Abstract
Neuroimaging studies have elucidated some of the underlying physiology of spontaneous and voluntary eye blinking; however, the neural networks involved in eye blink suppression remain poorly understood. Here we investigated blink suppression by analyzing fMRI data in a block design and event-related manner, and employed a novel hypothetical time-varying neural response model to detect brain activations associated with the buildup of urge. Blinks were found to activate visual cortices while our block design analysis revealed activations limited to the middle occipital gyri and deactivations in medial occipital, posterior cingulate and precuneus areas. Our model for urge, however, revealed a widespread network of activations including right greater than left insular cortex, right ventrolateral prefrontal cortex, middle cingulate cortex, and bilateral temporo-parietal cortices, primary and secondary face motor regions, and visual cortices. Subsequent inspection of BOLD time-series in an extensive ROI analysis showed that activity in the bilateral insular cortex, right ventrolateral prefrontal cortex, and bilateral STG and MTG showed strong correlations with our hypothetical model for urge suggesting these areas play a prominent role in the buildup of urge. The involvement of the insular cortex in particular, along with its function in interoceptive processing, help support a key role for this structure in the buildup of urge during blink suppression. The right ventrolateral prefrontal cortex findings in conjunction with its known involvement in inhibitory control suggest a role for this structure in maintaining volitional suppression of an increasing sense of urge. The consistency of our urge model findings with prior studies investigating the suppression of blinking and other bodily urges, thoughts, and behaviors suggests that a similar investigative approach may have utility in fMRI studies of disorders associated with abnormal urge suppression such as Tourette syndrome and obsessive-compulsive disorder.
Keywords: Eye blinking, urge suppression, fMRI, electrooculography
Introduction
Eye blinking is a complex natural motor behavior that occurs without volition, as during spontaneous or reflexive blinking, but is also under voluntary control, as during intentional blinking. Spontaneous blinking can be volitionally suppressed; however, this is always associated with an increasing buildup of urge to blink that, like other bodily urges such as breathing and urinating, cannot be sustained for extended periods of time. While a number of groups have used functional neuroimaging techniques to investigate brain activations associated with the spontaneous and voluntary aspects of eye blinking (Bodis-Wollner et al., 1999; Hanakawa et al., 2008; Kato and Miyauchi, 2003; Tsubota et al., 1999; Yoon et al., 2005), brain regions involved in the suppression of eye blinking and the consequent buildup of the urge to blink are less well defined.
Yoon et al. used functional MRI (fMRI) and an electrooculography (EOG) recording system to investigate brain activity—as measured by an increase in blood oxygen level dependent (BOLD) signal—involved in blink suppression in a block design manner and spontaneous eye blinking in an event-related manner (Yoon et al., 2005). Blink suppression and spontaneous blinking both increased activity in the bilateral occipital and parahippocampal cortices, while activity in the right precentral gyrus appeared specific to blink suppression and in the medial frontal gyrus (MeFG) to spontaneous blinking. However, since this study involved only 15-second blink suppression blocks, the block design findings may just reflect motor recruitment associated with the task performance of blink suppression rather than a buildup of urge.
In a positron emission tomography (PET) study by our group, the contrasting of 60-second blocks of blink suppression with similar blocks of spontaneous blinking revealed prominent activations within the insular cortex and anterior cingulate cortex (ACC), as well as primary facial motor areas, supplementary motor area (SMA), and superior temporal gyrus (STG) (Lerner et al., 2008). These results supported a prominent role for a limbic structure such as the insular cortex in blink suppression. The insular cortex has also been frequently reported to be activated during the suppression of other bodily natural urges such as when inhibiting the urge to void one’s bladder (Kuhtz-Buschbeck et al., 2005; Kuhtz-Buschbeck et al., 2009; Seseke et al., 2006; Zhang et al., 2005), when normal breathing is suppressed (Banzett et al., 2000; McKay et al., 2008), and when the urge to cough is suppressed (Mazzone et al., 2011). These studies have also reported on activations during urge suppression in other brain regions such as the ACC and SMA (Banzett et al., 2000; Kuhtz-Buschbeck et al., 2005; Kuhtz-Buschbeck et al., 2009; Mazzone et al., 2011; McKay et al., 2008; Zhang et al., 2005), frontal regions including the precentral gyrus and inferior frontal gyrus (Banzett et al., 2000; Kuhtz-Buschbeck et al., 2005; Kuhtz-Buschbeck et al., 2009; Mazzone et al., 2011; McKay et al., 2008) and temporal and parietal areas such as the STG, supramarginal gyrus, and precuneus (Kuhtz-Buschbeck et al., 2005; McKay et al., 2008; Zhang et al., 2005). The findings of prominent insular cortex activity across the various forms of bodily urge inhibition, however, along with its known anatomical organization and widespread connectivity to frontal, temporal, and parietal cortical areas of the brain (Shelley and Trimble, 2004), suggest this brain region in particular plays a key role in the buildup of urge sensations during suppression of natural bodily functions.
In the present study, the neural correlates of blink suppression were investigated using fMRI and EOG in an attempt to identify brain activations associated with the buildup of the natural bodily urge to blink. By using fMRI we are able to build on our prior PET study of blink suppression by analyzing the data in a block design manner, and by using EOG we could monitor task performance and at the same time investigate activations associated with the act of blinking in an event-related analysis. While previous fMRI studies of blink and other bodily urge suppression such as those referenced above have employed experimental designs with blocks under 30 seconds, we wished to explore blink suppression over 60 seconds as was used in our PET study. Since the sense of an urge to blink would be expected to increase over time and be much greater at the end of 60 seconds compared to the beginning of the suppression block, we questioned whether a standard block design analysis would adequately capture the neural activations associated with an increasing buildup of urge over this length of time. Although important advances in modeling hemodynamic responses during fMRI have been made that will lead to more accurate estimation of time varying neural responses (Havlicek et al., 2011), these can be statistically intensive and have not been applied to the study of time-varying internal experiences that develop over a period on the order of minutes. Thus, we also analyzed our data with a novel time-varying hemodynamic response model to simulate a buildup of urge over 60 seconds of blink suppression.
Based on the limited prior eye blink suppression studies, we hypothesized that our event-related and block analysis would reveal activations similar to those previously described such as in the occipital cortices, ACC, or SMA. We further hypothesized that our hypothetical model for the buildup of urge would reveal significant activations associated with blink suppression in brain areas similar to those found in our prior blink suppression PET study including the insular cortex, ACC, primary and secondary facial motor areas, and STG, and that those activations associated with the buildup of urge would not be detected using a standard block analysis.
Methods
Participants
We studied 15 healthy volunteers (9F, 6M) aged 29.7 ± 7.9 years (Mean ± SD). Fourteen were right-handed and one was left-handed. No subjects had a history of a psychiatric illness and none were taking antidepressants, antipsychotics, or any other neuroactive medication. All participants had normal or corrected-to-normal vision and normal neurological examinations. Subjects who wore glass or contact lenses were fitted with MRI-compatible vision correction lenses. The study was approved by the Combined Neurosciences Institutional Review Board of the National Institutes of Health, and all participants gave their written informed consent before participation.
MRI Scanning
Images were acquired with a 3T scanner and 8-channel head coil (GE Signa, Milwaukee, WI, USA). The coil was foam-padded to restrict head motion and improve subject comfort. Functional T2*-weighted images were acquired using gradient echo, echo planar imaging (EPI), and the functional imaging acquisition parameters were: matrix size = 64 × 64, FOV = 22 cm × 22 cm, TR = 1000 ms, TE = 30 ms, flip angle = 70°, bandwidth = 250 kHz. Each acquisition comprised 17 slices (except 5 runs collected from the first 5 subjects that contained 14 slices) covering most of the brain except for the cerebellum (3.3×3.3 mm2 nominal in-plane resolution, 5.0 mm thick slices, 0.5 mm gap). The scanning parameters used were chosen to keep brain volume scan times to within 1 second without the use of acquisition acceleration techniques. To improve the signal-to-noise ratio in areas prone to susceptibility artifacts, high-order shimming was applied to lessen the field inhomogeneities during data collection. A high-resolution anatomical scan was acquired for each subject for superposition of functional maps upon brain anatomy and to allow for image normalization to a standardized brain space using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence (matrix size = 256×256, FOV = 22×22 cm2, 1 mm3 isotropic resolution, TR = 10 ms, TE = 4.96 ms, flip angle = 19°).
Scanning Task
All participants underwent one functional scanning run that consisted of two experimental tasks: 1) rest during which subjects focused on a fixation cross, but were instructed to relax and blink normally (30 seconds), and 2) eye blink suppression during which subjects fixated on the word “GO” and were instructed to try and inhibit any blinking (60 seconds). Scanning began with a 30 second rest block, which was followed by 3 blink suppression blocks alternating with 3 rest blocks. Room lights in the MR scanner room were dimmed such that the main sources of light during scanning were from the display projector and what light entered through the control room window. Based on prior luminance measurements made for the MR scanner room used in this study, the room luminance was < 0.2 cd/m2 and the maximum screen luminance was < 1100 cd/m2 (Fukunaga et al., 2006). In order to limit head movement and avoid and straining of eye position during the experiment, head position and the head coil mounted mirror were adjusted for each subject such that the location of our display stimulus was positioned in the center of their visual field with eyes in a comfortable, midline position (approximately when bottom of iris is at the level of the resting bottom eye lid). The visual display subtended an angle of approximately 5 degrees. Participants were instructed to try to stay focused on the display screen and return immediately to the mode of blink suppression in the case of any inadvertent blinks occurring. Six of the participants were asked to perform a second identical blink suppression functional scanning run (a separate set of three 60-second suppression blocks). The second scanning run did not begin until participants reported they did not feel any increased urge to blink and had returned to a normal rate of blinking. The blink suppression scanning runs were performed prior to continuing on to a completely separate fMRI study during the same scanning session.
Electrooculography
MRI-compatible surface electrooculography (EOG) Ag-AgCl electrodes (BIOPAC Systems, Inc., Goleta, CA, USA), and recording system (Brain Products, Munich, Germany) were used to detect and record eye blinks during the scanning runs. One electrode was placed just above the right eyebrow and one about 1 cm below the lower eyelid of the right eye. Electrodes were placed centrally above and below the subject’s eyeball to maximally depolarize from the vertical movement of the globe during blinking and minimize depolarization from horizontal eye movements. The reference was placed on the dorsum of the right hand. The impedance of each electrode was maintained at less than 5 kΩ by using skin cleansers and/or high conductance electrode gel. The EOG signal underwent amplification and A/D conversion and then was fed through an optical cable to a PC in the MR control room for real-time monitoring of blinks and further off-line analysis. Before starting the scanning run, each participant’s blink waveforms were inspected while they lay in the MR scanner to verify adequate detection of eye blinking.
MRI scanner artifact correction and band-pass filtering was performed on EOG waveforms acquired during fMRI scanning using the commercially available Brain Products software Vision Analyzer and previously described techniques (van Duinen et al., 2005) (Figure 1a). EOG was sampled at 5000 Hz, and, after MR scanner artifact correction, data were down-sampled to 250 Hz and a segment created for each TR. Signal time-courses were high-pass filtered (Butterworth Zero Phase filter, low cutoff 1.0Hz, 48dB/oct) keeping frequencies between 1 and 250 Hz to remove possible movement artifacts. EOG waveforms were then rectified and exported to Matlab (The MathWorks, Natick, MA, USA) in order to isolate blinks to be used in the event-related analysis (Figure 1b–d). A blink event was defined by a change in the EOG signal (µV) of at least 0.25 of the standard deviation away from the EOG signal mean calculated for each individual subject scan separately.
Figure 1.
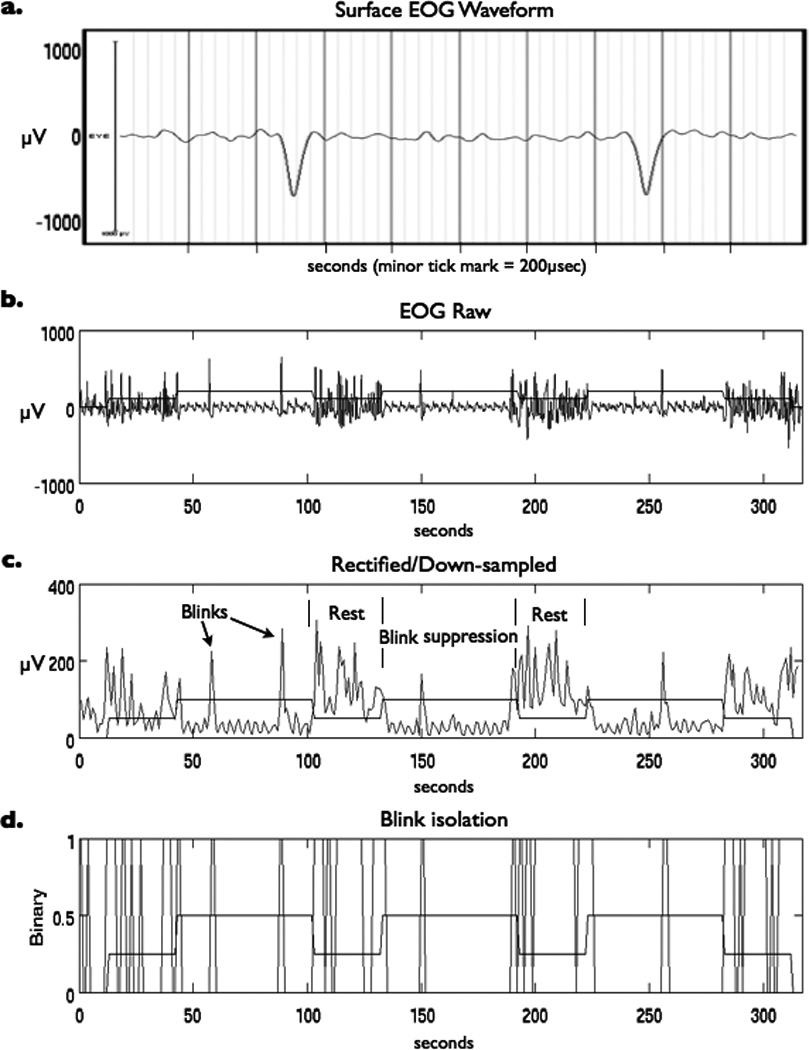
(a) Surface EOG waveform showing two blinks recorded during a MRI scanning session after scanner artifact correction and band-pass filtering. Additional EOG post-processing included (b) importation of time series into Matlab, (c) rectification and down sampling of signal, and (d) isolation of blink events based on signal exceeding threshold of 0.25 of the standard deviation above the mean. Task paradigm timing in relation to EOG recording of blinks is superimposed for reference.
Blinks identified using our signal threshold were then checked for accuracy against blinks identified by visual inspection of the EOG waveforms. In order to eliminate possible anticipatory blinks or reflexive blinks that may have been evoked by the changes on the visual display screen from analysis, blinks were excluded if they occurred within ±1 second of a transition between rest and GO task conditions. To test for a difference between the average number of blinks per minute during periods of blink suppression and periods of rest, as well as between the average number of blinks in the beginning and the end of rest periods that followed blink suppression, two-tailed, paired t-tests were performed and a significance threshold of p < 0.005 applied.
Urge Model
A hypothetical representation of time-course of neural activity associated with the buildup of the urge to blink during attempted blink suppression was designed (Figure 2). Our “URGE” model was based on the assumption that an urge to blink begins to develop at the onset of blink inhibition. Since spontaneous blinks occur within about 3 to 4 seconds of each other (Bentivoglio et al., 1997), the hypothetical threshold for the amount of urge required to trigger a spontaneous blink is depicted at 4 seconds. The buildup of urge during blink suppression over 60 seconds has been assumed to be linear. While involuntary blinks occurring while a subject is attempting to suppress them might lead to a transient decrease in the urge to blink, this was not incorporated into our rudimentary model as the number and timing of these blinks varied considerably among subjects and the degree to which such a blink would reduce the level of urge for an individual subject difficult to assess. Furthermore, subjects generally took about half of the following rest period (15 seconds) after the 60 seconds of blink suppression to return to a stable blink rate suggesting that brief blinks occurring during suppression would be unlikely to reset the urge or reduce the sense of urge significantly during the prolonged period of active blink inhibition. As urge is the driving force behind eye blinking in our model, we hypothesized that the increased blink urge built up over 60 seconds therefore required a similar amount of time to dissipate. A release of urge lasting 15 seconds after 60 seconds of blinks suppression was also suggested by post-hoc analysis looking at the time it took for the total blink counts to stabilize when all blink counts were evaluated at the group level (see Figure 4).
Figure 2.
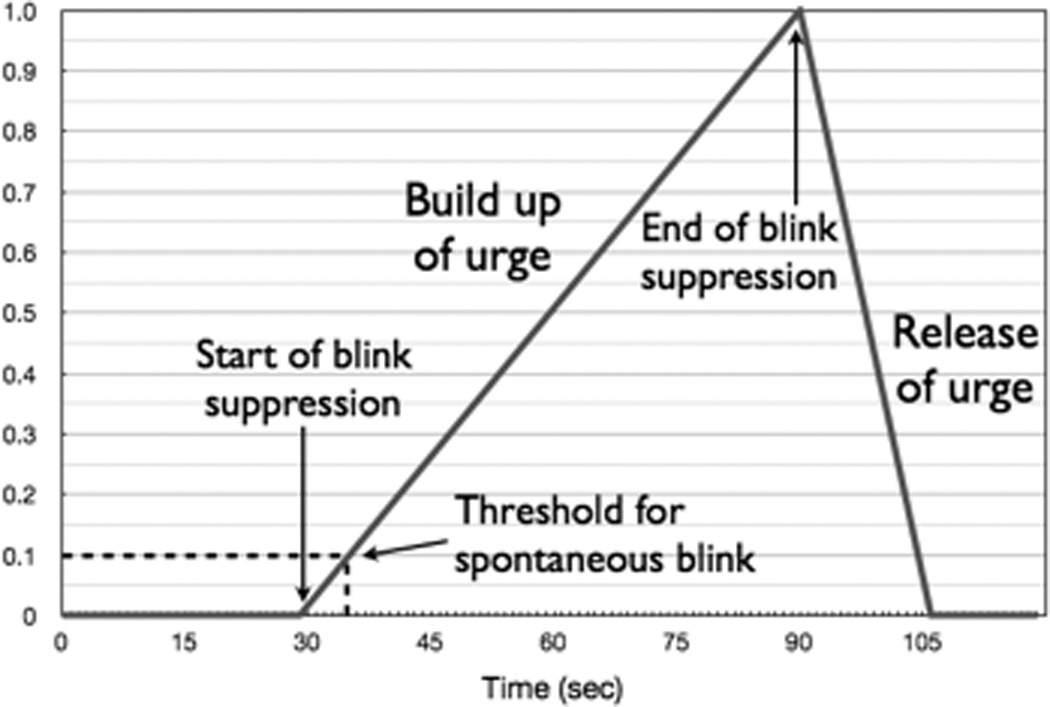
Hypothetical hemodynamic response model for task with linear buildup of urge to blink during 60 sec of suppression and 15 sec release of urge following end of blink suppression.
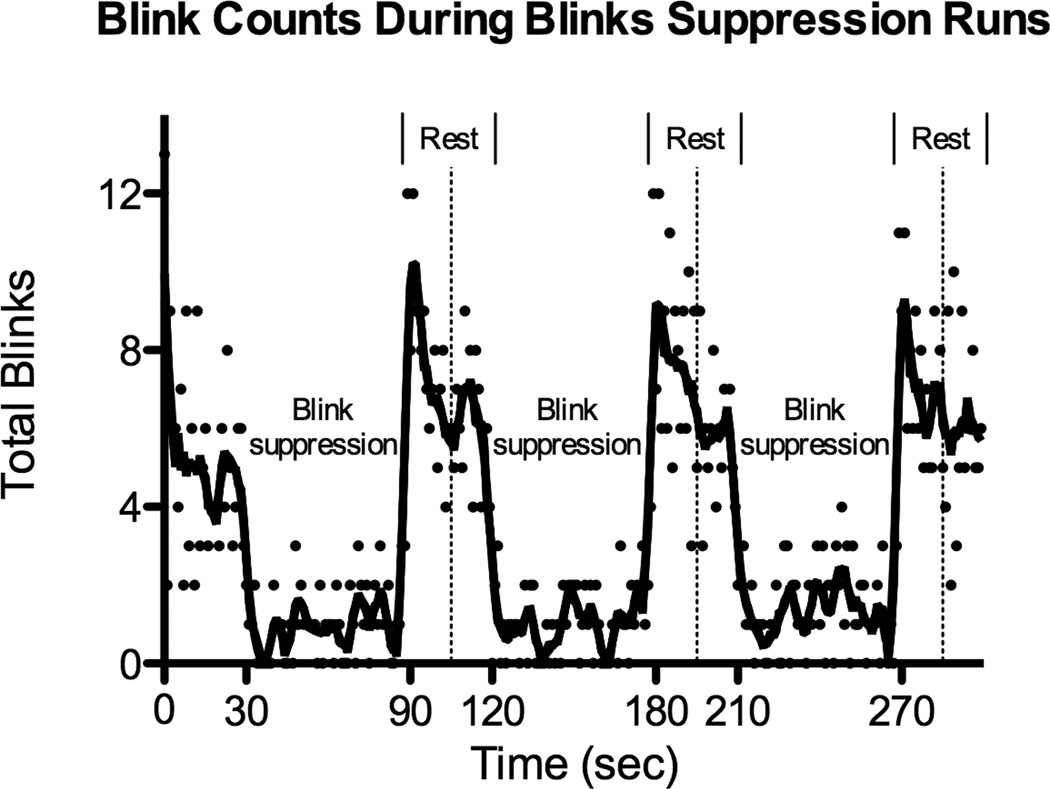
Figure 4.
Total number of detected blinks for all 18 scanning runs summed at each TR (1 sec). Each dot represents the total blink count at each time point, and the fit line was generated using a smoothing polynomial and averaging of the four nearest neighbors. Alternating 60 sec suppression blocks with 30 sec rest blocks are labeled and a dotted vertical line marks the middle of each rest block.
Image Analysis
Image analysis was performed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). The first 10 scans of each session were excluded from data analysis to account for T1 equilibration effects and subject scanner acclimation. EPI scans were corrected for slice timing and images were realigned to the last volume collected (closest to anatomical scan acquisition) and co-registered to the high-resolution MPRAGE anatomical images for each subject. Images were spatially smoothed using an isotropic 8-mm full width at half-maximum Gaussian filter to accommodate individual anatomical variability and normalized to have a mean of 100. The imagers were then analyzed within a general linear model (GLM).
Three different regressors were used in a single GLM analysis: 1) a standard boxcar block-design model (“BLOCK”) with the rise of BOLD signal associated with onset of the 60 second active periods of blink inhibition, 2) an event-related model using blinks as events (“EVENT”) with each blink event modeled as a delta function against a baseline that consisted of the remainder of the entire scanning run, and 3) our hypothetical URGE model. All regressors were convolved with a standard gamma-variate hemodynamic response function and entered along with covariates derived from head motion parameters into a design matrix in order to generate estimates for each regressor at every voxel. High-pass filtering was performed to remove any linear trend or other lower frequency content from data.
Individual subject statistical parametric maps were spatially normalized to Talairach and Tournoux space (Talairach and Tournoux, 1988) using the AFNI transformation program adwarp and group analysis performed using a one-sample t-test. Anatomical labels were derived from Talairach Daemon atlas (Lancaster et al., 2000) and cytoarchitectonic probability maps for the Montreal Neurological Institute Colin N27 brain (Eickhoff et al., 2005) distributed with AFNI. Statistical maps were corrected for multiple comparisons at the cluster level using Monte Carlo based simulations (AFNI program AlphaSim) for an overall corrected significance level of p < 0.005. Laterality of the insular cortex activations identified with our URGE model was investigated by comparing mean t values in the right and left insula with a two-tailed t-test and applying a significance threshold of p < 0.005.
ROI Analysis
ROI analysis was performed after individual subject EPI scans were co-registered to their anatomical MPRAGE images and transformed to Talairach-Tournoux space. The Talairach Daemon brain atlas (Lancaster et al., 2000) was used to define 17 anatomical brain regions corresponding to the insular cortex, inferior frontal gyrus (IFG), precentral gyrus, ACC, MeFG, STG, middle temporal gyrus (MTG), inferior parietal lobe (IPL), supramarginal gyrus and precuneus (Figure 3) to further investigate the role of these structures in eye blink suppression. BOLD signal time-course data for each subject scan were extracted for each ROI, normalized, and averaged across all scans. Normalized ROI BOLD time course data were compared to our hypothesized URGE regressor function convolved with a gamma-variate hemodynamic response function. BOLD signal data was plotted against the hypothesized urge response for each time point and goodness of fit for the model analyzed using linear regression with GraphPad Prism 5 software (www.graphpad.com). The fit between the BOLD data and the URGE model was then determined by linear correlation analysis and a good fit defined as a regression line demonstrating a coefficient of determination (R2) > 0.5. An analogous comparison was made between measured BOLD signal responses and the BLOCK regressor.
Figure 3.
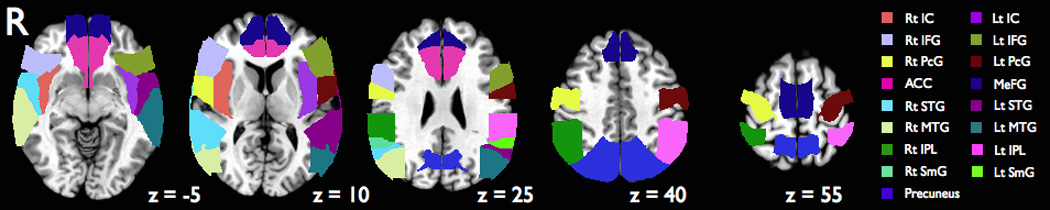
A priori ROIs derived from Talairach atlas used for ROI analysis (see text for details). ACC, anterior cingulate cortex; IC, insular cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; Lt, left; MeFG, medial frontal gyrus; MTG, middle temporal gyrus; PcG, precentral gyrus; Rt, right; SmG, supramarginal gyrus; STG, superior temporal gyrus.
Results
Behavior
Nine participants completed a single blink suppression scanning run and 6 completed two scanning runs. All participants reported experiencing an urge to blink during the suppression blocks. The data from one participant (two scanning runs) were excluded after she reported after the scanning that she closed her eyes during rest blocks and the data from one scanning run of a participant were excluded because of a scan interruption leading to a total of 18 scanning runs from 14 subjects being included in the final analysis.
The average number of blinks in the first 30-second rest period before any active suppression blocks was 6.3 ± 2.1 (mean ± SD) or 12.6 ± 4.1 blinks per minute. All of the subjects had increased blink rates at the beginning of the 30 seconds of rest compared to the end of the 30 seconds of rest following the suppression blocks and a significantly different number of blinks was seen between the first 15 seconds of these rest blocks compared to the last 15 seconds (p < 0.005) suggesting there was a buildup of urge over the suppression blocks. The average number of isolated blinks from all 18 included scanning runs was 3.1 ± 2.3 blinks per minute during periods of blink suppression and 16.3 ± 3.0 blinks per minute during periods of rest, a strongly significant difference (p < 10−11) further supporting that participants suppressed blinking during the task blocks. Figure 4 shows the total number of blinks for all scanning runs at each TR (1 second) and a superimposed fit line using a 4th order smoothing polynomial and averaging of four nearest neighbors (GraphPad Prism 5). In addition to clearly showing a decreased number of blinks during suppression periods, Figure 4 also showed that the increased blinking at the onset of rest took roughly half of the rest block (15 seconds) to stabilize.
Activation analyses
Using the BLOCK analysis, significant activations during blink suppression were only found in the bilateral middle occipital gyrus (MOG) (Figure 5, Table 1). Significant deactivations were also identified in the posterior cingulate cortex (PCC) and neighboring lingual gyrus and precuneus. The EVENT analysis revealed significant activations limited to the primary and secondary visual cortices including the cuneus, lingual gyrus, and calcarine gyrus.
Figure 5.
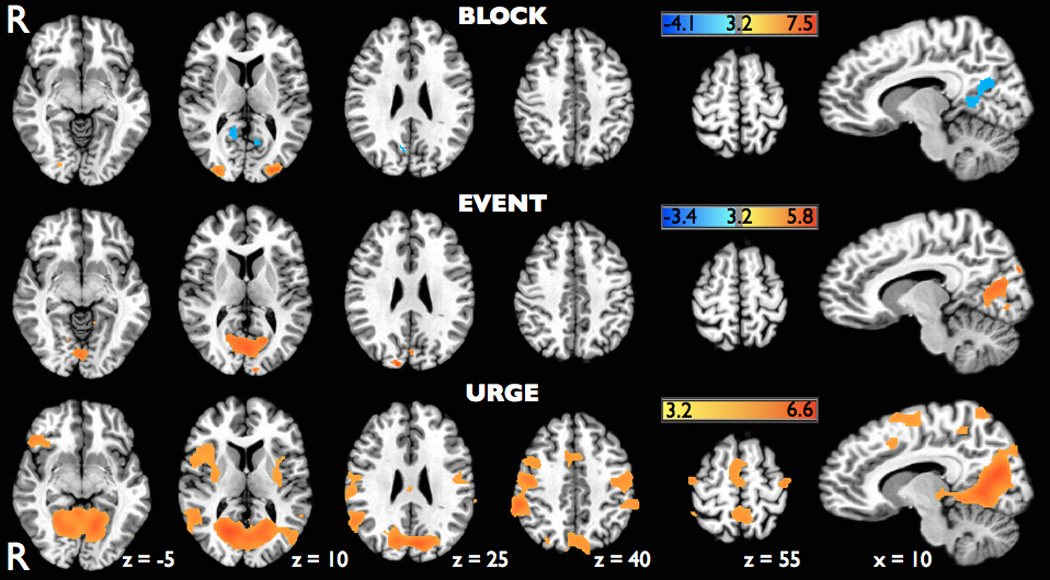
Axial brain slices with superimposed statistical parametric maps on the MNI Colin N27 brain showing significant activations (red) and deactivations (blue) identified using a standard block design analysis (BLOCK), an event-related analysis of blinks (EVENT), and a hypothetical hemodynamic response function for urge (URGE) during the blink suppression scanning runs. Parametric maps were all thresholded at t = 3.2 (p<0.005, corrected).
Table 1.
Activations identified during blink suppression using three different analysis methods
| Analysis method |
Cluster size (voxels) |
Side | Region (Brodmann area) | Talairach coordinates | Peak t value |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| BLOCK | 187 | R | Precuneus/posterior cingulate cortex (29, 30, 31), lingual gyrus (17, 18) | 11 | −61 | 20 | −3.86 |
| 166 | R | Middle occipital gyrus (18) | 29 | −85 | 8 | 4.90 | |
| 121 | L | Middle occipital gyrus (18) | −28 | −91 | 5 | 6.63 | |
| 89 | L | Lingual gyurs (17, 18), posterior cingulate cortex (29, 30) | −7 | −52 | 3 | −3.94 | |
| EVENT | 753 | L/R | Cuneus/calcarine gyrus/lingual gyrus (17, 18) | 14 | −88 | 26 | 4.84 |
| URGE | 5148 | L/R | Lingual gyrus/cuneus/calcarine gyrus (17, 18, 19), precuneus (31, 7), posterior cingulate cortex (29, 30) | −16 | −58 | 2 | 6.50 |
| R | Inferior parietal lobe/supramarginal gyrus (40) | 56 | −37 | 39 | 5.17 | ||
| R | Precentral gyrus (4), postcentral gyrus (3, 1, 2) | 49 | −11 | 47 | 4.97 | ||
| R | Superior/middle temporal gyrus (22, 39) | 52 | −50 | 20 | 4.89 | ||
| R | Inferior frontal gyrus (47, 45) | 49 | 28 | −4 | 4.57 | ||
| R | Middle frontal gyrus/precentral gyrus (9, 6) | 38 | 4 | 38 | 3.99 | ||
| R | Insula (13) | 38 | 19 | 5 | 3.98 | ||
| R | Putamen | 31 | −10 | 6 | 3.91 | ||
| 961 | L | Postcentral gyrus (3, 1, 2), precentral gyrus (4) | −52 | −13 | 47 | 4.27 | |
| L | Inferior parietal lobe/supramarginal gyrus (40) | −59 | −35 | 39 | 3.98 | ||
| L | Putamen | −29 | −8 | 5 | 3.70 | ||
| L | Insula (13) | −37 | 8 | 3 | 3.68 | ||
| L | Superior/middle temporal gyrus (22) | −56 | −47 | 14 | 3.62 | ||
| 526 | L/R | Medial frontal gyrus (6), middle cingulate cortex (24, 32) | 14 | 8 | 62 | 4.12 | |
| 293 | L/R | Precuneus (7) | −1 | −52 | 59 | 4.25 | |
| 74 | R | Thalamus | 2 | −19 | 20 | 3.85 | |
When the blink suppression runs were analyzed using our proposed hypothetical URGE model of a steadily increasing neural response to the buildup of urge during blink inhibition, prominent activations were seen in the insular cortex, right greater than left, the right IFG corresponding to the ventrolateral prefrontal cortex (VLPFC), and the middle cingulate cortex. At our statistical threshold of t = 3.2, the activation cluster size in the right insula (3,978 mm3; peak t = 8.05, mean t = 5.70) was much greater than in the left insula (720 mm3; peak t = 3.74, mean t = 3.38), and the mean t values within the clusters were significantly different (p < 0.0001). Robust activations were also identified in a number of bilateral motor-associated brain regions including primary motor and somatosensory cortex, premotor cortex, and SMA in locations consistent with previously described face motor areas (Chainay et al., 2004; Fox et al., 2001; Morecraft et al., 2004). Significant activations were also seen in other motor-associated areas such as the bilateral putamen and right thalamus and right dorsolateral prefrontal cortex (DLPFC). Additionally, signification bilateral activations were also seen in the STG extending inferiorly to the MTG, parietal regions primarily including the supramarginal gyrus and precuneus, and in medial occipital regions overlapping with those visual cortex areas identified in the EVENT analysis.
ROI Analysis
Figure 6 shows the brain regions that demonstrated strong correlations between extracted BOLD time courses averaged across all subjects and our hypothesized URGE model that has been convolved with a standard hemodynamic response function. These included the bilateral insular cortex (right: R2 = 0.59; left: R2 = 0.59), right IFG (R2 = 0.60), bilateral STG (right: R2 = 0.57; left: R2 = 0.54), and bilateral MTG (right: R2 = 0.68; left: R2 = 0.61). The fit of the left IFG was just below our threshold for a good fit (R2 = 0.48), and the MeFG fit showed a trend toward a good fit (R2 = 0.37), but the remaining ROIs showed correlations of R2 < 0.17 (Figure 7) despite the presence of significant activations identified in these areas in our URGE analysis. An identical correlation analysis using the BLOCK regressor revealed no good fits between the BOLD time series and the BLOCK model (all R2 < 0.22).
Figure 6.
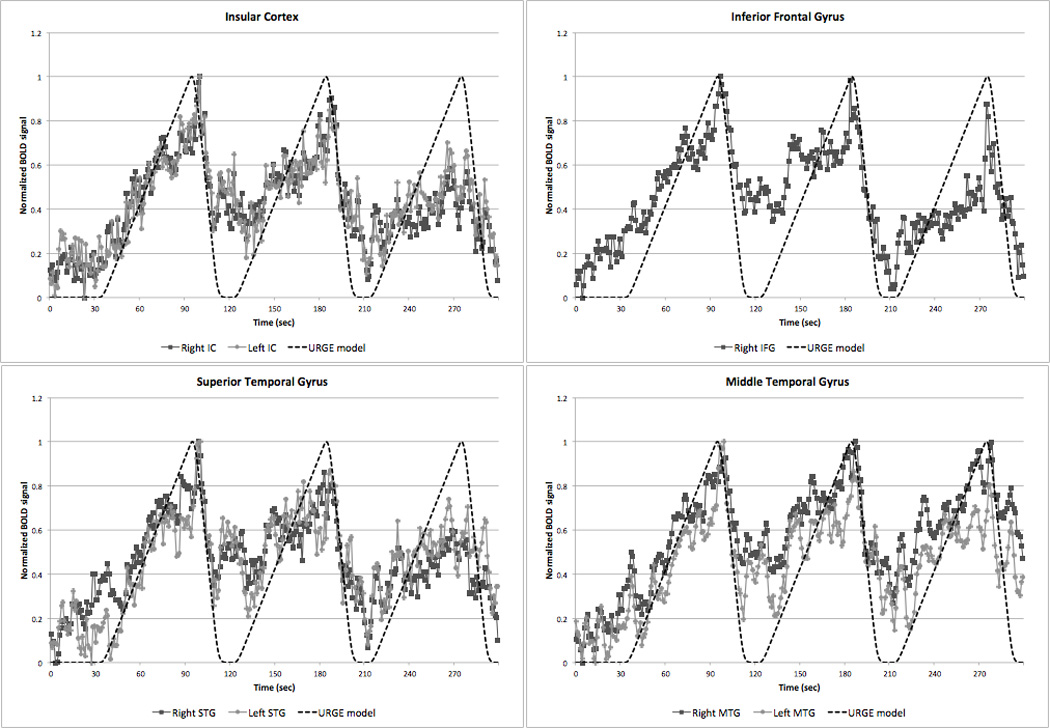
BOLD signal time courses from those ROIs that showed a strong correlation (R2 > 0.5) with our hypothetical URGE model (see text). IC, insular cortex; IFG, MTG, middle temporal gyrus; STG, superior temporal gyrus.
Figure 7.
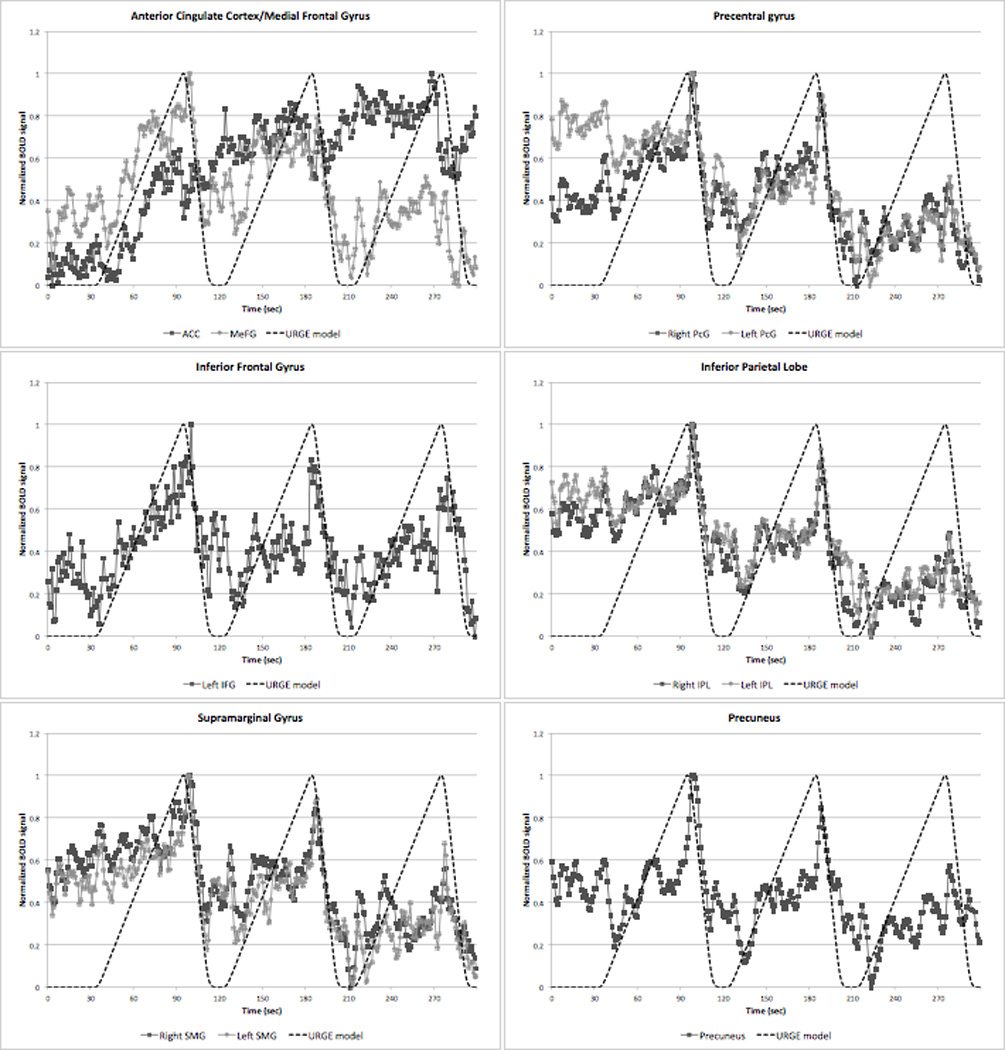
BOLD signal time courses from those ROIs that failed to show a strong correlation (R2 ≤ 0.5) with our hypothetical URGE model (see text). ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; IPL, inferior parietal lobe; MeFG, medial frontal gyrus; PcG, precentral gyrus; SMG, supramarginal gyrus.
Discussion
In this study, three separate fMRI imaging data analysis techniques were applied to the same data from a blink suppression task to try and identify the neural networks involved in the buildup of urge during eye blink suppression. Brain activations associated with blink suppression were investigated with a standard block design analysis, with an event-related analysis using EOG to detect individual blinks, and with a hypothetical neural response model of urge to try and more accurately investigate activations associated with a buildup urge over 60-second blink suppression blocks.
Block design and event-related findings
Using the standard BLOCK analysis, activations were only identified within the MOG. Activations in overlapping and/or neighboring occipital regions have previously been associated with blink suppression (Chung et al., 2006; Kato and Miyauchi, 2003; Yoon et al., 2005), as well as with voluntary blinking (Bodis-Wollner et al., 1999; Hanakawa et al., 2008; Kato and Miyauchi, 2003) and spontaneous blinking (Yoon et al., 2005), even when the investigation that was carried out in a dark environment (Tsubota et al., 1999). The MOG activation may be explained in these studies by recruitment of the extrastriate body area, which has been associated with goal-directed movements of limbs (Astafiev et al., 2004) and could similarly be activated during volitional suppression of eye blinking. MOG activation in our study, however, could have also stemmed from visualization of the word GO displayed during the active task period. Future blink suppression investigations may benefit from the use of a dark environment and auditory clues for task paradigm instructions to help better elucidate the role of the MOG. Whatever the underlying cause of MOG activations might be, their sustained activation over the 60 second suppression task periods detected with our BLOCK analysis suggest they are more likely to be task-related rather than associated with a buildup of urge.
Our BLOCK analysis also revealed deactivations in occipital cortex more rostral to that identified in the EVENT analysis and extending into the PCC and precuneus. While these deactivations could be associated with blink suppression, they may also just reflect activation of the default mode network during the rest blocks (Buckner et al., 2008; Fox and Raichle, 2007). Activation of other core nodes of the default mode network such as the medial prefrontal cortex, medial temporal lobes, and lateral parietal cortex, however, were not present arguing against significant activation of this task-negative network.
The use of EOG in our study proved a reliable way to ensure that subjects were successfully inhibiting blinking during the suppression blocks. EOG was also useful in evaluating how long after suppression ended that blink rates remained elevated, which helped support the tapering release of urge over 15 seconds used in our urge model. Monitoring blinks with EOG further allowed for our EVENT analysis, which revealed activations isolated to the visual cortices—a finding consistent with a number of prior imaging studies showing spontaneous blinks primarily activate visual cortices (Bodis-Wollner et al., 1999; Kato and Miyauchi, 2003; Yoon et al., 2005). Since spontaneous blinks in our study were identified under a number of different conditions including during attempted blink inhibition and following prolonged periods of blink suppression, the activations identified in the visual cortex may represent a common brain region activated by a variety of blink types. Further study of spontaneous blinks assessed in isolation is needed to better understand whether spontaneous blinks occurring under different experimental conditions are associated with a different underlying physiology.
Buildup of urge during blink suppression
Using our URGE model revealed robust activations within a large number of bilateral brain regions that have been previously associated with urge suppression including the insular cortex, right IFG or VLPFC, IPL, STG, and primary and secondary facial motor areas. To explore further which of these brain areas demonstrated activation changes most consistent with a steady buildup urge, BOLD time-series were extracted in an extensive ROI analysis examined for goodness of fit with our URGE model. The strongest correlations were seen with the bilateral insular cortex, right IFG, and bilateral STG and MTG, suggesting these structures in particular play a key role in the buildup of urge.
The finding of prominent involvement of the insular cortex during blink suppression is consistent with the findings of our prior PET study (Lerner et al., 2008). A key role for the insular cortex in urge suppression has also been suggested by a number of other studies of bodily urge suppression such as those investigating bladder control (Kuhtz-Buschbeck et al., 2005; Kuhtz-Buschbeck et al., 2009; Seseke et al., 2006; Zhang et al., 2005), air hunger (Banzett et al., 2000; McKay et al., 2008), and cough suppression (Mazzone et al., 2011). The anatomical organization and connectivity of the insular cortex (Shelley and Trimble, 2004), in addition to an increasing number of functional imaging studies identifying its role in urge suppression, support its function as an integration center for internal and external body state sensory information and as such may play a principal role in the sensing of physiological urges (Craig, 2002). Furthermore, there is increasing evidence that asymmetry exists in the processing of emotional valence of body states with the left hemisphere predominantly associated with parasympathetic activity (positive feelings and appetitive behaviors) and the right hemisphere predominantly associated with sympathetic activity (negative feelings and aversive behaviors) (Craig, 2005). This asymmetry may help explain the pattern of right lateralization in our findings as blink suppression that might be considered as creating an unpleasant sensation of buildup of urge.
Identification of VLPFC activation with our URGE model is also not surprising given this brain region’s known role in controlling attention (D'Esposito et al., 2000), and involvement of the right VLPFC in particular in inhibitory control (Aron et al., 2004). In addition to being associated with suppressing emotions and memories (Depue et al., 2007; Quirk and Beer, 2006), as well as with resisting temptations (Knoch and Fehr, 2007), the right VLPFC has been found to be important in motor response inhibition (Goghari and MacDonald, 2009; Levy and Wagner, 2011; Rubia et al., 2003). One study compared inhibition of hand and eye movements in the same subjects and found similar activations within the right VLPFC during both (Leung and Cai, 2007). In addition to the right VLPFC activation, eye and hand movement inhibition in that study were also accompanied by activation within the insular cortex, premotor cortex, SMA, IPL and DLPFC—areas similarly found activated during blink suppression using our URGE model. The co-activation of the VLPFC with motor-associated cortices during movement inhibition, along with its general inhibitory function in the cognitive control of a wide range of thoughts and behavior, suggest its primary role may be related to the suppression of the blinking urge as opposed to being central to the buildup of the urge sensation itself.
Significant activations in the facial primary and secondary motor regions as well as the middle cingulate cortex were also seen in the URGE analysis. Face area motor-associated brain region activations were similarly seen during our blink suppression PET study (Lerner et al., 2008), and the precentral gyrus has previously been reported to be activated during shorter blocks of blink suppression by a another group (Yoon et al., 2005). Activation of motor-related regions could be the result of increasing recruitment by subjects of facial muscles such as the levator palpebrae and frontalis muscles in order to counter the buildup of urge to blink during the prolonged period of blink inhibition. The EOG electrode placed above the right eyebrow, however, did not detect increasing muscle activity during the blink suppression blocks despite its proximity to the frontalis muscle. Furthermore, ROI analysis of the precentral gyrus did not reveal steady increases in BOLD signal during prolonged blink suppression as might be expected to accompany the buildup of urge, nor did it reveal a steady decrease in activation that might suggest a release of urge following the blink suppression blocks. Rather, a rapid peaking of activation that began at the onset of the rest blocks following suppression blocks was observed (see Figure 7). Similar peaking at the beginning of the rest blocks following suppression was visible in the parietal ROIs including the IPL, supramarginal gyrus, and precuneus. Given the burst of strong blinks that occurred in subjects once the blink suppression was released, the precentral and parietal peaks most likely represent motor activity related to these bursts of blinks. Future studies of blinking suppression may benefit from the use of additional EOG electrodes to record activity from more facial muscles in order to try and better explain the activations seen in the facial motor network during blink suppression.
The MTG and STG in our URGE analysis were also found to be activated in our URGE analysis and showed BOLD signal changes during blink inhibition that were highly correlated with our URGE model. Temporal areas such as the STG were also seen in our PET study of blink suppression (Lerner et al., 2008), and have been associated with blinking that occurs during attempted blink inhibition (Chung et al., 2006). There are also some imaging studies that suggest these temporal regions are involved in cognitive as well as motor response inhibition (Bernal and Altman, 2009; Horn et al., 2003). Given the location of temporal cortex activations within parietal-temporal-occipital association areas of the brain, however, it seems more likely these activations are related to an integrative role during blink suppression rather acting as a primary source of the buildup of an urge sensation or directing control of blink inhibition. The activations identified in the parietal cortex by our URGE model might similarly be related to an associative role of this brain region, though increasing activations in parietal cortex during blink suppression could also be related to its involvement in eye gaze fixation and visual attention (Culham and Kanwisher, 2001).
Limitations
The blink suppression fMRI scans for this study were collected immediately prior to a longer, completely separate fMRI study and as a consequence the scanning acquisition parameters may not have been optimal. Specifically, the voxel size was more anisotropic than would be ideal, which can reduce or distort BOLD signal in cortical regions near air-filled sinuses, and whole brain volumes were not collected leading to the lack of cerebellar evaluation and potential partial volume effects. In regards to our imaging analysis, one potential issue is that the baseline condition used for our BLOCK analysis often contained clustered and sometimes more forceful spontaneous blinks after the prolonged blink suppression blocks. This finding may represent carry over effects of urge, which is accounted for in our hypothetical model of urge but could lead to contamination of the baseline condition in the BLOCK analysis. Likewise, the baseline condition for the EVENT analysis includes active periods of blink suppression and therefore is not free from the effects of the buildup of urge either. Longer rest blocks and repeated scans investigating blinks without blocks of blinks suppression could help limit these issues in future studies.
In this study, we designed a hypothetical model for the buildup of urge to investigate neural activations that we postulated would steadily increase over time during active suppression of a natural bodily urge. The validity of this URGE model has not been verified and its use here is intended to be only a first approximation. As the model does not account for an individual’s experience of urge, the presence of inter-subject variability in the experience of the buildup of urge associated with prolonged blink suppression, as well as the amount and rapidity of relief experienced during rest blocks, could confound our results. Nevertheless, our BOLD time-series ROI analysis revealed strong correlations with our hypothetical model in brain regions strongly implicated in urge suppression not identified with a standard block design analysis suggesting that our URGE model was a reasonable approximation for the time-varying neural responses associated with urge sensation during 60 seconds of blink suppression. Further investigation will be needed to better characterize the neural responses that result during the inhibition of bodily urges and better clarify the roles of brain regions such as insular cortex and VLPFC in the buildup of urge.
Future directions
Although the actual underlying brain mechanisms associated with the buildup of urge are likely to be more complicated than our simplified hypothetical URGE model, the consistency of our findings to prior investigations of blink suppression and suppression of other natural bodily urges helps support its applicability to the study of time-varying internal experiences. Thus, the concepts employed here may have application in future studies of brain changes during the buildup (and taper) of natural bodily urges. Future research incorporating the subjective experience during blink suppression, or individual changes in physiological measures of stress such as heart rate variability or galvanic skin response, could help improve on our model for the buildup of urge and better account for an individual’s experience of urge during the experiment.
Since blinking is often one of the earliest manifestations and most common tics in Tourette syndrome, and blinks have similar characteristics to other motor tics (Peterson and Leckman, 1998; Shapiro et al., 1988), the study of blinking and blink suppression might lead to insight into the neural circuits associated with this disorder. Indeed, the buildup of the urge to blink during blink inhibition and the relief that accompanies their eventual performance could also serve as a good model for the buildup of uncomfortable sensations that commonly precede tics and the temporary relief experienced by their release. Hence, the study of blinking and blink suppression may lead to greater insight into the neural circuits associated with Tourette syndrome. This is supported by a recent large fMRI study that reported disturbances in frontostriatal, cingulate, and temporal lobe activity in children and adults with Tourette syndrome during blocks of blink inhibition when compared to healthy subjects (Mazzone et al., 2010).
Our findings here and in our prior blink suppression PET study, in conjunction with prior neuroimaging studies demonstrating insular cortex involvement in the suppression of thoughts, behavior, and other natural bodily urges, suggest this cortical region plays a prominent role in urge sensation and suppression. This may help explain why the insular cortex appears to play a role in the pathophysiology of conditions associated with disordered suppression such as Tourette syndrome (Bohlhalter et al., 2006; Fahim et al., 2009; Lerner et al., 2007) and other neuropsychiatric disorders including obsessive compulsive disorder, post-traumatic stress disorder, and eating disorders (Nagai et al., 2007; Nishida et al., 2011). Future fMRI studies employing analytical approaches that utilize time-varying neural response models such as presented here might reveal new insights into the suppression of blinking or other natural urges as well as cognitive and behavioral suppression. For example, similar methods could be used to investigate whether a common neural network is involved in Tourette syndrome and other neurological and psychiatric disorders associated with abnormal suppressive mechanisms such as obsessive-compulsive disorder, eating disorders, addiction, phobias, and post-traumatic stress disorder.
Acknowledgement
The authors would like to thank Rick Reynolds for his technical assistance. This work was made possible by the Intramural Research Program of the NINDS/NIH.
Abbreviations
- ACC
anterior cingulate cortex
- AFNI
Analysis of Functional NeuroImages software
- BOLD
blood oxygen level dependent
- DLPFC
dorsolateral prefrontal cortex
- EOG
electrooculography
- EPI
echo planar imaging
- fMRI
functional MRI
- GLM
general linear model
- IFG
inferior frontal gyrus
- IPL
inferior parietal lobe
- MeFG
medial frontal gyrus
- MFG
middle frontal gyrus
- MOG
middle occipital gyrus
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- MTG
middle temporal gyrus
- PET
positron emission tomography
- SMA
supplementary motor area
- STG
superior temporal gyrus
- VLPFC
ventrolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
B.D.B, S.G.H. and M.H. designed the study. B.D.B, S.G.H. and B.M. collected and analyzed the data. B.D.B interpreted data and drafted and revised the manuscript. S.G.H. and M.H. assisted with data interpretation and revision of the manuscript. All authors read and approved the final manuscript. The study was performed in the Human Motor Control Section of the NINDS/NIH in Bethesda, Maryland.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nature Neuroscience. 2004;7(5):542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport. 2000;11(10):2117–2120. doi: 10.1097/00001756-200007140-00012. [DOI] [PubMed] [Google Scholar]
- Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P, Albanese A. Analysis of blink rate patterns in normal subjects. Movement Disorders. 1997;12(6):1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- Bernal B, Altman N. Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: An fMRI study. International Journal of Neuroscience. 2009;119(10):1848–1880. doi: 10.1080/00207450802333029. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Bucher SF, Seelos KC. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology. 1999;53(8):1800–1805. doi: 10.1212/wnl.53.8.1800. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, Wurzman R, Hallett M. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(Pt 8):2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network - Anatomy, function, and relevance to disease. Year in Cognitive Neuroscience 2008. 2008:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chainay H, Krainik A, Tanguy ML, Gerardin E, Le Bihan D, Lehericy S. Foot, face and hand representation in the human supplementary motor area. Neuroreport. 2004;15(5):765–769. doi: 10.1097/00001756-200404090-00005. [DOI] [PubMed] [Google Scholar]
- Chung JY, Yoon HW, Song MS, Park H. Event related fMRI studies of voluntary and inhibited eye blinking using a time marker of EOG. Neuroscience Letters. 2006;395(3):196–200. doi: 10.1016/j.neulet.2005.10.094. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends in Cognitive Sciences. 2005;9(12):566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11(2):157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317(5835):215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fahim C, Yoon U, Sandor P, Frey K, Evans AC. Thinning of the Motor-Cingulate-Insular Cortices in Siblings Concordant for Tourette Syndrome. Brain Topography. 2009;22(3):176–184. doi: 10.1007/s10548-009-0105-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox PT, Huang A, Parsons LM, Xiong JH, Zamarippa F, Rainey L, Lancaster JL. Location-probability profiles for the mouth region of human primary motor-sensory cortex: Model and validation. Neuroimage. 2001;13(1):196–209. doi: 10.1006/nimg.2000.0659. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu RX, Deckers RHR, Leopold DA, Duyn JH. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic Resonance Imaging. 2006;24(8):979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW. The neural basis of cognitive control: Response selection and inhibition. Brain and Cognition. 2009;71(2):72–83. doi: 10.1016/j.bandc.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. The representation of blinking movement in cingulate motor areas: A functional magnetic resonance imaging study. Cerebral Cortex. 2008;18(4):930–937. doi: 10.1093/cercor/bhm129. [DOI] [PubMed] [Google Scholar]
- Havlicek M, Friston KJ, Jan J, Brazdil M, Calhoun VD. Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. Neuroimage. 2011;56(4):2109–2128. doi: 10.1016/j.neuroimage.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Kato M, Miyauchi S. Functional MRI of brain activation evoked by intentional eye blinking. Neuroimage. 2003;18(3):749–759. doi: 10.1016/s1053-8119(03)00005-3. [DOI] [PubMed] [Google Scholar]
- Knoch D, Fehr E. Resisting the power of temptations - The right prefrontal cortex and self-control. In: Balleine BW, Doya K, Odoherty J, Sakagami M, editors. Reward and Decision Making in Corticobasal Ganglia Networks. 2007. pp. 123–134. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: A functional magnetic resonance imaging study. Journal of Urology. 2005;174(4):1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JR, Gilster R, van der Horst C, Hamann M, Wolff S, Jansen O. Control of bladder sensations: An fMRI study of brain activity and effective connectivity. Neuroimage. 2009;47(1):18–27. doi: 10.1016/j.neuroimage.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas ES, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Boudreau EA, Hanakawa T, Pagan F, Mari Z, Bara-Jimenez W, Aksu M, Garraux G, Simmons JM, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68(23):1979–1987. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, Bara-Jimenez W, Aksu M, Sato S, Murphy DL, et al. Involvement of Insula and Cingulate Cortices in Control and Suppression of Natural Urges. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Cai WD. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. Journal of Neuroscience. 2007;27(37):9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Year in Cognitive Neuroscience. 2011:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Shan Y, Blair C, Gunter BC, Wang ZS, Marsh R, Peterson BS. An fMRI Study of Frontostriatal Circuits During the Inhibition of Eye Blinking in Persons With Tourette Syndrome. American Journal of Psychiatry. 2010;167(3):341–349. doi: 10.1176/appi.ajp.2009.08121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ. Investigation of the Neural Control of Cough and Cough Suppression in Humans Using Functional Brain Imaging. Journal of Neuroscience. 2011;31(8):2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Adams L, Frackowiak RSJ, Corfield DR. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. Neuroimage. 2008;40(4):1824–1832. doi: 10.1016/j.neuroimage.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Rossing WR. The motor cortex and facial expression: New insights from neuroscience. Neurologist. 2004;10(5):235–249. doi: 10.1097/01.nrl.0000138734.45742.8d. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: A review of recent literature. European Psychiatry. 2007;22(6):387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Nishida S, Narumoto J, Sakai Y, Matsuoka T, Nakamae T, Yamada K, Nishimura T, Fukui K. Anterior insular volume is larger in patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011 doi: 10.1016/j.pnpbp.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF. The temporal dynamics of ties in Gilles de la Tourette syndrome. Biological Psychiatry. 1998;44(12):1337–1348. doi: 10.1016/s0006-3223(98)00176-0. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20(1):351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P. Voluntary pelvic floor muscle control - An fMRI study. Neuroimage. 2006;31(4):1399–1407. doi: 10.1016/j.neuroimage.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Shapiro AK, Shapiro ES, Young JG, Feinberg TE. Gilles de la Tourette Syndrome. New York: Raven Press; 1988. [Google Scholar]
- Shelley BP, Trimble MR. The insular lobe of Reil - its anatamico-functional, behavioural and neuropsychiatric attributes in humans - A review. World Journal of Biological Psychiatry. 2004;5(4):176–200. doi: 10.1080/15622970410029933. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional system: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Tsubota K, Kwong KK, Lee TY, Nakamura J, Cheng HM. Functional MRI of brain activation by eye blinking. Experimental Eye Research. 1999;69(1):1–7. doi: 10.1006/exer.1999.0660. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Zijdewind I, Hoogduin H, Maurits N. Surface EMG measurements during fMRI at 3T: Accurate EMG recordings after artifact correction. Neuroimage. 2005;27(1):240–246. doi: 10.1016/j.neuroimage.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yoon HW, Chung JY, Song MS, Park HW. Neural correlates of eye blinking; improved by simultaneous fMRI and EOG measurement. Neuroscience Letters. 2005;381(1–2):26–30. doi: 10.1016/j.neulet.2005.01.077. [DOI] [PubMed] [Google Scholar]
- Zhang H, Reitz A, Kollias S, Summers P, Curt A, Schurch B. An fMRI study of the role of suprapontine brain structures in the voluntary voiding control induced by pelvic floor contraction. Neuroimage. 2005;24(1):174–180. doi: 10.1016/j.neuroimage.2004.08.027. [DOI] [PubMed] [Google Scholar]