Abstract
Recent studies have identified caveolin-1, a protein best known for its functions in caveolae, in apical endocytic recycling compartments in polarized epithelial cells. However, very little is known about the regulation of caveolin-1 in the endocytic recycling pathway. To address this question, in the current study we compared the relationship between compartments enriched in sub-apical caveolin-1 and Rab11a, a well-defined marker of apical recycling endosomes, using polarized MDCK cells as a model. We show that caveolin-1-containing vesicles define a compartment that partially overlaps with Rab11a, and that the distribution of subapical caveolin-1 and Rab11a show a similar dependence on microtubule disruption. Mutants of the Rab11a effector, Rab11-FIP2 also altered the localization of caveolin-1. These findings indicate that caveolin-1 is coordinately regulated with Rab11a within the apical recycling system of polarized epithelial cells, suggesting that the two proteins are components of the same pathway.
Keywords: Caveolin-1, Rab11a, Rab11-FIP2, apical recycling, MDCK, polarized epithelial cells
Introduction
Endocytic recycling of membrane from the apical surface of polarized epithelial cells is a closely regulated mechanism that is critical to polarized secretory processes in most epithelial cells. The internalized apical membrane and cargo pass through the apical early endosomal system, a common recycling endosome (CRE)1, the apical recycling endosome (ARE) and finally reinsert into the apical membrane. Membrane and proteins undergoing basal to apical transcytosis can enter the apical recycling system though the basal early endosome to the CRE [1-5].
Apical recycling and basal-apical transcytosis are controlled by members of the Rab small GTPase family and their effectors. One of these Rabs is Rab11a, a member of the Rab11 subfamily of small GTPases that is well established as a regulator of the recycling system in polarized cells. Rab11a is associated with vesicles in the pericentrosomal compartment beneath the apical plasma membrane and is concentrated in the ARE [3, 6, 7]. Rab11a interacts with and is regulated by specific interacting proteins, including a group of proteins, the Rab11 Family Interacting Proteins (Rab11-FIPs). This growing family of Rab11-FIPs currently includes the multiply spliced Rab11-FIP1 family (including RCP) [8-10], Rab11-FIP2 [8], Rab11-FIP3 [8], Rab11-FIP4 [11] and Rab11-FIP5 (pp75/Rip11) [12]. Mutations of many of the Rab11-FIP proteins can alter the apical recycling pathway in Madin-Darby canine kidney (MDCK) cells, a widely used model of polarized epithelial cells to study endocytic recycling and transcytosis, as well as other cells [8, 12, 13].
The protein caveolin-1 is best known for its association with caveolae, flask-shaped invaginations of the plasma membrane that are abundant in certain cell types, including many epithelial cells [14]. Caveolin-1 associates with cell membranes via a putative 33-amino acid intramembrane domain that forms a hairpin-like structure with cytoplasmically oriented N- and C-termini [15]. In addition to its role in the formation of caveolae, caveolin-1 functions in the scaffolding of signaling proteins, cholesterol binding and homeostasis, and regulation of endocytic trafficking [16-25]. Interestingly, several recent studies have now identified caveolin-1 in receptor recycling compartments in polarized epithelial cells [26-30]. In one such study, transferrin receptor-enriched basolateral recycling endosomes immunoisolated from polarized MDCK cells were found to contain caveolin-1 in addition to other markers of lipid rafts [26]. Another study used subcellular fractionation to show that caveolin-1 is enriched in a receptor-recycling compartment and in early sorting endosomes of rat hepatocytes [27]. More recently, we identified several novel pools of caveolin-1 in the subapical region of MDCK cells, a localization characteristic of ARE [31]. Sub-apically localized caveolin-1 was subsequently shown to be located in proximity to internalized dIgA in the apical region of MDCK cells [29]. Furthermore, we identified caveolin-1 in a proteomic analysis of immunoisolated H/K-ATPase containing tubulovesicles from human gastric mucosa, an amplified apical recycling system devoted to the regulated apical recycling of the H/K-ATPase [28].
Taken together, these findings suggest that caveolin-1 is a component of the endocytic recycling system of polarized epithelial cells. However, very little is currently known about the regulation of caveolin-1 in endocytic recycling in polarized cells. To address this question, in the current study we compared the relationship between compartments enriched in sub-apical caveolin-1 and Rab11a, a well-defined marker of apical recycling endosomes, in polarized MDCK cells.
Materials and Methods
Cell culture and drug treatments
Stable MDCK cell lines expressing EGFP-Rab11a and either wild type or mutant versions of EGFP-Rab11-FIP2 were as previously described [13, 32]. In brief, the EGFP-Rab11a cell line was generated in MDCK cells [6], whereas the EGFP-Rab11-FIP2 cell lines were generated in T23 cells (MDCK cells containing the rabbit pIgA receptor [33] and the TET off system [34]). Cells were grown in DMEM supplemented with 10% FBS (GIBCO), penicillin-streptomycin, 2 mM L-glutamine, 0.1 mM MEM nonessential amino acids (GIBCO-BRL) and 0.5 mg/ml G418 sulfate (Cellgro). Media for the EGFP-Rab11-FIP2 stable cell lines also contained 0.25 ng/ml hygromycin (Invitrogen). In the EGFP-Rab11-FIP2 stable cell lines, expression of the EGFP chimeras was inhibited with doxycycline (20 ng/ml; Calbiochem). To induce EGFPRab11-FIP2 protein expression, cells were plated without doxycycline in tetracycline-screened FBS (HyClone) medium. All cell lines were plated at confluence on to 0.4-μm Transwell filters (Costar) and allowed to polarize for 5-7 days unless indicated. MDCK cells were transfected at the time of plating on to transwells with caveolin-1-GFP [35] using Effectine (Qiagen). For the microtubule dependence studies cells were treated with either 33 μM of nocodazole (Calbiochem) or 7.5 μM of taxol (paclitaxel; Alexis Biochemical) for 4 hr at 37° C
Immunofluorescence of MDCK cell lines
Cells grown on Transwells were washed twice with PBS (OmniPur, EMD) and then fixed in 4% paraformaldehyde/PBS for 20 min at room temperature. The cells were washed three times with PBS and either stained immediately or stored at 4° C in PBS for later staining. Cells were blocked and extracted in BE buffer (10% normal donkey serum, PBS containing either 0.3% Triton X-100 or 5μg/ml filipin as indicated) for 30 min at room temperature, then washed one time with PBS. For flotillin immunostaining experiments, cells were first fixed in paraformaldehyde and then extracted in prechilled methanol [36].
Primary antibodies used for immunostaining included rabbit anti-Rab11a (VU57 [28], used at 1:500), rabbit anti-caveolin-1 pAb (BD Biosciences, #610059, used at 1:200), mouse anti-EEA1 EEA1 (BD Biosciences #610457, used at 1:200), mouse anti-flotillin-1 (BD Biosciences #610820, used at 1:100), and mouse anti-flotillin-2 (BD Biosciences #610383, used at 1:200). Primary antibodies were diluted in antibody buffer (1% normal donkey serum (Jackson ImmunoResearch), 0.05% tween-20, PBS) and incubated with the cells for 2 hr at room temperature. Cells were washed three times in PBS-T (0.05% Tween-20, PBS) then incubated with Cy-3 and Cy-5 labeled species-specific secondary antibodies against rabbit or mouse IgG (Jackson ImmunoResearch) for 1 hr at room temperature. In some experiments Alexa-647-phalloidin (Invitrogen) was included in the secondary antibody incubations (1:200 dilution). Cells were washed three times with PBS-T, then once with PBS. The filters were cut out of the transwells, rinsed in water, and then mounted with ProLong Gold with DAPI (Invitrogen). Cells were imaged on an Olympus FV-1000 (Vanderbilt Cell Imaging Resources Core) using a 100X lens. Twenty 0.5 μm thick Z-sections were imaged per stack.
Results
The distribution of caveolin-1 within the subapical regions of MDCK cells is dependent on cell polarity and fixation conditions
Caveolin-1 displays different epitopes depending on its localization within cells [31, 37, 38]. In addition, the antigenicity of caveolin-1 is strongly dependent on fixation and permeabilization conditions [31, 38]. In a previous study, we identified caveolin-1 in the sub-apical region of MDCK cells using three separate antibodies directed against the N-terminus of the protein [31]. The appearance of the subapical pool of caveolin-1 detected by a commercially available rabbit polyclonal antibody in filipin-permeabilized and Triton X-100 permeabilized cells was most similar to that previously reported for known markers of the apical recycling system. We thus utilized these staining conditions to study further subapical caveolin-1.
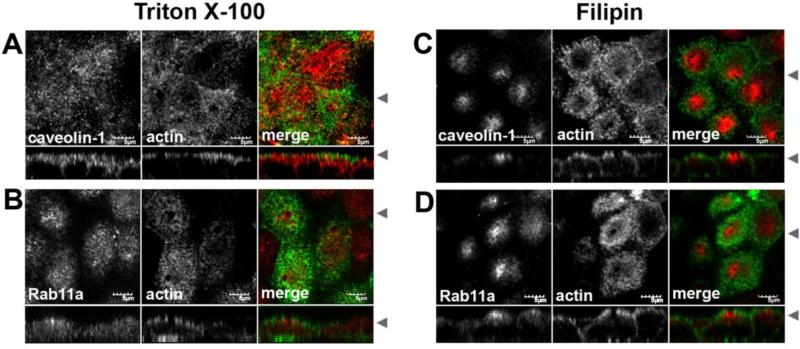
Our previous study examined caveolin-1 in partially polarized cells grown on glass coverslips [31]. We therefore first sought to test whether a similar population of caveolin-1 is present in fully polarized cells, examining the staining patterns of caveolin-1 in MDCK cells grown to full polarity on Transwells (Figure 1). As we had observed previously, the staining pattern varied for caveolin-1 depending upon the conditions used for permeabilization. In Triton X-100 extracted cells, caveolin was present in vesicles in the apical portion of the cell just below the F-actin (Figure 1A). This subapical pool was much more pronounced when filipin was used (Figure 1C). Caveolin-1 staining could also be seen extending along the basal and lateral membranes, consistent with the known distribution of caveolae in MDCK cells [39, 40]. This basolateral pool of caveolin-1 was relatively faint in filipin permeabilized cells (Figure 1C), but was much more apparent following Triton X-100 extraction (Figure 1A). These data demonstrate that caveolin-1 is enriched in the sub-apical region of fully polarized MDCK cells, and also confirm our previous findings that the apparent distribution of caveolin-1 varies depending on permeabilization conditions [31]. Endogenous Rab11a also exhibited differential distributions depending on the detergent used for the extraction. When Triton X-100 was used, the Rab11a vesicles were spread out just under the apical actin web, while with filipin, the Rab11a marked vesicles were more centrally condensed (Figure 1D).
Figure 1. The apparent distribution of caveolin-1 in the apical region of MDCK cells is dependent on fixation conditions.
MDCK cells were grown for 4-5 days post-confluence on Transwell filters. Paraformaldehyde fixed cells were extracted with Triton X-100 (A,B) or filipin (C,D), then co-stained for actin (green in merged) and either caveolin-1 (red in merged in A and C) or Rab11a (red in merge in B and D). Arrowheads indicate where the XY and XZ slices were taken. Bar=10 μM
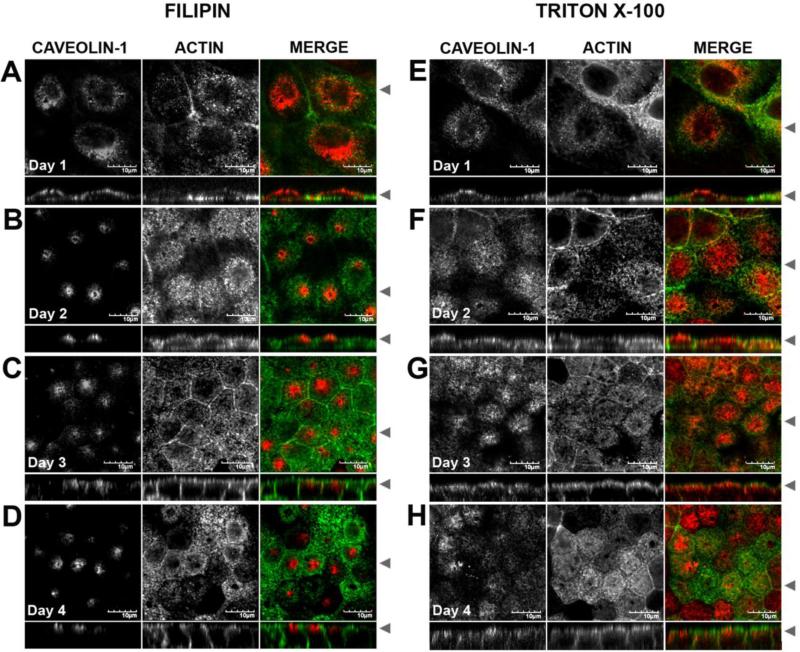
Although caveolin-1 staining patterns in fully polarized MDCK cells were generally consistent with our previous report [31], we noted slight differences that suggested that the morphology of the subapical compartments enriched in caveolin-1 maybe dependent on cell polarity. To test this directly, we performed a time course on cells grown on Transwells (Figure 2). The cells were plated at confluence then fixed at 1, 2, 3 or 4 days post-confluence and permeabilized with either filipin (Figure 2 A-D) or Triton X-100 (Figure 2 E-H). At day one, caveolin-1 staining was spread apically through the cells as well as slightly down the lateral walls (Figure 2 A, E). By day 3, caveolin-1 was associated with apical pericentrosomal vesicles that also extended down the lateral cell wall in the Triton X-100 permeabilized cells (Figure 2 G), and became concentrated in a subapical spot in the filipin permeabilized cells (Figure 2C). These staining patterns were similar to those we reported previously for cells grown on coverslips for 5 days [31]. At day 4, the caveolin-1 spread out apically and laterally in the Triton X-100 permeabilized cells (Figure 2H), and remained concentrated in the subapical region of the filipin-permeabilized cells (Figure 2D). This analysis also revealed a shift in the localization of caveolin-1 relative to actin over time. Caveolin-1 staining was present at the same level as actin staining at the apical surface at day 1. As the cells polarized and became taller, the caveolin-1 pool became distinct from the apical actin web. Thus, both the morphology and distribution of caveolin-1 containing compartments in the subapical region of MDCK cell varies in a cell-polarity and fixation-dependent manner.
Figure 2. The subapical caveolin-1 compartment is polarity dependent.
MDCK cells were grown on Transwell filters for 1 day (A, E), 2 days (B,F), 3 days (C,G) or 4 days (D,H) post confluence. Paraformaldehyde fixed cells were extracted with filipin (A-D) or with Triton X-100 (E-H) then co-stained for caveolin-1 (red in merged) and actin (green in merged). Arrowheads indicate where the XY and XZ slices were taken. Bar=10 μM.
Subapical caveolin-1 overlaps with the distribution of Rab11a, but not EEA1
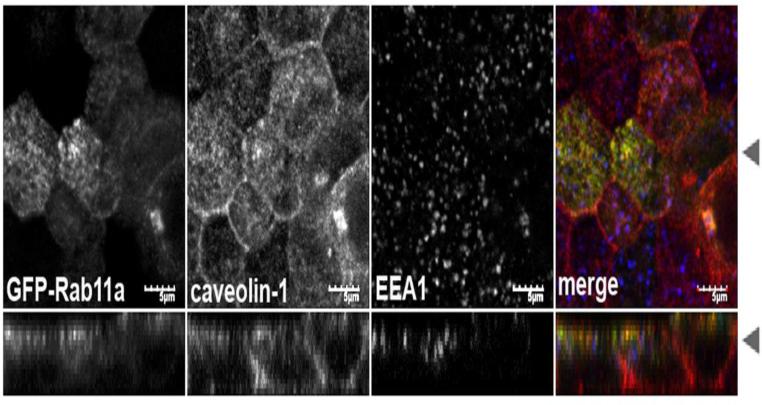
The staining pattern seen in Figure 1 for caveolin-1 and endogenous Rab11a in the subapical region were similar, consistent with the possibility that they reside within the same compartment. Since both the caveolin and Rab11a antibodies were raised in rabbit, we could not perform direct colocalization studies with the endogenous proteins. To examine directly the relationship between Rab11a and caveolin-1 containing vesicles, we utilized two approaches. First, we compared their localizations in a stable EGFP-Rab11a-expressing MDCK cell line [32]. As expected, the distribution of subapical caveolin-1 closely resembled the apical recycling compartment and partially colocalized with GFP-Rab11a, but not EEA1, a marker of early endosomes (Figure 3). Compared to the parental MDCK cells (Figure 1B), the subapical pool of caveolin-1 was slightly more aggregated in cells overexpressing EGFP-Rab11a and the lateral staining was more prominent, suggesting that overexpression of Rab11a may subtly alter the subcellular distribution of caveolin-1 containing compartments. As a second approach, we expressed caveolin-1-EGFP [35] in MDCK cells and compared its localization with that of endogenous Rab11a in filipin permeabilized cells (Figure 4). Like endogenous caveolin-1, caveolin-1-GFP was distributed along the basolateral surface. However, although caveolin-1-GFP was found in the apical region of cells, it was not concentrated in a subapical cluster and did not colocalize well with endogenous Rab11a, suggesting caveolin-1-GFP is not correctly targeted to this compartment under these conditions. Therefore, for further studies we focused primarily on studies of endogenous caveolin-1 in cells expressing GFP-tagged version of Rab11 or its modulators.
Figure 3. Caveolin-1 does not colocalize with the early endosomal marker EEA1, but partially co-localizes with GFP-Rab11a.
MDCK cells grown for 4-5 days post confluence on Transwell filters were fixed with paraformaldehyde and extracted with Triton X-100. GFP-Rab11a (green in merge) MDCK cells were co-stained for caveolin-1 (red in merge) and EEA1 (blue in merge). Arrowheads indicate where the XY and XZ slices were taken. Bar=5 μM.
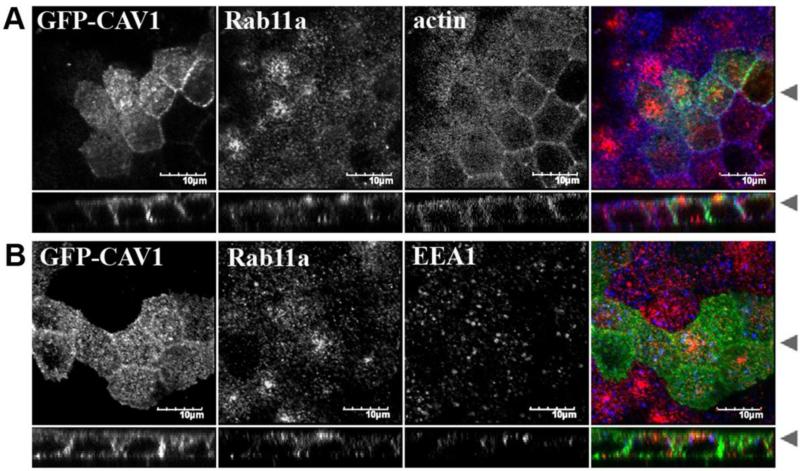
Figure 4. Caveolin-1-GFP does not colocalize with Rab11a in polarized MDCK cells.
MDCK cells plated on Transwell filters were transiently transfected with caveolin-1-GFP (green) and grown for an additional 4-5 days post confluence prior to fixation, permeabilization in filipin, and labeling for (A) endogenous Rab11a (red) and actin (blue), or (B) endogenous Rab11a (red) and EEA1 (blue). Bar = 10 μm.
Subapical caveolin-1 shows a similar dependence as GFP-Rab11a on microtubule manipulation
Endocytic trafficking between the apical or basal early endosome to the common endosome and the apical recycling system is blocked by treatment with the microtubule depolymerizing agent, nocodazole [41]. In fact, one of the most striking features of the Rab11a-positive apical recycling endosomal compartment in MDCK cells is its microtubule dependence. When microtubules are disrupted with nocodazole, the Rab11a compartment is dispersed, whereas stabilization of the microtubules with taxol causes accumulation of Rab11a in the subapical “corners” of cells [6]. Caveolin-1 has also been reported to undergo microtubule-dependent transport [42]. Therefore, to investigate further the relationship between caveolin-1 and Rab11a-containing vesicles, we directly compared the effects of microtubule disruption on the subcellular distribution of the two proteins.
As shown previously, nocodazole dispersed EGFP-Rab11a throughout the cell, whereas taxol caused an accumulation of EGFP-Rab11a in subapical “corners” of cells (Figure 5). Remarkably, the subapical pool of caveolin-1 redistributed in a similar manner following nocodazole and taxol treatment, and showed strong colocalization with EGFP-Rab11a in cell corners in taxol-treated cells. Similar results were observed with cells extracted with filipin (data not shown). Thus, subapical caveolin-1 has a similar microtubule dependence as that of Rab11a, implying that the subcellular distribution of Rab11a- and caveolin-1-containing vesicles within the apical recycling compartment are regulated in a coordinated manner.
Figure 5. Subapical caveolin-1 exhibits a similar dependence on microtubules as GFP-Rab11a.
A stable MDCK cell line expressing GFP-Rab11a (green) grown for 4-5 days post confluence on Tranwell filters, was left untreated (A), or treated with either nocodazole (B) or taxol (C), then fixed and extracted with Triton X-100. The cells were then costained for caveolin-1 (red) and actin (blue). In the non-treated panel (A), one cell (indicated by the white arrowhead) was enlarged and in shown in the insert. For clarity, only the GFP-Rab11a (green) and caveolin-1 (red) channels were used for the insert in the merge panel. Black arrowheads indicate where the XY and XZ slices were taken. Bar=5 μM.
Mutants of Rab11-FIP2 alter the localization of caveolin-1
To examine further the regulation of subapical caveolin-1, we next tested the role of the Rab11 effector Rab11-FIP2, a known regulator of apical recycling and transcytosis, in this process [13, 43]. To do so, we took advantage of a previously identified series of Rab11-FIP2 mutants that block discrete trafficking steps within the apical recycling pathway [13, 43]. These include a carboxyl-terminal truncation mutant missing the Rab binding domain (ΔRAB) that causes the mutant FIP2 to become cytosolic and leads to the dispersal of Rab11a-containing vesicles [43]. In addition, an amino-terminal truncation mutant missing the C2 domain (ΔC2) [13, 43] and another mutant containing 2 point mutations, S229A/R413G (SARG) [13] both collapse the apical recycling system, but at different steps [44].
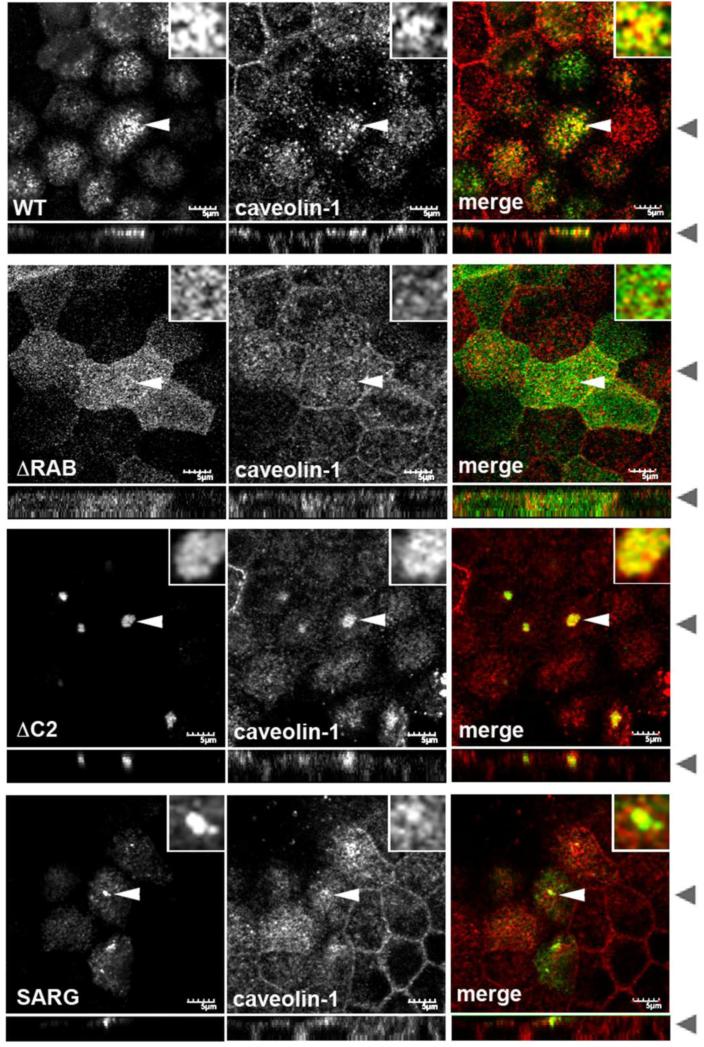
To examine the effects of these mutants, MDCK cells stably expressing EGFP-tagged versions of wild type or mutant Rab11-FIP2 were stained for endogenous caveolin-1 (Figure 6). Consistent with the overlap in Rab11a and caveolin-1 in the subapical region (Figure 1), caveolin-1 was also present in close proximity to wild type EGFP-Rab11-FIP2 in the subapical region of the cells (Figure 6). On close inspection, the two proteins resided on intertwining tubulovesicular membranes (see Figure 6 insets). In cells expressing EGFP-Rab11-FIP2(ΔRAB), the subapical population of caveolin-1 became dispersed, similar to the previously described effect of this mutant on Rab11a [43]. Expression of the mutant EGFP-Rab11-FIP2(ΔC2) also resulted in a dramatic redistribution of caveolin-1, reminiscent of its effects on Rab11a distribution [13, 43]. In particular, caveolin-1 was pulled into the collapsed apical recycling system (Figure 6), where caveolin-1 and Rab11-FIP2(ΔC2) appeared to reside on the coalesced tubulovesicular membranes (inset). Finally, cells expressing the EGFP-Rab11-FIP2(SARG) mutant also showed altered caveolin-1 distribution, with a sub-population of caveolin-1 redistributing to the collapsed structures induced by the SARG mutant. Thus, like Rab11a, subapical caveolin-1 is regulated by Rab11-FIP2.
Figure 6. The distribution of subapical caveolin-1 is altered by Rab11-FIP2 mutants that affect the subcellular distribution of Rab11a vesicles.
Stable MDCK cell lines expressing GFP-Rab11-FIP2 wild type (WT), a C-terminal truncation minus the Rab binding domain (ΔRAB), a N-terminal truncation minus the C2 domain (ΔC2) or a double point mutation (SARG) were grown on Transwells for 4-5 days post confluence, fixed with paraformaldehyde, extracted with Triton X-100 and stained for caveolin-1 (second column, red in merge). The GFP-FIP2 constructs are in the first column (green in merge). White arrowheads indicate the cell that was enlarged for the insert. Black arrowheads indicate where the XY and XZ slices were taken. Similar results were observed with cells extracted with filipin. Bar=5 μm.
Flotillin-positive endosomal compartments are not regulated by Rab11-FIP2
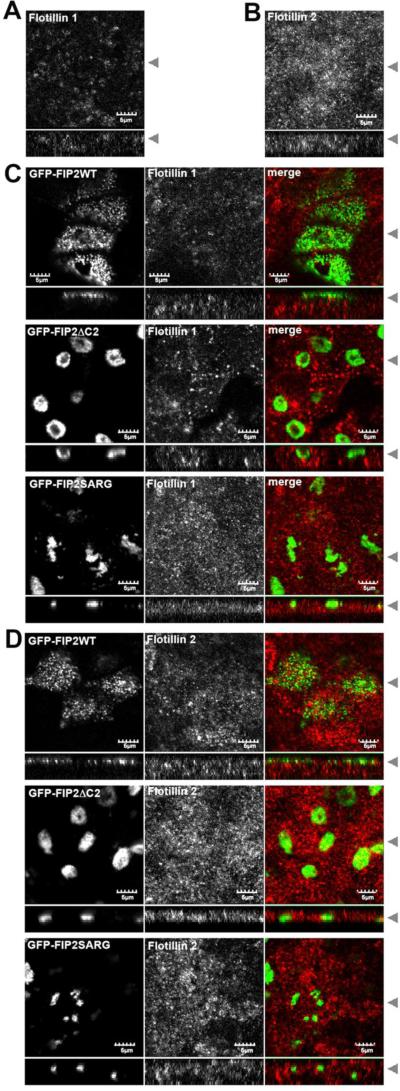
The finding that caveolin-1 and Rab11a are coregulated by Rab11-FIP2 raised the question of whether other raft-associated proteins are part of the same pathway. A number of recent studies have identified flotillin as a marker of a raft-related endocytic pathway [45], and flotillin has also been identified as a component of transferrin-containing recycling endosomes in MDCK cells [26]. We therefore tested for possible regulatory role of Rab11-FIP2 in controlling flotillin in polarized cells by examining the distribution of endogenous flotillin-1 and flotillin-2 in the Rab11-FIP2 cells. Both proteins were localized to punctate vesicular structures scattered throughout the cytoplasm (Figure 7 A,B) . Unlike the case of caveolin-1 (Figure 6), the localization of flotillin-1 and flotillin-2 was not altered in cells expressing mutant forms of Rab11-FIP2 (Figure 7C, D)). This suggests that flotillin-1 and flotillin-2 are contained within endosomal compartments that are regulated independently of Rab11-FIP2.
Figure 7. The subcellular distribution of flotillin is unaffected by Rab11-FIP mutants.
(A) MDCK cells were grown on Transwells for 4-5 days post confluence, fixed with paraformaldehyde, extracted with methanol, and stained for endogenous flotillin-1. (B) As in A except cells were stained for endogenous flotillin-2. (C) Stable MDCK cell lines expressing GFP-Rab11-FIP2 wild type (WT), a N-terminal truncation minus the C2 domain (ΔC2) or a double point mutation (SARG) were grown on Transwells for 4-5 days post confluence, fixed with paraformaldehyde, extracted with methanol, and stained for endogenous flotillin-1 (second column, red in merge). The GFP-FIP2 constructs are shown in the first column (green in merge). Black arrowheads indicate where the XY and XZ slices were taken. (D) As in C, except cells were stained for endogenous flotillin-2. Bar=5 μm.
Discussion
Although caveolin-1 has previously been identified in endosomal recycling compartments of polarized epithelial cells [26-29], little is known about how this population of caveolin-1 is regulated. To address this question, in the current study we took advantage of our previous observations that caveolin-1 in this region of the cell can be detected using specific N-terminal caveolion-1 antibodies and fixation conditions [31] to examine specifically the relationship between subapical caveolin-1 and Rab11a, a well-established marker of the apical recycling system.
Our results indicate that subapical caveolin-1 vesicles share several common features with Rab11a-positive apical recycling endosomes in polarized MDCK cells. First, the morphology of both compartments demonstrates a similar dependence on fixation conditions. Second, caveolin-1 staining substantially overlaps with GFP-Rab11a in this region of the cell, and moreover the localization of both proteins is altered in a similar fashion in response to microtubule disruption by either nocodazole or taxol treatment. Finally, expression of mutants of Rab11-FIP2, an effector of Rab11, led to substantial changes in caveolin-1 localization that mirror those previously reported for Rab11a. Thus, caveolin-1 and Rab11a-containing vesicles are regulated in a coordinated fashion.
The finding that caveolin-1 intersects with Rab11a is at first glance surprising given that clathrin-dependent endocytic vesicles have classically been thought to represent the predominate mechanism by which traffic through the Rab11a controlled pathway originates. However, it is increasingly recognized that multiple types of endocytic vesicles can merge in the sorting or common endosome and from there proceed along common pathways, including the Rab11a regulated apical recycling pathway [46]. We propose that the simplest explanation for our findings is that caveolin-1 is a component of apical recycling or transcytosing endosomes containing Rab11a. In support of this model, previous biochemical studies identified caveolin-1 in H/K-ATPase-containing vesicles immunoisolated from human gastric mucosa [28]. These vesicles, which are known to contain high levels of Rab11a, also contained caveolin-1 [28]. Some caveolin-1 may also be present in pathways leading to or from apical recycling endosomes. For example, transferrin-containing vesicles immunoisolated from MDCK cells also contain caveolin-1 [26]. Since transferrin-positive endosomes in polarized cells are not positive for Rab11a [3, 47, 48], it is possible that the presence of caveolin-1 in this compartment is the result of its trafficking to the common sorting endosome. The presence of caveolin-1 in compartments that feed into apical recycling endosomes could also explain why caveolin-1 only partially overlaps with Rab11a and Rab11-FIP2.
It is not yet clear if caveolin-1 itself recycles between the apical or basolateral surface and the apical recycling compartment, or if the subapical pool of caveolin-1 is regulated in an autonomous fashion. In non-polarized cells, where caveolar trafficking has been best studied, caveolin-1 undergoes microtubule-dependent trafficking to the pericentrosomal region [42]. Caveolae appear to retain their identity following internalization and are returned to the plasma membrane with their caveolin coat intact [49, 50]. Thus, internalized caveolae have the potential to recycle directly to the plasma membrane. More recent studies have also revealed that caveolin-1 can enter into endosomal and lysosomal compartments when the protein is not incorporated into caveolae [51]. As such, internalization of caveolae and caveolin-1 can be uncoupled. In MDCK cells, caveolin-1 is present on both the apical and basolateral surface, but caveolae per se are typically only found basolaterally. However, internalization of caveolin-1 and lipid raft-associated proteins from the apical plasma membrane has been observed under some conditions [39, 40]. As a result, caveolin-1 could potentially access apical recycling endosomes by transcytosis from the basolateral surface via internalization of caveolae, or from the apical surface by a caveolae-independent pathway.
Our results also suggest that the association of caveolin-1 with apical recycling compartments is controlled by cell polarity. One possible way this might occur is raised by the recent findings of Takahashi et al., who showed that cholesterol localization is dependent upon the confluence of non-polar cells and that Rab11 is involved in endocytosis of certain lipid subsets [52]. We speculate that caveolin-1 distribution may be controlled by a related mechanism.
The function of caveolin-1 in the apical recycling system remains to be determined. Given the role of caveolin-1 as an essential component of caveolae, it seems highly likely that caveolin-1 may function in regulating trafficking or sorting in this compartment. A previous study showed that caveolin-1 directly interacts with a cargo molecule of the apical recycling system, polymeric IgA [29]. Caveolin-1 and polymeric IgA receptor also co-localize in deep apical tubules in enterocytes, and this compartment may represent a site where apical externalization of transcytosed IgA occurs [30]. However, as IgA trafficking occurs in epithelial cells devoid of caveolin-1 [53], caveolin-1 does not appear to play an essential role in IgA transcytosis. It is also interesting to note that the endocytic recycling compartments of both non-polarized and polarized cells are highly enriched in cholesterol [26, 54-56]. As previous investigations have suggested that both caveolin-1 and Rab11 regulate cellular cholesterol transport and homeostasis [17, 52, 57-60], caveolin-1 may cooperate with Rab11 to control cholesterol trafficking through this compartment.
Conclusion
In conclusion, the results presented in this study suggest that caveolin-1 is a component of the apical recycling system of polarized MDCK cells. Similar to Rab11a, subapical caveolin-1 is dependent on microtubules and regulated by Rab11-FIP2. Given the many links between caveolin-1 and numerous cellular processes including cholesterol homeostasis, cell signaling, and mechanotransduction, it will be important to define in future studies which of these pathways depend on the function of caveolin-1 in the apical recycling system.
Acknowledgments
We thank Cathy Caldwell for assistance with cell culture and Drs. Gudrun Ihrke and Ann Hubbard for helpful discussions. Supported by NIH grants # DK70856, DK48370, and GM073846. Confocal images were generated in part through the use of the VUMC Cell Imaging Shared Resource (supported by National Institutes of Health [NIH] grants CA-68485, DK-20593, DK-58404, HD-15052, DK-59637, and EY-08126). The funding sources had no role in the study design, collection, analysis or interpretation of data, writing the report, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: CRE, common recycling endosome; ARE, apical recycling endosome; MDCK, Madin Darby Canine Kidney; Rab11-FIPs, Rab11 Family Interacting Proteins
References
- 1.Mostov K, Apodaca G, Aroeti B, Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992;116:577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- 4.Golachowska MR, Hoekstra D, van ISC. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol. 2010;20:618–626. doi: 10.1016/j.tcb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung SM, Ruiz WG, Apodaca G. Sorting of membrane and fluid at the apical pole of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 2000;11:2131–2150. doi: 10.1091/mbc.11.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EK, Lapierre LA, Goldenring JR. Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem. 2001;276:39067–39075. doi: 10.1074/jbc.M104831200. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, Goldenring JR. The Rab11-FIP1/RCP gene codes for multiple protein transcripts related to the plasma membrane recycling system. Biochim Biophys Acta. 2006;1759:281–295. doi: 10.1016/j.bbaexp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay AJ, McCaffrey MW. Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain. J Biol Chem. 2002;277:27193–27199. doi: 10.1074/jbc.M200757200. [DOI] [PubMed] [Google Scholar]
- 11.Wallace DM, Lindsay AJ, Hendrick AG, McCaffrey MW. Rab11-FIP4 interacts with Rab11 in a GTP-dependent manner and its overexpression condenses the Rab11 positive compartment in HeLa cells. Biochem Biophys Res Commun. 2002;299:770–779. doi: 10.1016/s0006-291x(02)02720-1. [DOI] [PubMed] [Google Scholar]
- 12.Prekeris R, Davies JM, Scheller RH. Identification of a novel Rab11/25 binding domain present in Eferin and Rip proteins. J Biol Chem. 2001;276:38966–38970. doi: 10.1074/jbc.M106133200. [DOI] [PubMed] [Google Scholar]
- 13.Ducharme NA, Williams JA, Oztan A, Apodaca G, Lapierre LA, Goldenring JR. Rab11-FIP2 regulates differentiable steps in transcytosis. Am J Physiol Cell Physiol. 2007;293:C1059–1072. doi: 10.1152/ajpcell.00078.2007. [DOI] [PubMed] [Google Scholar]
- 14.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. USA. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol. Biol. Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291:H677–686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- 18.Martin S, Parton RG. Caveolin, cholesterol, and lipid bodies. Semin Cell Dev Biol. 2005;16:163–174. doi: 10.1016/j.semcdb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Ikonen E, Heino S, Lusa S. Caveolins and membrane cholesterol. Biochem Soc Trans. 2004;32:121–123. doi: 10.1042/bst0320121. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel A, Lisanti MP. The caveolin triad: caveolae biogenesis, cholesterol trafficking, and signal transduction. Cytokine Growth Factor Rev. 2001;12:41–51. doi: 10.1016/s1359-6101(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 21.van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13:92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 22.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 23.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng ZJ, Singh RD, Marks DL, Pagano RE. Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids. Mol Membr Biol. 2006;23:101–110. doi: 10.1080/09687860500460041. [DOI] [PubMed] [Google Scholar]
- 25.Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122:1713–1721. doi: 10.1242/jcs.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagescu R, Demaurex N, Parton RG, Hunziker W, Huber LA, Gruenberg J. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell. 2000;11:2775–2791. doi: 10.1091/mbc.11.8.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pol A, Calvo M, Lu A, Enrich C. The “early-sorting” endocytic compartment of rat hepatocytes is involved in the intracellular pathway of caveolin-1 (VIP-21) Hepatology. 1999;29:1848–1857. doi: 10.1002/hep.510290602. [DOI] [PubMed] [Google Scholar]
- 28.Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1249–1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- 29.Leyt J, Melamed-Book N, Vaerman JP, Cohen S, Weiss AM, Aroeti B. Cholesterol-sensitive modulation of transcytosis. Mol Biol Cell. 2007;18:2057–2071. doi: 10.1091/mbc.E06-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen GH, Pedersen J, Niels-Christiansen LL, Immerdal L, Danielsen EM. Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J. 2003;373:125–132. doi: 10.1042/BJ20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush WS, Ihrke G, Robinson JM, Kenworthy AK. Antibody-specific detection of caveolin-1 in subapical compartments of MDCK cells. Histochem Cell Biol. 2006;126:27–34. doi: 10.1007/s00418-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 32.Lapierre LA, Dorn MC, Zimmerman CF, Navarre J, Burnette JO, Goldenring JR. Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp Cell Res. 2003;290:322–331. doi: 10.1016/s0014-4827(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 33.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 34.Barth AI, Pollack AL, Altschuler Y, Mostov KE, Nelson WJ. NH2-terminal deletion of beta-catenin results in stable colocalization of mutant beta-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J Cell Biol. 1997;136:693–706. doi: 10.1083/jcb.136.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 36.de Diesbach P, Medts T, Carpentier S, D'Auria L, Van Der Smissen P, Platek A, Mettlen M, Caplanusi A, van den Hove MF, Tyteca D, Courtoy PJ. Differential subcellular membrane recruitment of Src may specify its downstream signalling. Exp Cell Res. 2008;314:1465–1479. doi: 10.1016/j.yexcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verkade P, Harder T, Lafont F, Simons K. Induction of caveolae in the apical plasma membrane of Madin-Darby canine kidney cells. J. Cell Biol. 2000;148:727–739. doi: 10.1083/jcb.148.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel U, Sandvig K, Van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J. Cell Sci. 1998;111:825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 41.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic. 2001;2:149–159. doi: 10.1034/j.1600-0854.2001.020301.x. [DOI] [PubMed] [Google Scholar]
- 42.Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 43.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J Biol Chem. 2002;277:50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 44.Ducharme N, Ham A, Lapierre L, Goldenring JR. Rab11-FIP2 influences multiple components of the endosomal system in polarized MDCK cells. Cellular Logistics in press. 2011 doi: 10.4161/cl.1.2.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 46.Welling PA, Weisz OA. Sorting it out in endosomes: an emerging concept in renal epithelial cell transport regulation. Physiology (Bethesda) 2010;25:280–292. doi: 10.1152/physiol.00022.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in madin-darby canine kidney cells by Rab11a and Rab25. J Biol Chem. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- 48.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr., Mercer JA, Bahler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Bauer M, Pelkmans L. A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 51.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi M, Murate M, Fukuda M, Sato SB, Ohta A, Kobayashi T. Cholesterol controls lipid endocytosis through Rab11. Mol Biol Cell. 2007;18:2667–2677. doi: 10.1091/mbc.E06-10-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarnataro D, Nitsch L, Hunziker W, Zurzolo C. Detergent insoluble microdomains are not involved in transcytosis of polymeric Ig receptor in FRT and MDCK cells. Traffic. 2000;1:794–802. doi: 10.1034/j.1600-0854.2000.011006.x. [DOI] [PubMed] [Google Scholar]
- 54.Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- 55.Hornick CA, Hui DY, DeLamatre JG. A role for retrosomes in intracellular cholesterol transport from endosomes to the plasma membrane. Am J Physiol. 1997;273:C1075–1081. doi: 10.1152/ajpcell.1997.273.3.C1075. [DOI] [PubMed] [Google Scholar]
- 56.Wustner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc. Natl. Acad. Sci. USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtta-Vuori M, Tanhuanpaa K, Mobius W, Somerharju P, Ikonen E. Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol Biol Cell. 2002;13:3107–3122. doi: 10.1091/mbc.E02-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu BB, Ge L, Xie C, Zhao Y, Miao HH, Wang J, Li BL, Song BL. Requirement of myosin Vb.Rab11a.Rab11-FIP2 complex in cholesterol-regulated translocation of NPC1L1 to the cell surface. J Biol Chem. 2009;284:22481–22490. doi: 10.1074/jbc.M109.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fielding CJ, Fielding PE. Relationship between cholesterol trafficking and signaling in rafts and caveolae. Biochim Biophys Acta. 2003;1610:219–228. doi: 10.1016/s0005-2736(03)00020-8. [DOI] [PubMed] [Google Scholar]