Abstract
Neural circuits associated with retinal ganglion cells have long been used as models for investigating the mechanisms that govern circuit development and function. Similar to neurons in the brain, retinal ganglion cells are subdivided into distinct classes based upon their morphology, physiology and patterns of connectivity. Newly developed transgenic tools in which individual classes of retinal ganglion cells are labeled with reporter proteins have recently provided a method to study the development of their class-specific circuitry. Here, we examine a single class of intrinsically photosensitive retinal ganglion cells and discuss their class-specific circuitry, as well as the cellular and molecular mechanisms that govern assembly of this circuitry.
Keywords: synaptic targeting, retina, synapse, axon guidance, melanopsin, olivary pretectal nucleus, suprachiasmatic nucleus, intergeniculate leaflet, ventral lateral geniculate nucleus
Class-specific wiring in the vertebrate visual system
A defining principle in neural development is that distinct classes of neurons are assembled into neural circuits with functionally different outputs. One of the best examples of such class-specific neuronal wiring occurs in the vertebrate retina whose function is to convey light-derived information to the brain.
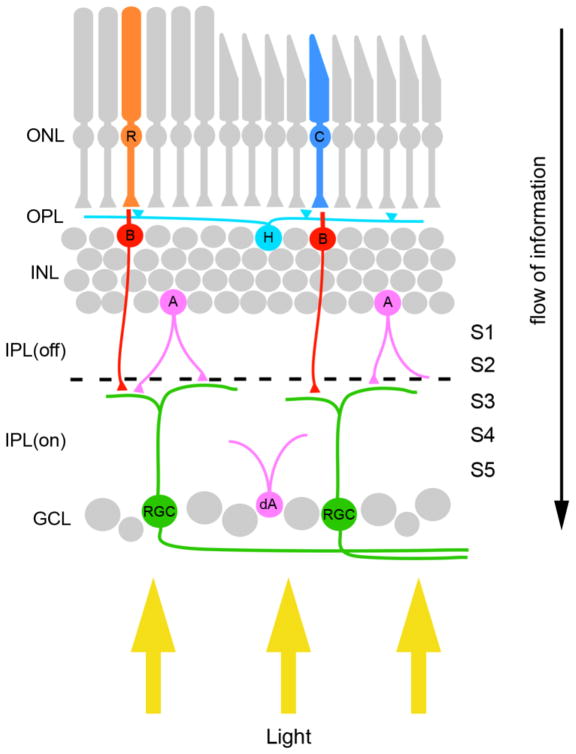
The retina is a layered structured that contains 5 main cell types: photoreceptors, bipolar cells, horizontal cells, amacrine cells and retinal ganglion cells (RGCs)(Figure 1)(1,2). Rod and cone photoreceptors, the main light sensitive cells in the retina, reside in the outer most layer of the retina – the outer nuclear layer (ONL)(Figure 1). Presynaptic terminals of these photoreceptors synapse onto dendrites of bipolar cells, whose somas reside in a second parallel cell layer termed the inner nuclear layer (INL) (Figure 1). Photoreceptors produce two different responses in bipolar cells depending on the neurotransmitter receptor type. Bipolar cells expressing metabotropic glutamate receptors are activated by photoreceptors following the onset or increase of a light stimulus; these bipolar cells are referred to as ON-bipolar cells. In contrast, bipolar cells expressing ionotropic glutamate receptors are activated following the decrement or removal of light stimuli and are therefore termed OFF-bipolar cells. Both classes of bipolar cells extend their axons into the inner plexiform layer (IPL), a cell-free retinal layer, where they synapse onto dendrites of RGCs (Figure 1). Bipolar cell-RGC synapses are segregated within the IPL such that terminals of OFF-bipolar cells reside in the outer 2/5 of the IPL, whereas ON-bipolar cell terminals reside in the inner 3/5 of the IPL. Because of the separation of OFF- and ON-bipolar inputs, the IPL is divided into “OFF” and “ON” divisions (Figure 1). Finally RGCs, the output neurons of the retina, project their axons to specific nuclei within the brain, termed retino-recipient nuclei. While light induced signals are transmitted through the three-neuron pathway described above (i.e. photoreceptor-bipolar cell-RGC pathways) it can also be influenced by synaptic input from inhibitory and modulatory interneurons, namely horizontal and amacrine cells (Figure 1).
Figure 1.
Basic wiring of the retina. The vertebrate retina contains five main cell types – photoreceptors (rods [R] and cones [C]), three types of interneurons (horizontal cells [H], amacrine cells [A], and bipolar cells [B]) and projection neurons (retinal ganglion cells [RGCs]). These cells are arranged in three distinct cell layers within the retina, the outer nuclear layer (ONL) which contains rod and cone photoreceptors, the inner nuclear layer (INL) which contains the three types of interneurons, and the ganglion cell layer (GCL) which contains both RGCs and displaced amacrine cells (dA). Synaptic connections between retinal neurons are confined to 2 synaptic layers – the outer plexiform layer (OPL) and inner plexiform layer (IPL). Black dashed line indicates the division of the inner plexiform layer into ON and OFF divisions of the IPL. Yellow arrows highlight the path of light through the retina. IPL(off) – OFF division of the inner plexiform layer; IPL(on) – ON division of the inner plexiform layer; S1-5 – sublaminae of the inner plexiform layer.
Circuit diagrams of the retina as described above (and illustrated in Figure 1) are vastly oversimplified. Each cell type can be sub-divided into distinct classes based upon morphology, connectivity and function, such that there may be over 100 different types of neurons in the retina alone (1–3). At present over 20 classes of RGCs have been identified in the mammalian retina (4) each having unique, stereotyped synaptic connections with distinct classes of bipolar neurons and amacrine cells (1–3). Therefore in addition to the ON and OFF divisions, the IPL can be divided into at least 5 parallel sublaminae (termed S1-S5) based on these stereotyped connections. Moreover, each class of RGC projects an axon to one or more of the ~20 different retino-recipient nuclei within the brain (e.g. 5–7). Despite the considerable attention that these cell types and their associated circuitry have received, we know relatively little about the cellular and molecular mechanisms that govern their development. The difficulty in labeling axons and dendrites belonging to a single class of RGC has, in large part, been to blame for this. To overcome this limitation several labs have harnessed the power of mouse genetics and created transgenic lines in which single classes of RGCs express reporter proteins (see 8–18). With these new tools at hand, our understanding of class-specific circuitry is rapidly expanding. In this review we highlight recent findings on the pattern and development of connectivity of a single, specialized class of RGC – the intrinsically photosensitive RGC (ipRGC).
Intrinsically photosensitive retinal ganglion cells
Until recently rods and cones had long been thought to be the only photoreceptive cells in the mammalian retina. This belief was challenged by a series of studies in which circadian photoentrainment, the ability to shift circadian rhythmicity in response to light-dark cycles, was preserved following rod and cone photoreceptor degeneration (19–22). The preservation of photoentrainment in the absence of rod and cone photoreceptors suggested that separate retinal circuits processed image forming and non-image forming responses to light (the former being responsible for vision and the later being responsible for light induced changes in behavior that do not require vision). Moreover, the preservation of photoentrainment in the absence of rods and cones hinted that additional light-sensitive cells existed in the retina. The identification of such a novel photoreceptive cell followed the discovery that the retina not only contains rhodopsin and photopsins (the photosensitive molecules in rods and cones, respectively) but also contains an opsin sensitive to blue light (23–25). This new opsin, termed melanopsin, is present in a small percentage of RGCs (~2–5%) that are intrinsically photosensitive, meaning that light directly leads to membrane depolarization and action potential firing (17,18,26,27). On the basis of their photosensitivity and projections to retino-recipient nuclei, these intrinsically photosensitive RGCs (ipRGCs) are thought to be irradiance detectors that code non-image forming responses to light essential for both circadian photoentrainment and pupillary light reflexes (17, 27–31). However, functions of ipRGCs are complex, as they can also activate retinal interneurons (32), modulate spontaneous retinal activity during early perinatal life (66), and even contribute to image-forming visual processing (18).
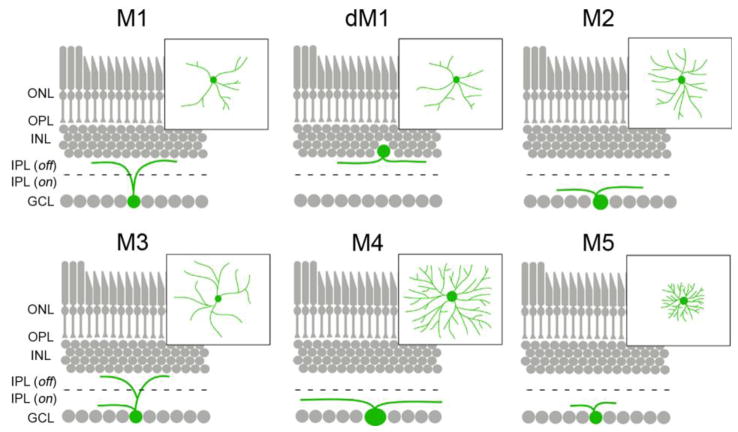
Since their initial discovery, ipRGCs have been subdivided into 5 distinct classes, termed M1–M5 ipRGCs. Such classification is based upon melanopsin expression level, dendritic morphology, sensitivity to light, intraretinal circuitry, and the pattern of their projections to retino-recipient nuclei (6,17,18,33–36)(Figure 2). M1 ipRGCs, which account for ~1% of all mouse RGCs, have sparsely branched dendritic arbors which stratify in S1 of the OFF division of the IPL(6,36) (Figure 2). M1 ipRGCs express highest levels of melanopsin among ipRGCs and show the greatest sensitivity to light (18,33,35,36). At least two findings suggest that M1 ipRGCs may not be a homogenous population of neurons. First, a cohort of M1 ipRGCs are displaced into the inner-most region of the INL(17)(Figure 2). Second, some but not all M1 ipRGCs express the transcription factor Brn3b (also called POU4F2) and axons from these Brn3B-positive M1 ipRGCs target different regions of brain than Brn3B-negative M1 ipRGCs (67). M2 ipRGCs are equally as abundant as M1s but express significantly lower levels of melanopsin and are only weakly photosensitive. In terms of their morphology, M2 ipRGCs differ from M1s in that they have slightly larger somas and more elaborate dendritic arbors that stratify in S5 of the ON division of the IPL (18,33–36) (Figure 2). Somas of both M1 and M2 ipRGCs are evenly spaced across the entire retina and their dendrites each tile the entire retina (except for the fovea in higher mammals [37]) such that their dendritic fields overlap to generate “photoreceptive nets” (29,34). There are considerably fewer M3 ipRGCs, raising the question of whether they are a distinct class of RGC or simply a variant of other ipRGCs. M3 ipRGCs are similar to M2s in terms of their level of melanopsin expression, sensitivity to light, soma size and dendritic fields (34,38), however their dendrites bifurcate to stratify in both S1 and S5 of the IPL (34,36,38) (Figure 2). Despite being intrinsically photosensitive M4 and M5 ipRGCs have been the least studied of all ipRGCs since they express the lowest levels of melanopsin and show little (if any) melanopsin immunoreactivity (18,34). M4 ipRGCs have large somas and large radiant dendritic arbors stratifying in the ON division of the IPL (18) (Figure 2). Lastly, M5 ipRGCs are weakly photosensitive and are readily distinguished from all other ipRGCs on the basis of their small dendritic fields and bushy dendritic arbors (18) (Figure 2). Since we focus on morphological differences of ipRGCs here, we encourage readers to view another recent review that describes physiological differences in ipRGCs in detail (68).
Figure 2.
Morphological differences between classes of ipRGCs. Stratification of the dendrites of each of the 5 classes of ipRGC (and displaced M1 ipRGCs [dM1]) are depicted in retinal cross-sections. Insets show the morphology of dendritic arborizations for each class as seen in retinal whole-mount preparations. Black dashed line indicates the division of the inner plexiform layer into ON and OFF divisions of the IPL. ONL – outer nuclear layer; OPL – outer plexiform layer; INL – inner nuclear layer, IPL(off) – OFF division of the inner plexiform layer; IPL(on) – ON division of the inner plexiform layer; GCL– ganglion cell layer.
With the identification and characterization of these 5 classes of ipRGCs efforts are underway to delineate their associated circuitry both at the level of the retina and within the brain. Thus far the greatest progress has been made in our understanding class-specific circuitry of M1 ipRGCS.
Intraretinal circuitry of M1 ipRGCs
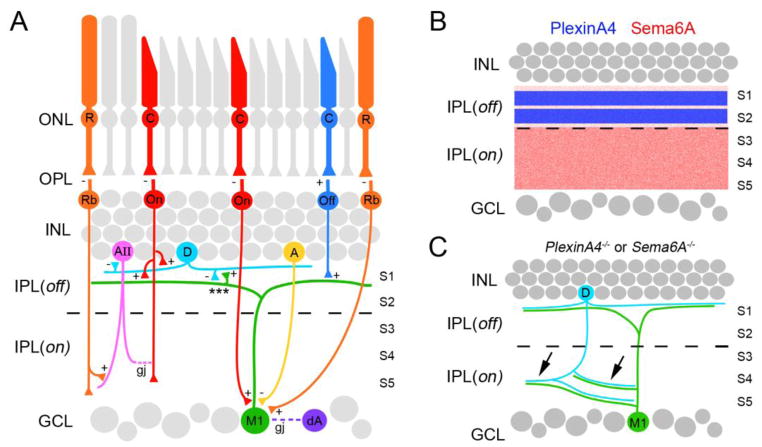
The unique morphology and high level of melanopsin expression of M1 ipRGCs has allowed their unambiguous identification using standard immunohistochemical and retrograde labeling techniques. This in turn has facilitated the use of ultrastructural and electrophysiological approaches to decipher M1 ipRGC circuitry within the retina. Such analyses have shown that M1 ipRGCs are not only intrinsically photosensitive, but are also incorporated into intraretinal circuits and are “extrinsically” activated by bipolar cells similar to conventional classes of RGCs (37,39,40). As described above, M1 ipRGCs arborize in the OFF division of the IPL and therefore receive input from OFF-bipolar cells (Figure 3A). However, physiological recordings have revealed that these OFF-bipolar inputs are only weak (40). Unexpectedly, these same studies demonstrated that M1 ipRGCs receive strong input from ON-bipolar cells, a surprising finding given the dendritic morphology of M1 ipRGCs (40). One possible explanation for this phenomenon is that ON-bipolar cells synapse onto M1 ipRGC somas and dendritic shafts within the ON division of the IPL (41) (Figure 3A), however receptive field mapping suggested that ON-bipolar inputs are present throughout entire M1 ipRGC dendritic arbors and not just near their somas (40). More revealing was the peculiar and remarkable discovery that a class of ON-bipolar cell synapses onto M1 ipRGC dendrites within the OFF-division of the IPL – a finding that challenges the prevailing thought that spatial segregation of inputs into ON or OFF divisions of the IPL is critical for the transmission of light stimuli (42–44) (Figure 3A). The functional significance of these unconventional ON-bipolar cell synapses remains unclear. Lastly, in addition to receiving information from cone photoreceptors via ON- and OFF-bipolar cells, M1 ipRGCs also receive input from a third type of bipolar cell, rod bipolar cells which are activated by rod photoreceptors (40). Synapses from rod-bipolar cells have been observed on the soma and proximal dendrites of M1 ipRGCs (45; but see 44), however they may also influence ipRGC activity through more conventional pathways that include amacrine cells (1) (Figure 3A).
Figure 3.
Development of intraretinal circuitry of M1 ipRGCs. A. Schematic diagram summarizes the synaptic inputs onto M1 ipRGC (M1) dendrites by Off-bipolar cells (Off), On-bipolar cells (On), rod-bipolar cells (Rb), dopaminergic amacrine cells (D) and inhibitory amacrine cells (A). Rod bipolar cells also exert influence on M1 ipRGC activity indirectly through AII amacrine cell-On bipolar cell circuitry. Gap junction (gj) coupling of M1 ipRGCs and displaced amacrine cells (dA) within the ganglion cell layer (GCL) is depicted with a purple dashed line. *** highlights reciprocal connections between dopaminergic amacrine cell and M1 ipRGCs dendrites, which allow M1 ipRGCs to exert influence over neurons in the INL. ‘+’ and ‘−’ denote whether excitatory or inhibitory receptors are present at synaptic sites. B. Distribution of PlexinA4 (blue) and Sema6A (red) in mouse retina. C. Genetic removal of PlexinA4 (PlexinA4−/−) or Sema6A (Sema6A−/−) leads to the mistargeting of dopaminergic amacrine cell (D) and M1 ipRGC (M) dendrites into the ON division of the inner plexiform layer (arrows). R- rod photoreceptor; C – cone photoreceptor; AII – AII amacrine cell; S1-5 – sublaminae of the inner plexiform layer; ONL – outer nuclear layer; OPL – outer plexiform layer; INL – inner nuclear layer, IPL(off) – OFF division of the inner plexiform layer; IPL(on) – ON division of the inner plexiform layer.
In addition to being activated by bipolar cells, M1 ipRGC activity is modulated by amacrine cells. Ultrastructural analyses and electrophysiological recordings have demonstrated that inhibitory amacrine cells synapse onto M1 ipRGCs (40,41) and dye-tracer experiments have shown that these ipRGCs are also gap junction coupled to displaced amacrine cells within the ganglion cell layer (GCL) (46) (Figure 3A). Thus far, the most well characterized input onto M1 ipRGCs originates from dopaminergic amacrine cells, a class of neuromodulatory retinal cells. Dendrites of these dopaminergic amacrine cells costratify with and form reciprocal connections with M1 ipRGC dendrites in the OFF-division of the IPL (32,36,38). Such reciprocal connections provide one mechanism through which M1 ipRGCs exert unconventional influence on information processing in the INL (Figure 3A).
How all of these stereotyped M1 ipRGC-specific circuits develop within the retina remains largely unclear. An attractive hypothesis is that synaptic specificity is driven by the targeting of axons and dendrites to specific sublaminae of the IPL (48,49). A recent study sought to test this hypothesis by examining whether semaphorins, a well-characterized family of axon guidance cues (50), and their receptors (neuropilins and plexins) contribute to laminar stratification and circuit formation of M1 ipRGCs and dopaminergic amacrine cells (51). Dopaminergic amacrine cells (and presumably other classes of cells whose dendrites arborize in S1 and S2 of the Off-division of the IPL) express PlexinA4, whereas transmembrane semaphorin6A (Sema6A), which acts as a repulsive ligand for neurons expressing PlexinA4 (52,53) is enriched in S3-S5 of the On-division of the IPL (Figure 3B)(51). Genetic deletion of either Plexin4A or Sema6A results in the misrouting of dopaminergic processes into the ON-division of the IPL (51) (Figure 3C). M1 ipRGC dendrites are also misrouted into the ON-division of the IPL in these mutants but remain co-ramified with the processes of dopaminergic amacrine cells. Since M1 ipRGCs express neither Sema6A nor PlexinA4 it is likely that the misrouting of their dendrites is an indirect consequence of aberrant laminar specification of dopaminergic amacrine processes (51)(Figure 3C). Whether other inputs onto M1 ipRGCs or dopaminergic amacrine cells (such as those from On-bipolar cells) correctly target mislocalized dendrites in these mutants remains unclear. Taken together these results suggest that synapse specificity remains intact in these mutants despite defects in sublaminar specificity and hint that intraretinal wiring requires different cues for directing sublaminar and synaptic specificity. Cues that direct intraretinal synaptic specificity of M1 ipRGCs have yet to be elucidated.
Targets of M1 ipRGC axons in the brain
While immunolabeling of M1 ipRGCs has proved useful for decoding intraretinal synaptic partners it cannot be employed to identify M1 ipRGC axons in brain. Although melanopsin is present on ipRGC axons within the retina it is absent from these axons once they exit the retina. It is for this reason that transgenic labeling techniques have been instrumental in assessing retino-recipient nuclei targeted by M1 ipRGCs. Typically individual classes of RGCs (or other types of cells) have been transgenically labeled by driving reporter protein expression with a class-specific promoter. To date no such class-specific promoter has been identified that distinguishes M1 ipRGCs from non-M1 ipRGCs, conventional RGCs or neurons within the brain. However, the generation of a transgenic mouse in which lacZ – the bacterial gene encoding beta-galactosidase – was inserted in place of the melanopsin gene (opn4) fortuitously created a tool that specifically labels M1 ipRGCs (6,17). Most likely, specificity of M1 ipRGC labeling in these mice results from poor sensitivity of beta-galactosidase and high expression of melanopsin by this class of neurons. These mice, termed opn4tau-lacZ/+ mice, have been essential in delineating retino-recipient nuclei innervated by M1 ipRGCs.
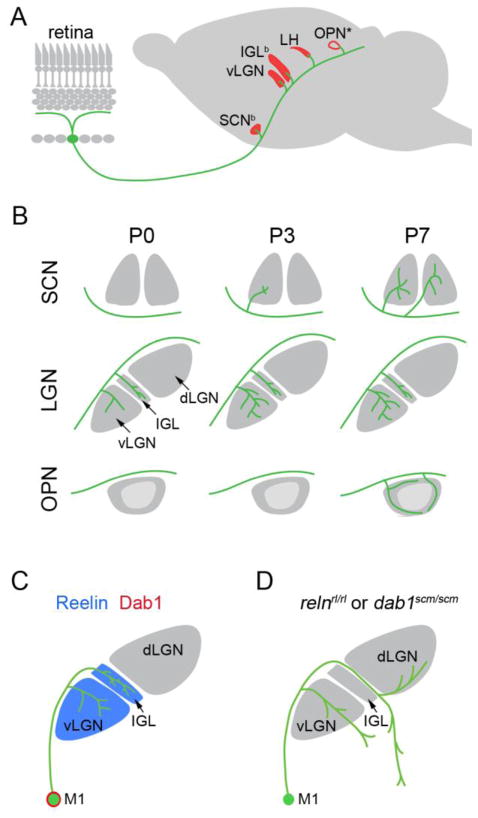
Five CNS nuclei receive dense projections from M1 ipRGCs – the suprachiasmatic nuclei (SCN), the ventral lateral geniculate nuclei (vLGN), the intergeniculate leaflet (IGL), the lateral habenula (LH) and the peripheral region of the olivary pretectal nucleus (OPN)(termed the OPN “shell”)(6,18,54,55)(Figure 4A). These nuclei are largely non-image forming visual nuclei whose functions include (but are not limited to) irradiance detection, pupillary light reflexes, and circadian photoentrainment. A number of other diencephalic and midbrain nuclei also receive input from M1 ipRGCs but the paucity of these projections suggest that only a small subset of M1 ipRGCs project to these regions. These regions include the superior colliculus, lateral hypothalamus, lateral posterior thalamic nucleus, lateral pre-optic nucleus, periaqueductal grey, bed nucleus of the stria terminalis, medial amygdaloid nucleus, perisupraoptic area, anterior hypothalamus, and the ventral subparaventricular zone (6). The role of M1 ipRGC input to these nuclei remains unclear but likely includes homeostatic and autonomic changes in response to light. Sparse M1 ipRGC terminal arbors have also been noted in the dorsal lateral geniculate nucleus (dLGN) at late postnatal ages (6,18). Although M1 and M2 ipRGCs both innervate SCN (33), projections from the different classes of ipRGCs are likely to be quite different (see 18). For example, the dorsal lateral geniculate nucleus (dLGN), central core of the OPN and superior colliculus are heavily innervated by non-M1 ipRGCs (18,55).
Figure 4.
Development of central projections of M1 ipRGCs, A. 5 nuclei (red) receive dense projections from M1 ipRGCs (green): suprachiasmatic nucleus (SCN), ventral lateral geniculate nucleus (vLGN); intergeniculate nucleus (IGL), lateral habenula (LH) and the olivary pretectal nucleus (OPN). ‘b’ denotes those nuclei that receive binocular input from M1 ipRGCs. ‘*’ denotes that only the outer ‘shell’ of the OPN is innervated by M1 ipRGCs. B. Development of M1 ipRGC innervation to the SCN, lateral geniculate nucleus (LGN, which is composed of the vLGN, IGL and dorsal LGN [dLGN]), and OPN at postnatal day 0,3 and 7 (P0, P3, P7 respectively) in mice. vLGN and IGL are innervated by M1 ipRGCs by P0, while SCN is innervated by P3 (although only the contralateral SCN is innervated by M1 ipRGC axons at this early age), and the ‘shell’ of the OPN is innervated by P7. C. Reelin (blue) is expressed in the ventral lateral geniculate nucleus (vLGN) and intergeniculate nucleus (IGL). M1 ipRGCs (M1) express disabled-1 (Dab1; red), an intracellular component of the reelin signaling pathway. D. M1 ipRGC axons are mistargeted in spontaneously generated mouse mutants lacking either Reelin (relnrl/rl) or Dab1 (dab1scm/scm).
A fascinating aspect of M1 ipRGC projections is that the timing of retino-recipient nuclei innervation is nuclei-specific despite single cells projecting to multiple nuclei (Figure 4B). For example, single M1 ipRGC axons project to both SCN and IGL (56) but innervate and arborize in IGL days before they do so in SCN (55)(Figure 4B). This discrepancy occurs despite M1 ipRGC axons encountering and bypassing the SCN before they innervate the IGL (Figure 4A, B). The timing of target innervation by M1 ipRGCs also differs from that of other non-M1 ipRGCs that co-innervate the same central nuclei. Non-M1 ipRGCs innervate the center of the OPN by birth, but M1 ipRGC axons do not arborize in the OPN “shell” until postnatal day 7 (P7), a time at which pupillary light reflexes emerge (55)(Figure 4B).
Mechanisms of M1 ipRGC central target selection
Since Roger Sperry’s seminal studies in the 1940s, the cellular and molecular mechanisms that guide RGC axons to central targets have receive considerable attention (57,58). At least three distinct mechanisms contribute to the targeting of RGC axons: topographic mapping, eye-specific segregation, and nuclei-specific targeting. Here we will briefly define and describe each of these although it remains an open question as to whether M1 ipRGC axons are topographically targeted or undergo eye-specific segregation from each other.
Thus far the most thoroughly characterized targeting mechanism of RGC axons is their sorting into topographically arranged maps (2,59). These maps convey the location of information in the visual field to spatially correlated regions of retino-recipient nuclei. Hence, RGCs that respond to adjacent elements in an animal’s field of vision will themselves be adjacent to each other in the retina and their axons will project to adjacent target cells in nuclei within the brain. However, retrograde injections into regions of non-image forming nuclei label a uniform distribution of M1 ipRGCs whose somas are not adjacent to one and other in the retina (56). Hence, it is unclear whether M1 ipRGCs are sorted topographically, or whether they need to be based upon their function in irradiance detection.
In many retino-recipient nuclei, RGC axons are also sorted into eye-specific domains, whereby axons from one eye are spatially segregated from those originating from the other eye. Such domains allow for visual maps of the two eyes to be in register and serve as building blocks for binocular vision. Input to most non-image forming retino-recipient nuclei originates from M1 ipRGCs that reside exclusively in the contralateral eye (6). Therefore while M1 ipRGC axons may be segregated into eye-specific domains with other classes of RGCs in these nuclei (for example in the OPN [6,18,55]) they are not segregated from other M1 ipRGC axons based upon their eye of origin. In nuclei that receive input from M1 ipRGCs from both eyes – namely the SCN and IGL (6,56) – M1 ipRGC axons do not appear segregated based on their eye of origin but rather appear widely dispersed in overlapping regions of the target nuclei.
The third mechanism of RGC axon targeting involves the ability of an axon to select and arborize in correct retino-recipient nuclei, or in many cases the correct layer (or domain) of a retino-recipient nucleus. It is noteworthy that although we consider nuclei- and layer-specific targeting a single mechanism for the purposes of this review, they may be two distinct targeting mechanisms. While an axon from a M1 ipRGC may not be in topographic register with others, it does target specific nuclei and in some cases (such as the OPN) highly discrete domains within a single nucleus (Figure 4A). Mechanisms underlying nuclei- or layer-specific target selection by M1s, or other RGCs for that matter, have yet to be elucidated. One attractive hypothesis that several groups are testing is whether all nuclei innervated by a single class of RGCs express the same molecule, or a set of molecules, which allow class-specific recognition of these targets. Identification of such molecular codes has been challenging due to the diverse molecular architecture and embryonic origin of target nuclei and the temporal complexity of target innervation (see that described above for M1 ipRGCs). An alternative possibility is that unique targeting cues are present in each nucleus and RGCs must express a repertoire of receptors to identify each appropriate target nuclei. As it remains unclear which of these possible mechanisms drives ipRGC targeting we undertook an unbiased screen to identify cues that target M1 ipRGC axons to vLGN and IGL and repel them from the adjacent dLGN (Figure 4C). We found reelin (Reln), an extracellular matrix molecule with known roles in directing neuronal migration and class-specific axonal targeting (60–63), is enriched in vLGN and IGL but absent from dLGN (54). Importantly, M1 ipRGCs express disabled-1 (Dab1), a necessary component of the reelin-signaling pathway (54,64,65)(Figure 4C). Mouse mutants lacking Reln or Dab1 have reduced M1 ipRGC arbors in IGL and misplaced arbors in inappropriate thalamic nuclei (54)(Figure 4D). Initially misrouted M1 ipRGC axons are correctly repelled from dLGN in the absence of either Reln or Dab1, however repellant cues in dLGN must eventually be down-regulated or degraded since a significant portion of M1 axons erroneously invade the medial aspect of dLGN at later postnatal ages (54)(Figure 4D). The targeting of axons from image-forming classes of RGCs appear unaffected by the absence of Reln or Dab1. While Reln appears necessary for M1 ipRGC projections to vLGN and IGL it is not expressed in the other main targets of M1 ipRGC axons and M1 projections to those nuclei appear unaltered in mutants lacking Reln (54). Together these studies reveal the first cue necessary for class-specific retinogeniculate targeting and suggest that M1 ipRGCs may use different mechanisms to target each nucleus.
Conclusion
Understanding how neural circuits form in the developing brain requires us to focus our attention on how individual classes of these neurons form specific, stereotyped circuits. In the retina there are over 20 distinct classes of RGC – each with unique class-specific circuitry. In this review we have highlighted the neural circuits associated with a single class of intrinsically photosensitive RGC. While our understanding of M1 ipRGC circuitry is quite remarkable given their relatively recent discovery, our understanding of the molecular mechanisms responsible for their assembly into specific circuits is still rudimentary. That being said, we know as much, if not more, about the cellular and molecular mechanisms governing M1 ipRGC wiring than any other single class of RGC. An obvious challenge facing this field is to continue the difficult task of unraveling the molecular signals that govern class-specific wiring in the retina and brain nuclei. A major question that remains to be resolved is whether conserved mechanisms of class-specific wiring exist or whether each class of neurons uses a unique set of mechanisms to establish its circuitry.
Acknowledgments
Work in our laboratories is supported by grants from the National Institutes of Health [NIH; EY012716 (WG) and EY021222 (MAF)], the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (MAF), the A.D. Williams Fund (MAF), and the VCU Presidential Research Incentive Fund (MAF).
References
- 1.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 2.Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 4.Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687. doi: 10.1002/cne.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscat L, Huberman AD, Jordan CL, Morin LP. Crossed and uncrossed retinal projections to the hamster circadian system. J Comp Neurol. 2003;466:513–524. doi: 10.1002/cne.10894. [DOI] [PubMed] [Google Scholar]
- 6.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager UC, Olsen JF. Origins of crossed and uncrossed retinal projections in pigmented and albino mice. J Comp Neurol. 1980;191:383–412. doi: 10.1002/cne.901910306. [DOI] [PubMed] [Google Scholar]
- 8.Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- 10.Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonehara K, Shintani T, Suzuki R, Sakuta H, Takeuchi Y, Nakamura-Yonehara K, Noda M. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS One. 2008;3:e1533. doi: 10.1371/journal.pone.0001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS One. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster RG, Provencio I, Hudson D, Fiske S, De Grip W, Menaker M. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 20.Provencio I, Wong S, Lederman AB, Argamaso SM, Foster RG. Visual and circadian responses to light in aged retinally degenerate mice. Vision Res. 1994;34:1799–1806. doi: 10.1016/0042-6989(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 21.Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., 3rd Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 22.Freedman MS, Lucas RJ, Soni B, von Schantz M, Munoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 23.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 26.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 27.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 28.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 30.Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 31.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 32.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 34.Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 37.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol. 2011;519:1492–1504. doi: 10.1002/cne.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Leon JA, Warren EJ, Allen CN, Robinson DW, Lane Brown R. Synaptic inputs to retinal ganglion cells that set the circadian clock. Eur J Neurosci. 2006;24:1117–1123. doi: 10.1111/j.1460-9568.2006.04999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 42.Hoshi H, Liu WL, Massey SC, Mills SL. ON inputs to the OFF layer: bipolar cells that break the stratification rules of the retina. J Neurosci. 2009;29:8875–8883. doi: 10.1523/JNEUROSCI.0912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grunert U, Jusuf PR, Lee SC, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci. 2010;28:39–50. doi: 10.1017/S095252381000026X. [DOI] [PubMed] [Google Scholar]
- 45.Ostergaard J, Hannibal J, Fahrenkrug J. Synaptic contact between melanopsin-containing retinal ganglion cells and rod bipolar cells. Invest Ophthalmol Vis Sci. 2007;48:3812–3820. doi: 10.1167/iovs.06-1322. [DOI] [PubMed] [Google Scholar]
- 46.Muller LP, Do MT, Yau KW, He S, Baldridge WH. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J Comp Neurol. 2010;518:4813–4824. doi: 10.1002/cne.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Contini M, Lin B, Kobayashi K, Okano H, Masland RH, Raviola E. Synaptic input of ON-bipolar cells onto the dopaminergic neurons of the mouse retina. J Comp Neurol. 2010;518:2035–2050. doi: 10.1002/cne.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 49.Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2011;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, Chedotal A, Mitchell KJ, Manabe T, Fujisawa H. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 53.Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su J, Haner CV, Imbery TE, Brooks JM, Morhardt DR, Gorse K, Guido W, Fox MA. Reelin is required for class-specific retinogeniculate targeting. J Neurosci. 2011;31:575–586. doi: 10.1523/JNEUROSCI.4227-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McNeill DS, Sheely CJ, Ecker JL, Badea TC, Morhardt D, Guido W, Hattar S. Development of melanopsin-based irradiance detecting circuitry. Neural Dev. 2011;6:8. doi: 10.1186/1749-8104-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- 57.Sperry RW. Optic Nerve Regeneration With Return of Vision in Anurans. J Neurophysiol. 1944;7:57–69. [Google Scholar]
- 58.Sperry RW. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 61.Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- 62.Borrell V, Del Rio JA, Alcantara S, Derer M, Martinez A, D’Arcangelo G, Nakajima K, Mikoshiba K, Derer P, Curran T, Soriano E. Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J Neurosci. 1999;19:1345–1358. doi: 10.1523/JNEUROSCI.19-04-01345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borrell V, Pujadas L, Simo S, Dura D, Sole M, Cooper JA, Del Rio JA, Soriano E. Reelin and mDab1 regulate the development of hippocampal connections. Mol Cell Neurosci. 2007;36:158–173. doi: 10.1016/j.mcn.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Sheldon M, Rice DS, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 65.Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 66.Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011 Jul 17; doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]