Abstract
Mast cells are hematopoietic immune cells located throughout the body, including within the brain. Reconstitution of mast cell deficient KitW-sh/W-sh mice has proven valuable in determining peripheral mast cell function. Here we study the brain mast cell population using a novel method of blood transfusion for reconstitution. We show that blood transfusion results in mast cells of donor origin in the WT mouse, including in the brain and are restricted to regions bearing host mast cells. In contrast, in KitW-sh/W-sh mice, transfusion results in mast cells in the pinna of the ear, but not the brain.
Keywords: Mast cells, brain, blood transfusion, KitW-sh/W-sh (sash) mouse
(1)INTRODUCTION
Mast cells are hematopoietic immune cells and are located throughout the body, including in the brain. The physiological function of peripheral mast cells has become better understood through reconstitution of these cells in mast cell deficient (KitW-sh/W-sh). Peripheral mast cell populations can be partially restored in KitW-sh/W-sh mice by intravenous injection of wild-type bone marrow-derived mast cells (BMMCs) (Grimbaldeston et al., 2005; Wolters et al., 2005). The results of such studies implicate peripheral mast cells in host defense and angiogenesis (Mallen-St Clair et al., 2004; Soucek et al., 2007; Liu et al., 2009). While peripheral mast cell reconstitution serves as a powerful tool to examine mast cell biology, the efficacy of central mast cell reconstitution is not clear. BMMCs injected into mast cell deficient KitW-sh/W-sh mice do not populate the brain (Grimbaldeston et al., 2005; Bennett et al., 2009). In rat, mature donor peritoneal mast cells (PMCs) injected intravenously cross the blood-brain barrier into the brain parenchyma within 1h (Silverman et al., 2000).
In normal physiology, mature mast cells are not found in the blood. Rather, mast cell precursors circulate in the blood, enter tissues and mature in their local microenvironment (Kitamura and Fujita, 1989; Chen et al., 2005). In the present study, we explore the possibility of adoptive transfer of mast cells by transplanting their precursors via blood transfusion into wild-type (WT) and mast cell deficient KitW-sh/W-sh mice using blood transfusion from Okabe mice. We show that mature donor-derived mast cells are found in WT mice within 2 weeks of transfusion, and remain resident there for at least 12 weeks: Their distribution coincides with the localization of resident host mast cells. In contrast, donor mast cells were not observed in KitW-sh/W-sh host brain, but were seen in the pinna of the ear.
(2)MATERIALS AND METHODS
Animals
Three lines of mice (Jackson Laboratories, Bar Harbor, ME) were used in these studies: (1) C57BL/6 male WT, (2) mast cell deficient C57BL/6-KitW-sh/W-sh mice (B6.Cg-KitW-sh/HNihrJaeBsmJ), and (3) Okabe mice (C57BL/6-Tg(CAG-EGFP)1Osb/J) that express green fluorescent protein (GFP) in all tissues except erythrocytes and hair (Okabe et al., 1997). Mice were housed in standard laboratory cages with ad libitum food and water in a 12:12 light-dark cycle at 22±1°C. All procedures were approved by the Institutional Animal Care and Use Committee at Columbia University.
Adoptive Transfer
Whole blood transfusion from Okabe to WT and KitW-sh/W-sh mice was performed. Blood (0.2ml/mouse) was collected from anesthetized Okabe mice (n=5) by cardiac puncture using a heparin-coated syringe, and immediately injected into the carotid artery of recipient WT (n=5) or KitW-sh/W-sh mice (n=4). WT controls (n=2) were injected with saline using a heparin coated syringe. WT mice were perfused 2 (n=2), 4 (n=1), 8 (n=1) or 12 (n=1) weeks following transfusion. KitW-sh/W-sh mice were perfused either 2 (n=2) or 12 (n=2) weeks following transfusion and immunohistochemistry (IHC) for avidin and GFP was performed.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital (200mg/kg) and perfused with 4% paraformaldehyde. Brains and ear pinna were post-fixed, cryoprotected and then cut into 50 μm thick coronal sections on a cryostat (Microm HM 500).
IHC was performed on every other brain section throughout the diencephalon as previously described (Silverman et al., 2000) with an antibody against GFP [rabbit anti-GFP (1:10,000 for 48h, #A6455, Molecular Probes, Carlsbad, CA) and CY2 conjugated donkey anti-rabbit (1:200 for 2h, Jackson Immunoresearch, West Grove, PA)] and CY3 conjugated egg white avidin to label mast cells (1:500 for 2h, Jackson Immunoresearch). Immunoreactivity was visualized using a fluorescent microscope (Nikon Eclipse E800) equipped with filter cubes to detect CY2 and Texas Red. Images were captured with a Q-Imaging Retiga EXi camera and software (Quantitative Imaging, Surrey, BC, Canada). To label mast cells, toluidine blue staining was performed on slide-mounted sections of ear pinna, as previously described (Asarian et al., 2002). Briefly, slides were washed in 60% EtOH for 2 min, stained with metachromatic acidic toludine blue (TB; Sigma, St. Louis, MO) for 10 min (4mg/ml TB in 60% EtOH, pH=2.0), washed briefly in distilled water, then dehydrated through a series of ethanols (50% for 15secs, 70% for 45secs, 2 × 95% for 1min, 2 × 100% for 1min) then cleared in Citrasolv (3 × 5min) and coverslipped with Permount.
(3)RESULTS
Blood transfusion results in donor mast cells in the brain of WT, but not KitW-sh/W-sh mice
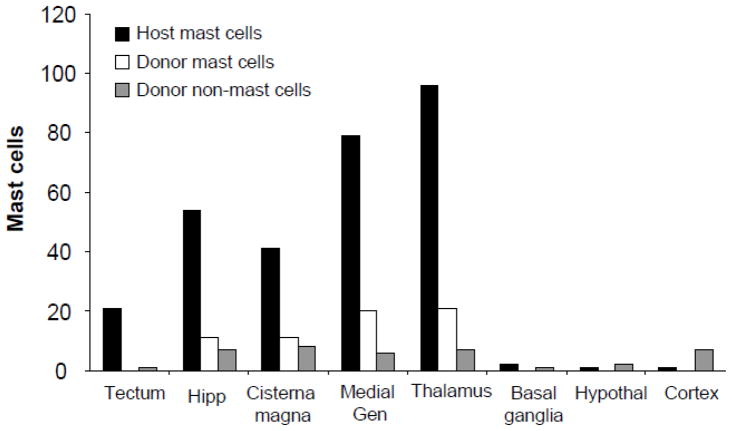
Host mast cells, identified by avidin and lack of GFP, were localized in the diencephalic and hippocampal parenchyma as well as choroid plexus and meninges of WT mice (Fig 1A). Donor cells identified by expression of GFP, were found throughout the brain of WT mice following whole blood transfusion (Fig 1A), but not in saline-injected WT controls (Fig 1B). Donor mast cells, identified by expression of both GFP avidin, were found in the brains of WT mice from 2–12 weeks following blood transfusion. There were no differences in number or distribution of donor cells or donor mast cells among time points following transfusion; therefore, the data from all time points were combined for the remainder of analysis.
Figure 1.
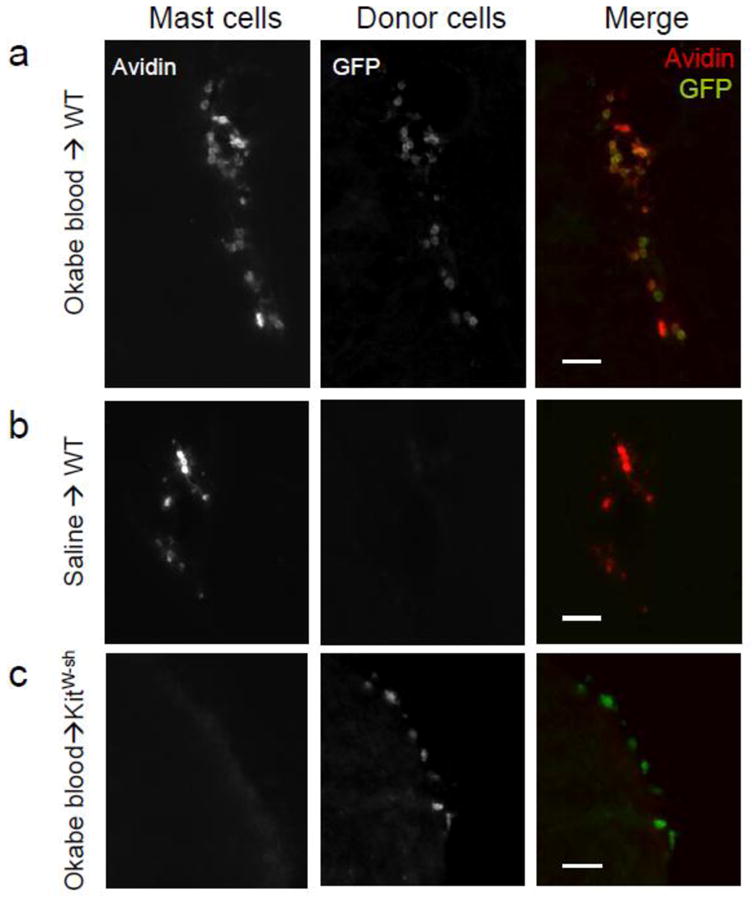
Staining for mast cells and donor cells is shown in WT mice transfused with Okabe blood (a) or saline (b), and a KitW-sh/W-sh mouse transfused with Okabe blood (c). Scale bars, 30μm.
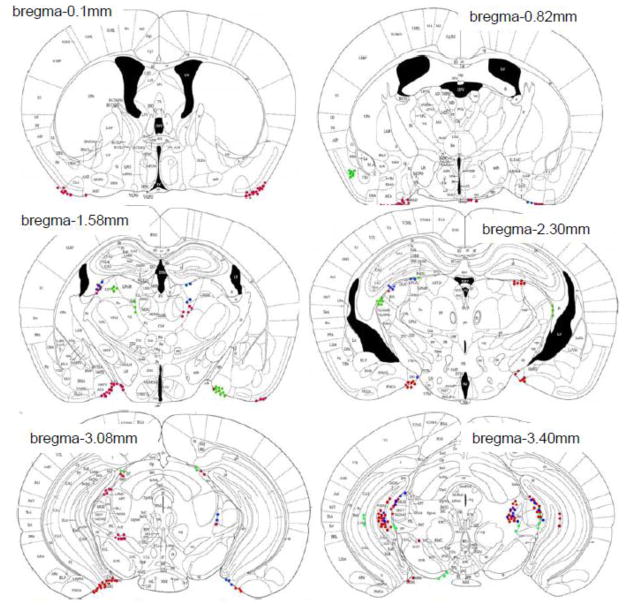
Transfused mast cells (GFP+ avidin+) were found only in brain areas that contained host mast cells (GFP− avidin+; Fig 2). In contrast, unidentified donor cells (GFP+ avidin−) were observed throughout the brain including in regions where mast cell occur infrequently, such as the cortex (Fig 3).
Figure 2.
The distribution of host mast cells (red), unidentified donor cells (green), and donor mast cells (blue) in WT mice transfused with Okabe blood is shown on representative atlas sections (Paxinos and Franklin, 2004).
Figure 3.
Number of host mast cells, donor mast cells, and unidentified donor non-mast cells in WT mice transfused with Okabe blood.
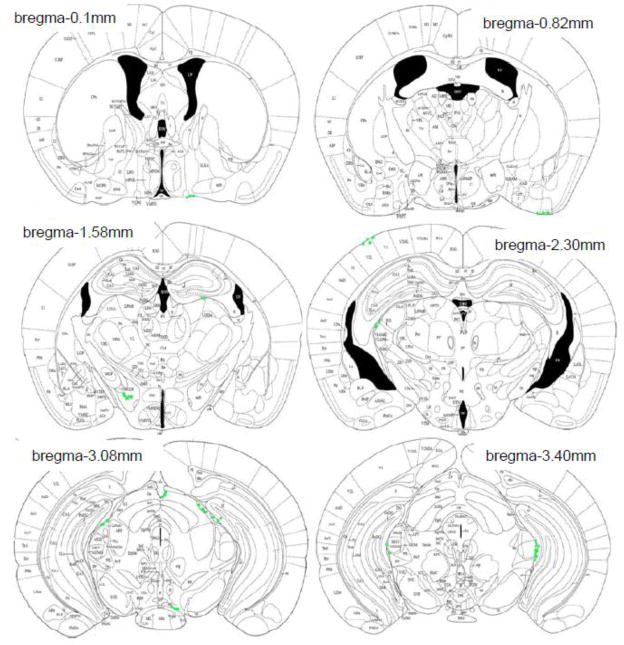
In host KitW-sh/W-sh mice, unidentified donor cells (GFP+ avidin−) were found in the brain at both 2 and 20 weeks following blood transfusion, but none were mast cells (Fig 1C). The distribution of unidentified donor cells in KitW-sh/W-sh mice was similar to that seen in the WT mouse brain. There were no differences in the distribution of GFP+ cells from 2 to 20 weeks following transfusion. GFP+ cells were found in the cortex, thalamus, hypothalamus and meninges and choroid plexus (Fig 4).
Figure 4.
The distribution of unidentified donor cells (green) in KitW-sh/W-sh mice transfused with Okabe blood is shown on representative atlas sections (Paxinos and Franklin, 2004).
As a control tissue, the skin of the ear pinna of mice was examined for the presence of mast cells following blood or saline transfusion (Fig 5). Following saline transfusion, mast cells were present in the ear pinna of WT (Fig 5a), but not KitW-sh/W-sh mice (Fig 5b). In contrast, following whole blood transfusion, mast cells were found in the ear pinna of KitW-sh/W-sh mice, (Fig 5c, d).
Figure 5.
Staining for mast cells with toluidine blue is shown in the ear pinna of a WT mouse (a), KitW-sh/W-sh mouse following saline transfusion (b), and mast cell deficient mouse following whole blood transfusion (c). A high-power image of one mast cell found in the ear pinna of a KitW-sh/W-sh mouse following whole blood transfusion is also shown (d). Scale bar 30 μm (a–c); 30 μm (d).
(4)Discussion
Mast cells leave the bone marrow and circulate in the blood in an immature state before entering tissue where they mature (Kitamura and Fujita, 1989). Using whole blood, the present results show that donor mast cells from Okabe mice occur in the brain of WT mice as early as 2 weeks, and as late as 12 weeks following transfusion. This is the first demonstration of whole blood transfusion as a method of adoptive transfer to introduce donor mast cells into host tissue, and may prove useful in studies of mast cells.
Our studies show that whole blood transfusion, a novel method for reconstitution, results in appearance of donor mast cells in the WT mouse brain. However, following comprehensive analysis of the entire diencephalon and mesencephalon, we confirm less detailed analyses of the cns by others, using different procedures, that brain mast cells are not reconstituted in KitW-sh/W-sh mice, We further show that this failure does not arise from their inability to migrate and mature in KitW-sh/W-sh mice, as mast cells are found in the ear pinna of these animals following whole blood transfusion. Additionally, unidentified non-mast cells of donor origin were found in the brain of KitW-sh/W-sh mice following blood transfusion, suggesting that migration of cells to the brain is not impaired. Although these unidentified cells were not examined in the present study, they may include microglia, dendritic cells and T-cells (Geissmann et al., 2003).
The lack of donor mast cells in the brain of KitW-sh/W-sh mice is consistent with results from other studies which used intravenous injection of BMMCs into KitW-sh/W-sh mice and also W/Wv mice, another mast cell deficient strain (Tanzola et al., 2003; Grimbaldeston et al., 2005; Bennett et al., 2009). However, intravenous injection of BMMCs does repopulate mast cells in peripheral tissues of mast cell deficient KitW-sh/W-sh and W/Wv mice, but the distribution of donor mast cells does not match the WT distribution (Tanzola et al., 2003; Grimbaldeston et al., 2005). For example, more donor mast cells were found in the stomach, lung and spleen, and fewer in the skin of reconstituted KitW-sh/W-sh mice compared to WT mice.
The lack of mast cell recruitment to the brain of KitW-sh/W-sh mice coupled with the recruitment of mast cells in the WT mouse specifically to brain regions containing host mast cells, suggests that host mast cells may play a role in recruiting donor mast cells to the brain. Of the large number of chemokines and other immune molecules that promote the migration of mast cells during adulthood and development (Lambracht-Hall et al., 1990), many are synthesized and released by mast cells. These mast cell-derived, mast cell chemoattractants include TGF-β, TNF-α NGF, IL-8, histamine and serotonin (Gruber et al., 1994; Nilsson et al., 1999; Sawada et al., 2000; Hofstra et al., 2003; Kushnir-Sukhov et al., 2006; Brzezinska-Blaszczyk et al., 2007). Mast cells in turn have cell surface molecules such as CD34 and CXCR2 that are implicated in mast cell mobility and trafficking (Nilsson et al., 1999; Drew et al., 2002; Blanchet et al., 2007). Possibly, injecting a cocktail of mast cell-derived mediators into the brain of KitW-sh/W-sh mice concurrently with whole blood transfusion will result in recruitment of donor mast cells.
In summary, the results reveal that mast cells in the brain hone to specific areas suggesting roles in the modulation of the function of these regions. In addition, these results present a simple method free of the need for cell-cultures, for adoptive transfer of mast cells in the mouse. Our comprehensive analysis of the location to which donor mast cells migrate in the brain may help further the understanding of mast cells and their migration to the brain will aid in studies of their functional role in this site (Nautiyal et al., 2008; Theoharides et al., 2008).
Acknowledgments
We’d like to thank Drs. Joseph LeSauter and Matthew Butler for their help with this project. Funding sources: F31 NIMH 084384 (to KMN), NSF IOS 05-54514 and R21 NIMH 067782 (to RS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asarian L, Yousefzadeh E, Silverman AJ, Silver R. Stimuli from conspecifics influence brain mast cell population in male rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol. 2009;182:5507–5514. doi: 10.4049/jimmunol.0801485. [DOI] [PubMed] [Google Scholar]
- Blanchet MR, Maltby S, Haddon DJ, Merkens H, Zbytnuik L, McNagny KM. CD34 facilitates the development of allergic asthma. Blood. 2007;110:2005–2012. doi: 10.1182/blood-2006-12-062448. [DOI] [PubMed] [Google Scholar]
- Brzezinska-Blaszczyk E, Pietrzak A, Misiak-Tloczek AH. Tumor necrosis factor (TNF) is a potent rat mast cell chemoattractant. J Interferon Cytokine Res. 2007;27:911–919. doi: 10.1089/jir.2006.0158. [DOI] [PubMed] [Google Scholar]
- Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew E, Merkens H, Chelliah S, Doyonnas R, McNagny KM. CD34 is a specific marker of mature murine mast cells. Exp Hematol. 2002;30:1211–1218. doi: 10.1016/s0301-472x(02)00890-1. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Kew RR. Transforming growth factor-beta 1 mediates mast cell chemotaxis. J Immunol. 1994;152:5860–5867. [PubMed] [Google Scholar]
- Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Fujita J. Regulation of mast cell differentiation. Bioessays. 1989;10:193–196. doi: 10.1002/bies.950100604. [DOI] [PubMed] [Google Scholar]
- Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- Lambracht-Hall M, Dimitriadou V, Theoharides TC. Migration of mast cells in the developing rat brain. Brain Res Dev Brain Res. 1990;56:151–159. doi: 10.1016/0165-3806(90)90077-c. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Mikovits JA, Metcalfe DD, Taub DD. Mast cell migratory response to interleukin-8 is mediated through interaction with chemokine receptor CXCR2/Interleukin-8RB. Blood. 1999;93:2791–2797. [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Sterotaxic Coordinates. San Diego, CA: Academic Press; 2004. [Google Scholar]
- Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood. 2000;95:2052–2058. [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Doyle R, Francis K, Conti P, Kalogeromitros D. Novel therapeutic targets for autism. Trends Pharmacol Sci. 2008;29:375–382. doi: 10.1016/j.tips.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]