 27.6 | 5.9 | 26.5 | 7.4 | ns |
| WRAT | 110.8 | 9.70 | 104.8 | 8.8 | ns |
| Parental Education (years) | 15.6 | 2.10 | 14.5 | 2.6 | ns |
| Gender (% male) | 63.1 | 75 | ns | ||
| Handedness (% right) | 89.5 | 85 | ns | ||
| SANS | 32.3 | 21.2 | |||
| SAPS | 15.7 | 15 | |||
| BPRS | 37.5 | 8.3 | |||
Note: ns = no significant group difference at p<.05, two-tailed
2.2. Task Procedures
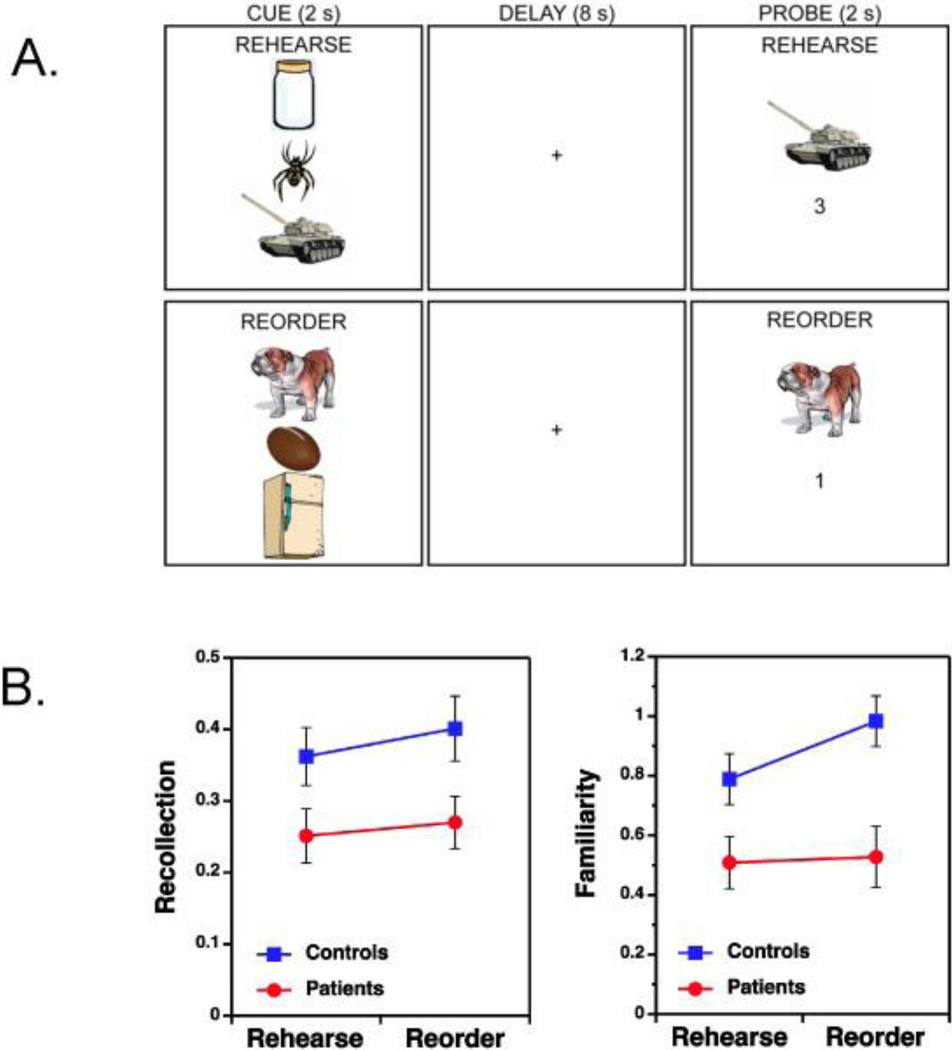
Stimuli consisted of 504 color Google® clip-art item drawings divided between 3 lists of 168 items each. Two lists were assigned to WM tasks, and the remaining list served as foils for LTM recognition. Lists were counterbalanced between subjects and across conditions. Stimuli were presented and responses recorded with Presentation® (version 11.1). During scanning, participants completed a pseudo-random sequence of 8 rehearse and 8 reorder runs, each consisting of 7 WM trials (Figure 1a). Each trial commenced with the presentation of a vertical array of three items (2 seconds), followed by a blank screen (8 seconds), and then a probe screen (2 seconds) consisting of one of the three items and a number (from 1–3). On rehearse trials, participants were instructed to maintain the order of the three items (from top to bottom). Upon presentation of the probe, they used a two-button response to indicate whether the number matched the serial order of the item (1=top, 2=middle, 3=bottom). On reorder trials, subjects were instructed to mentally reorder the 3 items according to physical weight (from lightest to heaviest), and subsequently to indicate whether the number on the probe screen matched the position of the item in the reordered memory set. Thus, rehearse and reorder trials were matched in terms of stimuli, responses, and timing parameters and differed primarily in terms of the demand to manipulate the sequence (i.e., relational processing demands). Trials were separated by a “jittered” inter-stimulus-interval (8–18 seconds) to better estimate event-related activity. Subjects were told to respond as quickly and accurately as possible and were required to complete practice tasks prior to scanning to ensure comprehension.
Figure 1.
(A) Experimental design of item-specific (Rehearse) and relational (Reorder) working memory tasks. In the Rehearse condition participants maintain the serial order (top to bottom) of the three objects during the delay and indicate on the probe trial whether the number matches the serial order of the item in the memory set. The Reorder condition requires participants to rearrange the order of the three objects based on their physical weight (from lightest to heaviest) and indicate during the probe trial whether the number matches the order of the object in the rearranged memory set. In this example, the correct Rehearse response is “yes” and the correct Reorder response is “no”.
(B) Bottom left figure illustrates the main effect of schizophrenia on recollection performance, with equivalent patient impairments across memory conditions. Bottom right figure illustrates the group by memory condition interaction for familiarity estimates, with disproportionate patient impairments in the relational (Reorder) memory condition.
Following scanning, subjects performed a subsequent LTM recognition task consisting of the 336 objects that were previously seen during the WM conditions and 168 new unstudied foils. They were instructed to indicate whether each item was “old” (left hand response) or “new” (right hand response) and rate confidence using one of three buttons (i.e., 3=high, 2=medium, 1=low). These 3-point confidence ratings were made with each hand, yielding a full 6-point confidence distribution ranging from highly confident old (i.e., left hand index finger) to highly confidant new (i.e., right hand index finger). Participants were given a practice task and instructed on the importance of using the full range of confidence ratings prior to testing.
2.3. MRI Acquisition
Data were acquired at the UC Davis Imaging Research Center on a 3-T Siemens Trio scanner (Erlangen, Germany) with a Siemens 8 channel phased array coil. After acquiring a rapid 3-plane localizer, trans-axial T2 weighted images were acquired with spatial resolution of 0.9 × 0.9 × 3.4 mm. Oblique axial T2* weighted images for fMRI were acquired using a single-shot EPI sequence. Functional images were acquired with blood oxygenation level dependent imaging (BOLD) using a 34-slice whole-brain, single-shot gradient-echo echo-planar sequence (TR 2000 ms, TE 27 ms, flip angle 90 degrees, FOV 220 × 220 mm, slice thickness 4 mm, no gap). Data were preprocessed using Statistical Parametric mapping (SPM5) including slice time correction, realignment to the median image, normalization to template space, and spatial smoothing (8 mm FWHM).
2.4. Statistical Analysis
Percent correct performance on WM probes was examined with analysis of variance (ANOVA) to test effects of group, task condition, and higher-order interactions. LTM recognition accuracy (d’) was calculated by subtracting standardized false alarm rates from standardized hit rates, and examined using the same ANOVA design. ROC curves were plotted and fitted with the dual-process signal detection model (Yonelinas, 1994) to derive estimates of familiarity (F) and recollection (R), which were assessed for effects of group, task condition and group by condition interactions using ANOVA. Modeling of the ROC data to obtain R and F parameter estimates revealed an excellent model fit in both patients [mean sum of square errors (SSE) = .0034] and controls (mean SSE = .0015), indicating that both groups were successful in distributing their confidence ratings. Because behavioral hypotheses were directional, significance levels were set at p<.05, one-tailed.
Subject-level fMRI analysis was performed using the general linear model (GLM) in VoxBo 1.8 (http://www.voxbo.org). BOLD responses during reorder and rehearse trials were modeled by convolving vectors of predicted neural activity corresponding to each phase (encoding, delay, and probe) of each trial type with a canonical hemodynamic response function. Separate covariates were included to model correct and incorrect WM trials, but only correct trials were examined in second-level analyses. A second analysis was performed to assess between-group differences in WM-related activity, while holding subsequent LTM performance constant. This analysis solely focused on trials with ‘high LTM memory’ performance (i.e., 2–3 items from each WM trial subsequently correctly recognized), and was limited to WM trials that participants performed correctly. Ideally, a factorial analysis of the effects of task (rehearse vs. reorder) and subsequent memory (high vs. low) would have been of interest. Unfortunately, there were too few participants with sufficient trials in each bin to perform such an analysis (i.e., controls tended to have too few “low LTM memory” trials). Nuisance covariates were orthogonalized to the design matrix, and included global signal changes, trial-specific shifts in baseline signal between scans, motion spikes, and an intercept. The design matrix also included a time-domain representation of low frequency (1/f) power and filters to remove frequencies >0.25 Hz and <0.02 Hz.
Parameter estimates from first-level GLM analyses (corresponding to cue- and delay-period covariates for rehearse and reorder trials) were entered into second-level one-sample and two-sample t-tests in SPM5 for the two contrasts of interest (reorder minus rehearse for correct WM, and reorder minus rehearse for high LTM). Because hypotheses concerned the PFC, search space was restricted with a frontal lobe mask from the WFU_PickAtlas (Maldjian et al., 2003), and a p<.05 cluster-level correction for multiple comparisons was established with AlphaSim using a voxel-wise threshold of p<.005 and extent threshold of 17 voxels. Mean beta values for functional regions of interest (ROIs) showing above-threshold activity in the PFC were calculated and used to illustrate task effects, any group by condition interactions, and to identify any correlations with medication dose or clinical symptoms (positive, disorganization, and negative symptom scores). For exploratory purposes, between-group SPM t-tests were repeated at the whole brain level using the same threshold to identify any group differences in the rest of the brain.
3. RESULTS
3.1. Behavioral Performance
Reaction times (RTs) revealed that all participants responded more quickly on rehearse (mean±standard deviation=1,701±648 msec) than reorder probes (1,854±796 msec) [F(1,37=16.9, p<.0005], with no group differences or interactions (both [F(1,37)<1]). Accuracy on WM probes was higher on rehearse (mean±standard deviation =86.5±12.0 percent correct) than reorder trials (78.1±13.3 percent correct) [F(1,37)=25.2, p<.0001], and controls were more accurate than patients (controls=86.4±10.4, patients=78.4±11.5 percent correct) [F(1,37)=5.14, p<.05], with no task by group interaction [F(1,37)<1].
Performance on the subsequent LTM test is summarized in Table 2. Reflecting the benefit of performing the more difficult relational processing task, recognition accuracy (d’) was higher for objects encoded on reorder than on rehearse trials [F(1,37)=34.6, p<.0001]. Although patient performance was below that of controls [F(1,37)=5.2, p<.05], there was no group by condition interaction [F(1,37)<1], suggesting that groups benefitted equally from relational versus item-specific encoding. Recollection was also higher following relational versus item encoding [F(1,37)=58.4, p<.01], and was greater in controls than patients [F(1,37)=5.2, p<.05], with no task by group interaction [F(1,37)<1] (Table 1). Familiarity showed a group by condition interaction [F(1,37)=3.1, p<.05], with patients having larger deficits following relational versus item-specific encoding (Figure 1b). Familiarity also showed main effects of task [F(1,37)=4.6, p<.05] and group [F(1,37)=9.7, p<.005].
Table 2.
Recognition task performance
| Controls (n-19) | Patients (n=20) | |||||
|---|---|---|---|---|---|---|
| Demographic Variable | Mean | SD | Mean | SD | p-value | Cohen's d |
| Rehearse D-Prime | 1.4 | 0.49 | 1.03 | 0.57 | <.05 | 0.69 |
| Reorder D-Prime | 1.59 | 0.48 | 1.2 | 0.56 | <.05 | 0.75 |
| Rehearse Recollection | 0.36 | 0.18 | 0.25 | 0.17 | 0.052 | 0.63 |
| Reorder Recollection | 0.4 | 0.19 | 0.26 | 0.16 | <.05 | 0.63 |
| Rehearse Familiarity | 0.79 | 0.37 | 0.51 | 0.38 | <.05 | 0.75 |
| Reorder Familiarity | 0.98 | 0.37 | 0.53 | 0.46 | <.002 | 1.08 |
Note: ns = no significant group difference at p<.05, two-tailed
Relationships between WM and LTM were examined using Spearman correlations. In both groups, better WM was associated with stronger familiarity performance for the rehearse condition (Controls: r=.52, p<.05; Patients: r=.69, p<.001). For the reorder condition, correlations with familiarity were significant for controls (r=.53, p<.05) but not for patients (r=.36, p=.11). However, when the magnitude of these correlations were compared between groups, there were no differences between patients and controls for either rehearse (Fisher’s Z=−.74, p=.45) or reorder conditions (Fisher’s Z=.57, p=.56). No correlations with WM were found with recollection estimates in either group.
3.2. fMRI Results
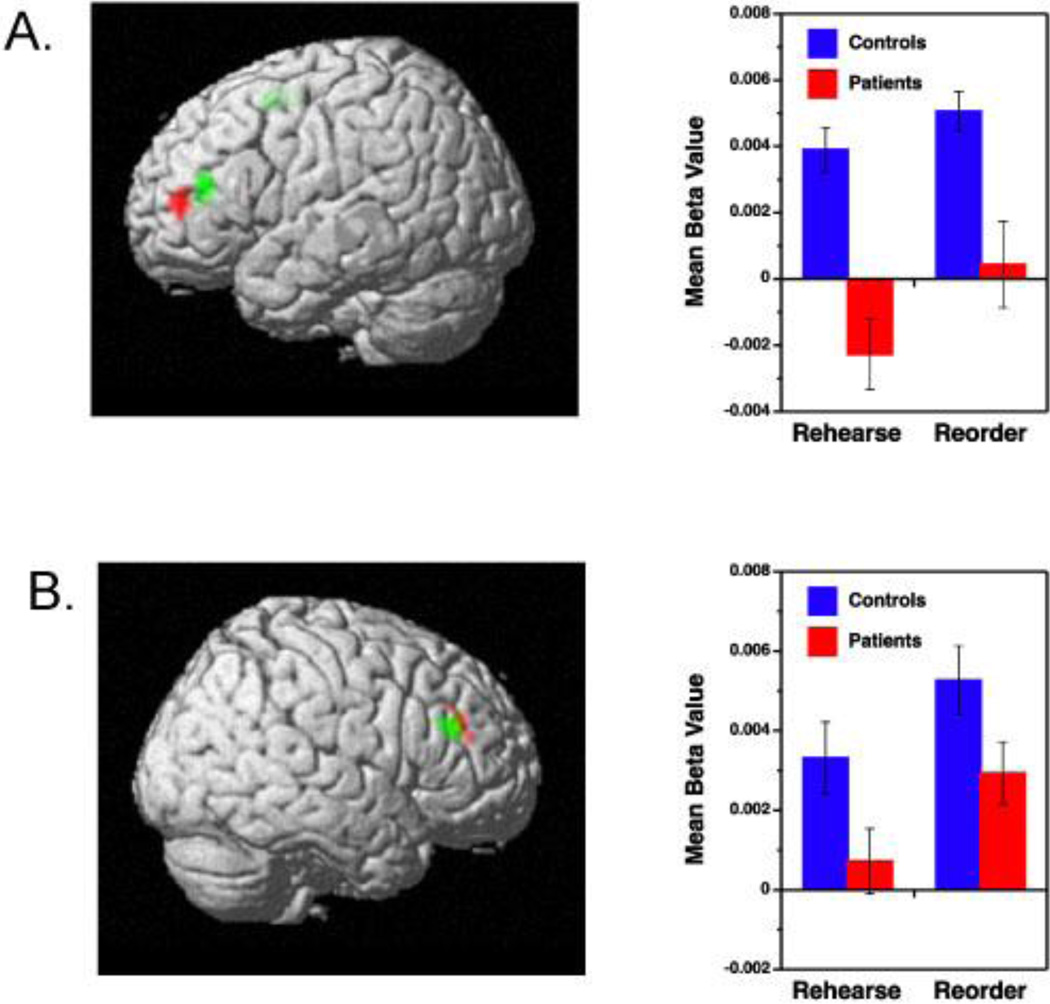
Images showed little motion across x, y and d dimensions (mean = 1.4±.49 mm translational and 0.1±.07 degrees of rotational motion) prior to motion correction, and no group differences for translational [F(1,37)=3.0, p>.10] or rotational domains [F(1,37)=1.0, p>.10]. Our first analysis investigated overall activation differences between correctly performed rehearse and reorder trials (Table 3, Figure 2a). Consistent with previous findings in healthy subjects (Blumenfeld & Ranganath, 2006), the increased relational processing demands of the reorder condition were associated with increased DLPFC activation in controls [Brodmann Area (BA) 9, 46], as well as in patients. Increases were also noted in the dorsal middle frontal gyrus (BA 6) in controls. Although level of DLPFC activity in the reorder-rehearse contrast appeared lower in patients (Figure 2b), there were no significant between-group differences. The only group difference was greater patient than control activation in the frontal pole (BA 10) (Table 3).
Table 3.
Local Cluster-Level Maxima of Blood-Oxygen-Level-Dependent fMRI Signal Change in Frontal Cortex During the Cue and Delay Period of the Reorder minus Rehearse Working Memory Task in Healthy Comparison Subjects and Patients With Schizophrenia
| Estimated | Coordinates2 | ||||
|---|---|---|---|---|---|
| Group and Region | Brodmann’s Area | Z Score1 | x | y | z |
| Comparison subjects (n=19) | |||||
| Left Middle Frontal Gyrus3 | 46 | 3.55 | −43 | 31 | 20 |
| Right Middle Frontal Gyrus3 | 9 | 3.36 | 49 | 29 | 30 |
| Left Middle Frontal Gyrus | 6 | 3.12 | −24 | 2 | 53 |
| Patients (n=20) | |||||
| Left Middle Frontal Gyrus3 | 46 | 3.63 | −45 | 42 | 18 |
| Right Middle Frontal Gyrus3 | 9 | 3.63 | 49 | 35 | 30 |
| Controls minus Patients | |||||
| No above-threshold differences | -- | -- | -- | -- | -- |
| Patients minus Controls | |||||
| Right Medial/Superior Frontal Gyrus | 10 | 3.73 | 16 | 51 | 9 |
| Left Middle Frontal Gyrus | 10 | 3.18 | −34 | 56 | 20 |
Note:
Peak activation in a cluster of seventeen voxels in which the difference in signal change exceeded a cluster-corrected p-value of 0.05;
Coordinates from the stereotaxic atlas of Talairach and Tournoux (1988);
Functional regions of interest (ROIs) used in correlational analyses.
Figure 2.
Prefrontal regions showing increased activation during reorder trials relative to rehearse trials for patients with schizophrenia (red color) and healthy controls (green color). Results are illustrated separately for left (A) and right hemisphere (B) and include corresponding regression coefficients (beta values) for Rehearse and Reorder memory conditions.
Exploratory between-group contrasts between reorder and rehearse trials on the whole-brain level did not reveal any additional areas of increased activation in controls versus patients, but, patients showed relatively greater activation than controls in several regions in temporal, occipital, and subcortical structures (Table 4). Thus, healthy participants responded to increased relational processing demands during WM through focal DLPFC increases, whereas patients engaged a more diffuse set of cortical and subcortical regions to meet the same processing demand.
Table 4.
Greater Patient versus Comparison subject Local Cluster-Level Maxima of Blood-Oxygen-Level-Dependent fMRI Signal Change in the Whole Brain During the Cue and Delay Period of the Reorder minus Rehearse Working Memory Task
| Estimated | Coordinates2 | ||||
|---|---|---|---|---|---|
| Group and Region | Brodmann’s Area | Z Score1 | x | y | z |
| Left Inferior Temporal Gyrus | 37 | 3.95 | −68 | −53 | −13 |
| Left Cuneus | 18 | 3.75 | −15 | −96 | 19 |
| Right Middle Temporal Gyrus | 37 | 3.75 | 57 | −63 | −1 |
| Right Medial Frontal Gyrus | 10 | 3.73 | 16 | 51 | 9 |
| Left Superior Temporal Gyrus | 22 | 3.61 | −65 | −59 | 19 |
| Left Middle Frontal Gyrus | 10 | 3.18 | −34 | 56 | 20 |
| Right Middle Occipital Gyrus | 18 | 3.15 | 24 | −103 | 8 |
| Left Caudate | -- | 3.10 | −18 | −37 | 14 |
| Left Putamen | -- | 3.08 | −20 | 16 | −8 |
Note:
Peak activation in a cluster of seventeen voxels in which the difference in signal change exceeded a cluster-corrected p-value of 0.05;
Coordinates from the stereotaxic atlas of Talairach and Tournoux (1988).
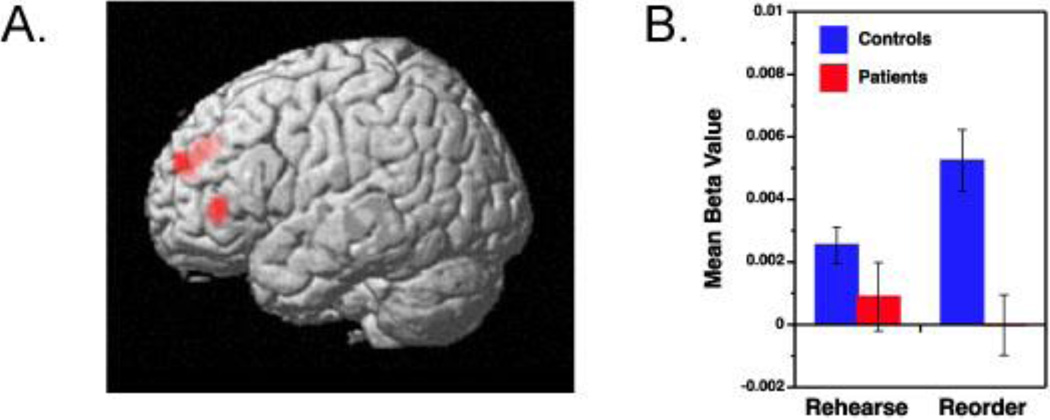
Our next analysis investigated the extent to which successful memory encoding was mediated by different brain regions across rehearse and reorder trials. As noted earlier, healthy young participants showed a relationship between DLPFC activation during reorder trials and subsequent LTM for items that were processed on these trials (Blumenfeld & Ranganath, 2006). To test whether a similar relationship might be evident in controls and patients, we contrasted activity between reorder trials for which participants subsequently showed high LTM performance (i.e., 2–3 cue items subsequently recognized) against rehearse trials with high LTM performance. Consistent with previous findings, this contrast in healthy controls revealed bilateral increases in DLPFC activation (BA 9, 46), along with increased activity in the left dorsal middle frontal gyrus (BA 6) (Table 5). In contrast, this analysis revealed no areas of suprathreshold activation in patients. A direct comparison revealed an interaction between task and patient group in DLPFC, such that the reorder-rehearse activity difference was significantly larger in controls than in patients (Figure 3a). There were no areas showing the reverse pattern. To illustrate this group by condition interaction, mean beta values for the left DLPFC ROI showing the group differences are plotted in Figure 3b.
Table 5.
Local Cluster-Level Maxima of Blood-Oxygen-Level-Dependent fMRI Signal Change in Frontal Cortex During the Cue and Delay Period of the Reorder minus Rehearse Working Memory Task for High Subsequent Memory in Healthy Comparison Subjects and Patients With Schizophrenia
| Estimated | Coordinates2 | ||||
|---|---|---|---|---|---|
| Group and Region | Brodmann’s Area | Z Score1 | x | y | z |
| Comparison subjects (n=19) | |||||
| Left Middle Frontal Gyrus | 46 | 3.72 | −46 | 36 | 26 |
| Right Middle Frontal Gyrus | 9 | 3.58 | 43 | 23 | 32 |
| Left Middle Frontal Gyrus | 6 | 3.39 | −32 | 5 | 55 |
| Left Middle Frontal Gyrus | 9 | 3.10 | −43 | 12 | 37 |
| Patients (n=20) | |||||
| No above-threshold differences | -- | -- | -- | -- | -- |
| Controls minus Patients | |||||
| Left Medial Frontal Gyrus | 9 | 3.39 | −12 | 41 | 32 |
| Left Inferior Frontal Gyrus | 46 | 2.98 | −43 | 35 | 10 |
Note:
Peak activation in a cluster of seventeen voxels in which the difference in signal change exceeded a cluster-corrected p-value of 0.05;
Coordinates from the stereotaxic atlas of Talairach and Tournoux (1988).
Figure 3.
(A) Left prefrontal regions showing greater control than patient activation for high subsequent LTM for Reorder minus Rehearse memory conditions.
(B) Corresponding regression coefficients (beta values) for Rehearse and Reorder memory conditions in patients (red) and controls (blue).
Exploratory whole-brain between-group analyses revealed interactions between task and group for high memory trials in the left middle temporal gyrus (BA 21; x=63, y=−56, z=5; Zscore=3.53) and inferior occipital cortex (BA 18; x=−46, y=−85, z=−7; Zscore=3.39). However, unlike the pattern observed in the DLPFC, the difference between reorder and rehearse trials in these regions was larger for patients than for controls.
Because the large spatial extent of the cluster-corrected threshold may have obscured hippocampal findings, a second exploratory analysis was performed after lowering the spatial extent to 5 voxels and employing a mask from the WFU_PickAtlas to isolate hippocampal activity differences. These modifications failed to reveal any task or group differences in hippocampal activity.
3.3. Clinical Correlation Results
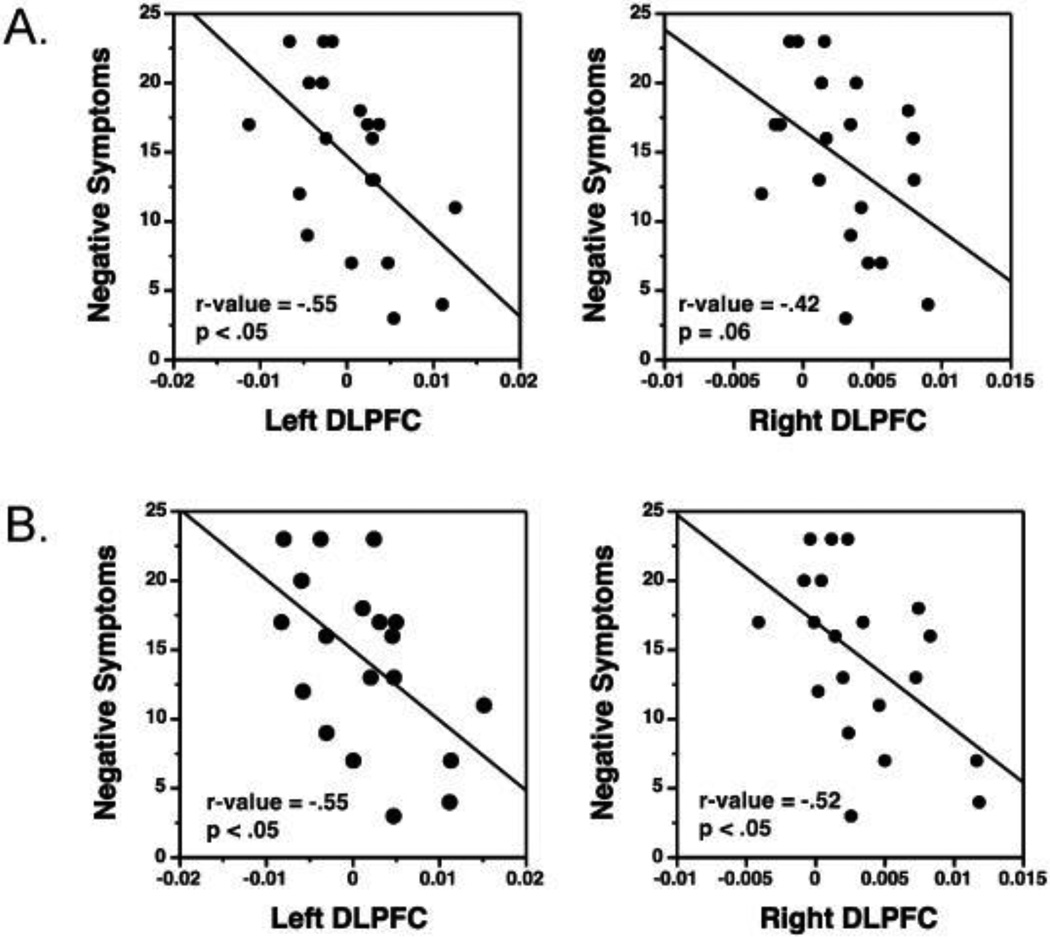
Examination of clinical correlations showed that increased left DLPFC activation during reorder versus rehearse conditions was associated with less severe negative symptoms in patients for both WM (r=−.55, p<.05) and high LTM analyses (r=−.55, p<.05). A similar result was obtained for the right DLPFC for the high LTM analysis (r=− .52, p<.05), but occurred only at a trend level for the WM analysis (r=−.42, p=.06). These results are illustrated by scatter plots in Figure 4. No correlations were observed between fMRI activity and medication dose or positive or disorganization symptoms.
Figure 4.
Scatter plots showing inverse correlations between DLPFC activation and negative symptoms in patients. Greater DLPFC activation in Reorder minus Rehearse conditions is associated with less severe negative symptoms. Results are illustrated separately for (A) working memory and (B) high subsequent LTM.
4. DISCUSSION
In the current study, we investigated the relationship between neural processing related to WM and LTM formation in patients with schizophrenia. Consistent with previous results, we found evidence for WM and LTM performance impairments in the patient sample. Against a background of generalized LTM deficits, patients showed a disproportionate impairment in recognition familiarity for items that were encoded on reorder trials (which emphasized processing of relational information in WM). Both patients and controls showed increased DLPFC activation during reorder trials relative to rehearse trials, but only in controls was this DLPFC increase associated with high levels of recognition performance. In contrast, patients remained behaviorally impaired for stimuli that were processed during reorder trials, recruited additional brain regions outside of the DLPFC, and failed to exhibit a link between DLPFC activity and successful LTM encoding. Moreover, those patients who were less successful in activating the DLPFC in response to increased relational processing demands also had more severe negative symptoms. Collectively, these results support the conclusion that, although schizophrenia patients can increase DLPFC activity in response to structured WM demands, this DLPFC activity is less beneficial to their LTM encoding and subsequent recognition performance. More specifically, there appears to be a decoupling in the relationship between DLPFC engagement during relational processing in WM and subsequent LTM performance in individuals with schizophrenia.
In a recent review, Van Snellenberg (Van Snellenberg, 2009) concluded that impairments in the control of information processing by the DLPFC may be a central mechanism of WM and LTM dysfunction in schizophrenia. Our results were partially consistent with this perspective. Our results revealed that patients could modulate DLPFC activation to perform a challenging task that requires relational processing in WM (although their WM performance was significantly impaired). However, patients appeared unable to translate this DLPFC recruitment into successful LTM performance.
As in studies of WM (Glahn et al., 2005) and executive function (Minzenberg et al., 2009), patients showed hyperactivation in the frontal pole and posterior association cortices that may have been compensatory in nature. To our knowledge, only one previous fMRI study (Barch et al., 2003) investigated the relationship between WM and LTM in schizophrenia patients, and found that patients showed deficits in DLPFC activation. The present results go beyond previous findings by demonstrating that: (1) healthy controls show a direct relationship between DLPFC functioning, relational processing in WM, and successful LTM encoding, and (2) this relationship is disrupted in schizophrenia patients.
A second objective of the present study was to determine the nature of patients’ LTM retrieval deficits. In healthy individuals, recognition can be supported by assessments of familiarity of studied items, or by recollection of contextual details associated with study events (Yonelinas, 2002). Typically, recollection and familiarity are estimated by using the “remember-know” paradigm to assess subjective experience during recognition, by comparing item and source recognition, or by using ROC analysis (as in the present study). Previous studies using remember-know and source memory methods have yielded mixed results, with some indicating that patients with schizophrenia have a specific recollection deficit (Bonner-Jackson et al., 2008; Huron et al., 1995; Thoma et al., 2006; van Erp et al., 2008), some suggesting a familiarity deficit (Weiss et al., 2008), and others suggesting that both processes are impaired (Lefebvre et al., 2010; Moritz & Woodward, 2006; Weiss et al., 2008). The present results are more consistent with the latter view, but they also indicate that patients’ memory deficits depend on how information is processed during encoding. That is, familiarity was disproportionately impaired following relational encoding (on reorder trials), as compared to item-specific encoding (on rehearse trials), and the fMRI data linked these deficits to prefrontal dysfunction.
This increased familiarity deficit in patients in the reorder condition might appear counter-intuitive since the reorder condition was more effortful, which should improve LTM performance, as it did in the control sample. However, we believe that patients were unable to benefit from the reorder task because of a fundamental deficit in their ability to form relational memory representations (Hannula et al., 2010; Ranganath, Minzenberg & Ragland, 2008; Williams et al., 2010). That is, preserved item-specific encoding processes in patients would lead familiarity-based recognition to be relatively preserved in the rehearse condition, whereas disrupted relational encoding processes would lead familiarity to be more severely impaired in the reorder condition. This ability of patients to utilize item-specific encoding processes was noted in several previous levels-of-processing studies examining overall recognition accuracy (Ragland et al., 2003; Bonner-Jackson et al., 2008). Use of the DPSD model in the current study extends previous findings by demonstrating that accuracy improvements appear to be driven by familiarity-based retrieval.
Considerable evidence from imaging studies of healthy individuals suggests that prefrontal activity is related to recollection and familiarity, and patients with prefrontal lesions show deficits in both processes (Ranganath, 2010). Accordingly, it is reasonable to suspect that the presence of familiarity deficits in schizophrenia may be related to the extent to which a task places demands on prefrontally-dependent control processes, and in particular, relational processing. Recollection deficits, on the other hand, may be related to dysfunction of both the prefrontal cortex and hippocampus (Preston et al., 2005; Ranganath & Blumenfeld, 2008; Tamminga et al., 2010). Imaging studies of healthy individuals have shown that encoding activity in the hippocampus is predictive of whether an item will be subsequently recollected, regardless of how it is processed during encoding (Davachi, 2006; Diana et al., 2007; Montaldi & Mayes, 2010). The present study was not designed in a manner that would allow us to differentiate between brain responses to subsequently recollected and non-recollected items, which might explain why we did not observe significant hippocampal activation in either group.
This study has a few potential limitations that may be worth considering. One possible concern is the greater difficulty of the reorder WM task. Because tasks that are more difficult also tend to have higher discriminating power [see (Chapman & Chapman, 1973)], the larger familiarity deficit in patients following the reorder task might reflect the greater sensitivity of the reorder condition to any generalized deficit in the patient sample. However, this is an unlikely explanation because the greater difficultly of the reorder WM task led to better LTM in healthy controls, suggesting that memory retrieval was easier following the relational encoding condition. The larger familiarity deficit in patients during this less demanding retrieval condition is inconsistent with a generalized deficit explanation of the group by encoding condition interaction. An additional limitation is that the WM paradigm did not provide a direct measure of reordering performance during the WM delay. Therefore, it is not possible to assess whether deficient semantic knowledge interfered with patients’ reordering performance and contributed to their LTM deficit in the reorder condition. However, there is a general consensus that semantic knowledge (or semantic store) is intact in schizophrenia (Elvev g & Storms, G., 2003), and test stimuli were chosen to be highly concrete to allow for any reduced semantic knowledge. Moreover, there were no differences in patient performance between the two WM tasks, indicating that the reordering demands did not substantially increase WM impairments. It is also worth noting that all but two patients were receiving medication, which may have influenced behavioral and imaging results. However, it is unlikely that patient deficits were artifactually related to antipsychotic medications, as there were no correlations with medication dose and since impaired memory and DLPFC dysfunction has been reported many times in both medicated and unmedicated patients (Snitz et al., 2005).
In summary, results support the conclusion that DLPFC mediated relational processing during WM is less beneficial to LTM memory in patients than in controls. Moreover, the DLPFC activation that was present in patients during WM was less focal - suggesting engagement of compensatory networks, and was inversely related to severity of negative symptoms - indicating the potential clinical significance of this DLPFC engagement. The current experimental design did not permit a test subsequent memory effects (i.e., hits versus misses), or a direct contrast between recollected and non-recollected items. These are both important areas for future task development. Finally, this study suggests that cognitive deficits in schizophrenia may reflect disrupted interactions between different memory processes and, more specifically, that it is important to consider both encoding and retrieval processes when developing behavioral or pharmacological interventions to remediate episodic memory in individuals with schizophrenia. One strategy may be to employ memory training procedures to capitalize on patients’ ability to encode item features to facilitate familiarity, while developing new treatments to restore relational processing and recollection.
Acknowledgements
Research was supported by National Institute of Mental Health grants R01MH084895, R01MH059883, and R01MH083734. We thank the participants for their time and effort and Joshua Phillips for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- Andreasen NC. Scale for the assessment of positive symptoms (SAPS) Iowa City: The University of Iowa; 1984. [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW, 3rd, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112(1):132–143. [PubMed] [Google Scholar]
- Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol. 2002;111(3):478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26(3):916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Yodkovik N, Csernansky JG, Barch DM. Episodic memory in schizophrenia: the influence of strategy use on behavior and brain activation. Psychiatry Res. 2008;164(1):1–15. doi: 10.1016/j.pscychresns.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychol Bull. 1973;79(6):380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Clare L, McKenna PJ, Mortimer AM, Baddeley AD. Memory in schizophrenia: what is impaired and what is preserved? Neuropsychologia. 1993;31(11):1225–1241. doi: 10.1016/0028-3932(93)90070-g. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Elevåg B, Storms G. Scaling and clustering in the study of semantic disruptions in patients with schizophrenia: a re-evaluation. Schizophr Res. 2003;63(3):237–246. doi: 10.1016/s0920-9964(02)00331-6. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C, Ramsay IS, Solomon M, Yoon J, Niendam TA, Carter CS, Ragland JD. Use of Eye Movement Monitoring to Examine Item and Relational Memory in Schizophrenia. Biological Psychiatry. 2010;68:610–616. doi: 10.1016/j.biopsych.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RR, Einstein GO. Relational and Item-Specific Information in Memory. Journal of Verbal Learning and Verbal Behavior. 1981;20(5):497–514. [Google Scholar]
- Huron C, Danion JM, Giacomoni F, Grange D, Robert P, Rizzo L. Impairment of recognition memory with, but not without, conscious recollection in schizophrenia. Am J Psychiatry. 1995;152(12):1737–1742. doi: 10.1176/ajp.152.12.1737. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Lefebvre AA, Cellard C, Tremblay S, Achim A, Rouleau N, Maziade M, et al. Familiarity and recollection processes in patients with recent-onset schizophrenia and their unaffected parents. Psychiatry Res. 2010;175(1–2):15–21. doi: 10.1016/j.psychres.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biol Psychiatry. 2006;60(11):1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161(1):116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20(11):1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS. The contribution of metamemory deficits to schizophrenia. J Abnorm Psychol. 2006;115(1):15–25. doi: 10.1037/0021-843X.15.1.15. [DOI] [PubMed] [Google Scholar]
- Overall JR, Gorham DR. The brief psychiatric rating scale. Journal of Operational Psychiatry. 1980;11:48–64. [Google Scholar]
- Preston AR, Shohamy D, Tamminga CA, Wagner AD. Hippocampal function, declarative memory, and schizophrenia: anatomic and functional neuroimaging considerations. Curr Neurol Neurosci Rep. 2005;5(4):249–256. doi: 10.1007/s11910-005-0067-3. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Moelter ST, McGrath C, Hill SK, Gur RE, Bilker WB, Siegel SJ, Gur RC. Levels-Of-Processing Effect on Word Recognition in Schizophrenia. Biological Psychiatry. 2003;54:1154–1161. doi: 10.1016/s0006-3223(03)00235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. Binding items and contexts: The cognitive neuroscience of episodic memory. Current Directions in Psychological Science. 2010;19(3):131–137. [Google Scholar]
- Ranganath C, Blumenfeld RS. Prefrontal Cortex and Memory. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. Vol. 3. Oxford: Academic Press; 2008. pp. 261–279. [Google Scholar]
- Ranganath C, Minzenberg M, Ragland JD. The Cognitive Neuroscience of Memory Function and Dysfunction in Schizophrenia. Biological Psychiatry. 2008;19:417–427. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA, Wong M. The remember response: subject to bias, graded, and not a process-pure indicator of recollection. Psychon Bull Rev. 2005;12(5):865–873. doi: 10.3758/bf03196778. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167(10):1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Thoma P, Zoppelt D, Wiebel B, Daum I. Recollection and familiarity in negative schizophrenia. Neuropsychologia. 2006;44(3):430–435. doi: 10.1016/j.neuropsychologia.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res. 2004;68(2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Lesh TA, Knowlton BJ, Bearden CE, Hardt M, Karlsgodt KH, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100(1–3):181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX. Working memory and long-term memory deficits in schizophrenia: is there a common substrate? Psychiatry Res. 2009;174(2):89–96. doi: 10.1016/j.pscychresns.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Weiss AP, Goff DC, Duff M, Roffman JL, Schacter DL. Distinguishing familiarity-based from source-based memory performance in patients with schizophrenia. Schizophr Res. 2008;99:208–217. doi: 10.1016/j.schres.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Must A, Avery S, Woolard A, Woodward ND, Cohen NJ, Heckers S. Biological Psychiatry. 68. 2010:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-Operating Characteristics in Recognition Memory - Evidence for a Dual-Process Model. Journal of Experimental Psychology-Learning Memory and Cognition. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]