Abstract
The nervous system relies on a highly specialized network of blood vessels for development and neuronal survival. Recent evidence suggests that both the central and peripheral nervous systems (CNS and PNS) employ multiple mechanisms to shape the vascular tree to meet its specific metabolic demands, such as promoting nerve-artery alignment in the PNS or the development the blood brain barrier in the CNS. In this article we discuss how the nervous system directly influences blood vessel patterning resulting in neuro-vascular congruence that is maintained throughout development and in the adult.
Keywords: Neuro-vascular development, vascular patterning, vessel ingression, blood brain barrier, arterial differentiation
1. Introduction
Blood vessels deliver oxygen and nutrients throughout the body, supporting organ development as well as metabolism and homeostasis. During embryogenesis, a primary capillary plexus undergoes extensive vascular remodeling and develops into a hierarchical vascular branching network. However, little is known about the anatomical template or environmental cues that control the highly stereotypic pattern of blood vessel branching in an organ-specific manner. The nervous system also forms a highly branched network reaching every organ in the body, and it relies on a dense network of blood vessels to supply oxygen and nutrients to meet its substantial metabolic demands, as well as neurotrophic factors for survival.
It has been recognized for hundreds of years that the vascular network closely associates with the neuronal network throughout development and in the adult. Among the many elaborate and intricate blood vessel patterning events taking place in the embryo, we focus on two vascular development models coordinated by the central and peripheral nervous systems (CNS and PNS). In this review, we discuss the cellular mechanisms and signaling pathways the CNS and PNS employ to directly interact with blood vessels, resulting in the formation of a specialized vascular network capable of supporting the nervous system. In the neural tube vascular patterning model, we discuss how CNS neural tube-derived signals regulate sprouting capillaries to form a stereotypic vessel ingression pattern (Figure 1) and acquire CNS vasculature-specific blood brain barrier (BBB) characteristics within the neural tube (Figure 2). In the limb skin vascular patterning model, we discuss how PNS nerve-derived signals direct arterial differentiation and patterns of angiogenic remodeling to establish the congruence of nerve and arterial vessel branching patterns (Figure 3). These two models demonstrate the action of neuronal signals on the formation of an architecturally complex vascular network.
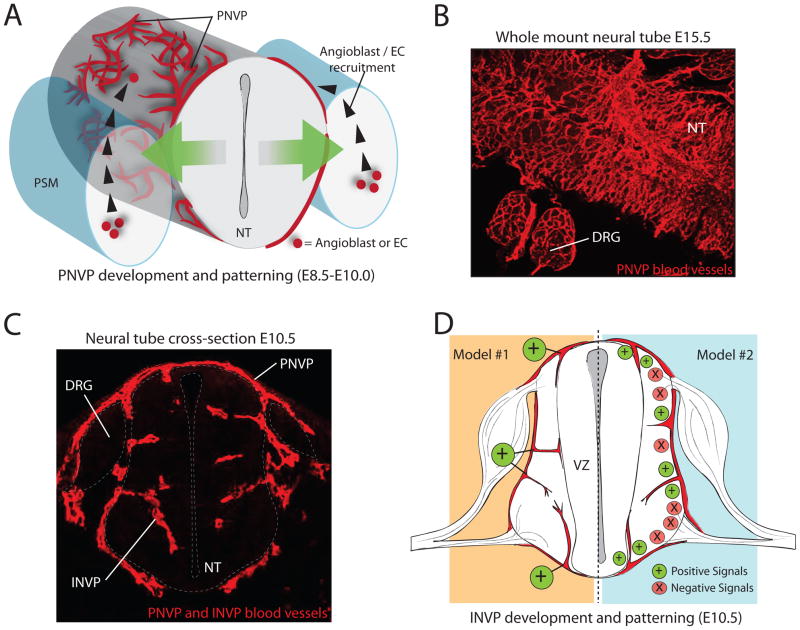
Figure 1. PNVP and INVP recruitment and patterning in the neural tube.
(A) Model of PNVP recruitment. At E8.5, angioblasts and ECs from the PSM respond to positive vessel patterning signals secreted from neural cells (green arrows) by differentiating, proliferating, and migrating to the surface of the neural tube. They surround the neural tube, forming a blood vessel plexus. (B) Immunofluorescence on a whole-mount, E15.5 NT (dorsal view), detecting blood vessels of the PNVP. PNVP vessels form a remodeled network on the surface of the NT and DRG. (C) Immunofluorescence on a cross-section of an E10.5 mouse embryo detecting both PNVP and INVP blood vessels. Blood vessels from the PNVP invade the neural tube at this stage, forming the INVP. (D) Two models for stereotypical vessel invasion during INVP formation. Model #1 depicts positive blood vessel patterning cues (such as matrix-binding VEGF-A) being localized to precise points of blood vessel invasion, whereas Model #2 depicts a balance of positive and negative blood vessel patterning cues to regulate the ingression pattern. Abbreviations: Peri-neural vessel plexus (PNVP), intra-neural vessel plexus (INVP), pre-somitic mesoderm (PSM), dorsal root ganglia (DRG), neural tube (NT), endothelial cell (EC), ventricular zone (VZ).
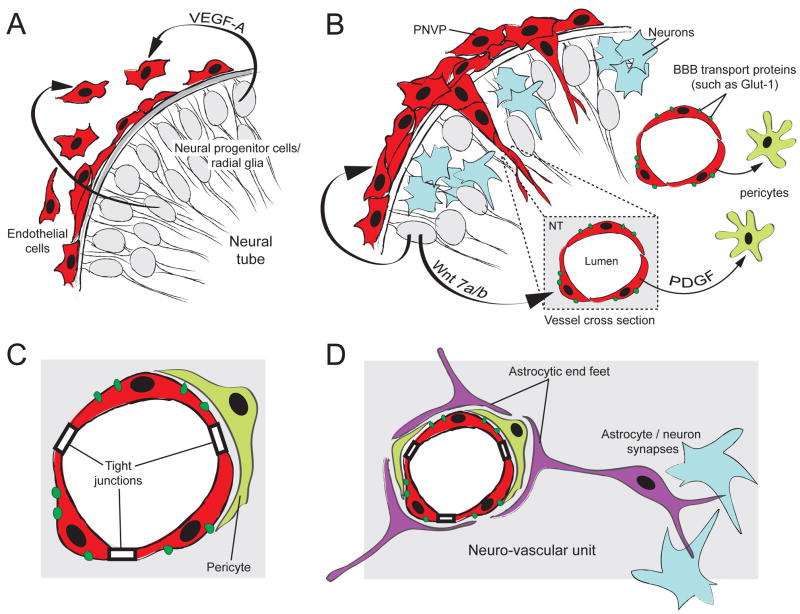
Figure 2. Model of early BBB and neuro-vascular unit formation.
(A) Neural progenitor cells (grey) secrete VEGF-A to recruit ECs to the surface of the neural tube. VEGF-A signaling from the neural tube is also required for subsequent blood vessel ingression into the neural tube (not shown). (B) At the onset of neurogenesis (differentiated neurons in blue), blood vessel sprouts originating from the PNVP invade the neural tube, forming the INVP. Wnt7 signaling is required for proper blood vessel ingression and induction of BBB-specific membrane transport proteins (such as Glut-1). Blood vessels recruit pericytes (light green) via the PDGF-B signaling pathway. (C) Pericyte recruitment is essential for stabilization of the BBB and for maintenance of tight junctions and Glut-1 expression. Pericytes are an important component of the neuro-vascular unit. (D) At the onset of gliogenesis, astrocytes (purple) differentiate and project cellular processes called end -feet to wrap around the CNS blood vessels. Astrocytes also form synaptic complexes with neurons. While some components of the BBB and the neuro-vascular unit develop before astrocytes differentiate, astrocytes are required for stabilization of the BBB and are critical components of the neuro-vascular unit.
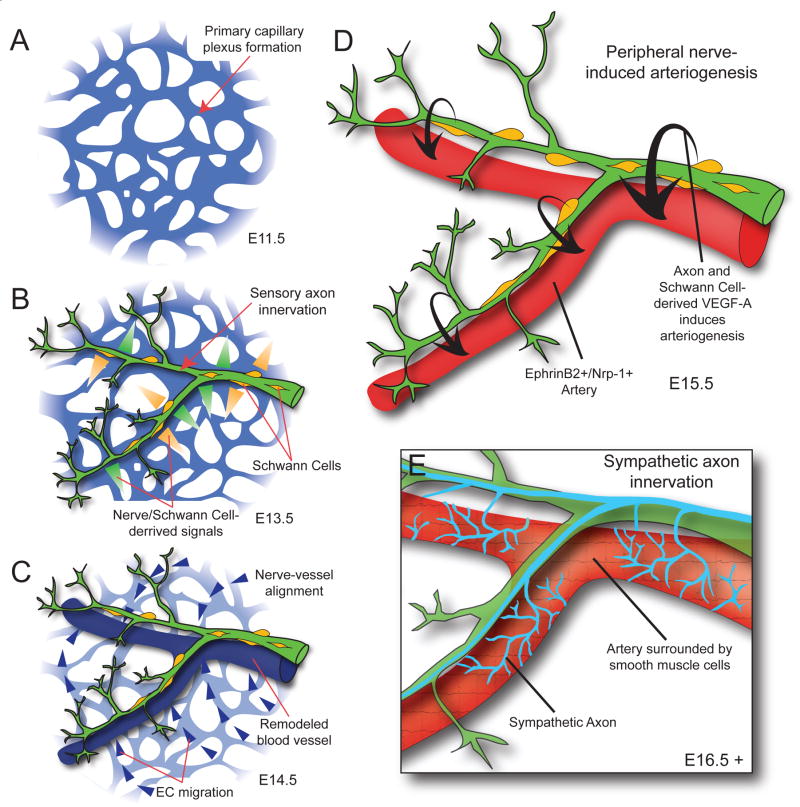
Figure 3. Model of blood vessel patterning by peripheral nerves in the developing limb skin.
(A) Blood vessels coalesce into a primary capillary plexus and begin to undergo remodeling by E11.5, before peripheral axon innervation in the limb. (B) Sensory axons, and associated Schwann cells, innervate the limb by E13.5 and secrete signals to pattern the primary vascular plexus. (C) In response to nerve and Schwann cell-derived signals, endothelial cells migrate toward and begin to align with peripheral nerves. (D) Blood vessels that have aligned with peripheral nerves undergo arteriogenesis in response to nerve and Schwann cell-derived VEGF-A. Aligned blood vessels upregulate arterial markers such as ephrinB2 and Nrp-1. Up-regulation of Nrp-1 is thought to increase endothelial cell response to VEGF-A signaling. (E) After sensory nerve-induced arteriogenesis is complete, sympathetic axons innervate the limb, utilizing the blood vessels and sensory nerves as a template for migrations. Sympathetic axons invade the arterial smooth muscle layer to regulate local control of vascular tone.
2. Blood vessel patterning in the developing spinal cord of the CNS
Vascularization of the embryonic spinal cord is crucial for CNS development and homeostasis. In early embryogenesis, there are no endothelial cells (ECs) or endothelial cell precursors (angioblasts) in the neuroectoderm, nor can cells within this tissue give rise to ECs [1–4]. Almost as soon as the anlagen CNS forms a tube, it begins communicating with the surrounding mesodermal tissue—where angioblasts and endothelial cells reside. In this narrow developmental window (E8.5–E10.0 in mouse or Day2–4 in avian embryos), the neural tube recruits angioblasts and ECs to coalesce into a ring of vessels, known as the peri-neural vessel plexus (PNVP) (Figure 1A, B). This is the first blood vessel patterning process coordinated by the neural tube of the CNS. As neural development proceeds within the neural tube, blood vessels invade the neuroectoderm via sprouting angiogenesis—forming an intra-neural vessel plexus (INVP) (Figure 1C). This is the second major blood vessel patterning event coordinated by the CNS. Both the PNVP and INVP acquire specific properties unique to CNS vasculature that form the blood brain barrier (BBB) [5–8] (Figure 2).
2.1 Formation of the peri-neural vessel plexus (PNVP)
The neural tube was identified as the source tissue for positive blood vessel patterning signals capable of inducing EC migration and directing PNVP formation. Ectopically grafted, mouse-derived neural tubes in avian hosts recruited a PNVP, whereas grafted acrylic beads, or notochords (an embryonic midline patterning structure) did not [9]. Furthermore, analysis of Tbx6 mutant mouse embryos, with defects in paraxial mesoderm specification resulting in the formation of multiple neural tubes lateral to the endogenous neural tube, revealed that these ectopic neural tubes also have the ability to recruit PNVPs [9, 10]. These experiments clearly demonstrate that the neural tube is the source of a diffusible blood vessel patterning signal coordinating PNVP formation. Furthermore, the ability of the neural tube to pattern a PNVP is not context dependent, as ectopic neural tubes induced patterning of ECs in multiple vascular beds.
2.2 Role of neural tube-derived VEGF-A in PNVP formation
A major component of the neural tube-derived signal is Vascular Endothelial Growth Factor-A (VEGF-A) (Figure 2A). VEGF-A is the most ubiquitous and potent angiogenic factor known, and has well-established pro-angiogenic effects on ECs and blood vessel patterning during development [11]. VEGF-A expression analysis in a LacZ reporter strain showed that VEGF-A was up-regulated in the neural tube just prior to PNVP recruitment and its expression became localized to the outer, pial surface of the neural tube as the PNVP developed [9]. Explanted mouse pre-somitic mesodermal tissue, a known source of angioblasts and ECs that colonize the PNVP in vivo, formed a blood vessel plexus when cultured in a collagen matrix either with VEGF-A protein or avian neural tubes; however, this plexus did not form when PSM is cultured with neural tubes in the presence of VEGF-A inhibitors [9]. This experiment demonstrated that the neural tube stimulates blood vessel plexus formation, and this effect is dependent on VEGF-A signaling.
VEGF-A modulates blood vessel patterning in several major ways. Proper VEGF-A expression levels in the developing embryo are required for embryonic survival. Inactivation of one vegf-a allele resulted in embryonic lethality due to cardiovascular defects in mice between E11–12 [12, 13]. Conversely, global increases in VEGF-A expression, produced by insertion of a modified vegf-a gene into the endogenous locus, resulted in embryonic lethality due to cardiovascular defects and vessel overgrowth at E12.5 [14]. Reduction of CNS-derived VEGF-A in mice resulted in early postnatal lethality, reduced blood vessel density in the brain, increased neuronal apoptosis, and degeneration of the cerebral cortex [15, 16]. These studies did not analyze effects on PNVP formation; however, there is evidence to suggest that altered CNS-derived VEGF-A levels can perturb PNVP patterning. Avian neural tubes electroporated with human vegf-a cDNA in a gain of function experiment, exhibited an increase in PNVP vessel thickness [17]. Neural tubes electroporated with high amounts of sflt1 transgene, a soluble flt1 (also known as vegfr1) to trap VEGF-A, resulted in missing sections of the PNVP or thinner PNVP vessels in some instances (J. M. James, unpublished data).
VEGF-A can also act both as a long-range and short-range vessel patterning cue to locally guide vessel sprouts [18–20]. It achieves this signaling range because it is alternatively spliced into at least 6 isoforms, three of which are abundantly expressed in the mouse CNS: VEGF120, VEGF164, and VEGF188 [21]. Each isoform interacts differently with the extracellular matrix via heparin binding sites [22]. VEGF188 has two heparin-binding sites and is the least soluble, providing short-range or precise vessel patterning, while VEGF120 has no matrix-binding sites, is diffusible, and can provide long-range vessel pattering signals. VEGF165 has one heparin-binding site and maintains intermediate properties [23]. Mice expressing only one of the three major isoforms had intraneural vessel patterning defects (refer to section 2.4); however, PNVP formation around the spinal cord and hindbrain regions of the neural tube appeared normal in these mutants (J. M. James, unpublished data, [19]). Taken together, the level of neural tube-derived VEGF-A is important for the PNVP formation whereas matrix-binding properties of VEGF-A may not be required for the recruitment of ECs and angioblasts to form the PNVP.
2.3 Formation of the intra-neural vessel plexus (INVP)
The PNVP begins forming approximately one day before angiogenic sprouts invade the neural tube. Vessel sprouts entering the neural tube do so in highly stereotypical locations (Figure 1C, D) [2, 4, 5, 24]. In the developing chick embryo, sixteen distinct blood vessels form reproducibly along the dorsal-ventral axis (transverse plane) of the neural tube before the neural tube becomes uniformly vascularized. Each vessel forms along both spatial and temporal axes, and earlier vessels act as a scaffold onto which subsequent vessels can anastomose with or sprout from [24]. These patterning events take place at regular intervals along the length of the chick neural tube. The discovery that a hierarchal system of intraneural blood vessel growth and patterning exists, suggests that neural tube angiogenesis is highly regulated by neural tube-derived signals.
Many signaling molecules with known roles in vascular patterning are expressed in the neural tube. Perhaps it is best to think of signals regulating INVP development as layers of complexity. In this section, we first discuss how neural tube-derived VEGF-A is important for blood vessel ingression, proper vascular density, and fine–tuning of the blood vessel pattern. Second, recent evidence shows that Wnt subfamily members promote acquisition of BBB characteristics in intra-neural vessels. Lastly, neural cells associate with blood vessels to develop neuro-vascular units, which function to pattern and stabilize the INVP, as well as maintain the integrity of the BBB. Each signaling layer builds on the one before it, giving us an overall picture of how a reproducible blood vessel pattern is achieved and maintained within the neural tube.
2.4 Role of neural tube-derived VEGF-A in INVP formation
In the avian spinal cord, inhibition of VEGF-A-signaling via electroporation of a soluble flt1 transgene (sflt1) resulted in an almost complete lack of vessel invasion [17]. Sequestering VEGF-A or inhibiting VEGF-A signaling in vitro and in vivo also profoundly disrupted INVP formation in dorsal root ganglia (DRG) of the PNS, indicating that VEGF-A signaling is crucial for INVP formation in both the CNS and PNS [25, 26]. Genetic studies in mice that express a single VEGF-A isoform provided evidence that VEGF-A isoform expression influences the INVP pattern. Vegf120/120 neural tubes displayed delayed ingression and reduced sprout number. Vegf188/188 neural tubes had hyper-branched, thin vessels, while vegf165/165 mutants had phenotypically normal blood vessels (J. M. James, unpublished data). These observations are consistent with reports describing vessel branching and morphogenesis defects within the hindbrain as well as globally in the isoform mutant mice [19, 20]. In avian neural tubes, over-expression of matrix-binding VEGF-A resulted in supernumerary and ectopic vessel sprout formation, whereas over-expression of soluble, non-matrix-binding VEGF-A did not [17]. These results suggest that matrix-binding VEGF-A can give precise patterning information to the blood vessels, directing vessel ingression patterns, and that matrix-binding VEGF-A partially directs the exact timing of blood vessel invasion into the neural tube.
Neuropilin-1 (NRP1) is also important for mediating isoform-specific VEGF-A signaling. NRP1, a co-receptor for VEGF-A, is expressed in ECs and has been shown to enhance VEGF-Flk1 interactions in vitro by forming a receptor complex with Flk1 (also known as VEGFR2) [27]. NRP1 is also highly expressed on neuronal axons and acts as a co-receptor for Semaphorin molecules, forming a complex with Plexin receptors [28]. Although NRP1 can bind all three major VEGF-A isoforms, VEGF120 is too small to bridge the gap between Flk1 and NRP1 [29], thus precluding complex formation. The lack of the Flk1-NRP1-VEGF complex is thought to partially account for the severity of blood vessel defects in the vegf120/120 mice. Conventional nrp1 mutant mice displayed normal blood vessel ingression and blood vessel density within the embryonic hindbrain, however there were defects in lateral blood vessel branching as vessel sprouts interface the ventricular zone [30]. Endothelial-specific deletion of nrp1 had a slightly different effect, resulting in the formation of large, un-branched vessels within the neural tube [31], similar to the vessel phenotype in vegf120/120 mutants. Taken together, VEGF-A/NRP1 signaling in ECs is important for proper INVP formation in the CNS.
Though much is known about the importance of VEGF-A signaling in proper development of the CNS vasculature, we still do not know if matrix-binding VEGF-As are localized to precise points of blood vessel ingression and branching, thus directing intricate vessel patterning (Figure 1D, model #1). Another possible mechanism to explain stereotypic blood vessel ingression is that VEGF-A-mediated positive spatial signals are balanced by negative spatial signals that prevent blood vessel ingression from the PNVP (Figure 1D, model #2). Since ectopic expression of sFlt-1 inhibited INVP formation in the neural tube [17] and vessel-derived sFlt-1 was required for local sprout guidance [32], local sFlt1 may neutralize VEGF-A secreted from the neural tube to inhibit vessel ingression.
Recent studies demonstrated that molecules regulating axonal guidance and patterning also regulate blood vessel patterning, and the tip cell of a growing vessel acts much like an axonal growth cone [33, 34]. Repulsive axon guidance molecules may coordinate with VEGF-A signaling to pattern blood vessel ingression in the neural tube. Netrin signaling through UNC5 receptor homodimers or UN C5-DCC receptor heterodimers provides repulsive cues to ECs [35, 36]. Netrins inhibited VEGF-A-induced EC migration in vitro and VEGF-A-driven angiogenesis in vivo. Semaphorin 3 (Sema3) subfamily members are expressed in the neural tube and also act as repulsive cues in axon guidance and neuronal cell migration [37], as well as EC migration. Sema3s signal through Plexin/NRP1 receptor complexes expressed on axons and ECs. Given that NRP1 acts as a VEGF-A and a Sema3 co-receptor, the inhibitory effect of Sema3s on angiogenic sprouting and EC migration was attributed to Sema3/VEGF-A competition for NRP1 binding [38–40]. However, recent biochemical studies demonstrated that Sema3A and VEGF-A bind to distinct domains of NRP1 [41, 42] and induce NRP1 endocytosis through different pathways, suggesting that these NRP1 ligands function separately, rather than competitively. An alternative molecular mechanism for explaining the effects of Sema3s on vascular development is suggested by the endothelial expression of plxnd1 in both mouse and zebrafish embryos. Paracrine Sema3 (Sema3E in mouse) signals from the somites blocked ingression of plexinD1 expressing ECs from the dorsal aorta via a repulsive mechanism, resulting in the patterned segmental vasculature between somite boundaries [43]. An intriguing idea is that these repulsive guidance signals in combination with a VEGF-A-mediated positive guidance signal control the precise vessel ingression pattern in the neural tube (Figure 1D, model #2). However, the requirement for axon guidance signals in neuronal development makes it difficult to distinguish whether defective INVP formation and patterning in mutants lacking these guidance genes is due to the genetic perturbation of the guidance cues to ECs or to secondary effects from a disruption of neuronal patterning. To circumvent this problem, EC-specific deletion of cell-autonomous components of these signaling pathways is required.
2.5 Role of neural tube-derived Wnts in INVP formation
The canonical Wnt signaling pathway regulates crucial aspects of cardiovascular development [44]. Wnt7a and Wnt7b are highly expressed by the embryonic spinal cord, whereas canonical Wnt signaling is active in both PNVP and INVP ECs. The CNS-specific deletion of wnt7a and wnt7b as well as EC-specific deletion of β-catenin in mice, resulted in severe CNS-specific hemorrhage due to dilated PNVP and defective INVP ECs and pericytes in the neural tube [45, 46]. These results indicated that canonical Wnt signaling in ECs is important for PNVP integrity and blood vessel ingression to form the INVP (Figure 2B).
CNS-specific hemorrhage observed upon CNS ablation of wnt7a and wnt7b as well as EC-specific ablation of β-catenin highlights the role of canonical Wnt signaling in BBB development. The BBB prevents movement of molecules and ions between blood circulation and the CNS. CNS ECs are joined at their apical surface by a belt-like ring of tight junctions to prevent para-cellular transport. Transplantation studies suggested that BBB formation is not intrinsic to CNS ECs. Instead, the CNS directly induces the BBB characteristics in ECs by interaction with the CNS cells such as neural progenitors during early developmental stages [47]. Wnt7 subfamily signaling is sufficient to induce BBB characteristics in ECs based on Wnt7a induction of Glut1 (a glucose transporter) and other BBB-specific influx transporters in cultured ECs and ectopic Wnt7a induction of Glut1 in non-CNS vasculature in mice carrying a wnt7a transgene [45, 46] (Figure 2B). As expected, both neuronal wnt7a/7b mutants and endothelial β-catenin mutants had reduced expression of the BBB-specific influx transporter in the PNVP and INVP [46].
An important question for future studies is how canonical Wnt signaling interacts with other signaling pathways like the VEGF-A pathway to control PNVP and INVP formation in the neural tube. Canonical Wnt signaling appears to have dual functions in patterning the CNS vasculature: one in regulating blood vessel ingression and also a role in BBB development. The gain-of-function manipulation of wnt7a induced a profound effect on Glut1 induction in non-CNS vasculature, but did not display ectopic or enhanced vessel ingression, although the loss of function manipulation of both wnt7a and wnt7b had severe defects in vessel ingression [46]. These observations raise an intriguing possibility that Wnt7s may be permissive signals, required for VEGF-A-mediated vessel ingression to proceed normally, whereas Wnt signaling is instructive for acquisition of BBB characteristics in ECs.
2.6 Establishment of neuro-vascular units for BBB development
ECs forming the BBB require interactions with other cell types in the CNS parenchyma such as pericytes (PCs), neurons, and astrocytes in order to maintain blood vessel integrity and BBB characteristics (Figure 2C, D). Collectively, these cell complexes are called neuro-vascular units. Disruption of these units leads to mis-pattering of the CNS blood vessels and severe intra-neural hemorrhage. One major mechanism regulating formation of functional neuro-vascular units is integrin-mediated, TGFβ signaling. Three TGFβ isoforms (TGFβ1, TGFβ2, TGFβ3) are secreted from cells in an inactive form, associating with the Latent Associated Peptide (LAP). Integrins are transmembrane cell-adhesion proteins, composed of an alpha (α) and beta (β) subunit, that are required to cleave the LAP from latent TGFβ. Within the context of the developing CNS vasculature, TGFβ1 and TGFβ3 activation by αv/β8 integrin is required for proper neuro-vascular development. Both TGFβ and αv/β8 integrin are expressed by radial glial cells as ECs begin to invade the neural tube. Mice carrying mutations in both tgfβ1 and tgfβ3 genes developed cerebral hemorrhages from E11.5 and subsequent embryonic lethality [48, 49]. Interestingly, mice with mutations in αv or β8 integrin also developed cerebral hemorrhages [50, 51]. These studies strengthen the evidence supporting a critical role for TGFβ signaling in neuro-vascular unit formation; however, which components in the neuro-vascular unit are influenced by TGFβ signaling remains unclear.
PCs are recruited to the endothelium during vascular development. Evidence from in vitro experiments as well as genetic studies in mice has suggested that while TGFβ signaling appears to be critical for early PC differentiation, platelet-derived growth factor (PDGF)/PDGF receptor-β (PDGFR-β) signaling seems to be involved in later proliferation and migration of these cells (Figure 2B). Because the EC-specific pdgfb mutant exhibited reduced PC recruitment and brain microhemorrhages [52], this suggests that proper PC recruitment is important for the formation of the neuro-vascular unit. Recent exciting studies suggest that PC recruitment to CNS vasculature, prior to astrocyte development, is necessary for the BBB formation [53, 54]. PCs strengthen EC barrier formation through regulation of tight junction structure during embryogenesis (Figure 2C). While much is known about the cellular dynamics of PC recruitment and function in the neuro-vascular unit, the origin and specification of PCs in the CNS vasculature remain elusive.
Here we propose one intriguing model to explain CNS vascular development: ECs are recruited by neural tube-derived VEGF-A to form the PNVP (Figure 2A). PNVP ECs are induced to express BBB specific genes, such as Glut1, by interaction with CNS cells such as neural progenitors. This process seems to be largely regulated by canonical Wnt signaling (Figure 2B). Further, functional EC integrity to form the BBB is regulated by PCs and subsequently other components of the neuro-vascular unit such as astrocytes (Figure 2C, D). Stereotypic patterning of the INVP is regulated by a combination of positive spatial cues (VEGF-A and Wnt7a/7b) and unidentified negative spatial cues (Figure 1D).
Communication between blood vessels and CNS tissues (e.g. neural tube) during early embryonic development is crucial for the formation of CNS vasculature. The potential for therapeutic manipulation in instances of BBB impairment such as multiple sclerosis (MS), hereditary hemorrhagic telangiectasia (HHT), and brain tumors, underscores the need to better understand what signaling pathways regulate the CNS vasculature during development.
3. Blood vessel patterning by peripheral nerves
Both blood vessels and nerves develop intricate branching networks that invade almost every organ in the body. The two networks share several anatomical and functional characteristics and are often patterned similarly in peripheral tissues. This parallel pattern of blood vessels and nerves raised a fundamental question about whether their branching patterns are established independently or coordinately. Does the branching pattern of peripheral nerves determine that of blood vessels, or vice versa? Is the branching pattern of both blood vessels and nerves controlled by shared guidance cues from the surrounding tissues? Here we focus on our developing limb skin vascular patterning model that illuminates the developmental events in neuronal control of blood vessel patterning.
3.1 Control of arterial branching patterns by peripheral nerves
Innervation occurs later than vascular formation in tissues (Figure 3A, B). The vascular system develops by two processes: vasculogenesis and angiogenesis. In vasculogenesis, endothelial precursor cells called angioblasts arise within the lateral mesoderm and then differentiate and coalesce to form the primary vascular plexus; angiogenesis then promotes sprouting and remodeling of this primitive vascular network to form a well-defined hierarchical branching network. Timing of innervation in peripheral tissues depends on the neuronal subtype. Sensory and sympathetic neurons arising from the neural crest extend their axons from dorsal root ganglia and sympathetic ganglia, respectively; motor neurons from the ventral spinal cord project their axons to the periphery. Axon projection over large distances to reach distal target tissues in a highly stereotypic manner is orchestrated by precise environmental cues including axon guidance molecules (netrins, semaphorins, ephrins and Slits) and growth factors like neurotrophins. In the embryonic limb skin, sensory and motor neurons invade at approximately E13.5, after a primary capillary plexus is established (Figure 3A, B). Subsequently, the pattern of sensory/motor axons provides a spatial template for the pattern of arterial vessel branching (Figure 3C, D), whereas post-ganglionic sympathetic axon innervation of the autonomic nervous system occurs along remodeled arteries at a later stage, after the establishment of a hierarchical arterial vascular network (Figure 3E) [55]. As a result, the congruence of blood vessel and nerve patterns is established in developing skin (Figure 4A–D) and is maintained in the adult (Figure 4E–H). Functionally, the congruence of these patterns facilitates efficient delivery of oxygen, nutrients and survival factors for nerves, and importantly, mediates sympathetic axon innervation of arterial smooth muscles for local control of vascular tone.
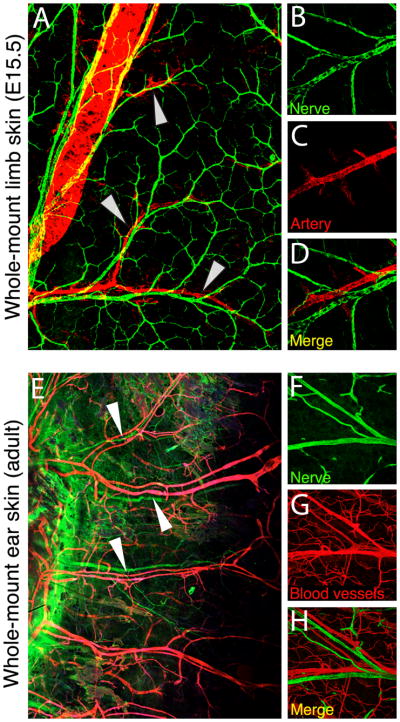
Figure 4. Neuro-vascular congruence in the embryonic and adult skin.
(A–E) Immuno-fluorescence detecting arteries (red) and nerves (green) in whole-mount limb skin preparations from E15.5 mouse embryos (A, 10X and B–D, 20X magnification). Nerves and vessels closely align (arrowheads). (E–H) Immuno-fluorescence detecting blood vessels (red) and nerves (green) in whole-mount ear skin preparations from adult mice (E, 10X and F–H, 20X magnification). Nerve-vessel alignment is maintained in the adult (arrowheads).
Genetic studies in mice explained how the congruence of blood vessel and peripheral nerve patterns is established in the embryonic limb skin. Mutant mice (neurogenin1/neurogenin2 double mutants) lacking peripheral sensory (and motor) axons and associated Schwann cells, displayed altered patterning of remodeled vessels and defective arterial differentiation. Hierarchal blood vessel branching was disrupted, though remodeling of some large and small vessels did occur. Further, arterial marker expression in branched vessels was greatly diminished in these mutants [55]. In sema3a mutants with abnormal peripheral nerve patterning in the limb skin, remodeled arteries were also re-routed. Branched vessels aligned with the mis-patterned nerves and began to express arterial markers. These results demonstrated that the peripheral nerve branching pattern directs blood vessel patterning and arterial differentiation. These results also rule out the possibility that limb skin blood vessels and nerves pattern independently through a shared patterning mechanism such as Sema3A signaling. If Sema3A or other guidance molecules control the branching of arteries independently of nerves, one would expect that in the absence of nerves the pattern of blood vessel branching would remain normal. Since the mutant lacking such nerves displayed defective vascular branching morphogenesis, this is clearly not the case. Taken together, the congruence of blood vessels and peripheral nerve branching patterns in the limb skin vasculature model is established through nerve-derived signals.
3.2 Role of nerve-derived VEGF-A in arterial differentiation
Mutant mice (erbB3 mutants) that do not develop Schwann cells displayed defects in nerve-vessel alignment as well as arterial differentiation, indicating that these cells also provide signals to direct arterial differentiation and patterning of arterial branching. VEGF-A is highly expressed in peripheral nerves in the embryonic skin. Evidence from in vitro culture experiments suggested that VEGF-A derived from DRG sensory neurons or Schwann cells could induce expression of arterial markers such as ephrinB2 and NRP1 in isolated ephrinB2-negative ECs, independently of its ability to induce proliferation or cell survival. However, this effect was diminished by exposure to a soluble VEGF receptor. VEGF-A also up-regulates the expression of NRP1, which is specific for arterial ECs [25, 55]. NRP1 appears to amplify VEGF-A-mediated arterial differentiation, perhaps due to increased Flk1-NRP1-VEGF signaling.
Genetic ablation of vegf-a in the peripheral nerves and nrp1 in ECs resulted in the lack of arterial differentiation [25]. These studies strongly suggested that nerve-derived VEGF-A is required for arterial differentiation in the limb skin in vivo. Consistent with these findings, mice carrying a vegf164 transgene driven by a myosin heavy chain promoter, increased ephrinB2+ arterial vascular networks and concurrently decreased EphB4+ venous vascular networks in the heart [56]. Injection of vegf-a mRNA in zebrafish embryos induced ectopic arterial marker expression in the posterior cardinal vein [57]. These data provide strong evidence that VEGF-A signaling promotes arterial differentiation by inducing arterial specific genes in ECs.
There is an interesting question about whether the process of arterial differentiation is instructed or selected by nerve-derived VEGF-A. In culture, at most 50% of isolated ephrinB2-negative ECs can be induced to express arterial markers by VEGF-A or by co-culture with DRG neurons or Schwann cells [55]. Exposure to a soluble form of Delta, a Notch ligand that also induces ephrinB2 in vitro (Y. Mukouyama, unpublished data) as well as in vivo [57, 58], produces no further increase in the proportion of cells expressing arterial markers. One interpretation of these observations is that additional signals such as hemodynamic force and oxygen tension might be required to instruct arterial differentiation of uncommitted cells. Indeed, experimental evidence indicated that prolonged exposure to shear stress both in vitro and in vivo drives arterial differentiation by up-regulating arterial markers, such as ephrinB2, and by down-regulating venous markers, such as EphB4 [59, 60]. Alternatively, ephrinB2-negative ECs may be heterogeneous and ~50% of the cells are pre-specified for an arterial fate and can express arterial markers in response to VEGF-A. The other cells that do not respond to VEGF-A are not determined for an arterial fate, or they may be committed to a venous fate. Further studies are needed to determine whether there are intrinsic differences between the VEGF-A-responsive and non-responsive EC subpopulations.
Intriguingly, and somewhat unexpectedly, blood vessels still remodeled and aligned with peripheral nerves in conditional knockout mice lacking vegf-a in the peripheral nerves or nrp1 in ECs [25]. This indicated that nerve-derived signals other than VEGF-A exist to coordinate vascular remodeling and nerve-vessel alignment.
3.3 Role of Nerve-derived signals to control the vascular branching pattern
Emerging evidence has shown that blood flow is critical for vascular remodeling and subsequent branching network formation. Indeed, mutant mice lacking either sensory nerves or Schwann cells have remodeled vasculature, but they fail to develop nerve-vessel alignment and arterial characteristics in the limb skin. Thus, nerve-derived signals are required for alignment but not for vascular remodeling in the skin.
Why do only a subset of vessels become aligned with nerves during vascular remodeling and subsequently undergo arterial differentiation? In the instruction model, all primary capillary vessels are initially able to become aligned with nerves and the alignment is simply determined by their initial proximity to nerves at the time of innervation. Nerve-derived VEGF-A promotes arterial differentiation and NRP1 amplifies the VEGF-A effect (Figure 3D). Alternatively, in the selection model, a subset of vessels may be pre-specified to align with nerves due to the selective expression of a receptor for a nerve-derived alignment signal. Since alignment favors VEGF-A-induced arterial differentiation, this mechanism would ensure that nerves are always associated with arteries. Further studies to identify nerve-derived signals for vessel alignment will help to distinguish these two models.
We have very recently discovered that the nerve-derived C-X-C motif chemokine ligand (CXCL)12 (also known as SDF-1) regulates nerve-vessel alignment (W. L., manuscript in submission). CXCL12 is highly expressed in migrating Schwann cells in the peripheral nerves, whereas its receptor CXCR4 is expressed by a subset of vessels in the primary capillary plexus. Interestingly, mutants lacking either cxcl12 or cxcr4 exhibit disruption of arterial vessel remodeling and nerve-vessel alignment. Since these mutants exhibit an almost identical phenotype in nerve-vessel alignment and arterial differentiation to erbB3 mutants lacking Schwann cells in the peripheral nerves [55], Schwann cells appear to be a source of the alignment signal for the arterial vascular network. In general, G-protein-coupled receptors like CXCR4 interact with G-proteins to control the intracellular signal transduction events that regulate cell migration [61]. Therefore, CXCL12/CXCR4 signaling may be central to the cellular mechanisms of vascular branching networks. Further studies will reveal the dynamic cellular and molecular events underlying the nerve-mediated arterial branching network.
3.4 Remaining questions in neuro-vascular congruence
We have provided evidence of the physical proximity and cooperative patterning of the developing peripheral nerves and arterial vasculature. However, what controls venous differentiation and patterning of venous branching still remains to be answered. Recent genetic studies in mice and zebrafish suggest that COUP-TFII and PI3K/Akt signaling suppress Notch activation to promote venous cell fate (reviewed in [62]). Furthermore, in early vascular development in zebrafish, BMP signaling mediates venous sprouting from the axial vein independently of VEGF-A signaling [63]. More investigation is required to properly establish venous specification, differentiation and patterning during mammalian development.
In this review, neuro-vascular congruence is mainly indicated by the responsiveness of vascular development to signals secreted by peripheral sensory nerves in the developing skin. Interestingly, however, a reciprocal guidance event is observed in the patterning of peripheral sympathetic nerves. Vessel-derived guidance cues direct the pattern of neuronal innervation. Arterial vascular smooth muscle cells (VSMCs) secrete factors that stimulate sympathetic axon extension along arteries (reviewed in [64]). Sympathetic neurons from the superior cervical ganglia extend axons along the external carotid arteries en route to final target tissues. In this case, arterial VSMCs secrete endothelins to mediate sympathetic axon growth [65]. Artemin, a member of the GDNF family of ligands, and NT-3, a neurotrophic factor are also expressed by arterial VSMCs and act as guidance signals that stimulate sympathetic axon extension along arteries [66, 67]. Future efforts will establish additional molecules that act as guidance cues to control pattern of sympathetic axons.
4. Concluding remarks
Our understanding of early neuro-vascular development has advanced in recent years on two major fronts. First, within the CNS vasculature model, a growing body of work has demonstrated that neural tube-derived signals regulate sprouting angiogenesis, forming a stereotypic pattern of CNS vasculature with BBB characteristics. Second, within the limb skin vasculature model, nerve-derived signals regulate arterial differentiation and vascular branching in peripheral tissues resulting in nerve-artery alignment. These vasculature models provide insights into understanding how blood vessels in different tissues acquire specialized branching patterns and properties. However, major questions remain unanswered. For example, how are these specialized interactions maintained in the adult? And how to they become deficient in instances of injury or the development of disease? We know that diseases of the vascular system can also affect the nervous system, and vice versa; however, specific mechanisms remain to be determined. In the coming years, we hope to identify more genes important for neuro-vascular development in the embryo, but also develop adult model systems of neuro-vascular congruence (such as the adult ear skin mod el) to begin to answer these challenging questions.
Highlights.
Neural tube-derived signals regulate sprouting capillaries to form a stereotypic vessel ingression pattern.
Neural tube-derived signals induce blood brain barrier characteristics in the neural tube vasculature.
Peripheral nerve-derived signals direct arterial differentiation in the developing limb skin.
Peripheral nerve-derived signals regulate patterns of angiogenic remodeling to establish nerve-artery alignment.
Acknowledgments
We thank W. Li for providing unpublished results, K. Perkins for providing unpublished images, and K. Gill for laboratory management. Thanks also to R. Adelstein and M. Conti for their helpful comments and editorial advice on the manuscript, and members of Laboratory of Stem Cell and Neuro-Vascular Biology for thoughtful discussion. None of the authors have any financial or other conflicts of interests. This work was supported by the Intramural Research Program of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart PA, Wiley MJ. Structural and histochemical features of the avian blood-brain barrier. J Comp Neurol. 1981;202:157–167. doi: 10.1002/cne.902020203. [DOI] [PubMed] [Google Scholar]
- 2.Nakao T, Ishizawa A, Ogawa R. Observations of vascularization in the spinal cord of mouse embryos, with special reference to development of boundary membranes and perivascular spaces. Anat Rec. 1988;221:663–677. doi: 10.1002/ar.1092210212. [DOI] [PubMed] [Google Scholar]
- 3.Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097–1103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- 4.Kurz H, Gartner T, Eggli PS, Christ B. First blood vessels in the avian neural tube are formed by a combination of dorsal angioblast immigration and ventral sprouting of endothelial cells. Dev Biol. 1996;173:133–147. doi: 10.1006/dbio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 5.Bar T. The vascular system of the cerebral cortex. Adv Anat Embryol Cell Biol. 1980;59(I–VI):1–62. doi: 10.1007/978-3-642-67432-7. [DOI] [PubMed] [Google Scholar]
- 6.Risau W, Hallmann R, Albrecht U, Henke-Fahle S. Brain induces the expression of an early cell surface marker for blood -brain barrier-specific endothelium. EMBO J. 1986;5:3179–3183. doi: 10.1002/j.1460-2075.1986.tb04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risau W, Hallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood -brain barrier. Dev Biol. 1986;117:537–545. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- 8.Risau W, Wolburg H. Development of the blood -brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- 9.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- 10.Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 11.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 14.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 15.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 16.Raab S, Beck H, Gaumann A, Yuce A, Gerber HP, Plate K, et al. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 17.James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P, Ng YS, Nuyens D, Theilmeier G, Brusselmans K, Cornelissen I, et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat Med. 1999;5:495–502. doi: 10.1038/8379. [DOI] [PubMed] [Google Scholar]
- 19.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, et al. Spatially restricted patterning cues provided by heparin -binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 23.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feeney JF, Jr, Watterson RL. The development of the vascular pattern within the walls of the central nervous system of the chick embryo. J Morphol. 1946;78:231–303. doi: 10.1002/jmor.1050780205. [DOI] [PubMed] [Google Scholar]
- 25.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 26.Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18:1952–1954. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- 27.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin -1 is expressed by endothelial and tumor cells as an isoform -specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 28.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 29.Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, et al. Neuropilin -1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- 30.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 31.Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2(5):a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 35.Larrivee B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428–441. doi: 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- 36.Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagri A, Tessier-Lavigne M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv Exp Med Biol. 2002;515:13–31. [PubMed] [Google Scholar]
- 38.Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes Semaphorin3A and Neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 39.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin -1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoji W, Isogai S, Sato-Maeda M, Obinata M, Kuwada JY. Semaphorin3a1 regulates angioblast migration and vascular development in zebrafish embryos. Development. 2003;130:3227–3236. doi: 10.1242/dev.00516. [DOI] [PubMed] [Google Scholar]
- 41.Appleton BA, Wu P, Maloney J, Yin J, Liang WC, Stawicki S, et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and VEGF binding. EMBO J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, et al. Vascular endothelial growth factor and semaphorin induce neuropilin -1 endocytosis via separate pathways. Circ Res. 2008;103:71–79. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gay CM, Zygmunt T, Torres-Vazquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev Biol. 2011;349(1):1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parmalee NL, Kitajewski J. Wnt signaling in angiogenesis. Curr Drug Targets. 2008;9:558–564. doi: 10.2174/138945008784911822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 47.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood -brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 48.Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122:227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Enge M, Bjarnegård M, Gerhardt H, Gustafsson E, Kalén M, Asker N, et al. Endothelium-specific platelet-derived growth factor-beta ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood -brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 55.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 56.Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci U S A. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 58.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 59.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 60.Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, et al. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 2009;106:203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 61.Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 63.Wiley DM, Kim JD, Hao J, Hong CC, Bautch VL, Jin SW. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol. 2011;13(6):686–692. doi: 10.1038/ncb2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 65.Makita T, Sucov HM, Gariepy CE, Yanagisawa M, Ginty DD. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35(2):267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- 67.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]