Abstract
Introduction
It is important to identify all circulating metabolites, including free fluoride, for accurate pharmacokinetic modeling of [18F]-labeled radiotracers. We sought to determine the most efficient method to detect and quantify low levels of free [18F]fluoride in biological samples.
Methods
Low levels of [18F]fluoride were analyzed using two methods: A) an ion-exchange cartridge and gamma counting; and B) radio-HPLC, to compare the detection limits of these two analytical methods. Twenty microliters of [18F]fluoride solution was loaded onto an ion-exchange cartridge then eluted with 20% MeCN/water (5 mL) and radioactivity trapped in the cartridge counted on a gamma counter. [18F]-Fluoride was also determined in plasma and urine from mice injected with [18F]-labeled thymidine analogues using method A.
Results
The detection sensitivity of method A was 9.4-fold higher than that of method B (0.075±0.004 nCi vs. 0.71±0.02 nCi). Using method A, [18F]fluoride was determined in plasma for [18F]FLT, [18F]FMAU, [18F]FEAU and N3-[18F]FPrT as 1.4±0.31% (n=4), 0.17±0.49% (n=3), 4.88±1.62% (n=3) and 12.94±0.48% (n=4), respectively. The amount of [18F]fluoride determined in the urine was 11.49±1.60% (n=4) from [18F]FLT, 5.36±2.34% (n=3) from [18F]FMAU, 13.57±1.96% (n=3) from [18F]FEAU, and 11.19±1.98% (n=4) from N3-[18F]FPrT.
Conclusion
Low levels of [18F]fluoride in biological samples can be detected and quantified using an ion-exchange cartridge and gamma counting. This methodology is simple, accurate and superior to the standard use of radio-HPLC on a C18 column for metabolite analysis; and it should be useful in pharmacokinetic modeling for animal imaging studies using an [18F]-labeled radiotracer and PET.
Keywords: PET, Radiopharmaceuticals, [18F]fluoride, ion exchange cartridge
1. Introduction
Positron emission tomography (PET) is a noninvasive imaging modality for the in vivo imaging of various metabolic processes [1], such as cellular proliferation [2], determination of herpes simplex virus thymidine kinase (HSV1-tk) reporter gene expression [3], expression of various receptors [4], and for the assessment of treatment response [5]. The in vivo catabolism of radiolabeled compounds is well documented; for example, many [18F]-labeled compounds are known to be catabolized in vivo into smaller species, including [18F]fluoride [6, 7]. Only a few positron-emitting radiopharmaceuticals are not catabolized. Therefore, in most instances, labeled metabolites in the plasma should be analyzed to determine the exact input function for quantitative PET measurements [8-10].
Free [18F]fluoride, which is produced by in vivo catabolism and defluorination of an [18F]-labeled compounds, may partially remain in circulating blood and partially absorbed into bone; the rest is excreted through renal clearance into urine. In order to develop accurate pharmacokinetic modeling of an [18F]-labeled radiotracer, it is necessary to measure all radioactive species, including free [18F]fluoride circulating in blood, during PET imaging. In the current study, we investigated the use of an ion-exchange cartridge and gamma counting method to measure low levels of free [18F]fluoride. For comparison, we used radio-HPLC method on a C18 reverse-phase column that is commonly used for metabolite analysis of [18F]-labeled compounds. Analysis of free [18F]fluoride using flash chromatography on a C18 reverse-phase cartridge was also performed for comparison. We determined the lowest limit of detection by the a) ion-exchange cartridge trapping and gamma counting method and b) radio-HPLC detection method. Furthermore, we determined the recovery efficiency of [18F]fluoride in the ion-exchange cartridge and that from the radio-HPLC system.
Analysis of the free 18F]fluoride was extended to the biological samples from mice injected with the [18F]-labeled pyrimidine nucleosides analogues to detect and quantify low levels of fluoride derived from catabolism and defluorination. We report the advantages of an ion-exchange cartridge for detection and quantification of low levels of free [18F]fluoride in biological samples.
2. Materials and methods
2.1 Reagents and instrumentation
All reagents and solvents were purchased from Aldrich Chemical Co. (Milwaukee, WI) and used without further purification. Purified water (resistivity 18.2 MΩcm-1) was prepared using the Millipore Milli-Q Gradient water purification system. Ion-exchange cartridges were purchased from ABX (Radeberg, Germany). Solid-phase extraction cartridges (C18, 100 mg) were purchased from Alltech Associates (Deerfield, IL).
Radioactivity was counted on a Packard Cobra II auto-gamma counting system E500300 (Perkin-Elmer, Waltham, MA). High performance liquid chromatography (HPLC) analysis was performed on an 1100 series pump (Agilent, Germany) with a built-in UV detector operated at 254 nm and a dual (BGO HPLC coincidence) radioactivity detector B-FC-4100 (Bioscan, Washington, DC) using an analytical C18 column (10×250 mm, Econosil; Alltech). A solution of 10% acetonitrile (MeCN)/10 mM Na-acetate or 10% MeCN/10 mM Na-phosphate was used as eluting solvent for the HPLC analytical studies on the C18 column. A solution of 10% MeCN/40 mM Na-bicarbonate or 10% MeCN/10 mM Na-acetate was used for flash chromatographic analysis on the C18 cartridge.
2.2 Radiosynthesis of the [18F]-labeled compounds
[18F]Fluoride (K[18F]) was purchased from Cyclotope Inc. (Houston, TX). [18F]-Labeled compounds were synthesized according to published methods: [18F]FLT [11]; [18F]FMAU [12]; [18F]FEAU [13]; and N3-[18F]Fluoropropyl thymidine (N3-[18F]FPrT) [14].
2.3: Dilution of the radiotracers
For each compound, including [18F]fluoride, dilute solutions of various concentrations were prepared, and a known volume of each solution was counted on a gamma counter to identify the proper activity concentration within its optimal range of counting efficiency, to avoid saturation of the counter. All radioactive compounds were diluted to a concentration in the range of 5-15×106 cpm/mL, and 20 μL of each solution was counted in a gamma counter to identify the actual activity concentration suitable for counting without saturation of the counter. Twenty microliters of this solution was used for analysis on ion-exchange or C18 cartridge and HPLC.
2.4: Activation of the ion-exchange cartridge
All ion-exchange cartridges were activated according to the procedure described in the manufacturer’s instructions using a clean plastic syringe. The cartridge was activated by passing with 1 ml of ethanol followed by 1 ml of distilled water; then, after 5 minutes, excess solvent was removed by pushing air with a syringe through the cartridge. Similarly, the C18 cartridges were activated and equilibrated with the eluting solvent (10% MeCN/10 mM Na-phosphate).
2.5: Loading and elution of the sample on the ion exchange cartridge and C18 cartridge
A 20-μl solution of each radioactive compound (described above) was loaded onto the ion-exchange cartridge. The radioactivity was eluted from the cartridge with a solution of 20% MeCN in water (except where otherwise mentioned) using a 5-ml plastic syringe. Cartridges were washed 5 times with 1 ml of the eluting solvent (20% MeCN/water), and fractions were collected in scintillation vials. Radioactivity in each fraction was counted on a gamma counter, along with the cartridge and a blank vial as background. Combined radioactivity in the washing was calculated and converted as the percent activity in the washing. The radioactivity in the cartridge was converted as the percent of radioactivity retained, and the combined radioactivity in the cartridge and washings was considered to be 100%. The C18 cartridge was eluted with 10% MeCN/10 mM Na-phosphate (5×1 mL); radioactivity in the cartridge and washing fractions was counted on a gamma counter.
2.6: Determination of recovery efficiency of [18F]fluoride using ion-exchange cartridge and HPLC system
A standard solution of K[18F] (20 μCi/10 mL) was prepared. Twenty microliters of this solution (in triplicate) was dispensed by a micro-syringe (HPLC syringe) into gamma counting vials and the radioactivity was counted on a gamma counter. This activity (cpm) was considered to be 100%. Twenty microliters of the same solution (in triplicate) was dispensed by the same micro-syringe onto an ion-exchange cartridge then eluted with 20% MeCN/water solution in 1 mL fractions (5 mL) to gamma counting vials and the radioactivity in the cartridge and washings was counted on a gamma counter. Total radioactivity (cpm) in the cartridge was divided by radioactivity counted in the counting vial (directly dispensed into the counting vial) and multiplied by 100, and this value was considered as percent recovery (trapped in the cartridge), and expressed as recovery efficiency. Similarly, the recovery efficiency from the HPLC system was determined as follows: 20 μL of the same fluoride solution was injected into the HPLC system, and the total volume of solvent (eluted) was collected. A known volume of this eluent was counted on a gamma counter then the activity (cpm) in the total eluent was calculated. This value (cpm) was divided by the total activity (cpm) counted in the directly dispensed counting vial then multiplied by 100, and this value was considered as percent recovery from the HPLC system. It should be noted that radioactivity trapped in the ion-exchange cartridge and that recovered from the HPLC system are considered to be recovery efficiency of the respective systems.
2.7: Determination of the limits of detection of the gamma counter and HPLC radioactivity detector
A standard solution of [18F]fluoride (20 μCi in 10 mL) was prepared as described above. From this solution a series of dilutions were made and a known volume (20 μL) of these dilute solutions was counted on the gamma counter. The radioactivity (cpm) counted on the gamma counter was plotted against radioactivity (nCi) to draw a standard curve, which was obtained as a straight line passing through the origin. From this graph the lowest limit of detection was calculated, based on the signal-to-noise ratio as 3.00 [15]. Similarly, another standard curve for the radioactivity (area under the peak) against radioactivity (nCi) was drawn for the HPLC analysis of the dilute solutions. A known volume (20 μL) of the dilute solutions was injected to the HPLC and the radioactive peak area was calculated for each dilute solutions. The peak area was plotted against radioactivity (nCi), which produced a straight line that did not pass through the origin, and from this graph the lowest limit of detection was calculated based on the signal-to-noise ratio as 3.00 [15].
2.8: Injection into animals and plasma processing
Animal studies were performed under an approved institutional animal protocol. Nude mice were injected with each radiotracer or K[18F] (100-150 μCi) through the tail vein (i.v.). Then, 1-2 hours after the time of injection, blood samples were collected directly from the heart using a syringe pretreated with heparin, then transferred to a centrifuge tube and kept on ice at 0°C. Blood samples were centrifuged at a rate of 13,400 RPM for 15 min. The supernatant (plasma) was transferred to an eppendorff centrifuge tube. The protein content from the plasma was separated by two different methods. Method 1: The plasma (50-100 μL) was transferred to an eppendorff centrifuging tube, and MeCN (3x volumes) was added to the tube, then mixed well (vortexed) for 20 seconds. The mixture was cooled in ice for 5 min and then centrifuged at 13,400 rpm for 15 min. The supernatant was removed, an aliquot was used for gamma counting and the activity was converted to %ID/g. The remaining solution was used for analysis on an ion exchange cartridge or by HPLC, as described above. Method 2: The plasma (50-100 μL) was treated with same volume of trichloroacetic acid (TCA, 3N, 50-100 μL), kept on ice for 5 min and centrifuged at a rate of 13,400 RPM for 15 min. The supernatant was collected, an aliquot was counted for radioactivity and the radioactivity was converted to %ID/g. The rest of the material was neutralized (NaHCO3 solution) and analyzed on the ion-exchange cartridge or by HPLC. Urine samples were diluted with water to prepare the appropriate activity concentration, approximately 10×106 cpm/ml and then 20 μL of this solution was analyzed on the ion-exchange cartridge.
3. Results and discussion
Radiolabeled metabolites in plasma must be analyzed to determine the exact input function for quantitative PET measurements [8-10]. However, there is no suitable method to determine low levels of [18F]fluoride for the [18F]-labeled radiotracers; therefore, such a method would be highly useful for the accurate pharmacokinetic modeling of [18F]-labeled compounds.
For metabolite analysis, it is necessary to isolate plasma from blood and remove the protein content. The recovery efficiency of radioactivity ([18F]fluoride or other species) in blood samples was estimated using two different methods. After removal of the red blood cells by centrifugation, the plasma was isolated and processed by the use of either MeCN or trichloro acetic acid (TCA). The recovery of [18F]fluoride using MeCN precipitation was 88.64±5.19% and that using TCA precipitation was 82.52±7.07%. Thus, the MeCN precipitation method was more efficient and superior than the TCA precipitation method.
The recovery efficiency of the radio-HPLC system and an ion-exchange cartridge system was determined using [18F]fluoride. The recovery efficiency of radioactivity from the HPLC system (column, guard column and injector) was 83±3%, and that of the ion-exchange cartridge system was 97±1%. These results suggest that when the HPLC system is used, approximately 17% of the radioactivity remains uncounted due to non-specific binding of [18F]fluoride in the HPLC system. For ion-exchange cartridge trapping and gamma counting only about 3% of radioactivity remains uncounted. To compare the detection sensitivity of the gamma counter with that of HPLC radioactivity detector, standard curves for both methods were plotted to determine the differences in lower limits of detection (Fig. 1).
Figure 1.
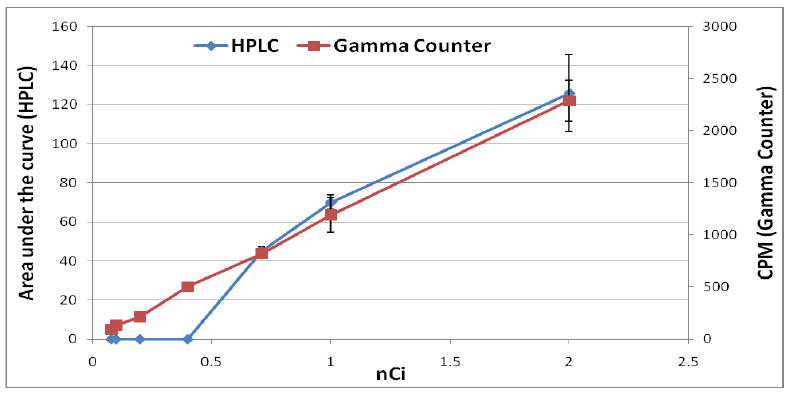
Standard curves for detection of [18F]fluoride radioactivity trapped on an ion-exchange cartridge then counted on the gamma counter (red) or calculated area under the curve derived by the HPLC radioactivity detector (blue).
The lowest limit of detection of the gamma counter based measurement of radioactivity in the ion-exchange cartridge was 0.075±0.004 nCi, whereas that for the HPLC detector was 0.71±0.02 nCi, on the basis of signal-to-noise ratio of 3.0 [15]. This means that the sensitivity of the ion-exchange cartridge and gamma counter based method is 9.4-times higher than that of the radio-HPLC. According to the manufacturer’s specification, the lowest limit of detection (LLOD) of the gamma counter and radio-HPLC detector is 0.048 nCi and 0.09 nCi, respectively; i. e. gamma counter is approximately 2-fold higher sensitive than the HPLC detector. However, our experimental LLOD of gamma counter is 0.075 nCi, 1.6-fold higher than the manufacturer’s specified value; and that for HPLC detector is 0.71 nCi, 7.8-flod higher than the manufacturer’s specified value. In the case of HPLC, large difference in LLOD is possibly due to the fact that the manufacturer determined the LLOD as a single point counting as opposed to our flow counting. Furthermore, lower recovery efficiency (83%) from the HPLC, measurement of the area under the curve and difference between LLOD and limit of quantitation (LOQ) may cause this significant difference. Thus, low levels of radioactivity ([18F]fluoride) can be better detected and quantified using an ion-exchange cartridge and gamma counter as opposed to detection (area under the curve) by the radio-HPLC detector.
The ion-exchange cartridge approach was chosen for this study because it traps and concentrates [18F]fluoride very efficiently (99.41±0.42%) and the whole cartridge can be placed directly into a counting vial for gamma counting of radioactivity. This cartridge can trap only anionic species, including fluoride, and the neutral molecules can pass through, except for some non-specific binding or interaction of the neutral compound. In most of the previous studies for kinetic modeling and compartmental analysis [16-21] for interpretation of the PET images of the [18F]-labeled compounds, metabolite analyses were performed on HPLC using a C18 reverse phase column, relying on the percent of the parent compound and any other metabolites that can be easily detected by the HPLC radioactivity detector. In our experience, the C18 column generally does not produce a sharp peak for radioactive fluoride when eluted with MeCN/water; the free fluoride elutes very slowly, producing a broad peak that cannot be detected by the radioactivity detector of the HPLC. As a result, the free fluoride present at low levels can easily be missed. However, when a Na-phosphate buffer was used, the [18F]fluoride could be detected with a significant amount of activity injected to the HPLC. A similar approach, such as flash chromatography on a C18 cartridge (100 mg) revealed results similar to those observed by HPLC using a C18 column. The C18 cartridge showed that >96% of [18F]fluoride could be eluted with a buffer (Fig. 2A). However, when analyzed by HPLC, the amount of [18F]fluoride was too low and remained in the noise level of the HPLC chromatogram. On the other hand, an ion-exchange cartridge can hold any trace amount of [18F]fluoride, and its holding efficiency is > 99% (Fig.2B). Therefore, very low levels of fluoride can be trapped on an ion-exchange cartridge and detected by a gamma counter.
Figure 2.
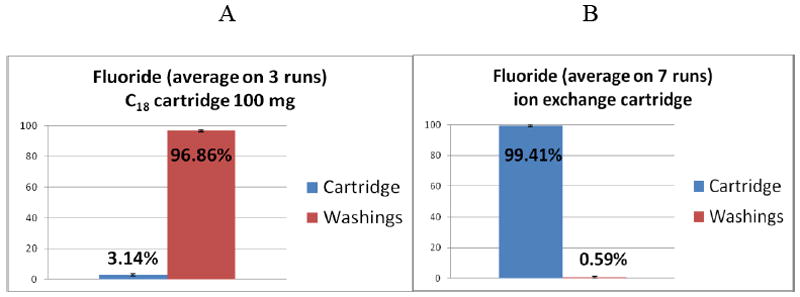
Percent radioactivity retained in the cartridge and in the wash: A, C18 cartridge, 96.86% activity washed out; B, ion-exchange cartridge, 99.41% activity retained. Bars: averages ± standard deviation.
If an ion exchange cartridge is used for analysis of plasma or urine samples from animal species, low levels of circulating fluoride can be easily detected and quantified, and more efficiently than on a C18 column or cartridges. In animal blood or plasma samples, it is easy to underestimate the presence of low levels of [18F]fluoride, especially when analyzed by HPLC on a C18 column. The trapping profile of the anion exchange cartridge for [18F]fluoride derived from in vivo catabolism and defluorination in plasma and urine samples is provided in Table 1.
Table 1.
Percent radioactivity (18F-fluoride), derived from [18F]-labeled compounds estimated by in the ion-exchange cartridge and gamma counting. Data: average ± standard deviation.
| Compounds | % Fluoride in plasma | % Fluoride in urine | No of expts |
|---|---|---|---|
| [18F]FLT | 1.4±0.31 | 11.49±1.6 | 4 |
| [18F]FMAU | 0.89±0.29 | 5.36±2.34 | 3 |
| [18F]FEAU | 4.88±1.62 | 13.57±1.96 | 3 |
| N3[18F]FPrT | 12.93±.48 | 11.19±1.98 | 4 |
Table 1 demonstrates that animals injected with [18F]FLT, [18F]FMAU, [18F]FEAU, and [18F]FPrT had 1.4±0.31%, 0.89±0.29%, 4.88±1.62% and 9.31±3.11% of [18F]fluoride in plasma. The free fluoride in urine due to in vivo defluorination was 11.49±1.6% for [18F]FLT, 5.36±2.34% for [18F]FMAU, 13.57±1.96% for [18F]FEAU, and 11.19±1.98% for N3-[18F]FPrT. Compared with plasma, urine had approximately 8.0 times higher fluoride for [18F]FLT, 6.0 times higher for [18F]FMAU and 2.8 times higher for [18F]FEAU. Amount of [18F]fluoride from [18F]FLT in both plasma and urine was higher than that from [18F]FMAU. Analysis of urine and plasma on an ion-exchange cartridge suggests that part of the free fluoride is excreted through renal clearance that goes to the urine, whereas some fluoride remains in circulating blood prior to accumulation into bone.
Figure 3 demonstrates the amount of [18F]fluoride derived from each compound and retained in the ion-exchange cartridge; the values shown are net radioactivity trapped after subtraction of the nonspecific binding of each compound.
Figure 3.
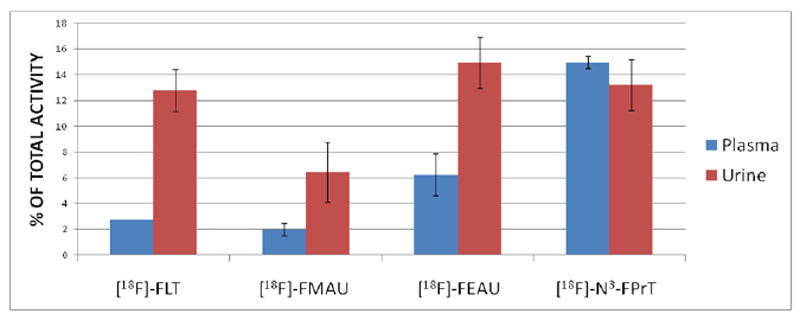
Percent of free [18F]fluoride produced from the corresponding compounds due to catabolism and defluorination in plasma and urine samples. Results are averages with standard deviation.
Urine had greater amount of [18F]fluoride retained in the cartridge as compared to plasma for [18F]FLT, [18F]FMAU, and [18F]FEAU; however, [18F]fluoride for N3-[18F]FPrT was comparable in plasma and urine. The amount of [18F]fluoride in the plasma and urine samples in mice injected with [18F]FLT was approximately 2-fold higher than that of [18F]FMAU, which suggests that [18F]FMAU is relatively more stable in vivo in mice. Although the levels of [18F]fluoride were quite low, they may be sufficient to accumulate in bone. In previous studies, it was reported that [18F]FLT was incorporated into bone marrow at much higher rates than [18F]FMAU in dogs [16, 19, 20]. According to our results, it is conceivable that the total accumulation of radioactivity in dog’s bone marrow may have been a combination of [18F]FLT in the marrow and [18F]fluoride in the bone. However, further studies are required to establish whether [18F]FLT uptake in marrow is a combination of both the parent compound [18F]FLT and [18F]fluoride in the bone.
Our analysis of the free [18F]fluoride (K[18F]) and [18F]fluoride from the [18F]-labeled compounds in plasma and urine on the ion-exchange cartridge, C18 column, and C18 cartridge suggests that the ion-exchange cartridge with gamma counting is the best tool to detect and estimate low levels of [18F]fluoride. Low levels of fluoride in plasma can be easily missed during metabolite analysis by HPLC on a C18 column, because levels of [18F]fluoride can be below the limit of detection by a HPLC radioactivity detector; as a result, the presence of fluoride can be missed and data misinterpreted. Therefore, neither the C18 column on an HPLC nor a C18 cartridge on a flash chromatography system is suitable for accurate detection and quantification of circulating [18F]fluoride in plasma or urine samples, especially at low levels. By contrast, an ion exchange cartridge traps the fluoride ion very effectively (>99%), so any trace of circulating [18F]fluoride can be trapped and measured accurately on a gamma counter. Therefore, this approach using ion-exchange cartridge with gamma counting should be highly useful for plasma or urine analysis of [18F]fluoride in biological samples.
4. Conclusions
The method using an ion-exchange cartridge and gamma counting provides an accurate and efficient determination of low levels of [18F]fluoride in biological samples. This method is superior to the conventional radio-HPLC with a C18 reverse phase column and could be widely used for quantification of [18F]fluoride in plasma and urine samples after administration of various [18F]-labeled pyrimidine nucleoside analogues and other [18F]-labeled imaging agents.
Acknowledgments
This work was supported by start-up funds from The University of Texas M. D. Anderson Cancer Center to Drs. Mian M. Alauddin and Juri G. Gelovani and the NCI CCSG Core Grant CA 106672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosé C, Dose J, Avril N. Positron emission tomography for the diagnosis of breast cancer. Nucl Med Comm. 2002;37:613–8. doi: 10.1097/00006231-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Vasselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3′-deoxy-3′-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: co-relation of 18F-FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res. 2002;8:3315–23. [PubMed] [Google Scholar]
- 3.Soghomonyan S, Hajitou A, Rangel R, Trepel M, Pasqualini R, Arap W, et al. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat Protoc. 2007;2:416–23. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 4.Yeh HH, Ogawa K, Balatoni J, Mukhapadhyay U, Pal A, Gonzales-Lepera C, et al. Molecular Imaging of Active Mutant L858R EGFR Kinase Expressing Non Small Cell Lung Carcinomas using PET/CT with [18F]F-PEG6-IPQA. PNAS. 2020;108(4):1603–8. doi: 10.1073/pnas.1010744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostakoglu L, Goldsmith SJ. [18F]-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. J Nucl Med. 2003;44:224–39. [PubMed] [Google Scholar]
- 6.Ikenish R, Kitarawa T, Nishiuchi M. Gas chromatographic method for the determination of fluoride ion in biological samples. I. Fluoride level in monkey. Chem Pharm Bull. 1988;36:662–9. doi: 10.1248/cpb.36.662. [DOI] [PubMed] [Google Scholar]
- 7.Ikenishi R, Kitagawa T. Gas chromatographic method for the determination of fluoride ion in biological samples. II. Stability of fluorine-containing drugs and compounds in human plasma. Chem Pharm Bull. 1988;36:810–4. doi: 10.1248/cpb.36.810. [DOI] [PubMed] [Google Scholar]
- 8.Ishiwata K, Itou T, Ohyama M, Yamada T, Mishina M, Ishi K, et al. Metabolite analysis of [11C]flumazenil in human: Assessment as the standardized value for quantitative PET studies. Anals Nucl Med. 1998;12:55–9. doi: 10.1007/BF03165418. [DOI] [PubMed] [Google Scholar]
- 9.Ishiwata K, Hatazawa J, Kubata K, Kameyama M, Ito M, Matsuzawa T, et al. Metabolic fate of L-[methyl-11C]methionine in human plasma. Eur J Nucl Med. 1989;15:655–69. doi: 10.1007/BF00251681. [DOI] [PubMed] [Google Scholar]
- 10.Ishiwata K, Yanai K, Iwata R, Takahashi T, Hatazaowa J, Itoh M, et al. Analysis of plasma metabolites during human PET studies with three receptor ligands, [11C]YmM 09151-2, [11C]doxepin and [11C]pyrilamine. Tokohu J Exp Med. 1996;178:129–36. doi: 10.1620/tjem.178.129. [DOI] [PubMed] [Google Scholar]
- 11.Martin SJ, Eisenbarth JA, Wagner-Utermann U, Mier W, Henze M, Pritzkow H, et al. A new precursor for the radiosynthesis of [18F]FLT. Nucl Med Biol. 2002;29:263–73. doi: 10.1016/s0969-8051(01)00289-x. [DOI] [PubMed] [Google Scholar]
- 12.Alauddin MM, Conti PS, Fissekis JD. Synthesis of [18F]-labeled 2′-deoxy-2′-fluoro-5-methyl-1-β-D-arabinofuranosyluracil ([18F]FMAU) J Labelled Comp Radiopharm. 2002;45:583–90. [Google Scholar]
- 13.Alauddin MM, Conti P, Fissekis J. A general synthesis of [18F]-labeled 2′-deoxy-2′-fluoro-1-β-D-arabinofuranosyluracil nucleosides. J Labelled Comp Radiopharm. 2003;46:583–90. [Google Scholar]
- 14.Mukhopadhyay U, Soghomonyan S, Yeh H, Flores L, Shavrin A, Volgin AY, et al. N3-Substituted thymidine analogues V: Synthesis and preliminary PET imaging of N3-[18F]fluoroethyl thymidine and N3-[18F]fluoropropyl thymidine. Nucl Med Biol. 2008;35:697–705. doi: 10.1016/j.nucmedbio.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Currie Lloyd A. Limits for qualitative detection and quantitative determination: Application to radiochemistry. Anal Chem. 1968;40:586–593. [Google Scholar]
- 16.Sun H, Mangner TJ, Collins JM, Muzik O, Douglas K, Shields AF. Imaging DNA synthesis in vivo with [18F]-FMAU and PET. J Nucl Med. 2005;46:292–6. [PubMed] [Google Scholar]
- 17.Tehrani OS, Muzik O, Heilbrun LK, Douglas KA, Lawhorn-Crews JM, Sun H, et al. Tumor imaging using 1-(2’-deoxy-2’-18F-fluoro-β-D-arabinofuranosyl)thymine and PET. J Nucl Med. 2007;48:1436–41. doi: 10.2967/jnumed.107.042762. [DOI] [PubMed] [Google Scholar]
- 18.Shields AF, Grierson JR, Dohmen BM, Machulla H-J, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [18F]FLT and positron emission tomography. Nat Med. 1998;4:1334–1336. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 19.Kenny LM, Vigushin DM, Al-Nahash A, Osman S, Luthra SK, Shousha S, et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by 18F-fluorothymidine positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 2005;65:10104–112. doi: 10.1158/0008-5472.CAN-04-4297. [DOI] [PubMed] [Google Scholar]
- 20.Shields AF, Grierson JR, Muzik O, Stayanoff JC, Lawhorn-Crews JM, et al. Kinetics of 3-Deoxy-3-[F-18]Fluorothymidine Uptake and Retention in Dogs. Mol Imag Biol. 2002;4:83–89. doi: 10.1016/s1095-0397(01)00070-x. [DOI] [PubMed] [Google Scholar]
- 21.Visvikis D, Francis D, Mulligan1 R, Costa DC, Croasdale I, Luthra SK, et al. Comparison of methodologies for the in vivo assessment of 18FLT utilization in colorectal cancer. Eur J Nucl Med Mol Imaging. 2004;31:169–178. doi: 10.1007/s00259-003-1339-2. [DOI] [PubMed] [Google Scholar]


