1. Mn essentiality and toxicity
Mn is an essential ubiquitous trace element required for normal growth, development and cellular homeostasis [1]. Specifically, Mn is important in bone formation, fat and carbohydrate metabolism, blood sugar regulation, and calcium absorption. In humans and animals, Mn functions as a required cofactor of several enzymes necessary for neuronal and glial cell function, as well as enzymes involved in neurotransmitter synthesis and metabolism [2, 3, 4]. Furthermore, in vitro data has implicated Mn in the induction of stellate process formation by astrocytes [5]. Mn exists in various chemical forms including oxidation states (Mn2+, Mn3+, Mn4+ Mn6+, Mn7+), salts (sulfate and gluconate), and chelates (aspartate, fumarate, succinate). The versatile chemical properties of Mn have enabled its industrial usage in making glass and ceramics, adhesives, welding, paint, gasoline anti-knock additives (methylcyclopentadienyl manganese tricarbonyl (MMT), and many others. While uncommon, Mn deficiency can contribute to birth defects, impaired fertility, bone malformation, weakness, and enhanced susceptibility to seizures [6, 7]. The routes of Mn exposure are mainly through dietary intake, dermal absorption, and inhalation. Moreover, Mn in the diet is found mostly in whole grains, nuts, and seeds, tea, legumes, pineapple, and beans. Despite its essential role in multiple metabolic functions, excessive Mn exposure can accumulate in the brain and has been associated with dysfunction of the basal ganglia system that causes a severe neurological disorder similar to PD [8].
Mn levels in brain tissue average approximately 1–2 µg/g dry weight. The concentration of Mn in the brain varies across brain regions under excessive exposures. Importantly, the highest Mn levels are found in the globus pallidus in humans and in the hypothalamus in rats [9, 10]. Excessive and prolonged exposure to Mn resulting from occupations such as welding and mining, inhalation of combustion products from the anti-knock agent in fuel (MMT) and highly concentrated Mn concentrations in ground/well water leads to Mn accumulation in the dopamine-rich regions of the basal ganglia. In fact, spectroscopy in rats has demonstrated that mitochondria in the basal ganglia accumulate the highest amount of Mn following exposure [11, 12]. This causes a clinical disorder referred to as manganism that is characterized by a set of extrapyramidal symptoms resembling idiopathic Parkinson’s disease (IPD) including anorexia, apathy, and muscle and joint pain. Shortly after the onset of these symptoms, patients also exhibit memory loss, compulsive behavior, visual impairment, illusions and delusions, and disorientation, which is clinically referred to as locura manganica, or manganese madness [13]. Mn overload affects two vital organs, the brain and lungs; the latter results from inhalation [14, 15]. Elevated Mn levels in the brain have been associated with impairment in iron homeostasis, excitotoxicity, mitochondrial dysfunction, oxidative stress, induction of protein aggregation, and alteration in the homeostatic conditions of other divalent metals that share similar transporter systems with Mn. Although the relationship between increased Mn levels and its disruptive effects on the neurochemistry of neurotransmitters has been debated, elevated Mn has been suggested to alter concentrations of γ-aminobutyric acid (GABA), dopamine, and glutamate neurotransmitters in the brain [7, 16].
1a. Mn biology and tissue homeostasis
Although copper and magnesium can substitute for Mn as a cofactor for some enzymes, a subset of enzymes with roles in neuron and/or glial function only in the presence of Mn. These discrete Mn-binding proteins (manganoproteins) include glutamine synthetase, superoxide dismutase 2 (SOD2), arginase, pyruvate decarboxylase, and serine/threonine phosphatase [10, 17, 18].
Glutamine synthetase (GS), the most abundant manganoprotein, is predominantly expressed in astrocytes, where it converts glutamate to glutamine. Because GS contains four Mn ions per octamer [19], Mn has been proposed to regulate GS activity. In fact, insufficient manganese has been proposed to increase glutamate trafficking, glutamatergic signaling, and excitotoxicity [20]. Furthermore, it has been proposed that the increased susceptibility to seizures observed in individuals with Mn deficiency may be due in part to diminished GS levels and/or activity [21]. SOD2 is a mitochondrial enzyme that detoxifies superoxide anions through the formation of hydrogen peroxide (H2O2). Although the concentration of Mn within neurons is low (<10−5 M), their high mitochondrial energy demands is correlated with a propensity of increased SOD2 in neurons compared to glia [9, 14]. Furthermore, loss of SOD2 activity increases the susceptibility to mitochondrial inhibitor induced toxicity and causes oxidative stress [22].
Arginase regulates elimination of ammonia from the body by converting L-arginine, synthesized from ammonia, to L-ornithine and urea as part of the urea cycle. Moreover, in the brain, L-arginine is converted to nitric oxide by neuronal nitric oxide synthetase. Proper regulation of arginase promotes neuronal survival by impairing nitric oxide signaling [23, 24]. Pyruvate carboxylase is an essential enzyme required for glucose metabolism that interacts with Mn to generate oxaloacetate, a precursor of the tricarboxylic acid (TCA) cycle [25]. Interestingly, in the brain, pyruvate carboxylase is predominantly expressed in astrocytes [26, 27]. Protein phosphatase 1 is essential for glycogen metabolism, cell progression, regulation synthesis and release of neurotrophins, which promote neuronal survival, and synaptic membrane receptors and channels [28].
Intricate regulation of Mn absorption and tissue specific accumulation is crucial for it to properly regulate these enzymes, so understanding Mn’s essential roles and toxicity in the brain requires knowledge of its regulation in the periphery. Three major factors have been postulated to modulate plasma Mn levels. First, given that the main source of Mn is diet, tight regulation of gastrointestinal absorption of Mn is crucial. Second, following Mn absorption and a concomitant increase in plasma Mn levels, transport of Mn to target organs, including the liver, is necessary to prevent Mn-induced toxicity in the periphery. Finally, although the liver detoxifies substances, Mn must be further eliminated from the plasma via shuttling to bile [14]. The absorption of Mn by the gastrointestinal tract is highly dependent on the quantity of ingested Mn and net accumulated levels in the plasma. In vivo experiments in mice and rats have defined the range (1–3.5%) of GI absorption of Mn [29, 30]. While Mn is transported by simple diffusion in the large intestine, Mn is absorbed by active transport in the small intestine [14]. Mn excretion into bile is likely active as well because it depends on concentration gradients [31].
A plethora of plasma proteins or ligands have been implicated as specific Mn carrier proteins, including transglutaminase, beta1-globulin, album, and transferrin [32, 33]. In fact, approximately 80% of plasma Mn is bound to beta1-globulin [32]. Despite the demonstration that Mn preferentially binds to albumin in the plasma of both rabbits and humans, emerging evidence has provided evidence for weaker binding of Mn to albumin compared to Cd and Zn [34, 35].
Intracellular Mn2+ is sequestered in the mitochondria of the brain and liver via the Ca2+ uniporter [36, 37]. Mitochondria are the primary pool of Mn in the cell, however, nuclei have also been implied (remains debatable) to preferentially accumulate this metal [26, 38, 39]. The efflux of mitochondrial Mn2+ is mediated preferentially through an active but slow Na+-independent mechanism; a Na+-dependent mechanism also contributes minimally (reviewed in [10]). This slow Mn efflux has been suggested to account for the net accumulation of Mn in mitochondria. Nevertheless, the cytoplasmic Fe2+ exporter, ferroportin-1, has been reported to transport Mn. Interestingly, ferroportin-1 surface localization and protein expression is perturbed following Mn exposure [40].
1b. Mn transporters in the brain
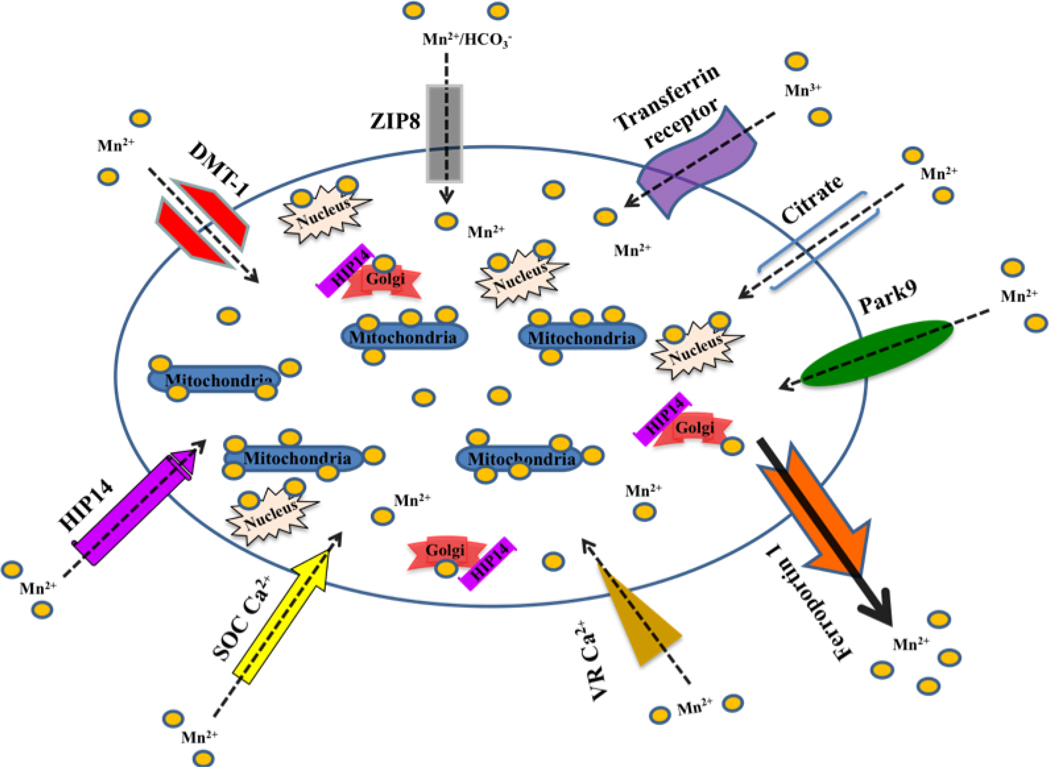
Mn can cross the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCB) through several carriers and in different oxidation states. Given the essential physiological functions of Mn and the neurotoxicity associated with Mn overload, Mn absorption, transport, and tissue levels are stringently regulated. Under normal physiological conditions, Mn is efficiently transported across the blood-brain barrier (BBB) in both the developing fetus and adults [14, 41]. Although Mn transport across the BBB has been actively investigated, which transporter systems are primarily responsible have not been conclusively determined. However, over the past three decades, several Mn transporter systems have been characterized, including active and facilitated diffusion modes of Mn transport [42, 43, 44]. Emerging reports have indicated that Mn can be transported via the divalent metal transporter 1 (DMT1), the transferrin (Tf) receptor (TfR) that mediates trivalent Fe uptake, the divalent metal/bicarbonate ion symporters ZIP8 and ZIP14, various calcium channels, the solute carrier-39 (SLC39) family of zinc transporters, park9/ATP13A2, the magnesium transporter hip14 and the transient receptor potential melastatin 7 (TRPM7) channels/transporters. While the tissue-specific expression of each of the aforementioned Mn transporters is yet to be determined, it is likely that optimal tissue Mn levels are maintained through the involvement of all the above and other unknown Mn transporters. In addition, other cellular processes may regulate the activity of the above transporters in response to Mn deficiency or overload. Of all the above listed polyvalent transporters, DMT1 and TfR are the most extensively documented [44]. Interestingly, a small fraction of plasma Mn is bound to transferrin (Tf), an iron-binding protein, while approximately 80% of plasma Mn is associated with albumin and beta1-globulin[32].
The divalent metal transporter 1 (DMT1) is a member of the family of natural resistance-associated macrophage proteins (NRAMP) and crucial for the maintenance of essential metal homeostasis in the brain [45, 46, 47]. It is best known for its ability to regulate Fe homeostasis in the gastrointestinal lumen [47]. DMT1 is also known as the divalent cation transporter (DCT1) due to its ability to competently transport divalent metals including Zn2+, Mn2+, Co2+, Cd2+, Cu2+, Ni2+, Pb2+, and Fe2+ across the plasma membrane into the cytosol [45, 48]. Both alternative splice isoforms of DMT1 are predominantly localized at the plasma membrane; however, one also localizes to late endosomes and lysosomes while the other localizes to early endosomes [49]. Because only one isoform contains an iron-response element (IRE), subcellular localization depends on Fe concentration [10, 46].. The relative high affinity of DMT1 for Mn has been well established both in vivo and in vitro. Specifically, mutations in the DMT1 gene of Belgrade rats and microcytic anemia mice result in significant decreases in Mn and Fe tissue levels.[50, 51, 52] Furthermore, magnetic resonance imaging (MRI) in a recent study demonstrated the consistency of the cross-BBB transport mechanism(s) of Mn and Fe, suggesting that they are shared [53]. Finally, DMT1-mediated metal transport across rat brain endothelial cells in culture has been reported to be pH, temperature, and Fe-dependent [54, 55].
The TfR is the major cellular receptor for Tf-bound Fe, but because Tf can also bind trivalent Mn, TfR can also mediate Mn transport. Once Mn3+ is internalized through the endocytic pathway, it is reduced to Mn2+ and transported through DMT1 to the cytosol. Mn binding to Tf is time-dependent and Tf receptors are also present on the surface of cerebral capillaries [44, 56]. Additionally, the TfR is an active transporter that is pH and Fe-dependent [56]. Both in vivo and in vitro studies have reported that Mn is efficiently transported via the TfR. For example, a spontaneous mutation in a murine gene linked to the TfR, referred to as hypotransferrinemic, results in a drastic serum TfR deficiency, impairs Mn transport, and disrupts Fe deposition [57, 58]. Interestingly, autioradiography reports have indicated that the TfR is generally localized in gray matter, but not the Fe-abundant white matter tracts in humans and rodents [59, 60, 61].
The zinc- interacting protein 8 (ZIP8) and 14 (ZIP14) proteins are divalent metal/bicarbonate ion symporters known to transport Mn, Zn, and Cd under normal conditions [62, 63]. ZIP8 and ZIP14 are members of the SLC39 family of genes [63, 64] that are glycosylated and expressed on the apical surface of brain capillaries. Mn uptake through ZIP8 or Zip14 is driven by extracellular carbonate (HCO3−). The expression of Zip8 and Zip14 in the brain is lower than that in the liver, duodenum, and testis [65]. In addition, voltage-gated Ca2+ channels, including L and P-type channels [66], such as ligand-gated Ca2+ channels; store-operated Ca2+ channels (SOCCs) [67], and the ionotropic glutamate receptor Ca2+ channels [68] have been implicated in Mn transport across the BBB.
Over the past decade, there has been increasing interest in the exploration and identification of novel candidate Mn transporters, including Hip14 and the product of the park9 gene. Emerging experimental data has indicated that huntingtin-interacting protein 14 and 14L (Hip14, Hip14L) mediates transport of Mn2+ and other divalent metals (Mg2+, Sr2+, Ni2+, Ca2+, Ba2+, Zn2+) across cell membranes [69, 70]. Hip14 is the mammalian ortholog of the ankyrin repeat protein 1 (Akr1p) that is primarily expressed in neurons of the brain. Hip14 is involved in the palmitoylation of several neuronal proteins including huntingtin (HTT) [49]. In addition, it is required for endo- and exocytosis, as well as targeting of cysteine string protein (CSP) and synaptosomal-associated protein 25 (SNAP25) to the synapse [71, 72]. Hip14 is predominantly expressed in the presynaptic terminal, Golgi apparatus, and vesicular structures localized in the axon, dendrites, and soma of neurons [73]. Biochemical studies including yeast-two-hybrid screens have demonstrated that the interaction between Hip14 and HTT is inversely correlated with the poly-Q length in the HTT protein [72].
Interestingly, Gitler and colleagues have recently reported that the park9 gene responsible for early-onset Parkinsonism also transports Mn [70]. The park9 gene encodes a putative P-type transmembrane ATPase (ATP13A2) protein. Although the exact function of park9 is unknown, it is generally thought to be a shuttle for cations, including Mn, across the cell. Biochemical studies have demonstrated that the highest and lowest park9 mRNA levels are localized within the substantia nigra and cerebellum, respectively [74].
Although Mn inhibits the choline transporter at the BBB, it has been suggested that the choline transporter may mediate Mn transport during periods of high throughput. In addition, the choline transporter has a higher affinity for Mn compared to the other metals (Cd2+ and Al3+) that it transports [75, 76, 77]. Mn transport across the choline transporter is sodium-independent, carrier-mediated, and saturable [56].
TRPM7 is ubiquitously expressed in vertebrates and functions as an active Ca2+ selective transporter and a serine/threonine protein kinase. Furthermore, the kinase activity is important for its metal transport function. Specifically, the transporter operates by regulating intracellular Ca2+ levels and Mg2+ homeostasis through the creation of an inward current, thus contributing to the establishment of a cellular membrane potential. The relative permeability of cations through TRPM7 has been reported to be as follows: Zn2+, Ni2+>Ba2+, Co2+> Mg2+> Mn2+> Sr2+> Cd2+> Ca2+. Physiological levels of Mg2+ and Ca2+ are necessary for maintaining the permeability of TRPM7 to Mn2+, Co2+, and Ni2+ [56].
The homomeric purinoreceptors, including P2X and P2Y, have been suggested to participate in Mn transport. These receptors are ATP-dependent and ubiquitously expressed on endothelial cells [78, 79, 80]. Purinoreceptors have a relatively lower affinity for Mn than for the other divalent metals they transport (Ca> Mg> Ba> Mn) [56]. Finally, the citrate transporter has also been implicated in Mn transport across the BBB [81].
1c. Methods for detecting Mn in biological specimens
Over the past two decades, several analytical methods have been developed for detecting Mn levels and monitoring Mn homeostasis in biological samples. Most methods require digestion of all organic matrix prior to analysis, a recently developed method has successfully measured trace concentrations of metals without sample digestion [82]. In older methods, the biological sample type determines the method of digestion to be used. For example, blood or saliva may be digested by an ion exchange resin while tissue samples would require acid (nitric or sulfuric) digestion. Regardless of the sample preparation method, exogenous Mn can contaminate biological samples and affect the accuracy of measurements, especially if Mn levels are low. The current methods used for measuring Mn levels in biological specimens include atomic absorption spectroscopy (AAS), atomic emission spectroscopy (AES), inductively coupled plasma-atomic emission spectrometry (ICP-AES) and mass spectrometry (ICP-MS), neutron activation analysis, x-ray fluorimetry, spectrophotometry, and radioactive trace assays.
AAS and ICP-MS are the most common methods used for measuring Mn levels in biological samples. AAS analysis requires aspiration of the sample into a flame or graphite furnace (GFAAS), where a photoelectric detector measures levels of elements. GFAAS is most used for the determination of very low analyte levels in solid samples [83]. ICP-MS and ICP-AES are similarly sensitive methods for measuring levels of multiple elements, including Mn, in both liquid and solid biological specimens. In fact, ICP-AES and ICP-MS can often measure analytes at the part per trillion levels [84].. Both types of samples require manipulation prior to measurement: while measurements of analytes in solid samples require a laser ablation system, liquid samples are introduced into the ICP by a nebulizer for analyte (Mn) measurements. In contrast, neutron activation analysis minimizes potential contamination of biological Mn levels through minimal sample handling and no reagent usage. Furthermore, neutron activation analysis also has a low detection limit; it is capable of accurate detection of analyte concentrations as low as 4ng/g [83]. However, none of the above Mn detection methods can distinguish between different Mn compounds or oxidation states.
In spite of the several advantages that these Mn detection techniques provide, for example, multi-elemental analysis, excellent specificity, extremely high sensitivity and limited chemical interference, their expense and duration makes them unfeasible for assessing Mn transport kinetics on a large scale.. A less expensive, more rapid quantitative approach has recently been developed in which fura-2 is loaded into living cells to assess intracellular metal ion concentrations by rapid and time-dependent quenching of fura-2 fluorescence [48, 85, 86, 87, 88, 89]. This method, still has a very low throughput, prohibiting pharmacological and toxicological concentration-response curve experiments and other experimental approaches requiring multiple samples to be analyzed. However, a newer approach, the cellular fura-2 Mn extraction assay (CFMEA), enables quantitative measurements of extracted Mn levels in multiwell plate format [90, 91]
2. An overview of the role for Mn and other metals in neurodegeneration
In the past decade, there has been a growing interest in understanding the metabolism of neurotoxic metals and their influence on various neurodegenerative diseases, including manganism, Wilson’s disease (WD), PD, and Alzheimer’s disease (AD). These metals (see below) likely also contribute to Huntington’s disease (HD), though fewer studies have investigated the link. Occupational and environmental exposures (see section 1) to neurotoxic metals, including Mn2+, Hg2+, Cu2+, Zn2+, As2+, Cr2+, Pb2+, and Al3+ have been associated with neurodegeneration and modulation of the age of disease onset and severity in neurodegenerative diseases. The brain is capable of efficiently regulating these metals under physiological conditions; however, excessive exposure can cause them to accumulate in the brain. The distribution of metals throughout the brain is not uniform, and accumulation in specific brain regions reflects neurotoxicity; for example, Mn accumulation and neurotoxicity in the globus pallidus results in manganism. Alterations in metal homeostasis have been suggested to cause neurodegeneration via association of metals with proteins and subsequent induction of aggregate formation. In addition, metals can cause neurodegeneration through a vicious cycle by disrupting mitochondrial function, which depletes ATP, induces ROS production, and ultimately causes cell death by apoptotic and/or necrotic mechanisms.
Acute exposure of a transgenic C. elegans model of PD to Mn has recently been reported to result in degeneration of dopaminergic neurons [92]. Experimental studies in PD postmortem brain tissues have found strong evidence for a link between oxidative stress and PD, specifically, increased Fe levels, markers of oxidative stress and lipid peroxidation in the substantia nigra. Interestingly, pharmacological chelation of elevated Fe levels in an MPTP-induced neurotoxicity model of PD prevents and delays degeneration of midbrain dopaminergic neurons [93]. These studies indicate the potential neurotoxic contributions of Fe and Mn in PD neuropathology. On the other hand, Zn has been proposed to serve as a cofactor for several enzymes and be involved in normal neurological function because it is present at significant levels (10 µM) in the brain [94]. While some brain Zn is associated with proteins, the neocortex and hippocampus possess a substantial amount of chelatable Zn [94, 95, 96, 97]. The defined physiological functions of Zn are currently unclear, but it has been suggested to help stabilize glutamate-containing vesicles at the synapse of secretory cells [98, 99]. In addition, in vitro experimental studies have demonstrated that Zn attenuates NMDA-induced toxicity [100]. However, intracerebroventricular administration of Zn in rats causes epileptic seizure-induced hippocampal neurodegeneration [101].
In fact, other studies have also shown that Mn and other metals (e.g. Cu, Al, Zn) can interact and promote amyloid fibrillogenesis and aggregation of proteins such as prion protein (PrP) and α-synuclein [102]. These proteins bind metals, which contributes to their altered conformational state, solubility, and aggregation [103, 104, 105, 106, 107]. However, in vitro analysis of PrP aggregates has demonstrated that Mn can promote aggregation independent of the PrP metal binding site [106]. In AD, Cu has been shown to bind Aβ with high affinity and modulate its conformational state and peptide length [108, 109]. Other in vitro studies have demonstrated that Aβ interacts with Fe and Zn to promote amyloidogenesis. Interestingly, these findings have been corroborated in AD postmortem brains that show significantly elevated Fe and Zn in the neocortex and amyloid plaque deposits [109]. All these studies indicate that metal interactions with PrP, α-synuclein, and Aβ proteins can cause cell death by inducing the formation of aberrant and toxic aggregates as well as activating redox cycling via Fenton and Haber-Weiss reactions, which depletes production of cellular antioxidants, including glutathione, thus increasing levels of ROS (HO., O2.−, or H2O2). The generation of these highly reactive species can cause oxidative stress that damages lipids, proteins, and DNA and further deplete ATP to cause cell death. These pathophysiological mechanisms, including excitotoxicity, oxidative stress, protein aggregation, mitochondrial dysfunction, and altered metal homeostasis, are strikingly similar to those underlying most common neurodegenerative diseases, including PD, AD, and HD.
3. Manganese exposure and Parkinson’s disease
3a. Mangansim vs. PD
Manganism was first described in 1837 by Couper [110], who observed five patients working in an ore-crushing facility who presented with muscle weakness, bent posture, whispering speech, limb tremor and salivation (see section 1) [111]. Manganism’s psychological symptoms occur early during intoxication and are characterized by hallucinations, psychoses and a myriad of behavioral disturbances. Later, motor deficits develop, encompassing the extrapyramidal system: gait dysfunction with a propensity to fall backward, postural instability, bradykinesia, rigidity, micrographia, mask-like facial expression and speech disturbances [111]. Unlike the resting tremor characteristic of PD, manganism features less frequent and kinetic tremor, if tremor is present at all. Exposure to high Mn levels may also causes dystonias characterized by plantar flexion of the foot, which causes a “cock-walk,” and facial grimacing. Noticeably, the symptoms of Mn intoxication, once established, usually become progressive and irreversible, reflecting permanent damage of neurologic structures.
Though generally described as toxicity to the basal ganglia, manganism is known to affect other CNS region, such as the cortex and hypothalamus [112]. Human manganism at the morphological level is characterized by neuronal loss and reactive gliosis in the globus pallidus and substantia nigra pars reticulata (SNpr) without Lewy bodies, the intraneuronal protein aggregates that distinguish PD[112]. Though rarely reported, damage to the striatum (caudate nucleus and putamen) and subthalamic nucleus may occur, while the substantia nigra pars compacta (SNpc) [113] is less likely to be affected. In contrast, idiopathic PD is predominantly characterized by neuronal loss in the SNpc[114].
3b. Human exposure to Mn and relationship to PD
A recent editorial [116] proposed that radiotracer imaging techniques should be used to investigate the integrity of the dopaminergic system in asymptomatic workers exposed to Mn and highlights the need to assess motor signs in addition to cognitive and behavioral symptoms. PET with [18F]FDOPA [117] in a small study with very high average blood Mn in workers and gender imbalance among groups showed that active and asymptomatic welders have presynaptic nigrostriatal dopaminergic dysfunction with different anatomical localization from that generally observed in PD, affecting the caudate more than the putamen. The welders also had significantly lower Unified Parkinson's Disease Rating Scalesubsection 3 scores than those of the control group, indicating that their occupation led to motor impairment.
3c. α-synuclein and Mn-related protein aggregation
Mn, and certain other essential and toxic metals, can directly increase fibril formation by α-synuclein. Though α-synuclein‘s function is still unclear, these fibrils form the intra-cytoplasmic inclusions (Lewy bodies and Lewy neurites) found in idiopathic Parkinson’s disease, dementia with Lewy bodies (DLB) and multiple system atrophy (MSA), disorders classified as synucleinopathies. It is known that both genetic and environmental factors affect synuclein pathology (reviewed by Eller and Williams, 2011) [40]. Thus, Mn seems to act jointly with α-synuclein in inducing neuronal cell death [119]. It has also been suggested that some metals, including Mn, can act synergistically, even at low concentrations, with certain herbicides to promote α-synuclein misfolding and aggregation [120].
Mn also increases the expression of α-synuclein in vitro [121, 122], and chronic Mn exposure leads to aggregation of α-synuclein in vivo in neurons and glial cells of - non-human primates [123]. A genetic interaction between α-synuclein and PARK9 has been reported in yeast and because PARK9, which could encode a metal cation transporter, seems to protect cells from Mn toxicity, this could provide a mechanism linking genetic and environmental causes of neurodegeneration [70]. Various mechanisms mediated by Mn could converge on α-synuclein in vivo, potentially linking Mn to Parkinson’s disease [124]. Overexpression of α-synuclein in human cells seems to facilitate Mn-induced neurotoxicity through activation of the transcription factor NF-κB, the kinase p38 MAPK, and apoptotic signalling cascades, thus possibly playing a role in dopaminergic cell death [125]. It has also been recently suggested that chronic exposure to Mn could decrease striatal dopamine turnover in transgenic mice expressing human α--synuclein [126].
3d. Mitochondrial dysfunction, Mn and PD
Studies of postmortem PD brains show damage to the SN consistent with generation of reactive oxygen and nitrogen species (ROS, RNS), characterized by lipid peroxidation, protein oxidation, 3-nitrotyrosine formation, DNA oxidation, DNA breaks, and a decrease in the activities of the ROS scavenging enzymes glutathione peroxidase and superoxide dismutase. ROS and RNS lead to mitochondrial damage, and mitochondrial dysfunction has been observed in a plethora of PD models, including 1-methyl-4-phenylpyridium ions (MPP+) [the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)], rotenone, and 6-OHDA. Mitochondria have been posited to play a central role in the etiology of both PD and manganism, which has raised several hypotheses, namely, that (1) dopaminergic neuron mitochondria may be selectively vulnerable to toxins that cause mitochondrial dysfunction; (2) neurons within the SN produce endogenous mitochondrial toxin(s); or (3) SN mitochondria inherently possess defects in enzymes, such as complex I, that cause impaired energy metabolism. It is noteworthy that overexpression of wild type human α-synuclein in C. elegans increases vulnerability to mitochondrial complex I inhibitors, such as rotenone, fenperoximate, pyridaben and stigmatellin [127]. α-Synuclein overexpression has also been recently shown to enhance Mn-induced neurotoxicity via the NF-κB-mediated pathway [125] in a mesencephalic cell line (MES 23.5). Mn also induces the overexpression of α-synuclein in PC12 cells via ERK activation [121], and chronic exposure to Mn decreases striatal dopamine turnover in human α-synuclein transgenic mice [126].
PD and Mn-induced neurotoxicity are analogous in numerous mechanistic ways at the mitochondrial level. Interestingly, the primary storage site for intracellular Mn2+ is within the mitochondria, where it is taken up via the Ca2+ uniporter [38, 128]. Both MPP+ (a model toxin for experimental PD) and Mn activate heme oxygenase-1 [129], resulting in oxidative mitochondrial damage. Mitochondrial impairment, oxidative stress and increased α-synuclein aggregation have been linked in both Mn exposure and various experimental PD models [130, 131, 132]. A link between familial PD-related genes and Mn is also well established; for example, Mn treatment is known to upregulate ER-stress factors, including parkin. Notably, parkin mutations are associated with early onset PD and the E3 ubiquitin ligase it encodes is neuroprotective and is up-regulated upon oxidative stress [133]. Parkin also upregulates PINK1[134], the first protein shown to directly link mitochondrial abnormalities to a PD phenotype.
4. Mn and HD
Huntington’s disease (HD) is a progressive neurodegenerative disorder that begins late in life, with a median age of onset at 39, and was first described by George Huntington in 1872. HD is inherited in an autosomal dominant pattern, and its prevalence is approximately 5 in 100,000 worldwide and 1 in 10,000 in the United States [135]. HD is characterized by motor impairment, cognitive deterioration, emotional disturbance, and psychiatric deficits. Motor symptoms begin with chorea, postural imbalance, dystonia, incoordination, and oculomotor apraxia and progress to akinesia at later stages. HD patients also exhibit emotional disturbances, including depression, temper, apathy, and irritability, and cognitive deficits in short-term memory, attention, and learning. In fact, it has been reported that the cognitive and emotional features of HD precede motor symptoms by several years [136, 137]. HD is caused by an expansion in the glutamine-encoding triplet repeat (CAG) of the normal HTT gene [76, 135, 138]; having more than 36 repeats is pathogenic. Importantly, it has been reported that there is an inverse correlation between age of disease onset and repeat length [139]. Furthermore, the longer the CAG repeat length the greater influence repeat length has over determining age of onset, but for repeat lengths less than 50, only about 44% of the variation in age of onset is due to repeat length [140]. In contrast, individuals with at least 60 repeats invariably exhibit symptoms by 20 years of age. Environmental factors (i.e. non-familial effects) are suspected to contribute to a substantial portion of the residual variance in age of onset [140].
4a. A role for environmental factors in HD
Over a decade after the identification of the causative HD mutation, there have been conflicting reports linking complete or incomplete penetrance of HD to triplet repeat expansion length. Environmental factors have also been suggested to contribute to the residual variation in age of onset, perhaps even more so than genetic factors [140, 141]. Moreover, Gómez-Esteban and others have implicated environmental influences that modify age of disease onset and clinical presentation in monozygotic twin studies, as twins have the same number of repeats [142, 143, 144, 145]. Such twin studies, however, fail to reveal the nature of the environmental factors involved. Furthermore, animal models of HD have provided further support for the influence of environmental factors on HD onset and progression [141, 146].
4b. Links between HTT function and metals
There are several known functional links between the HTT gene and metals. For example, wildtype HTT is important for iron metabolism and oxidative energy production, as evidenced by the decreased hemoglobin and altered iron endocytosis in Htt deficient zebrafish [147]. In fact, Fe and Cu levels are elevated in the corpus striatum of postmortem brain tissue from HD patients and animal models [148, 149]. In addition, HD postmortem brains exhibit alterations in Mn-dependent enzyme activity [3]. Furthermore, animal models have also demonstrated an increase in microglial ferritin, an intracellular iron storage protein [150]. Interestingly, Fox and colleagues have reported that wildtype Htt protein interacts with Cu and decreases its solubility [151]. Finally, the formation of inclusion bodies by CAG expansion in mutant Htt protein fragments may be associated with iron-dependent oxidative events [152]. All these studies strongly suggest that wildtype Htt is necessary for proper metal homoeostasis in the brain.
4c. A role for altered metal homeostasis and toxicity in HD neuropathology
The clinical progression of HD is associated with elevated Fe and Cu in the corpus striatum [148, 149]. Several Mn-dependent enzymes, including arginase, glutamine synthetase, pyruvate decarboxylase, and Mn superoxide dismutase 2 (SOD2) are altered in human HD postmortem brains and toxicant models of HD [3, 22, 153, 154, 155, 156]. Also, data from animal models of HD have demonstrated a significant increase in microglial ferritin (an intracellular iron storage protein) levels [150]. Interestingly, Fox et al. have recently reported that the Htt protein interacts with Cu and decreases the solubility of wildtype Htt protein [151]. However, the cellular effects of Cu or other metal ions on Htt function, proteolytic processing to generate N-terminal fragments, aggregation of fragments, and formation of mutant Htt inclusion bodies remain unknown. Finally, emerging evidence indicates that inclusion bodies formed by CAG expansion in mutant Htt protein fragments are associated with iron-dependent oxidative events, opening the possibility that other redox-reactive metal ions, such as Mn, may influence polyglutamine aggregation [152]. In essence, several studies have suggested that oxidative stress, mitochondrial dysfunction, excitotoxicity, and alterations in iron homeostasis are crucial steps in both Mn neurotoxicity and HD neuropathology. Importantly, chronic exposure to Mn in animal models leads to its significant accumulation in the striatum, providing an opportunity for Mn and HTT to interact within the neurons most vulnerable to HD pathology. Given the strong association between protein aggregation, metal ions and neurodegeneration, it is highly rational to speculate that metals may modulate HD pathophysiology.
4d. Discovery of a disease-toxicant interaction between HD and Mn exposure
To identify metals that share pathophysiological mechanisms with HD and thus potentially modify the age of disease onset, severity, and neuropathology, Bowman and colleagues performed a disease-toxicant screen using a striatal cell line model of HD [157]. They tested the potential of Mn2+, Fe3+, Cu2+, Zn2+, Pb2+, Cd2+, Co2+, and Ni2+ to modify cell survival in an established knock-in immortalized murine striatal cell line model of HD that expresses either wildtype HTT (STHdhQ7/Q7) or the polyQ-expanded form of the protein (STHdhQ111/Q111) [158, 159, 160, 161, 162, 163, 164]. This study revealed a novel gene-environment interaction between expression of mutant HTT and Mn. Specifically, acute Mn exposure of the cultured striatal cells unexpectedly decreased the vulnerability of mutant expressing cells (STHdhQ111/Q111) to Mn cytotoxicity compared to wild-type (STHdhQ7/Q7) [165]. Furthermore, total intracellular Mn levels following Mn exposure by GFAAS in STHdhQ7/Q7 and STHdhQ111/Q111 cells were significantly lower in mutant than wild type cells. Moreover, the mutant HTT– Mn interaction was corroborated in vivo using the YAC128Q mouse model of HD; these mice accumulated less Mn in the striatum than wild-type animals following subcutaneous Mn injections [165, 166]. Furthermore, basal Mn levels were significantly lower in mutant than wild-type cells [165]., as was Mn uptake as indicated by CFMEA [91]. Importantly, the discovery of a disease-toxicant interaction between glutamine-expanded HTT protein and Mn establishes the significance of deciphering how mutant HTT protein modulates Mn transport and transporter systems to elicit decreased susceptibility to Mn toxicity.
5. Mn and Amyotrophic Lateral Sclerosis (ALS)
Mn overload has also been implicated in ALS. This link was first described by Voss, who documented a Mn smelter who developed occupational manganism and bulbar ALS in Germany [167]. Subsequently, a Mn miner also affected by occupational manganism and showing some neurological signs of motor neuron disease was reported in Cuba and recovered after treatment [168]. ALS also occurred among Mn miners in Guam [169], and has been observed among welders, as indicated by small and large epidemiological studies in these workers [170, 171, 172, 173]. ALS arising in a patient affected by liver cirrhosis, a condition known to imply Mn overload due to impaired biliary excretion of the metal [174], has also been observed [175]. Mn overload has been reported in pathological and analytical studies of Guamanian and sporadic ALS cases [176, 177, 178, 179, 180]; the last two studies also showed an increase in MnSOD levels in motor neurons of affected subjects. Indeed, environmental data from the Western Pacific endemic foci of ALS, including the Kii peninsula in Japan, support a role for Mn in its prevalence in these areas [181, 182] and contribute to what is generally called “the mineral hypothesis” of the ALS/Parkinson’s Dementia Complex (ALS/PDC).
The alternative “vegetal hypothesis” [183, 184, 185] focuses on cycads, which require high amounts of Mn [186]. However, high levels of Mn have been recently documented in the leaves of Guamanian Pandanus tectorius, a plant traditionally used for food, fiber and medicine [187, 188]. Considering that several plants, particularly species in the Western Pacific, hyperaccumulate Mn [189, 190], the two major environmental hypotheses on ALS/PDC could actually converge instead of being mutually exclusive. The recent detection of genetic variants of two melastatins, TRPM2 and 7, in Guamanian ALS/Parkinson’s Dementia Complex patients [198, 199, 200] appears interesting, as TRPM7 is strongly activated by Mn [201] and Guam is known to be a Mn-rich environment [188]. Therefore, these melastatins could mediate the accumulation of Mn in Guamanian ALS/PDC patients.
A considerable percentage of ALS patients show T1-weighted hyperintensity at MRI, a neuroradiological sign compatible with Mn overload [191, 192], along the motor system [193, 194, 195, 196, 197], but to date, no studies have directly quantified Mn levels in these brain areas in ALS patients. Further, Mn overload induces apoptosis (reviewed by [202]), which contributes to motor neuron disease [203].
Finally, ALS [204] has been occasionally reported among subjects affected by Mn-ephedrone syndrome, a severe motor disorder [205] thought to be mostly due to severe manganism developing in drug abusers who inject themselves intravenously. While to date there is not definitive proof that Mn can cause ALS, these observations collectively indicate that in certain conditions, the metal could cause or trigger motor neuron disease in some individuals, through a mechanism that could involve members of the melastatin ion channel family.
6. Mn and prion diseases
A growing body of literature indicates that Mn triggers misfolding and aggregation of the PrP in vitro, and that animals and/or humans with prion disease show increased Mn levels in blood, brain and liver [206, 207, 208, 209]. The PrP influences Mn uptake and protects against Mn-induced oxidative stress and apoptosis [210]. Many observations suggest that Mn overload could play a role in prion diseases, and the main ones are summarized here. Mn increases intracellular PrP levels [211] and induces PrP misfolding and proteinase-resistance [212] at micromolar dosages and physiological pH [104]. High Mn levels are detected in the central nervous system and blood of humans and animals affected by prion diseases [206, 207, 209]. Mn also causes the Prionics® test to indicate the presence of transmissible spongiform encephalopathies (TSE)- related PrPSc under UVA irradiation/reducing conditions [213]. Brain MRI of a Creutzfeldt-Jakob disease (CJD) patient has detected T1-weighted hyperintensity in the globi pallidi, compatible with Mn overload [214].
Evidence from responses to treatment also seems to support the Mn/prion disease connection: the metal chelator EDTA reverses Mn-induced aggregation of the prion protein in vitro [107] and CDTA, another polyaminocarboxylic chelator with strong affinity for Mn, significantly increases survival of mice inoculated with the human-derived, mouse-adapted, prion strain M1000 [215]. The link between Mn and prion disease has been recently comprehensively reviewed [216]. Additionally, both Mn overload and prion diseases cause MAPK activation and apoptosis [217, 218]. To date there is no definitive proof that Mn overload can trigger prion disease, as the observed high Mn levels in organs and tissues of affected subjects and animals could simply be an epiphenomenon of prion disease and whether Mn triggers PrP misfolding in vivo is uncertain. Nonetheless, these multidisciplinary data don not exclude a causal relationship between Mn and prion disease.
Studying other disorders possibly associated with prion disease could prove useful to detect whether essential metal imbalances, particularly those involving Fe, Cu, and Mn, could be involved. At least one CJD patient has simultaneously presented with hepatic encephalopathy, which is known to induce Mn overload (Camacho-Muñoz, Hernández-Ramos and Ortega-Martínez De Victoria L 2001) [219]. Similarly, a case of sporadic CJD recently came to our attention in which the patient was clinically diagnosed with hemochromatosis (not genetically confirmed) (EHH, unpublished data). Hemochromatosis involves not only Fe overload, which may be related to prion disease [220], but also excess Mn [206]. This observation could support a possible relationship between hemochromatosis and prion disease [206]. Very recently, a decrease in cerebrospinal fluid transferrin has been suggested to be a diagnostic marker of prion diseases [221], which could also harmonize with the above hypothesis, as Mn-transferrin is one of the forms in which the metal accesses the brain (review by Yokel, 2009).
7. Mn and Alzheimer’s disease (AD)
Chronic Mn treatment of macaques [222] induces up-regulation of amyloid-like protein 1, confirmed by immunohistochemistry, and diffuse amyloid-beta plaques in the frontal cortex, potentially supporting a link between advanced–stage manganism and dementia, as occasionally reported [223]. However, this study involved a limited sample and some variability in age, Mn exposure, dosage, and treatment duration. Also, these animals were repeatedly anesthetized to allow intravenous injections and neuroradiological studies [224], raising the possibility that general anesthesia could have altered gene expression. However, the potential link between Mn and Alzheimer’s disease [123] appears of great interest and deserves to be investigated further.
8. Future directions
Studies over the past several decades have greatly improved understanding of the health risks associated with exposure to Mn and its signs and refined understanding of Mn transport and molecular mechanisms of cellular neurodegeneration. Several Mn transporters have been identified and the complex interrelationship between Mn and Fe, as well as other divalent metals, has been elucidated. Neurotoxic mechanisms common to Mn and other mitochondrial poisons have also been identified. Yet while manganism and PD, as well as other neurological disorders, display distinct neurological symptoms during their early phases, their multiple striking similarities at the clinical, physiological, cellular, and molecular levels suggest that their etiologies share common neurodegenerative pathways. As reviewed herein, Mn may play a role in many neurodegenerative disorders, including PD, HD, AD, ALS, and prion disease, all of which rely on similar intracellular mechanisms involving oxidative stress, mitochondrial impairment, and protein aggregation. Future studies will lead to better understanding of the many facets of Mn homeostasis, the interplay between genes and Mn insult, and the molecular mechanisms of Mn-induced neurodegeneration.
Figure 1. Identified and putative Mn transporters.
These illustrated Mn transporters have been demonstrated to facilitate Mn trafficking (uptake, storage, efflux) between the extra- and intra-cellular milieu. Each of these transporter proteins has also been implicated in the transport of other metals.
Acknowledgements
This review was partially supported by grants from the NIH/NIEHS, RO1ES016931 (ABB) and RO1ES10563 (MA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Erikson KM, Syversen T, Aschner J, Aschner M. Interactions between excessive manganese-exposure and dietary iron-deficiency in neurodegeneration. EnvironToxicol Pharmacol. 2005;19:415–421. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 2.Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. doi: 10.1016/s0197-0186(03)00037-8. http://www.ncbi.nlm.nih.gov/pubmed/12742094. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth J. Changes in nine enzyme markers for neurons, glia, and endothelial cells in agonal state and Huntington's disease caudate nucleus. J Neurochem. 1986;47:583–587. doi: 10.1111/j.1471-4159.1986.tb04539.x. http://www.ncbi.nlm.nih.gov/pubmed/2874190. [DOI] [PubMed] [Google Scholar]
- 4.Hurley LS, Keen CL. Manganese in Trace elements in human health and animal nutrition. In: Underwood E, Mertz W, editors. New York: Academic Press; 1987. pp. 185–225. [Google Scholar]
- 5.Liao SL, Chen CJ. Manganese stimulates stellation of cultured rat cortical astrocytes. Neuroreport. 2001;12:3877–3881. doi: 10.1097/00001756-200112210-00004. http://www.ncbi.nlm.nih.gov/pubmed/11742202. [DOI] [PubMed] [Google Scholar]
- 6.Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, Clegg MS. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20:213–223. http://www.ncbi.nlm.nih.gov/pubmed/10385885. [PubMed] [Google Scholar]
- 7.Aschner M, Shanker G, Erikson K, Yang J, Mutkus LA. The uptake of manganese in brain endothelial cultures. Neurotoxicology. 2002;23:165–168. doi: 10.1016/s0161-813x(02)00056-6. http://www.ncbi.nlm.nih.gov/pubmed/12224757. [DOI] [PubMed] [Google Scholar]
- 8.Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. http://www.ncbi.nlm.nih.gov/pubmed/17466353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987;67:858–901. doi: 10.1152/physrev.1987.67.3.858. http://www.ncbi.nlm.nih.gov/pubmed/3299411. [DOI] [PubMed] [Google Scholar]
- 10.Bowman AB, Erikson KM, Aschner M. Manganese - The two faces of essentiality and neurotoxicity. In: Huang S, editor. Metals and Neurodegeneration. Kerala, India: Research Signpost; 2010. [Google Scholar]
- 11.Morello M, Canini A, Mattioli P, Sorge RP, Alimonti A, Bocca B, Forte G, Martorana A, Bernardi G, Sancesario G. Sub-cellular localization of manganese in the basal ganglia of normal and manganese-treated rats An electron spectroscopy imaging and electron energy-loss spectroscopy study. Neurotoxicology. 2008;29:60–72. doi: 10.1016/j.neuro.2007.09.001. http://www.ncbi.nlm.nih.gov/pubmed/17936361. [DOI] [PubMed] [Google Scholar]
- 12.Liccione JJ, Maines MD. Manganese-mediated increase in the rat brain mitochondrial cytochrome P-450 and drug metabolism activity: susceptibility of the striatum. J Pharmacol Exp Ther. 1989;248:222–228. http://www.ncbi.nlm.nih.gov/pubmed/2913273. [PubMed] [Google Scholar]
- 13.Finkelstein Y, Milatovic D, Aschner M. Modulation of cholinergic systems by manganese. Neurotoxicology. 2007;28:1003–1014. doi: 10.1016/j.neuro.2007.08.006. http://www.ncbi.nlm.nih.gov/pubmed/17920128. [DOI] [PubMed] [Google Scholar]
- 14.Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15:333–340. doi: 10.1016/s0149-7634(05)80026-0. http://www.ncbi.nlm.nih.gov/pubmed/1956602. [DOI] [PubMed] [Google Scholar]
- 15.agency USep, editor. USEPA. Health assessment document for manganese. Cincinnati: EPA; 1984. [Google Scholar]
- 16.Seth PK, Chandra SV. Neurotransmitters and neurotransmitter receptors in developing and adult rats during manganese poisoning. Neurotoxicology. 1984;5:67–76. http://www.ncbi.nlm.nih.gov/pubmed/6144084. [PubMed] [Google Scholar]
- 17.Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. http://www.ncbi.nlm.nih.gov/pubmed/12505649. [DOI] [PubMed] [Google Scholar]
- 18.Christianson DW. Structural chemistry and biology of manganese metalloenzymes. Prog Biophys Mol Biol. 1997;67:217–252. doi: 10.1016/s0079-6107(97)88477-5. http://www.ncbi.nlm.nih.gov/pubmed/9446936. [DOI] [PubMed] [Google Scholar]
- 19.Wedler FC, Denman RB. Glutamine synthetase: the major Mn(II) enzyme in mammalian brain. Curr Top Cell Regul. 1984;24:153–169. doi: 10.1016/b978-0-12-152824-9.50021-6. http://www.ncbi.nlm.nih.gov/pubmed/6149889. [DOI] [PubMed] [Google Scholar]
- 20.Maciejewski PK, Rothman DL. Proposed cycles for functional glutamate trafficking in synaptic neurotransmission. Neurochem Int. 2008;52:809–825. doi: 10.1016/j.neuint.2007.09.015. http://www.ncbi.nlm.nih.gov/pubmed/18006192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49 Suppl 2:42–52. doi: 10.1111/j.1528-1167.2008.01492.x. http://www.ncbi.nlm.nih.gov/pubmed/18226171. [DOI] [PubMed] [Google Scholar]
- 22.Andreassen OA, Dedeoglu A, Friedlich A, Ferrante KL, Hughes D, Szabo C, Beal MF. Effects of an inhibitor of poly(ADP-ribose) polymerase, desmethylselegiline, trientine, and lipoic acid in transgenic ALS mice. Exp Neurol. 2001;168:419–424. doi: 10.1006/exnr.2001.7633. http://www.ncbi.nlm.nih.gov/pubmed/11259130. [DOI] [PubMed] [Google Scholar]
- 23.Ash DE, Cox JD, Christianson DW. Arginase: a binuclear manganese metalloenzyme. Met Ions Biol Syst. 2000;37:407–428. http://www.ncbi.nlm.nih.gov/pubmed/10693141. [PubMed] [Google Scholar]
- 24.Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci. 2006;26:8512–8516. doi: 10.1523/JNEUROSCI.0728-06.2006. http://www.ncbi.nlm.nih.gov/pubmed/16914676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mildvan AS, Scrutton MC, Utter MF. Pyruvate carboxylase. VII. A possible role for tightly bound manganese. J Biol Chem. 1966;241:3488–3498. http://www.ncbi.nlm.nih.gov/pubmed/5919681. [PubMed] [Google Scholar]
- 26.Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23:756–771. doi: 10.1097/01.WCB.0000056062.25434.4D. http://www.ncbi.nlm.nih.gov/pubmed/12796724. [DOI] [PubMed] [Google Scholar]
- 27.Yu AC, Drejer J, Hertz L, Schousboe A. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem. 1983;41:1484–1487. doi: 10.1111/j.1471-4159.1983.tb00849.x. http://www.ncbi.nlm.nih.gov/pubmed/6619879. [DOI] [PubMed] [Google Scholar]
- 28.Fong NM, Jensen TC, Shah AS, Parekh NN, Saltiel AR, Brady MJ. Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J Biol Chem. 2000;275:35034–35039. doi: 10.1074/jbc.M005541200. http://www.ncbi.nlm.nih.gov/pubmed/10938087. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg DM, Campbell WW. Studies in Mineral Metabolism with the Aid of Induced Radioactive Isotopes: IV-Manganese. Proc Natl Acad Sci U S A. 1940;26:448–452. doi: 10.1073/pnas.26.7.448. http://www.ncbi.nlm.nih.gov/pubmed/16588380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack S, George JN, Reba RC, Kaufman RM, Crosby WH. The Absorption of Nonferrous Metals in Iron Deficiency. J Clin Invest. 1965;44:1470–1473. doi: 10.1172/JCI105253. http://www.ncbi.nlm.nih.gov/pubmed/14334611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klaassen CD. Biliary excretion of manganese in rats, rabbits, and dogs. Toxicol Appl Pharmacol. 1974;29:458–468. doi: 10.1016/0041-008x(74)90117-3. http://www.ncbi.nlm.nih.gov/pubmed/4283708. [DOI] [PubMed] [Google Scholar]
- 32.Foradori AC, Bertinchamps A, Gulibon JM, Cotzias GC. The discrimination between magnesium and manganese by serum proteins. J Gen Physiol. 1967;50:2255–2266. doi: 10.1085/jgp.50.9.2255. http://www.ncbi.nlm.nih.gov/pubmed/6064150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotzias GC, Borg DC, Bertinchamps A. Clincal experiences with manganese. In: Seven MJ, Johnson LA, editors. Metal binding in medicine. Philadelphia: Lippincott Co; 1960. pp. 50–57. [Google Scholar]
- 34.Scheuhammer AM, Cherian MG. The influence of manganese on the distribution of essential trace elements. II. The tissue distribution of manganese, magnesium, zinc, iron, and copper in rats after chronic manganese exposure. J Toxicol Environ Health. 1983;12:361–370. doi: 10.1080/15287398309530433. http://www.ncbi.nlm.nih.gov/pubmed/6655740. [DOI] [PubMed] [Google Scholar]
- 35.Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840:163–169. doi: 10.1016/0304-4165(85)90115-1. http://www.ncbi.nlm.nih.gov/pubmed/3995083. [DOI] [PubMed] [Google Scholar]
- 36.Gunter TE, Puskin JS. Manganous ion as a spin label in studies of mitochondrial uptake of manganese. Biophys J. 1972;12:625–635. doi: 10.1016/S0006-3495(72)86108-3. http://www.ncbi.nlm.nih.gov/pubmed/4337705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liccione JJ, Maines MD. Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther. 1988;247:156–161. http://www.ncbi.nlm.nih.gov/pubmed/2902211. [PubMed] [Google Scholar]
- 38.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20:445–453. http://www.ncbi.nlm.nih.gov/pubmed/10385903. [PubMed] [Google Scholar]
- 39.Kalia K, Jiang W, Zheng W. Manganese accumulates primarily in nuclei of cultured brain cells. Neurotoxicology. 2008;29:466–470. doi: 10.1016/j.neuro.2008.02.012. http://www.ncbi.nlm.nih.gov/pubmed/18400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eller M, Williams DR. alpha-Synuclein in Parkinson disease and other neurodegenerative disorders. Clin Chem Lab Med. 2011;49:403–408. doi: 10.1515/CCLM.2011.077. http://www.ncbi.nlm.nih.gov/pubmed/21342025. [DOI] [PubMed] [Google Scholar]
- 41.Mena I, Honuchi K, Lopez G. Factors enhancing entrance of manganese into brain iron deficiency and age. J Nucl Med. 1974;15 [Google Scholar]
- 42.Rabin O, Hegedus L, Bourre JM, Smith QR. Rapid brain uptake of manganese(II) across the blood-brain barrier. J Neurochem. 1993;61:509–517. doi: 10.1111/j.1471-4159.1993.tb02153.x. http://www.ncbi.nlm.nih.gov/pubmed/7687654. [DOI] [PubMed] [Google Scholar]
- 43.Murphy VA, Wadhwani KC, Smith QR, Rapoport SI. Saturable transport of manganese(II) across the rat blood-brain barrier. J Neurochem. 1991;57:948–954. doi: 10.1111/j.1471-4159.1991.tb08242.x. http://www.ncbi.nlm.nih.gov/pubmed/1861159. [DOI] [PubMed] [Google Scholar]
- 44.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Res Bull. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. http://www.ncbi.nlm.nih.gov/pubmed/8293318. [DOI] [PubMed] [Google Scholar]
- 45.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. http://www.ncbi.nlm.nih.gov/pubmed/9242408. [DOI] [PubMed] [Google Scholar]
- 46.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. http://www.ncbi.nlm.nih.gov/pubmed/12572663. [DOI] [PubMed] [Google Scholar]
- 47.Mackenzie B, Hediger MA. SLC11 family of H+-coupled metal-ion transporters NRAMP1 and DMT1. Pflugers Arch. 2004;447:571–579. doi: 10.1007/s00424-003-1141-9. http://www.ncbi.nlm.nih.gov/pubmed/14530973. [DOI] [PubMed] [Google Scholar]
- 48.Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. http://www.ncbi.nlm.nih.gov/pubmed/12750164. [DOI] [PubMed] [Google Scholar]
- 49.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. http://www.ncbi.nlm.nih.gov/pubmed/18565586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra V, Pandav R. Gene-environment interaction in Alzheimer's disease: a potential role for cholesterol. Neuroepidemiology. 1998;17:225–232. doi: 10.1159/000026175. http://www.ncbi.nlm.nih.gov/pubmed/9705582. [DOI] [PubMed] [Google Scholar]
- 51.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. http://www.ncbi.nlm.nih.gov/pubmed/9448300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. http://www.ncbi.nlm.nih.gov/pubmed/9241278. [DOI] [PubMed] [Google Scholar]
- 53.Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103:116–124. doi: 10.1093/toxsci/kfn019. http://www.ncbi.nlm.nih.gov/pubmed/18234737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitsanakis VA, Piccola G, Aschner JL, Aschner M. Characteristics of manganese (Mn) transport in rat brain endothelial (RBE4) cells, an in vitro model of the blood-brain barrier. Neurotoxicology. 2006;27:60–70. doi: 10.1016/j.neuro.2005.06.004. http://www.ncbi.nlm.nih.gov/pubmed/16169084. [DOI] [PubMed] [Google Scholar]
- 55.Fitsanakis VA, Piccola G, Aschner JL, Aschner M. Manganese transport by rat brain endothelial (RBE4) cell-based transwell model in the presence of astrocyte conditioned media. J Neurosci Res. 2005;81:235–243. doi: 10.1002/jnr.20560. http://www.ncbi.nlm.nih.gov/pubmed/15948148. [DOI] [PubMed] [Google Scholar]
- 56.Fitsanakis VA, Piccola G, Marreilha dos Santos AP, Aschner JL, Aschner M. Putative proteins involved in manganese transport across the blood-brain barrier. Hum Exp Toxicol. 2007;26:295–302. doi: 10.1177/0960327107070496. http://www.ncbi.nlm.nih.gov/pubmed/17615110. [DOI] [PubMed] [Google Scholar]
- 57.Dickinson TK, Devenyi AG, Connor JR. Distribution of injected iron 59 and manganese 54 in hypotransferrinemic mice. J Lab Clin Med. 1996;128:270–278. doi: 10.1016/s0022-2143(96)90028-1. http://www.ncbi.nlm.nih.gov/pubmed/8783634. [DOI] [PubMed] [Google Scholar]
- 58.Trenor CC, 3rd, Campagna DR, Sellers VM, Andrews NC, Fleming MD. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–1118. http://www.ncbi.nlm.nih.gov/pubmed/10910930. [PubMed] [Google Scholar]
- 59.Morris CM, Candy JM, Oakley AE, Taylor GA, Mountfort S, Bishop H, Ward MK, Bloxham CA, Edwardson JA. Comparison of the regional distribution of transferrin receptors and aluminium in the forebrain of chronic renal dialysis patients. J Neurol Sci. 1989;94:295–306. doi: 10.1016/0022-510x(89)90238-4. http://www.ncbi.nlm.nih.gov/pubmed/2614472. [DOI] [PubMed] [Google Scholar]
- 60.Hill JM, Ruff MR, Weber RJ, Pert CB. Transferrin receptors in rat brain: neuropeptide-like pattern and relationship to iron distribution. Proc Natl Acad Sci U S A. 1985;82:4553–4557. doi: 10.1073/pnas.82.13.4553. http://www.ncbi.nlm.nih.gov/pubmed/2989832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mash DC, Pablo J, Flynn DD, Efange SM, Weiner WJ. Characterization and distribution of transferrin receptors in the rat brain. J Neurochem. 1990;55:1972–1979. doi: 10.1111/j.1471-4159.1990.tb05784.x. http://www.ncbi.nlm.nih.gov/pubmed/2230804. [DOI] [PubMed] [Google Scholar]
- 62.Fujishiro H, Doi M, Enomoto S, Himeno S. High sensitivity of RBL-2H3 cells to cadmium and manganese: an implication of the role of ZIP8. Metallomics. 2011;3:710–718. doi: 10.1039/c1mt00020a. http://www.ncbi.nlm.nih.gov/pubmed/21509381. [DOI] [PubMed] [Google Scholar]
- 63.Himeno S, Yanagiya T, Fujishiro H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie. 2009;91:1218–1222. doi: 10.1016/j.biochi.2009.04.002. http://www.ncbi.nlm.nih.gov/pubmed/19375483. [DOI] [PubMed] [Google Scholar]
- 64.Andresen JM, Gayan J, Cherny SS, Brocklebank D, Alkorta-Aranburu G, Addis EA, Cardon LR, Housman DE, Wexler NS. Replication of twelve association studies for Huntington's disease residual age of onset in large Venezuelan kindreds. J Med Genet. 2007;44:44–50. doi: 10.1136/jmg.2006.045153. http://www.ncbi.nlm.nih.gov/pubmed/17018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. http://www.ncbi.nlm.nih.gov/pubmed/18270315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucaciu CM, Dragu C, Copaescu L, Morariu VV. Manganese transport through human erythrocyte membranes. An EPR study. Biochim Biophys Acta. 1997;1328:90–98. doi: 10.1016/s0005-2736(97)00039-4. http://www.ncbi.nlm.nih.gov/pubmed/9315607. [DOI] [PubMed] [Google Scholar]
- 67.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. http://www.ncbi.nlm.nih.gov/pubmed/11805119. [DOI] [PubMed] [Google Scholar]
- 68.Kannurpatti SS, Joshi PG, Joshi NB. Calcium sequestering ability of mitochondria modulates influx of calcium through glutamate receptor channel. Neurochem Res. 2000;25:1527–1536. doi: 10.1023/a:1026602100160. http://www.ncbi.nlm.nih.gov/pubmed/11152381. [DOI] [PubMed] [Google Scholar]
- 69.Goytain A, Hines RM, Quamme GA. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport. J Biol Chem. 2008;283:33365–33374. doi: 10.1074/jbc.M801469200. http://www.ncbi.nlm.nih.gov/pubmed/18794299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. http://www.ncbi.nlm.nih.gov/pubmed/19182805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohyama T, Verstreken P, Ly CV, Rosenmund T, Rajan A, Tien AC, Haueter C, Schulze KL, Bellen HJ. Huntingtin-interacting protein 14, a palmitoyl transferase required for exocytosis and targeting of CSP to synaptic vesicles. J Cell Biol. 2007;179:1481–1496. doi: 10.1083/jcb.200710061. http://www.ncbi.nlm.nih.gov/pubmed/18158335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singaraja RR, Hadano S, Metzler M, Givan S, Wellington CL, Warby S, Yanai A, Gutekunst CA, Leavitt BR, Yi H, Fichter K, Gan L, McCutcheon K, Chopra V, Michel J, Hersch SM, Ikeda JE, Hayden MR. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum Mol Genet. 2002;11:2815–2828. doi: 10.1093/hmg/11.23.2815. http://www.ncbi.nlm.nih.gov/pubmed/12393793. [DOI] [PubMed] [Google Scholar]
- 73.Stowers RS, Isacoff EY. Drosophila huntingtin-interacting protein 14 is a presynaptic protein required for photoreceptor synaptic transmission and expression of the palmitoylated proteins synaptosome-associated protein 25 and cysteine string protein. J Neurosci. 2007;27:12874–12883. doi: 10.1523/JNEUROSCI.2464-07.2007. http://www.ncbi.nlm.nih.gov/pubmed/18032660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. http://www.ncbi.nlm.nih.gov/pubmed/16964263. [DOI] [PubMed] [Google Scholar]
- 75.Lorkovic H, Feyrer A. Manganese ions inhibit acetylcholine receptor synthesis in cultured mouse soleus muscles. Neurosci Lett. 1984;51:331–335. doi: 10.1016/0304-3940(84)90398-7. http://www.ncbi.nlm.nih.gov/pubmed/6521960. [DOI] [PubMed] [Google Scholar]
- 76.Bates GP. History of genetic disease: the molecular genetics of Huntington disease - a history. Nat Rev Genet. 2005;6:766–773. doi: 10.1038/nrg1686. http://www.ncbi.nlm.nih.gov/pubmed/16136077. [DOI] [PubMed] [Google Scholar]
- 77.Lockman PR, Roder KE, Allen DD. Inhibition of the rat blood-brain barrier choline transporter by manganese chloride. J Neurochem. 2001;79:588–594. doi: 10.1046/j.1471-4159.2001.00589.x. http://www.ncbi.nlm.nih.gov/pubmed/11701762. [DOI] [PubMed] [Google Scholar]
- 78.Xiang Z, Burnstock G. Expression of P2X receptors in rat choroid plexus. Neuroreport. 2005;16:903–907. doi: 10.1097/00001756-200506210-00006. http://www.ncbi.nlm.nih.gov/pubmed/15931059. [DOI] [PubMed] [Google Scholar]
- 79.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. http://www.ncbi.nlm.nih.gov/pubmed/12270951. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka N, Kawasaki K, Nejime N, Kubota Y, Nakamura K, Kunitomo M, Takahashi K, Hashimoto M, Shinozuka K. P2Y receptor-mediated Ca(2+) signaling increases human vascular endothelial cell permeability. J Pharmacol Sci. 2004;95:174–180. doi: 10.1254/jphs.fpj03036x. http://www.ncbi.nlm.nih.gov/pubmed/15215641. [DOI] [PubMed] [Google Scholar]
- 81.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. http://www.ncbi.nlm.nih.gov/pubmed/12564377. [DOI] [PubMed] [Google Scholar]
- 82.Stupar J, Doolinsek F, Simicic J, Bizjak MBB. Trace element analysis of the hair of duke mirko petrovic-njegos – a possible means of clarification of his death. Trace Elements Electrolytes. 2005;22:118–126. [Google Scholar]
- 83.Dale Marcy A, Drake PL. Development of a field method for measuring manganese in welding fume. J Environ Monit. 2007;9:1199–1204. doi: 10.1039/b705252a. http://www.ncbi.nlm.nih.gov/pubmed/17968446. [DOI] [PubMed] [Google Scholar]
- 84.Jarvis KE, Gray AL, Houk RS. Handbook of inductively coupled plasma mass spectrometry. New York: Chapman and Hall; 1992. [Google Scholar]
- 85.Fasolato C, Hoth M, Penner R. Multiple mechanisms of manganese-induced quenching of fura-2 fluorescence in rat mast cells. Pflugers Arch. 1993;423:225–231. doi: 10.1007/BF00374399. http://www.ncbi.nlm.nih.gov/pubmed/8321625. [DOI] [PubMed] [Google Scholar]
- 86.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. http://www.ncbi.nlm.nih.gov/pubmed/3838314. [PubMed] [Google Scholar]
- 87.Merritt JE, Jacob R, Hallam TJ. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989;264:1522–1527. http://www.ncbi.nlm.nih.gov/pubmed/2536366. [PubMed] [Google Scholar]
- 88.Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem. 2000;275:35738–35745. doi: 10.1074/jbc.M005387200. http://www.ncbi.nlm.nih.gov/pubmed/10942769. [DOI] [PubMed] [Google Scholar]
- 89.Snitsarev VA, McNulty TJ, Taylor CW. Endogenous heavy metal ions perturb fura-2 measurements of basal and hormone-evoked Ca2+ signals. Biophys J. 1996;71:1048–1056. doi: 10.1016/S0006-3495(96)79305-0. http://www.ncbi.nlm.nih.gov/pubmed/8842241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwakye GF, Li D, Bowman AB. Novel high-throughput assay to assess cellular manganese levels in a striatal cell line model of Huntington's disease confirms a deficit in manganese accumulation. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.01.002. http://www.ncbi.nlm.nih.gov/pubmed/21238486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwakye GF, Li D, Kabobel OA, Bowman AB. Cellular fura-2 manganese extraction assay (CFMEA) Curr Protoc Toxicol. 2011;Chapter 12(Unit12):18. doi: 10.1002/0471140856.tx1218s48. http://www.ncbi.nlm.nih.gov/pubmed/21553393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Finley EJ, Avila DS, Chakraborty S, Aschner M. Insights from Caenorhabditis elegans on the role of metals in neurodegerative diseases. Metallomics. 2010:271–279. doi: 10.1039/c0mt00064g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. http://www.ncbi.nlm.nih.gov/pubmed/12670420. [DOI] [PubMed] [Google Scholar]
- 94.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. http://www.ncbi.nlm.nih.gov/pubmed/2689380. [DOI] [PubMed] [Google Scholar]
- 95.Frederickson CJ, Kasarskis EJ, Ringo D, Frederickson RE. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J Neurosci Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. http://www.ncbi.nlm.nih.gov/pubmed/3600033. [DOI] [PubMed] [Google Scholar]
- 96.Danscher G, Howell G, Perez-Clausell J, Hertel N. The dithizone, Timm's sulphide silver and the selenium methods demonstrate a chelatable pool of zinc in CNS. A proton activation (PIXE) analysis of carbon tetrachloride extracts from rat brains and spinal cords intravitally treated with dithizone. Histochemistry. 1985;83:419–422. doi: 10.1007/BF00509203. http://www.ncbi.nlm.nih.gov/pubmed/3000994. [DOI] [PubMed] [Google Scholar]
- 97.Szerdahelyi P, Kasa P. Histochemistry of zinc and copper. Int Rev Cytol. 1984;89:1–33. doi: 10.1016/s0074-7696(08)61298-x. http://www.ncbi.nlm.nih.gov/pubmed/6206018. [DOI] [PubMed] [Google Scholar]
- 98.Zalewski PD, Millard SH, Forbes IJ, Kapaniris O, Slavotinek A, Betts WH, Ward AD, Lincoln SF, Mahadevan I. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. J Histochem Cytochem. 1994;42:877–884. doi: 10.1177/42.7.8014471. http://www.ncbi.nlm.nih.gov/pubmed/8014471. [DOI] [PubMed] [Google Scholar]
- 99.Pattison SE, Dunn MF. On the mechanism of divalent metal ion chelator induced activation of the 7S nerve growth factor esteropeptidase. Thermodynamics and kinetics of activation. Biochemistry. 1976;15:3696–3703. doi: 10.1021/bi00662a009. http://www.ncbi.nlm.nih.gov/pubmed/821523. [DOI] [PubMed] [Google Scholar]
- 100.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. http://www.ncbi.nlm.nih.gov/pubmed/2883728. [DOI] [PubMed] [Google Scholar]
- 101.Itoh M, Ebadi M. The selective inhibition of hippocampal glutamic acid decarboxylase in zinc-induced epileptic seizures. Neurochem Res. 1982;7:1287–1298. doi: 10.1007/BF00965899. http://www.ncbi.nlm.nih.gov/pubmed/7155279. [DOI] [PubMed] [Google Scholar]
- 102.Gaeta A, Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol. 2005;146:1041–1059. doi: 10.1038/sj.bjp.0706416. http://www.ncbi.nlm.nih.gov/pubmed/16205720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Binolfi A, Rasia RM, Bertoncini CW, Ceolin M, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO. Interaction of alpha-synuclein with divalent metal ions reveals key differences: a link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc. 2006;128:9893–9901. doi: 10.1021/ja0618649. http://www.ncbi.nlm.nih.gov/pubmed/16866548. [DOI] [PubMed] [Google Scholar]
- 104.Giese A, Levin J, Bertsch U, Kretzschmar H. Effect of metal ions on de novo aggregation of full-length prion protein. Biochem Biophys Res Commun. 2004;320:1240–1246. doi: 10.1016/j.bbrc.2004.06.075. http://www.ncbi.nlm.nih.gov/pubmed/15249223. [DOI] [PubMed] [Google Scholar]
- 105.Jobling MF, Huang X, Stewart LR, Barnham KJ, Curtain C, Volitakis I, Perugini M, White AR, Cherny RA, Masters CL, Barrow CJ, Collins SJ, Bush AI, Cappai R. Copper and zinc binding modulates the aggregation and neurotoxic properties of the prion peptide PrP106–126. Biochemistry. 2001;40:8073–8084. doi: 10.1021/bi0029088. http://www.ncbi.nlm.nih.gov/pubmed/11434776. [DOI] [PubMed] [Google Scholar]
- 106.Tsenkova RN, Iordanova IK, Toyoda K, Brown DR. Prion protein fate governed by metal binding. Biochem Biophys Res Commun. 2004;325:1005–1012. doi: 10.1016/j.bbrc.2004.10.135. http://www.ncbi.nlm.nih.gov/pubmed/15541389. [DOI] [PubMed] [Google Scholar]
- 107.Levin J, Bertsch U, Kretzschmar H, Giese A. Single particle analysis of manganese-induced prion protein aggregates. Biochem Biophys Res Commun. 2005;329:1200–1207. doi: 10.1016/j.bbrc.2005.02.094. http://www.ncbi.nlm.nih.gov/pubmed/15766554. [DOI] [PubMed] [Google Scholar]
- 108.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer's disease. J Biol Inorg Chem. 2010;15:61–76. doi: 10.1007/s00775-009-0600-y. http://www.ncbi.nlm.nih.gov/pubmed/19862561. [DOI] [PubMed] [Google Scholar]
- 109.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. http://www.ncbi.nlm.nih.gov/pubmed/20730621. [DOI] [PubMed] [Google Scholar]
- 110.Couper J. On the effects of black oxide of manganese which inhaled into the lungs. Br Ann Med Pharm. 1837;1:41–42. [Google Scholar]
- 111.Olanow CW. Manganese-induced parkinsonism and Parkinson's disease. Ann N Y Acad Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. http://www.ncbi.nlm.nih.gov/pubmed/15105268. [DOI] [PubMed] [Google Scholar]
- 112.Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. 1986;70:273–278. doi: 10.1007/BF00686083. http://www.ncbi.nlm.nih.gov/pubmed/3766127. [DOI] [PubMed] [Google Scholar]
- 113.Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. http://www.ncbi.nlm.nih.gov/pubmed/7936278. [DOI] [PubMed] [Google Scholar]
- 114.Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. http://www.ncbi.nlm.nih.gov/pubmed/10385886. [PubMed] [Google Scholar]
- 115.Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, Beal MF, Calne DB, Perl DP. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–498. doi: 10.1212/wnl.46.2.492. http://www.ncbi.nlm.nih.gov/pubmed/8614520. [DOI] [PubMed] [Google Scholar]
- 116.Martin WR. Fuming over Parkinson disease: are welders at risk? Neurology. 2011;76:1286–1287. doi: 10.1212/WNL.0b013e31821528ab. http://www.ncbi.nlm.nih.gov/pubmed/21471465. [DOI] [PubMed] [Google Scholar]
- 117.Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced uptake of [(1)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. http://www.ncbi.nlm.nih.gov/pubmed/21471467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. http://www.ncbi.nlm.nih.gov/pubmed/11553618. [DOI] [PubMed] [Google Scholar]
- 119.Pifl C, Khorchide M, Kattinger A, Reither H, Hardy J, Hornykiewicz O. alpha-Synuclein selectively increases manganese-induced viability loss in SK-N-MC neuroblastoma cells expressing the human dopamine transporter. Neurosci Lett. 2004;354:34–37. doi: 10.1016/j.neulet.2003.09.064. http://www.ncbi.nlm.nih.gov/pubmed/14698476. [DOI] [PubMed] [Google Scholar]
- 120.Andre C, Truong TT, Robert JF, Guillaume YC. Effect of metals on herbicides-alpha-synuclein association: a possible factor in neurodegenerative disease studied by capillary electrophoresis. Electrophoresis. 2005;26:3256–3264. doi: 10.1002/elps.200500169. http://www.ncbi.nlm.nih.gov/pubmed/16143978. [DOI] [PubMed] [Google Scholar]
- 121.Cai T, Yao T, Zheng G, Chen Y, Du K, Cao Y, Shen X, Chen J, Luo W. Manganese induces the overexpression of alpha-synuclein in PC12 cells via ERK activation. Brain Res. 2010;1359:201–207. doi: 10.1016/j.brainres.2010.08.055. http://www.ncbi.nlm.nih.gov/pubmed/20735995. [DOI] [PubMed] [Google Scholar]
- 122.Li Y, Sun L, Cai T, Zhang Y, Lv S, Wang Y, Ye L. alpha-Synuclein overexpression during manganese-induced apoptosis in SH-SY5Y neuroblastoma cells. Brain Res Bull. 2010;81:428–433. doi: 10.1016/j.brainresbull.2009.11.007. http://www.ncbi.nlm.nih.gov/pubmed/19932157. [DOI] [PubMed] [Google Scholar]
- 123.Guilarte TR. APLP1, Alzheimer's-like pathology and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Neurotoxicology. 2010;31:572–574. doi: 10.1016/j.neuro.2010.02.004. http://www.ncbi.nlm.nih.gov/pubmed/20188756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Covy JP, Giasson BI. alpha-Synuclein, leucine-rich repeat kinase-2, and manganese in the pathogenesis of parkinson disease. Neurotoxicology. 2011 doi: 10.1016/j.neuro.2011.01.003. http://www.ncbi.nlm.nih.gov/pubmed/21238487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prabhakaran K, Chapman GD, Gunasekar PG. alpha-Synuclein overexpression enhances manganese-induced neurotoxicity through the NF-kappaB-mediated pathway. Toxicol Mech Methods. 2011;21:435–443. doi: 10.3109/15376516.2011.560210. http://www.ncbi.nlm.nih.gov/pubmed/21417633. [DOI] [PubMed] [Google Scholar]
- 126.Peneder TM, Scholze P, Berger ML, Reither H, Heinze G, Bertl J, Bauer J, Richfield EK, Hornykiewicz O, Pifl C. Chronic exposure to manganese decreases striatal dopamine turnover in human alpha-synuclein transgenic mice. Neuroscience. 2011;180:280–292. doi: 10.1016/j.neuroscience.2011.02.017. http://www.ncbi.nlm.nih.gov/pubmed/21333719. [DOI] [PubMed] [Google Scholar]
- 127.Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, Liu L, Przedborski S, Wolozin B. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J Biol Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. http://www.ncbi.nlm.nih.gov/pubmed/16239214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mela L, Chance B. Spectrophotometric measurements of the kinetics of Ca2+ and Mn2+ accumulation in mitochondria. Biochemistry. 1968;7:4059–4063. doi: 10.1021/bi00851a038. http://www.ncbi.nlm.nih.gov/pubmed/5722269. [DOI] [PubMed] [Google Scholar]
- 129.Migheli R, Godani C, Sciola L, Delogu MR, Serra PA, Zangani D, De Natale G, Miele E, Desole MS. Enhancing effect of manganese on L-DOPA-induced apoptosis in PC12 cells: role of oxidative stress. J Neurochem. 1999;73:1155–1163. doi: 10.1046/j.1471-4159.1999.0731155.x. http://www.ncbi.nlm.nih.gov/pubmed/10461907. [DOI] [PubMed] [Google Scholar]
- 130.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. http://www.ncbi.nlm.nih.gov/pubmed/10934145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. http://www.ncbi.nlm.nih.gov/pubmed/12177198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. http://www.ncbi.nlm.nih.gov/pubmed/14645467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. http://www.ncbi.nlm.nih.gov/pubmed/16818890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Um JW, Stichel-Gunkel C, Lubbert H, Lee G, Chung KC. Molecular interaction between parkin and PINK1 in mammalian neuronal cells. Mol Cell Neurosci. 2009;40:421–432. doi: 10.1016/j.mcn.2008.12.010. http://www.ncbi.nlm.nih.gov/pubmed/19167501. [DOI] [PubMed] [Google Scholar]
- 135.Cowan CM, Raymond LA. Selective neuronal degeneration in Huntington's disease. Curr Top Dev Biol. 2006;75:25–71. doi: 10.1016/S0070-2153(06)75002-5. http://www.ncbi.nlm.nih.gov/pubmed/16984809. [DOI] [PubMed] [Google Scholar]
- 136.Paulsen JS, Zhao H, Stout JC, Brinkman RR, Guttman M, Ross CA, Como P, Manning C, Hayden MR, Shoulson I. Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001;57:658–662. doi: 10.1212/wnl.57.4.658. http://www.ncbi.nlm.nih.gov/pubmed/11524475. [DOI] [PubMed] [Google Scholar]
- 137.Harper PS. Huntington’s Disease. 2nd Edition. Vol. London: Elsevier Science; 1996. [Google Scholar]
- 138.MacDonald ME, Vonsattel JP, Shrinidhi J, Couropmitree NN, Cupples LA, Bird ED, Gusella JF, Myers RH. Evidence for the GluR6 gene associated with younger onset age of Huntington's disease. Neurology. 1999;53:1330–1332. doi: 10.1212/wnl.53.6.1330. http://www.ncbi.nlm.nih.gov/pubmed/10522893. [DOI] [PubMed] [Google Scholar]
- 139.Brinkman RR, Mezei MM, Theilmann J, Almqvist E, Hayden MR. The likelihood of being affected with Huntington disease by a particular age, for a specific CAG size. Am J Hum Genet. 1997;60:1202–1210. http://www.ncbi.nlm.nih.gov/pubmed/9150168. [PMC free article] [PubMed] [Google Scholar]
- 140.Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, Marder K, Penchaszadeh G, Roberts SA, Gayan J, Brocklebank D, Cherny SS, Cardon LR, Gray J, Dlouhy SR, Wiktorski S, Hodes ME, Conneally PM, Penney JB, Gusella J, Cha JH, Irizarry M, Rosas D, Hersch S, Hollingsworth Z, MacDonald M, Young AB, Andresen JM, Housman DE, De Young MM, Bonilla E, Stillings T, Negrette A, Snodgrass SR, Martinez-Jaurrieta MD, Ramos-Arroyo MA, Bickham J, Ramos JS, Marshall F, Shoulson I, Rey GJ, Feigin A, Arnheim N, Acevedo-Cruz A, Acosta L, Alvir J, Fischbeck K, Thompson LM, Young A, Dure L, O'Brien CJ, Paulsen J, Brickman A, Krch D, Peery S, Hogarth P, Higgins DS, Jr, Landwehrmeyer B. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci U S A. 2004;101:3498–3503. doi: 10.1073/pnas.0308679101. http://www.ncbi.nlm.nih.gov/pubmed/14993615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.van Dellen A, Grote HE, Hannan AJ. Gene-environment interactions, neuronal dysfunction and pathological plasticity in Huntington's disease. Clin Exp Pharmacol Physiol. 2005;32:1007–1019. doi: 10.1111/j.1440-1681.2005.04313.x. http://www.ncbi.nlm.nih.gov/pubmed/16445565. [DOI] [PubMed] [Google Scholar]
- 142.Gomez-Esteban JC, Lezcano E, Zarranz JJ, Velasco F, Garamendi I, Perez T, Tijero B. Monozygotic twins suffering from Huntington's disease show different cognitive and behavioural symptoms. Eur Neurol. 2007;57:26–30. doi: 10.1159/000097006. http://www.ncbi.nlm.nih.gov/pubmed/17108691. [DOI] [PubMed] [Google Scholar]
- 143.Friedman JH, Trieschmann ME, Myers RH, Fernandez HH. Monozygotic twins discordant for Huntington disease after 7 years. Arch Neurol. 2005;62:995–997. doi: 10.1001/archneur.62.6.995. http://www.ncbi.nlm.nih.gov/pubmed/15956172. [DOI] [PubMed] [Google Scholar]
- 144.Anca MH, Gazit E, Loewenthal R, Ostrovsky O, Frydman M, Giladi N. Different phenotypic expression in monozygotic twins with Huntington disease. Am J Med Genet A. 2004;124A:89–91. doi: 10.1002/ajmg.a.20328. http://www.ncbi.nlm.nih.gov/pubmed/14679593. [DOI] [PubMed] [Google Scholar]
- 145.Georgiou N, Bradshaw JL, Chiu E, Tudor A, O'Gorman L, Phillips JG. Differential clinical and motor control function in a pair of monozygotic twins with Huntington's disease. Mov Disord. 1999;14:320–325. doi: 10.1002/1531-8257(199903)14:2<320::aid-mds1018>3.0.co;2-z. http://www.ncbi.nlm.nih.gov/pubmed/10091627. [DOI] [PubMed] [Google Scholar]
- 146.Garcia M, Vanhoutte P, Pages C, Besson MJ, Brouillet E, Caboche J. The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module. J Neurosci. 2002;22:2174–2184. doi: 10.1523/JNEUROSCI.22-06-02174.2002. http://www.ncbi.nlm.nih.gov/pubmed/11896157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lumsden AL, Henshall TL, Dayan S, Lardelli MT, Richards RI. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet. 2007;16:1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 148.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. http://www.ncbi.nlm.nih.gov/pubmed/1832073. [DOI] [PubMed] [Google Scholar]
- 149.Dexter DT, Jenner P, Schapira AH, Marsden CD. Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. The Royal Kings and Queens Parkinson's Disease Research Group. Ann Neurol. 1992;(32 Suppl):S94–S100. doi: 10.1002/ana.410320716. http://www.ncbi.nlm.nih.gov/pubmed/1510387. [DOI] [PubMed] [Google Scholar]
- 150.Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G. Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington's disease. Glia. 2007;55:1074–1084. doi: 10.1002/glia.20526. http://www.ncbi.nlm.nih.gov/pubmed/17551926. [DOI] [PubMed] [Google Scholar]
- 151.Fox JH, Kama JA, Lieberman G, Chopra R, Dorsey K, Chopra V, Volitakis I, Cherny RA, Bush AI, Hersch S. Mechanisms of copper ion mediated Huntington's disease progression. PLoS One. 2007;2:e334. doi: 10.1371/journal.pone.0000334. http://www.ncbi.nlm.nih.gov/pubmed/17396163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Firdaus WJ, Wyttenbach A, Giuliano P, Kretz-Remy C, Currie RW, Arrigo AP. Huntingtin inclusion bodies are iron-dependent centers of oxidative events. FEBS J. 2006;273:5428–5441. doi: 10.1111/j.1742-4658.2006.05537.x. http://www.ncbi.nlm.nih.gov/pubmed/17116244. [DOI] [PubMed] [Google Scholar]
- 153.Chiang MC, Chen HM, Lee YH, Chang HH, Wu YC, Soong BW, Chen CM, Wu YR, Liu CS, Niu DM, Wu JY, Chen YT, Chern Y. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington's disease. Hum Mol Genet. 2007;16:483–498. doi: 10.1093/hmg/ddl481. http://www.ncbi.nlm.nih.gov/pubmed/17213233. [DOI] [PubMed] [Google Scholar]
- 154.Carter CJ. Glutamine synthetase activity in Huntington's disease. Life Sci. 1982;31:1151–1159. doi: 10.1016/0024-3205(82)90090-x. http://www.ncbi.nlm.nih.gov/pubmed/6128649. [DOI] [PubMed] [Google Scholar]
- 155.Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR. Mice transgenic for an expanded CAG repeat in the Huntington's disease gene develop diabetes. Diabetes. 1999;48:649–651. doi: 10.2337/diabetes.48.3.649. http://www.ncbi.nlm.nih.gov/pubmed/10078572. [DOI] [PubMed] [Google Scholar]
- 156.Josefsen K, Nielsen MD, Jorgensen KH, Bock T, Norremolle A, Sorensen SA, Naver B, Hasholt L. Impaired glucose tolerance in the R6/1 transgenic mouse model of Huntington's disease. J Neuroendocrinol. 2008;20:165–172. doi: 10.1111/j.1365-2826.2007.01629.x. http://www.ncbi.nlm.nih.gov/pubmed/18034868. [DOI] [PubMed] [Google Scholar]
- 157.Williams BB, Li D, Wegrzynowicz M, Vadodaria BK, Anderson JG, Kwakye GF, Aschner M, Erikson KM, Bowman AB. Disease-toxicant screen reveals a neuroprotective interaction between Huntington's disease and manganese exposure. J Neurochem. 2010a;112:227–237. doi: 10.1111/j.1471-4159.2009.06445.x. http://www.ncbi.nlm.nih.gov/pubmed/19845833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gines S, Ivanova E, Seong IS, Saura CA, MacDonald ME. Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington's disease knock-in striatal cells. J Biol Chem. 2003;278:50514–50522. doi: 10.1074/jbc.M309348200. http://www.ncbi.nlm.nih.gov/pubmed/14522959. [DOI] [PubMed] [Google Scholar]
- 159.Mao Z, Choo YS, Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington's disease knock-in striatal cells. Eur J Neurosci. 2006;23:1701–1710. doi: 10.1111/j.1460-9568.2006.04686.x. http://www.ncbi.nlm.nih.gov/pubmed/16623826. [DOI] [PubMed] [Google Scholar]
- 160.Milakovic T, Johnson GV. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280:30773–30782. doi: 10.1074/jbc.M504749200. http://www.ncbi.nlm.nih.gov/pubmed/15983033. [DOI] [PubMed] [Google Scholar]
- 161.Oliveira JM, Chen S, Almeida S, Riley R, Goncalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC. Mitochondrial-dependent Ca2+ handling in Huntington's disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. http://www.ncbi.nlm.nih.gov/pubmed/17065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seong IS, Ivanova E, Lee JM, Choo YS, Fossale E, Anderson M, Gusella JF, Laramie JM, Myers RH, Lesort M, MacDonald ME. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14:2871–2880. doi: 10.1093/hmg/ddi319. http://www.ncbi.nlm.nih.gov/pubmed/16115812. [DOI] [PubMed] [Google Scholar]
- 163.Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. http://www.ncbi.nlm.nih.gov/pubmed/11092756. [DOI] [PubMed] [Google Scholar]
- 164.Xifro X, Garcia-Martinez JM, Del Toro D, Alberch J, Perez-Navarro E. Calcineurin is involved in the early activation of NMDA-mediated cell death in mutant huntingtin knock-in striatal cells. J Neurochem. 2008;105:1596–1612. doi: 10.1111/j.1471-4159.2008.05252.x. http://www.ncbi.nlm.nih.gov/pubmed/18221365. [DOI] [PubMed] [Google Scholar]
- 165.Williams BB, Kwakye GF, Wegrzynowicz M, Li D, Aschner M, Erikson KM, Bowman AB. Altered manganese homeostasis and manganese toxicity in a Huntington's disease striatal cell model are not explained by defects in the iron transport system. Toxicol Sci. 2010;117:169–179. doi: 10.1093/toxsci/kfq174. http://www.ncbi.nlm.nih.gov/pubmed/20547568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dodd CA, Ward DL, Klein BG. Basal Ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. http://www.ncbi.nlm.nih.gov/pubmed/16393931. [DOI] [PubMed] [Google Scholar]
- 167.Voss H. Progressive bulbar paralysis and amyotrophic lateral sclerosis from chronic manganese poisoning. Arch Gewerbepathol Gewerbehyg. 1939;9:464–476. [Google Scholar]
- 168.Penalver R. Diagnosis and treatment of manganese intoxication; report of a case. AMA Arch Ind Health. 1957;16:64–66. http://www.ncbi.nlm.nih.gov/pubmed/13434499. [PubMed] [Google Scholar]
- 169.Yanagihara R. Heavy metals and essential minerals in motor neuron disease. Adv Neurol. 1982;36:233–247. http://www.ncbi.nlm.nih.gov/pubmed/7180685. [PubMed] [Google Scholar]
- 170.Gunnarsson LG, Bodin L, Soderfeldt B, Axelson O. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49:791–798. doi: 10.1136/oem.49.11.791. http://www.ncbi.nlm.nih.gov/pubmed/1463680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hakansson N, Gustavsson P, Johansen C, Floderus B. Neurodegenerative diseases in welders and other workers exposed to high levels of magnetic fields. Epidemiology. 2003;14:420–426. doi: 10.1097/01.EDE.0000078446.76859.c9. discussion 427–428. http://www.ncbi.nlm.nih.gov/pubmed/12843765. [DOI] [PubMed] [Google Scholar]
- 172.Herrero Hernández E, Casalone C, Bozzetta E, Iulini B, Martucci F, Manea B, Davari A, Ghiglione A, Spinelli P, Bergo V, Pira E, Caramelli M. NeuroPrion. Vol. 25. Turin, Italy, PA: 2006. Exploring the metal-triggered hypothesis in Scrapie and BSE; p. 139. [Google Scholar]
- 173.Strickland D, Smith SA, Dolliff G, Goldman L, Roelofs RI. Amyotrophic lateral sclerosis and occupational history. Arch Neurol. 1996;53:730–733. doi: 10.1001/archneur.1996.00550080044011. http://www.ncbi.nlm.nih.gov/pubmed/8759978733. [DOI] [PubMed] [Google Scholar]
- 174.Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–875. doi: 10.1002/ana.410360611. http://www.ncbi.nlm.nih.gov/pubmed/7998773. [DOI] [PubMed] [Google Scholar]
- 175.Herrero Hernández E, Chiò A, Carmellino C, Palmas A, Discalzi G, Davari A, Pavia G, Pira E. Occupational and environmental risk factors in amyotrophic lateral sclerosis: results from a case-control study in Piedmont (Northern Italy), 1998–2005. 28th International Congress On Occupational Health ICOH Milan; 11–16 June 2006; 2005a. Conference proceedings: 245. [Google Scholar]
- 176.Fitzmaurice PS, Shaw IC, Mitchell JD. Alteration of superoxide dismutase activity in the anterior horn in motoneuron disease patients. J Neurol Sci. 1995;129 Suppl:96–98. doi: 10.1016/0022-510x(95)00075-d. http://www.ncbi.nlm.nih.gov/pubmed/7595633. [DOI] [PubMed] [Google Scholar]
- 177.Kihira T, Mukoyama M, Ando K, Yase Y, Yasui M. Determination of manganese concentrations in the spinal cords from amyotrophic lateral sclerosis patients by inductively coupled plasma emission spectroscopy. J Neurol Sci. 1990;98:251–258. doi: 10.1016/0022-510x(90)90266-p. http://www.ncbi.nlm.nih.gov/pubmed/2243233. [DOI] [PubMed] [Google Scholar]
- 178.Liu Y, Brooks BR, Taniguchi N, Hartmann HA. CuZnSOD and MnSOD immunoreactivity in brain stem motor neurons from amyotrophic lateral sclerosis patients. Acta Neuropathol. 1998;95:63–70. doi: 10.1007/s004010050766. http://www.ncbi.nlm.nih.gov/pubmed/9452823. [DOI] [PubMed] [Google Scholar]
- 179.Mitchell JD, East BW, Harris IA, Pentland B. Manganese, selenium and other trace elements in spinal cord, liver and bone in motor neurone disease. Eur Neurol. 1991;31:7–11. doi: 10.1159/000116626. http://www.ncbi.nlm.nih.gov/pubmed/2015841. [DOI] [PubMed] [Google Scholar]
- 180.Miyata S, Nakamura S, Nagata H, Kameyama M. Increased manganese level in spinal cords of amyotrophic lateral sclerosis determined by radiochemical neutron activation analysis. J Neurol Sci. 1983;61:283–293. doi: 10.1016/0022-510x(83)90012-6. http://www.ncbi.nlm.nih.gov/pubmed/6644329. [DOI] [PubMed] [Google Scholar]
- 181.Yase Y. Neurologic disease in the western Pacific islands, with a report on the focus of amyotrophic lateral sclerosis found in the Kii peninsula, Japan. Am J Trop Med Hyg. 1970;19:155–166. doi: 10.4269/ajtmh.1970.19.155. http://www.ncbi.nlm.nih.gov/pubmed/5416288. [DOI] [PubMed] [Google Scholar]
- 182.Yase Y, Yoshida S, Kihira T, Wakayama I, Komoto J. Kii ALS dementia. Neuropathology. 2001;21:105–109. doi: 10.1046/j.1440-1789.2001.00303.x. http://www.ncbi.nlm.nih.gov/pubmed/11396674. [DOI] [PubMed] [Google Scholar]
- 183.Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. http://www.ncbi.nlm.nih.gov/pubmed/3603037. [DOI] [PubMed] [Google Scholar]
- 184.Whiting MG. Food Practices in Als Foci in Japan, the Marianas, and New Guinea. Fed Proc. 1964;23:1343–1345. http://www.ncbi.nlm.nih.gov/pubmed/14236144. [PubMed] [Google Scholar]
- 185.Whiting MG. Neurotoxicity of Cycads. An annotated bibliography for the years 1829–1989. No. 5. vol. 2. Lyonia: Occasional papers of the Harold L. Lyon Arboretum; 1989. pp. 201–270. [Google Scholar]
- 186.Hanes J. Year of the golden Cycads. The Cycad Newsletter. 2002:10–12. [Google Scholar]
- 187.Denton GR, Siegrist HG, Jano-Edwards JP. Trace elements in Pandanus (Pandanus tectorius) from a manganese-enriched wetland in Southern Guam: a possible Lytico-Bodig connection? J Toxicol Environ Health A. 2009(a);72:574–576. doi: 10.1080/15287390902733523. http://www.ncbi.nlm.nih.gov/pubmed/19296406. [DOI] [PubMed] [Google Scholar]
- 188.Denton GRW, Siegrist HG, Jano-Edwards JP. Metal deficiencies and imbalances in wetland plants from a manganese-enriched wetland in Southern Guam: a preliminary investigation. Progress in Environ Sci Tech. 2009(b):1853–1858. [Google Scholar]
- 189.Fernando D, Fernando D. Manganese hyperaccumulation by plants. The University of Melbourne, School of Botany. 2009:1–3. http://www.botany.unimelb.edu.au/botany/schoolresources/HotTopicsFeb2009.pdf. [Google Scholar]
- 190.Fernando DR, Mizuno T, Woodrow IE, Baker AJ, Collins RN. Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol. 2010;188:1014–1027. doi: 10.1111/j.1469-8137.2010.03431.x. [DOI] [PubMed] [Google Scholar]
- 191.Klos KJ, Ahlskog JE, Kumar N, Cambern S, Butz J, Burritt M, Fealey RD, Cowl CT, Parisi JE, Josephs KA. Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology. 2006;67:1984–1989. doi: 10.1212/01.wnl.0000247037.37807.76. http://www.ncbi.nlm.nih.gov/pubmed/17159105. [DOI] [PubMed] [Google Scholar]
- 192.Mirowitz SA, Westrich TJ. Basal ganglial signal intensity alterations: reversal after discontinuation of parenteral manganese administration. Radiology. 1992;185:535–536. doi: 10.1148/radiology.185.2.1410368. http://www.ncbi.nlm.nih.gov/pubmed/1410368. [DOI] [PubMed] [Google Scholar]
- 193.da Rocha AJ, Oliveira AS, Fonseca RB, Maia AC, Jr, Buainain RP, Lederman HM. Detection of corticospinal tract compromise in amyotrophic lateral sclerosis with brain MR imaging: relevance of the T1-weighted spin-echo magnetization transfer contrast sequence. AJNR Am J Neuroradiol. 2004;25:1509–1515. http://www.ncbi.nlm.nih.gov/pubmed/15502129. [PMC free article] [PubMed] [Google Scholar]
- 194.Lee YC, Markus R, Hughes A. MRI in ALS: corticospinal tract hyperintensity. Neurology. 2003;61:1600. doi: 10.1212/01.wnl.0000096015.48322.2a. http://www.ncbi.nlm.nih.gov/pubmed/14663049. [DOI] [PubMed] [Google Scholar]
- 195.Valentini MC, Venturi F, Calvo A, Ghiglione P, Herrero Hernandez E, Mutani R, Chio’ A for the Turin ALS Research Group (TARG) Specificity and sensitivity of hyperintensity of corticospinal tracts as neuroimaging sign of ALS. ALS and Other Motor Neuron Disorders. 2003;4 S1:161. [Google Scholar]
- 196.Valentini MC, Venturi F, Perrucci I, Calvo A, Herrero Hernandez E, Terreni A, Mutani R, Chio’ A for the Turin ALS Research Group (TARG) Specificity and sensitivity of corticospinal tracts hyperintensity as neuroimaging sign of ALS. Clin Neuropathol. 2005;24/23:152. [Google Scholar]
- 197.Waragai M, Shinotoh H, Hayashi M, Hattori T. High signal intensity on T1 weighted MRI of the anterolateral column of the spinal cord in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1997;62:88–91. doi: 10.1136/jnnp.62.1.88. http://www.ncbi.nlm.nih.gov/pubmed/9010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hermosura MC, Cui AM, Go RC, Davenport B, Shetler CM, Heizer JW, Schmitz C, Mocz G, Garruto RM, Perraud AL. Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci U S A. 2008;105:18029–18034. doi: 10.1073/pnas.0808218105. http://www.ncbi.nlm.nih.gov/pubmed/19004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hermosura MC, Garruto RM. TRPM7 and TRPM2-Candidate susceptibility genes for Western Pacific ALS and PD? Biochim Biophys Acta. 2007;1772:822–835. doi: 10.1016/j.bbadis.2007.02.008. http://www.ncbi.nlm.nih.gov/pubmed/17395433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. http://www.ncbi.nlm.nih.gov/pubmed/16051700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ryazanova LV, Dorovkov MV, Ansari A, Ryazanov AG. Characterization of the protein kinase activity of TRPM7/ChaK1, a protein kinase fused to the transient receptor potential ion channel. J Biol Chem. 2004;279:3708–3716. doi: 10.1074/jbc.M308820200. http://www.ncbi.nlm.nih.gov/pubmed/14594813. [DOI] [PubMed] [Google Scholar]
- 202.Shibata S, Maeda M, Furuta K, Suzuki M, Oh-Hashi K, Kiuchi K, Hirata Y. Neuroprotective effects of (arylthio)cyclopentenone derivatives on manganese-induced apoptosis in PC12 cells. Brain Res. 2009;1294:218–225. doi: 10.1016/j.brainres.2009.07.059. http://www.ncbi.nlm.nih.gov/pubmed/19643096. [DOI] [PubMed] [Google Scholar]
- 203.Martin LJ. Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol. 1999;58:459–471. doi: 10.1097/00005072-199905000-00005. http://www.ncbi.nlm.nih.gov/pubmed/10331434. [DOI] [PubMed] [Google Scholar]
- 204.Khatiashvili K, Akhvlediani M, Megrelishvili M, Janelidze N, Lobjanidze Movement disorder caused by injections of manganese containing compounds. Mov Disord. 2007;22 Suppl:16. [Google Scholar]
- 205.Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Zjablov G, Asser T, Aquilonius SM. Irreversible motor impairment in young addicts--ephedrone, manganism or both? Acta Neurol Scand. 2007;115:385–389. doi: 10.1111/j.1600-0404.2007.00818.x. http://www.ncbi.nlm.nih.gov/pubmed/17511846. [DOI] [PubMed] [Google Scholar]
- 206.Hesketh S, Sassoon J, Knight R, Brown DR. Elevated manganese levels in blood and CNS in human prion disease. Mol Cell Neurosci. 2008;37:590–598. doi: 10.1016/j.mcn.2007.12.008. http://www.ncbi.nlm.nih.gov/pubmed/18234506. [DOI] [PubMed] [Google Scholar]
- 207.Hesketh S, Sassoon J, Knight R, Hopkins J, Brown DR. Elevated manganese levels in blood and central nervous system occur before onset of clinical signs in scrapie and bovine spongiform encephalopathy. J Anim Sci. 2007;85:1596–1609. doi: 10.2527/jas.2006-714. http://www.ncbi.nlm.nih.gov/pubmed/17296770. [DOI] [PubMed] [Google Scholar]
- 208.Wolfe LL, Conner MM, Bedwell CL, Lukacs PM, Miller MW. Select tissue mineral concentrations and chronic wasting disease status in mule deer from North-central Colorado. J Wildl Dis. 2010;46:1029–1034. doi: 10.7589/0090-3558-46.3.1029. http://www.ncbi.nlm.nih.gov/pubmed/20688718. [DOI] [PubMed] [Google Scholar]
- 209.Wong BS, Chen SG, Colucci M, Xie Z, Pan T, Liu T, Li R, Gambetti P, Sy MS, Brown DR. Aberrant metal binding by prion protein in human prion disease. J Neurochem. 2001;78:1400–1408. doi: 10.1046/j.1471-4159.2001.00522.x. http://www.ncbi.nlm.nih.gov/pubmed/11579148. [DOI] [PubMed] [Google Scholar]
- 210.Choi CJ, Anantharam V, Saetveit NJ, Houk RS, Kanthasamy A, Kanthasamy AG. Normal cellular prion protein protects against manganese-induced oxidative stress and apoptotic cell death. Toxicol Sci. 2007;98:495–509. doi: 10.1093/toxsci/kfm099. http://www.ncbi.nlm.nih.gov/pubmed/17483122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Choi CJ, Anantharam V, Martin DP, Nicholson EM, Richt JA, Kanthasamy A, Kanthasamy AG. Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease. Toxicol Sci. 2010;115:535–546. doi: 10.1093/toxsci/kfq049. http://www.ncbi.nlm.nih.gov/pubmed/20176619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Brown DR, Hafiz F, Glasssmith LL, Wong BS, Jones IM, Clive C, Haswell SJ. Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J. 2000;19:1180–1186. doi: 10.1093/emboj/19.6.1180. http://www.ncbi.nlm.nih.gov/pubmed/10716918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Deloncle R, Guillard O, Bind JL, Delaval J, Fleury N, Mauco G, Lesage G. Free radical generation of protease-resistant prion after substitution of manganese for copper in bovine brain homogenate. Neurotoxicology. 2006;27:437–444. doi: 10.1016/j.neuro.2006.01.003. http://www.ncbi.nlm.nih.gov/pubmed/16481041. [DOI] [PubMed] [Google Scholar]
- 214.de Priester JA, Jansen GH, de Kruijk JR, Wilmink JT. New MRI findings in Creutzfeldt-Jakob disease: high signal in the globus pallidus on T1-weighted images. Neuroradiology. 1999;41:265–268. doi: 10.1007/s002340050744. http://www.ncbi.nlm.nih.gov/pubmed/10344511. [DOI] [PubMed] [Google Scholar]
- 215.Brazier MW, Volitakis I, Kvasnicka M, White AR, Underwood JR, Green JE, Han S, Hill AF, Masters CL, Collins SJ. Manganese chelation therapy extends survival in a mouse model of M1000 prion disease. J Neurochem. 2010;114:440–451. doi: 10.1111/j.1471-4159.2010.06771.x. http://www.ncbi.nlm.nih.gov/pubmed/20456001. [DOI] [PubMed] [Google Scholar]
- 216.Brown DR. Prions and manganese: A maddening beast. Metallomics. 2011;3:229–238. doi: 10.1039/c0mt00047g. http://www.ncbi.nlm.nih.gov/pubmed/21390367. [DOI] [PubMed] [Google Scholar]
- 217.Carimalo J, Cronier S, Petit G, Peyrin JM, Boukhtouche F, Arbez N, Lemaigre-Dubreuil Y, Brugg B, Miquel MC. Activation of the JNK-c-Jun pathway during the early phase of neuronal apoptosis induced by PrP106–126 and prion infection. Eur J Neurosci. 2005;21:2311–2319. doi: 10.1111/j.1460-9568.2005.04080.x. http://www.ncbi.nlm.nih.gov/pubmed/15932590. [DOI] [PubMed] [Google Scholar]
- 218.Lee HP, Jun YC, Choi JK, Kim JI, Carp RI, Kim YS. Activation of mitogen-activated protein kinases in hamster brains infected with 263K scrapie agent. J Neurochem. 2005;95:584–593. doi: 10.1111/j.1471-4159.2005.03429.x. http://www.ncbi.nlm.nih.gov/pubmed/16135077. [DOI] [PubMed] [Google Scholar]
- 219.Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346:270–274. doi: 10.1016/s0140-6736(95)92164-8. http://www.ncbi.nlm.nih.gov/pubmed/7630246. [DOI] [PubMed] [Google Scholar]
- 220.Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, Singh N. Abnormal brain iron homeostasis in human and animal prion disorders. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000336. e1000336. http://www.ncbi.nlm.nih.gov/pubmed/19283067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Singh A, Beveridge AJ, Singh N. Decreased CSF transferrin in sCJD: a potential premortem diagnostic test for prion disorders. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016804. e16804. http://www.ncbi.nlm.nih.gov/pubmed/21408069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem. 2008;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. http://www.ncbi.nlm.nih.gov/pubmed/18284614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Banta RG, Markesbery WR. Elevated manganese levels associated with dementia and extrapyramidal signs. Neurology. 1977;27:213–216. doi: 10.1212/wnl.27.3.213. http://www.ncbi.nlm.nih.gov/pubmed/557755. [DOI] [PubMed] [Google Scholar]
- 224.Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence for cortical dysfunction and widespread manganese accumulation in the nonhuman primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicol Sci. 2006;94:351–358. doi: 10.1093/toxsci/kfl106. http://www.ncbi.nlm.nih.gov/pubmed/16968886. [DOI] [PubMed] [Google Scholar]