Abstract
Ototoxicity is a dose-limiting side effect of chemotherapeutic treatment with cisplatin. In a series of experiments on neonatal rat cochlear organotypic cultures, the extent of damage induced by a broad range of cisplatin treatment concentrations was examined. Paradoxically, it was found that hair cell loss was greater following 48 h exposure to low (10, 50 and 100 μM) versus high (400 and 1000 μM) concentrations of cisplatin; these findings indicate that hair cells possess intrinsic resistance to high levels of extracellular cisplatin. Using cisplatin conjugated to Alexa Fluor 488, it was found that cisplatin is readily taken up by hair cells at low concentrations, but is largely excluded at high concentrations. Recent studies indicate that the major influx of cisplatin into hair cells occurs via the copper transporter, Ctr1, whereas ATP7A and ATP7B are copper pumps responsible for cisplatin sequestration and efflux. Using immunolabeling procedures for these copper trafficking proteins, it was found that Ctr1 and ATP7B were localized in the hair cells, whereas ATP7A showed extensive labeling in the pillar cells in the organ of Corti. Additional experiments confirmed the protective effect of copper sulfate and cimetidine in attenuating cisplatin-induced hair cell loss. However, because neither copper sulfate nor cimetidine provided complete protection against cisplatin, and high levels of copper sulfate itself were found to be ototoxic, it is suggested that future therapeutic efforts may benefit from a combination of pharmacological treatments which seek to not only limit the uptake of cisplatin into cochlear cells but also increase its efflux.
Keywords: cochlear organotypic cultures, cisplatin, outer hair cell, copper trafficking proteins, copper sulfate, cimetidine
1. INTRODUCTION
Cisplatin is a platinum-based chemotherapy drug widely used to treat a variety of malignant tumors, including ovarian, testicular, head and neck, and lung cancers. In addition to its potent anti-tumor action, cisplatin is highly toxic to the kidney, nervous system and inner ear (for review, see (Rybak et al., 2009)). Of these dose-limiting side effects, ototoxicity is of particular concern for children, as they appear to be at greater risk than adults (Coradini et al., 2007; Li et al., 2004), and the permanent hearing loss associated with cochlear damage can have considerable negative consequences on their language development (Brock et al., 1991; Skinner, 2004).
Cisplatin-induced ototoxicity is initiated by its uptake into the sensory hair cells, neurons and/or supporting cells in the inner ear. Recent studies on multiple cell systems, including an immortalized cochlear cell line (More et al., 2010), have identified copper transporter 1 (Ctr1) as the major influx transporter for platinum-based drugs, such as cisplatin (for review, see (Howell et al., 2010)), whereas the sequestering and efflux of these drugs occurs via two P-type ATPases, copper-transporting ATP7A and ATP7B (for review, see (Safaei, 2006)). In addition to Ctr1, the organic cation transporter, OCT2, has been localized in the mouse cochlea and implicated in cisplatin-induced ototoxicity (Ciarimboli et al., 2010).
It is well established that the uptake of cisplatin through Ctr1 can be competitively inhibited by increased extracellular copper (Ishida et al., 2002; More et al., 2010). Moreover, cimetidine, a histamine H2-receptor antagonist and a pharmacological inhibitor of OCT2, has been shown to attenuate cisplatin uptake in renal tubular cells (Pabla et al., 2009), thereby decreasing cisplatin-induced nephrotoxicity (Ciarimboli et al., 2005). In the present study, the extent of damage induced by a range of cisplatin treatment concentrations was examined, and then a comprehensive investigation of the potential protective effect of copper sulfate and cimetidine on cochlear organotypic cultures exposed to cisplatin was conducted. Neonatal rat cochlear explants were used to address this important chemotherapeutic question as this in vitro preparation allowed for the precise control of the concentration of cisplatin, copper sulfate and cimetidine. Preliminary findings of this work were presented in abstract form at the annual meeting of the Association for Research in Otolaryngology (Ding et al., 2009a; Ding et al., 2009b; Salvi et al., 2009).
2. MATERIALS AND METHODS
2.1 Animal Procedures
Sprague-Dawley rat pups purchased from Charles River Laboratories (Wilmington, MA) were used for this study. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of University at Buffalo, and conform to the guidelines issued by the National Institutes of Health.
2.2 Cochlear organotypic cultures
Our procedures for preparing cochlear organotypic cultures have been described in detail in earlier publications (Ding et al., 2002; McFadden et al., 2003; Wei et al., 2010). On postnatal day 3, rat pups were decapitated and their cochleae were carefully removed and placed in Hank’s Balanced Salt Solution (1X GIBCO, 14175, Invitrogen, Carlsbad, CA). The lateral wall was dissected away and the whole basilar membrane containing the organ of Corti and spiral ganglion neurons was isolated for transfer onto a collagen gel matrix. A drop (12 μl) of rat tail collagen (Type 1, BD Biosciences, 4236 Bedford, MA, 10×basal medium eagle, BME, Sigma B9638, 2% sodium carbonate, 9:1:1 ratio) was placed in the center of a 35 mm diameter culture dish (Falcon 1008, Becton Dickinson) and allowed to gel at room temperature. Afterward, 1.2 ml of serum-free medium consisting of 2 g bovine serum albumin (BSA, Sigma A-4919), 2 ml Serum-Free Supplement (Sigma I-1884), 4.8 ml of 20% glucose (Sigma G-2020), 0.4 ml penicillin G (Sigma P-3414), 2 ml of 200 mM glutamine (Sigma G-6392), and 190.8 ml of 1X BME (Sigma B-1522) were added to the culture dish. The cochlear explant was placed as a flat preparation on the surface of the collagen gel, and incubated overnight (Forma Scientific 3029, 37°C in 5% CO2). On day 2, the culture medium was removed and replaced with fresh medium.
2.3 Ctr1, ATP7A and ATP7B immunolabeling
Cochlear explants (n=6 per condition) were fixed in 10% phosphate buffered formalin (SF100-20, Fisher Scientific) for 30 min (4 °C), washed with 0.01 M phosphate buffered saline (PBS), and then incubated at 4 °C for 48 h with 1% Triton X100 and 5% horse serum in 0.01 M PBS containing Ctr1, ATP7A or AT7B primary antibody (goat-anti-CTR1, Santa Cruz, sc-18473; goat-anti-ATP7A, Santa Cruz, sc-30858, goat-anti ATP7B, Santa Cruz, sc-33826). Specimens were rinsed with 0.1 M PBS, incubated with the appropriate secondary antibody in PBS, rinsed again with 0.01 M PBS, and then immersed in TRITC-labeled phalloidin (Sigma P1951, 1:200 in PBS). Following these immunolabeling procedures, the cochlear explants were mounted on glass slides in glycerin and coverslipped. The immunoreactive products were observed under a confocal microscope (Zeiss LSM510).
2.4 Cisplatin and otoprotective treatments
To determine the relationship between cisplatin concentrations and the ensuing damage, cochlear explants were cultured for 48 h in fresh serum-free medium containing cisplatin at varying concentrations (0, 10, 50, 100, 400 or 1000 μM; n=6 per concentration). To ensure that cisplatin’s efficacy was not compromised at these concentrations, equivalent concentrations were tested on human retinoblastoma cancer cells with a known chemosensitivity to cisplatin (Di Nicolantonio et al., 2003). RB143 human retinoblastoma cells were grown in Dulbecco’s Modified Eagle’s Medium with 20% calf serum at 37 °C in 5% CO2/95% room air conditions. Cisplatin (0, 10, 50, 100, 400 and 1000 μM) was added at time zero. Cell number was evaluated at 24 and 48 h by manual cell counting with a hemocytometer chamber under 20X magnification.For each experiment, cell counts were carried out in triplicate.
In a separate series of experiments, the protective effects of copper sulfate and cimetidine on cisplatin-treated cultures were assessed. Cochlear explants (n=6 per treatment condition) were cultured with cisplatin alone (10 or 50 μM), copper sulfate alone (10, 50, 100, 200 or 500 μM), or the combination of cisplatin (10 or 50 μM) and copper sulfate (10, 50 or 100 μM) for 48 h. Furthermore, an additional group of cochlear organotypic cultures (n=6 per treatment condition) were treated with cisplatin alone (10 or 50 μM), cimetidine alone (10, 100, 2000 or 4000 μM) or cisplatin (10 or 50 μM) plus cimetidine (10, 100 or 2000 μM) for 48 h.
2.5 Culture histology: stereocilia and neurofilament labeling
At the end of a treatment experiment, hair cell stereocilia were stained for visualization using the following procedures. First, the cochlear tissue was fixed with 10% phosphate buffered formalin for 4 h. After fixation, specimens were rinsed in 0.01 M PBS, incubated in 0.25% Triton X-100 for 5 min and immersed in TRITC-labeled phalloidin (Sigma P1951, 1:200) in PBS for 30 min. Specimens were rinsed in 0.01M PBS, and mounted on glass slides in glycerin, coverslipped and examined using the confocal microscope.
A separate group of cochlear explants (n=4) were prepared for neurofilament labeling by first fixing the tissue for 30 min in 10% phosphate buffered formalin. Specimens were immersed overnight (4 °C) in a solution containing 20 μl of mouse anti-neurofilament 200 kD antibody (Sigma N0142, clone N52), 20 μl Triton X-100 (10%), 6 μl normal goat serum, and 154 μl of 0.01 M PBS. Specimens were then rinsed with 0.01 M PBS, immersed in a solution containing 2 μl of secondary anti-mouse IgG TRITC (Sigma T5393), 12 μl normal goat serum, 40 μl Triton X-100 (10%) and 345 μl of 0.01 M PBS, and then rinsed with 0.01 M PBS. To label the stereocilia and the cuticular plate of the hair cells, specimens were stained with Alexa Fluor 488 phalloidin (Invitrogen A12379). Finally, specimens were mounted on glass slides in glycerin, coverslipped and examined using the confocal microscope.
2.6 Cochleograms
For sensory hair cell counting, cochlear cultures whose stereocilia had been labeled (see above) were observed under a fluorescent microscope (Zeiss Axioskop) equipped with appropriate filters for TRITC (absorption: 544 nm, emission: 572 nm). The number of missing cochlear hair cells for each 0.24-mm segment was counted over the entire length of the organ of Corti as described previously (Qi et al., 2008). A cochleogram was constructed by plotting the percent inner (IHC) and outer hair cell (OHC) loss as a function of the percent distance from the apex of the cochlea. For analysis, individual cochleograms were grouped to generate a mean cochleogram for each treatment condition using custom software. Because each end of the basilar membrane is damaged during the surface preparation, comparisons between hair cell counts were measured between 20% and 80% of the distance from the apex of the cochlea.
2.7 Uptake of Alexa Fluor 488-labeled cisplatin
The accumulation of cisplatin in hair cells was monitored using a custom formulation of cisplatin conjugated to a fluorescent probe. Dye-labeled cisplatin derivative was obtained by conjugating Alexa Fluor 488 hydrazide (Invitrogen) to carbohydrate-linked platinum(II) complex cis-dichloro[(2-β-D-glucopyranosidyl)propane-1,3-diamine]platinum. The carbohydrate-linked cisplatin derivative was obtained according the methods described by Chen and colleagues (Chen et al., 1999) whereas the labeling of Alexa Flour 488 was performed according the manufacturer’s instructions. The carbohydrate-linked cisplatin derivative was first converted into an aldehyde, which could readily react with hydrazines, hydroxylamines or primary amine–containing compounds, such as Alexa Fluor 488 hydrazide. Cochlear cultures were treated with Alexa Fluor 488-cisplatin (10, 50, 100, 400 or 1000 μM; n=5 per concentration) or Alexa Fluor 488 alone for 48 h. For control experiments, unconjugated Alexa Fluor 488 (Alexa Fluor 488 alone) was prepared by inactivating a 10 mM stock of Alexa Fluor 488 carboxylic acid, succinimidyl ester in dionized water. The amine-reactive succinimide was inactivated by incubation at room temperature overnight in the dark. In all cases, after 48 h of treatment, the cochlear tissue was fixed (10% phosphate buffered formalin for 4 h) and then rinsed with 0.01M PBS and stained with TRITC-labeled phalloidin. Specimens were mounted on glass slides in glycerin, and once coverslipped, they were examined under the confocal microscope with appropriate filters for TRITC (absorption: 544 nm, emission: 572 nm) and for Alexa Fluor 488 fluorescent signals (excitation 488nm, emission 520 nm).
2.8 FM1-43 uptake
FM1-43, a small styryl dye that labels hair cells by passing through the mechano-electrical transduction channels in the stereocilia, was used to monitor the functional status of the transduction apparatus in cochlear cultures (Meyers et al., 2003). After 48 h of exposure to cisplatin (10, 50 or 1000 μM), cochlear tissue (n=4 per condition) was incubated for 30 min in freshly made FM1-43 (Invitrogen, 30 μg/ml in serum-free medium), then fixed with 10% phosphate buffered formalin for 40 min. Following fixation, the specimens were stained with Alexa Fluor 488-labeled phalloidin for 30 min, and mounted in glycerin on glass slides and coverslipped. Specimens were then examined under the confocal microscope using appropriate filters to detect the green fluorescence of phalloidin-labeled F-actin on the stereocilia bundles and cuticular plate of hair cells (excitation 488nm, emission 520 nm) and FM1-43’s red fluorescent properties (excitation 568 nm, emission 580 nm) which identified its uptake by the cochlear hair cells.
Additional experiments using FM1-43 were performed in which cochlear explants were treated with either a low (50 μM; n=6) or high concentration (1000 μM; n=6) of cisplatin for 18 h, after which time the medium containing cisplatin was purged, and the tissue was cultured with the normal serum-free medium for another 18 h. The level of FM1-43 uptake was assessed following this ‘recovery period’ using the procedures described above.
3. RESULTS
3.1 Localization of copper transporter Ctr1 and copper pumps ATP7A and ATP7B in the cochlea
The location of copper trafficking proteins in the cochlea was determined using immunolabeling with antibodies against Ctr1, ATP7A and ATP7B. Ctr1 or CTR1 are names for the rat and human copper transporter genes Slc31a1 and SLC31A1 (solute carrier family 31 (copper transporters) member 1). As shown in Fig. 1, the copper transport proteins were found in abundance in the organ of Corti (Fig. 1A-C), epithelium of the stria vascularis (Fig. 1D-F), and spiral ganglion neurons (Fig. 1G-I). Ctr1 and ATP7B were localized in the hair cells (Fig. 1A and 1C), whereas ATP7A showed extensive labeling in the pillar cells in the organ of Corti (Fig. 1B).
Fig. 1.

In the normal cochlea, the copper transporter Ctr1 and copper pumps ATP7A and ATP7B were localized in the organ of Corti (A-C), epithelium of the stria vascularis (D-F), and the spiral ganglion neurons (G-I). Representative confocal photomicrographs of neonatal rat cochlear organotypic cultures show immunolabeled (green) Ctr1 (A, D and G), ATP7A (B, E and H) and ATP7B (C, F and I). (A-C) Vertical plane images provide a surface view of the organ of Corti; Ctr1 (A) and ATP7B (C) were localized in the outer hair cells (OHC), whereas ATP7A (B) showed extensive labeling in the pillar cells (PC). (D-F) The marginal (MC), intermediate (IC) and basilar (BC) cell layers of the stria vascularis are shown in cross-section. (G-I) Note that yellow-labeled spiral ganglion neurons (SGN) are the result of overlap of green and red labeling. Scale bars shown in panels A, D, and G each represent 10 μm.
3.2 Cisplatin concentration-damage response relationship
Examination of the organ of Corti following 48 h of cisplatin exposure at various concentrations (10, 50, 100, 400 and 1000 μM) revealed an unusual concentration-damage response relationship; hair cell loss increased up to 50 μM cisplatin, but then dropped off precipitously at concentrations higher than 100 μM as shown in Fig. 2 and 3. Similarly, neurofilament staining showed that spiral ganglion neurons and auditory nerve fibers were destroyed at a low concentration of cisplatin (50 μM; Fig. 3B), but remained intact at a high concentration (1000 μM; Fig. 3C). These novel results led us to further investigate two possibilities: that at high concentrations, the cytotoxicity of cisplatin was compromised, or alternatively, that mechanisms intrinsic to the hair cells prevented intracellular accumulation of cisplatin.
Fig. 2.

Cochlear hair cell loss occurred after 48 h exposure to low, but not high concentrations of cisplatin. (A) Representative confocal photomicrographs showing inner (IHC) and outer hair cells (OHC) in the middle turn of the cochlear basilar membrane of control and cisplatin-treated (10, 50, 100, 400 or 1000 μM) cultures. Note the severe hair cell loss following 48 h exposure to 50 μM cisplatin (Cis50), whereas most hair cells were intact following high concentrations of cisplatin (Cis400 and Cis1000). Scale bar shown in upper left panel represents 10 μm. (B) Mean cochleograms for each treatment condition (control, 10, 50, 100, 400 or 1000 μM cisplatin) show the degree of hair cell loss as a function of the percent distance from the apex of the cochlea. Hair cell loss was fairly uniform throughout the length of the cochlea following exposure to 10, 50 and 100 μM cisplatin. The minor hair cell loss observed at each end of the basilar membrane in the control, 400 and 1000 μM cisplatin-treated cultures was an artifact associated with the surface preparation. (C) Comparison of the mean number of surviving hair cells (measured between 20% and 80% of the distance from the apex) for each treatment condition revealed unexpectedly that low concentrations of cisplatin (10, 50 and 100 μM) were more ototoxic than high concentrations (400 and 1000 μM).
Fig. 3.

Low, but not high concentrations of cisplatin destroyed spiral ganglion neurons (SGNs) and auditory nerve fibers (ANFs). (A-C) Representative confocal photomicrographs of control and cisplatin-treated (50 and 1000 μM) cochlear organotypic cultures showing green-labeled (Alexa Fluor 488-phalloidin) hair cells (upper three rows= OHCs; bottom row= IHCs), and red-labeled (anti-neurofilament 200 antibody) ANFs and SGNs. (B) Following 48 h exposure to 50 μM cisplatin, there was considerable damage to the ANFs and SGNs. (C) In contrast, no damage was observed after exposure to 1000 μM cisplatin. Scale bar shown in panel A represents 10 μm.
To determine whether the lack of cochlear damage induced by high concentrations of cisplatin occurred because its cytotoxic efficacy had been reduced (e.g., 1000 μM is approaching the aqueous solubility limit of cisplatin), the survival of cancer cells (human RB143 retinoblastoma cells) exposed to various cisplatin concentrations (0-1000 μM) was compared. Because a concentration-dependent decrease in the number of surviving cancer cells was observed as shown in Fig. 4, we conclude that cisplatin’s potency was not compromised at the high concentrations used in our cochlear organotypic culture experiments.
Fig. 4.
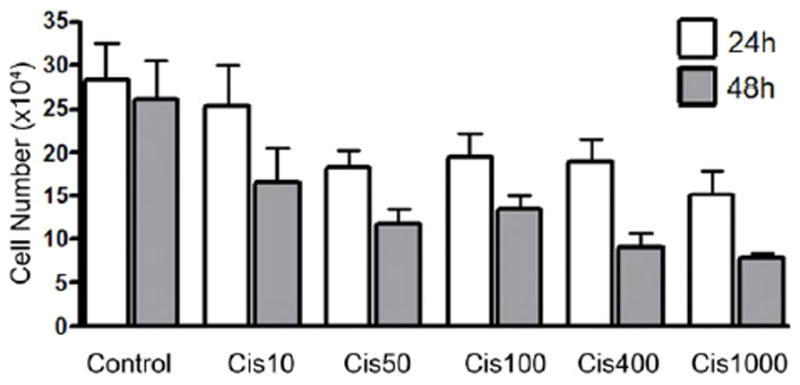
Cisplatin treatment caused a decrease in cell number of RB143 cells in a concentration-dependent manner. Human RB143 retinoblastoma cells were treated with 0-1000 μM of cisplatin, and cell number was measured at 24 and 48 h. As the cisplatin concentration increased, there was a decrease in cell number, which supports the conclusion that cisplatin’s cytotoxic potency was not compromised at the high concentrations used in the cochlear culture experiments.
3.3 Intracellular accumulation of cisplatin
The intracellular accumulation of cisplatin at different concentrations was monitored by conjugating it with a fluorescent probe, Alexa Fluor 488, and treating cochlear organotypic cultures for 48 h. Exposure at 50 μM resulted in considerable accumulation of Alexa Fluor 488-cisplatin in cochlear cells, with greater uptake in the OHCs than IHCs, but only limited uptake in supporting cells as shown in Fig 5A. These findings are consistent with previous studies showing that cisplatin preferentially damages OHCs (Cardinaal et al., 2000; van Ruijven et al., 2004). In contrast to the low concentration treatment, cochleae exposed to 1000 μM showed little accumulation of Alexa Fluor 488-cisplatin in the hair cells as shown in Fig 5B. Control experiments revealed that the hair cells failed to uptake the Alexa Fluor 488 when it was administered alone to the cochlear cultures (data not shown).
Fig. 5.
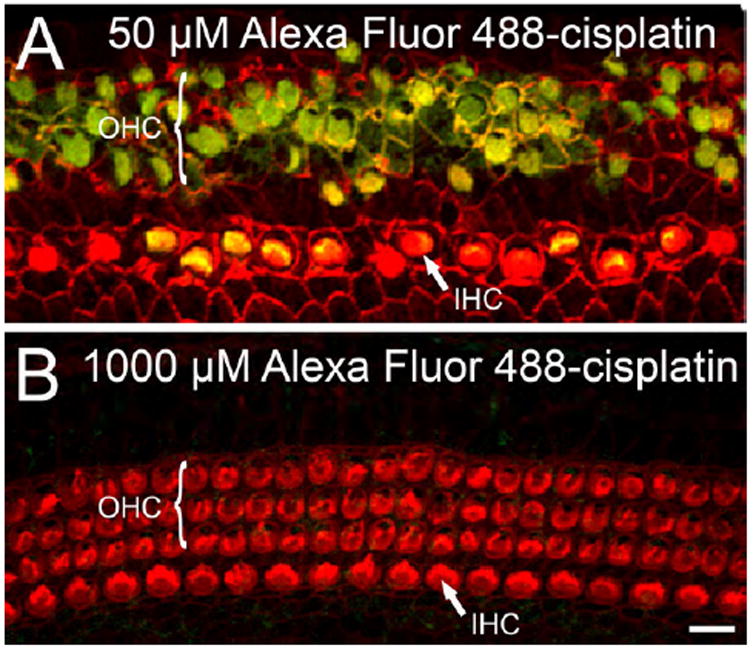
Accumulation of cisplatin in hair cells was greater following 48 h exposure to a low-versus high concentration. (A and B) Representative confocal photomicrographs show the intracellular accumulation of cisplatin conjugated to a fluorescent probe, Alexa Fluor 488 (green), in OHCs and IHCs. (A) At a concentration of 50 μM, Alexa Fluor 488-cisplatin accumulated preferentially in the OHCs. Consistent with this cisplatin accumulation, the OHCs showed significant structural damage. (B) Following treatment at 1000 μM, hair cells were intact and showed little accumulation of Alexa Fluor 488-cisplatin. Scale bar shown in panel B represents 10 μm.
3.4 Stereocilia damage induced by low- but not high concentrations of cisplatin
As shown in Fig. 6, FM1-43 was robustly taken up by normal (untreated) hair cells, but not the surrounding supporting cells (Fig. 6A). In contrast, FM1-43 uptake was almost completely blocked in OHCs and greatly reduced in the IHCs after 48 h treatment with 10 μM cisplatin as shown in Fig. 6B. When cochlear cultures were treated with 50 or 1000 μM cisplatin, FM1-43 was barely detectable within the hair cell cytoplasm as observed in Fig. 6C and 6D. Under these conditions, it is possible that FM1-43 failed to enter the hair cells because cisplatin 1) blocked the mechano-electrical transduction channel (Kimitsuki et al., 1993); 2) induced an intrinsic mechanism which restricted overall cellular uptake, and/or 3) damaged the stereocilia.
Fig. 6.
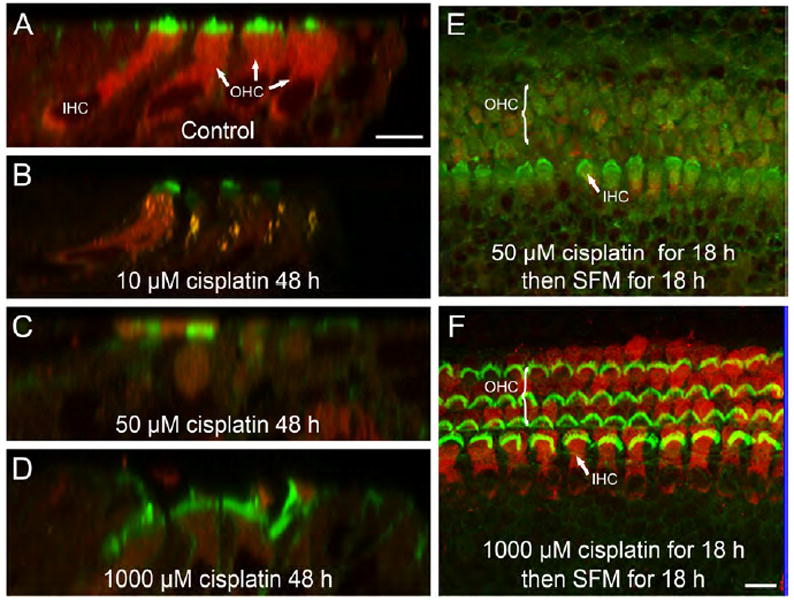
Stereocilia damage was induced by low, but not high concentrations of cisplatin. (A-D) Representative confocal micrographs show the uptake of FM1-43 (red) in IHCs and OHCs in control and cisplatin-treated (10, 50 and 1000 μM) cultures. Hair cell stereocilia are labeled green. (E and F) FM1-43 uptake was visualized after 18 h of cisplatin treatment (50 or 1000 μM) followed by 18 h of exposure to normal serum-free medium (SFM). Unlike hair cells exposed to 50 μM cisplatin (E), those treated with a high concentration of cisplatin (F) were able to uptake FM1-43 after the ‘recovery period’, which indicated that the stereocilia were not permanently damaged. Scale bars shown in panels A and F each represent 10 μm.
To determine if the extent of cisplatin-induced stereocilia damage differed between the low (50 μM) and high (1000 μM) concentration treatments, the uptake of FM1-43 was examined after the cisplatin-containing medium had been replaced with normal serum-free medium for an additional 18 h. Interestingly, cochlear explants exposed previously to a high concentration of cisplatin were capable of FM1-43 uptake after the ‘recovery period’ as shown in Fig. 6F, whereas little FM1-43 uptake was observed in cochlear explants exposed previously to a low concentration of cisplatin as shown in Fig. 6E. Thus, unlike a low concentration treatment of cisplatin (Fig. 2 and 6E), exposure to a high concentration of cisplatin failed to cause substantial hair cell loss (Fig. 2) or permanent damage to the stereocilia (Fig. 6F).
3.5 Copper sulfate and cisplatin-induced ototoxicity
To investigate whether co-administration of copper sulfate could protect cochlear organotypic cultures from cisplatin-induced damage, cochlear explants were treated for 48 h with 10 μM or 50 μM of cisplatin, 10-500 μM of copper sulfate, or the combination of cisplatin plus copper sulfate. Copper sulfate alone had no adverse effects at 200 μM or less, whereas exposure to 500 μM damaged the hair cells (data not shown). Specimens treated with 10 μM of cisplatin sustained ~20% hair cell loss; however, the addition of copper sulfate completely blocked the cisplatin-induced damage as shown in Fig. 7A. When cochlear explants were treated with 50 μM cisplatin, however, ~80% of the hair cells were destroyed, and co-administration of copper sulfate was ineffective at preventing this severe hair cell loss as shown in Fig. 7B.
Fig. 7.
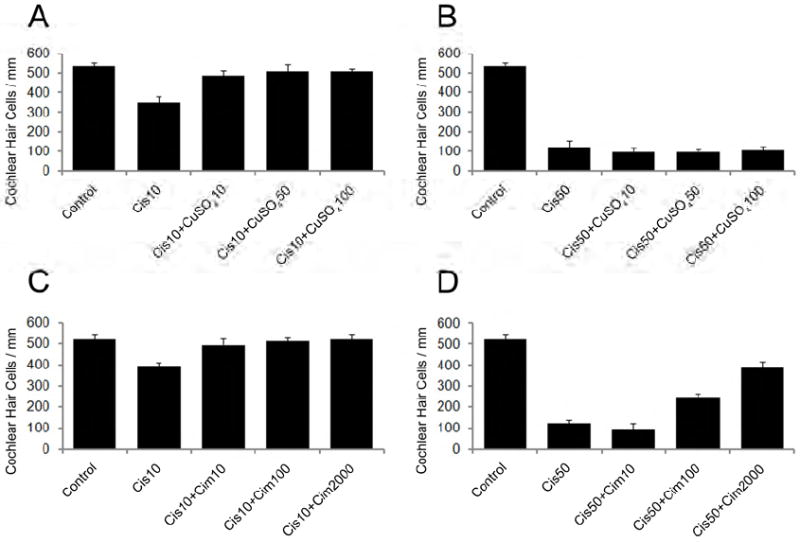
Copper sulfate (A-B) and cimetidine (C-D) provided some protection to hair cells when co-administered with cisplatin. The mean number of surviving hair cells was counted for cochlear explants (n=6 per condition) exposed for 48 h to 10 μM (A, C) or 50 μM (B, D) cisplatin, the combination of cisplatin plus copper sulfate (CuSO4; 10, 50 or 100 μM) (A,B), or the combination of cisplatin plus cimetidine (10, 100 or 2000 μM) (C,D).
3.6 Cimetidine and cisplatin-induced ototoxicity
To determine if cimetidine could protect against cisplatin-induced hair cell damage, cochlear explants were treated with 10 μM or 50 μM of cisplatin, cimetidine (10, 100, 2000 or 4000 μM), or the combination of cisplatin plus cimetidine. First, it was found that cimetidine concentrations at 2000 μM or less had no adverse effects on the hair cells; however, 4000 μM cimetidine disrupted hair cell stereocilia bundles (data not shown). Again, treatment of cochlear cultures with 10 μM or 50 μM of cisplatin damaged ~20% or ~80% of the hair cells, respectively (Fig. 7C and 7D). When cimetidine (10, 100 or 2000 μM) was co-administered to cultures exposed to 10 μM cisplatin, nearly 100% of the hair cells survived as shown in Fig. 7C. An otoprotective effect was also observed if cimetidine was co-administered with 50 μM cisplatin; in cultures co-treated with 100 μM of cimetidine, hair cell loss decreased to ~50%, and when the concentration of cimetidine was increased to 2000 μM, hair cell loss decreased to ~20% as shown in Fig. 7D.
4. DISCUSSION
In a series of experiments on neonatal rat cochlear explants, the extent of damage induced by a very broad range of cisplatin treatment concentrations was examined. Unexpectedly, it was found that hair cell loss was greater following exposure to low (50 μM) versus very high (1000 μM) concentrations of cisplatin. Similarly, SGNs and ANFs were destroyed after low, but not high concentrations of cisplatin. Cochlear cultures treated for 48 h with low concentrations of cisplatin (10-50 μM) showed considerable stereocilia bundle damage (Fig. 2A); when cisplatin was removed from the medium and cultured for an additional 18 h, the stereocilia failed to regenerate and the hair cell no longer took up FM1-43 (Fig. 6E). However, unlike a low concentration of cisplatin, exposure at a high concentration did not permanently damage the stereocilia, as evidenced by the hair cell’s ability to uptake the FM1-43 dye through the mechano-electrical transduction channel once cisplatin had been removed from the culture medium (Fig 6F). By conjugating cisplatin with Alexa Fluor 488, it was found that the minimal hair cell loss observed at high concentrations coincided with limited intracellular accumulation of cisplatin. Collectively, these results indicate that high levels of extracellular cisplatin are not sufficient to induce hair cell loss or stereocilia damage; rather, intracellular accumulation of cisplatin is necessary for ototoxicity and stereocilia damage to occur.
The copper transporter, Ctr1, has been identified as a major influx transporter for platinum-based drugs, such as cisplatin (Holzer et al., 2006; Larson et al., 2009). Consistent with a recent study on 3-4 week old mouse cochleae (More et al., 2010), Ctr1 was localized in the organ of Corti, epithelium of the stria vascularis, and spiral ganglion neurons of our postnatal day 3 rat cochlear organotypic cultures. That these structures contain Ctr1 in greater abundance than supporting cells may help explain why these structures represent the three major targets of cisplatin-induced cochlear damage (Ding et al., 2008; van Ruijven et al., 2004).
Additional experiments investigated whether cisplatin-induced hair cell damage in cochlear cultures could be reduced by co-administering copper sulfate, a Ctr1 competitive inhibitor, or cimetidine, an inhibitor of OCT2. Although the severe hair cell loss caused by 50 μM cisplatin was not attenuated, copper sulfate effectively blocked the damage induced by exposure to 10 μM cisplatin. In a recent study, More and colleagues (More et al., 2010) found that cisplatin uptake decreased by ~40% in mouse cochlear cultures co-incubated with cisplatin (10 μM) and copper sulfate (1 mM) for 2 h; findings which could underlie the otoprotective effects of copper sulfate observed in the present study. One must be cautious, however, not to over-extend the comparison between the present study and that by More and colleagues (More et al., 2010), as the concentration of copper sulfate they used for 2 h in their 12-d-old mouse cultures exceeded that which was found to be safe for hair cells in our rat cochlear cultures incubated for 48 h. Ultimately, similar to cells in other systems (Ishida et al., 2002), our results indicate that hair cells can be modestly protected from the ototoxic effects of cisplatin by co-administration of copper sulfate. In addition to copper sulfate, cimetidine is able to limit cisplatin uptake (Pabla et al., 2009). In the present study, co-administration of cimetidine provided considerable protection against cisplatin-induced hair cell loss in cochlear cultures. In support of this finding, Ciarimboli and colleagues (Ciarimboli et al., 2010) reported recently that ototoxicity was prevented in mice co-medicated with cisplatin and cimetidine.
While the present results suggest that copper sulfate and cimetidine may suppress cisplatin uptake through copper transporters, a more convincing test of this hypothesis would be to knockdown the expression of the transporters by RNA inhibition or the use of knockout mice lacking these transporters. The results from these organotypic culture studies suggest that cisplatin uptake into hair cells is a critical step in hair cell death and that round window application of copper sulfate, cimetidine or siRNA against Ctr1 to suppress the influx of cisplatin into hair cells may be effective methods of suppressing cisplatin ototoxicity without compromising its antitumor efficacy. Preliminary studies (unpublished results) from our lab suggest that application of copper sulfate to the round window membrane can suppress IHC damage in chinchillas treated with ototoxic doses of carboplatin, an analog of cisplatin.
In summary, using cochlear organotypic cultures, we have presented the first evidence of hair cells’ intrinsic resistance to high levels of extracellular cisplatin. These findings were initially discovered serendipitously in an effort to establish the full concentration-response range for cisplatin ototoxicity in postnatal cochlear cultures. Because these results were obtained using neonatal cochlear cultures, it is unclear if the resistance to high levels of cisplatin is due the immaturity of the hair cells or transduction channels. Since FM1-43 enters the hair cells through the transduction channel of our cochlear cultures as well as adult animals (Meyers et al., 2003), it seems unlikely that maturation of the transduction channels is responsible for this resistance. Regardless of the exact mechanism, the issue of hair cell maturation could be addressed in future studies that test the ototoxicity of cisplatin using adult organ cultures from the inner ear or vestibular system where it is possible to apply a wide range of cisplatin concentrations while avoiding problems associated with nephrotoxicity. In addition, we have confirmed the protective effect of copper sulfate and cimetidine in attenuating cisplatin-induced hair cell loss. Because neither copper sulfate nor cimetidine provided complete protection against cisplatin, and high levels of copper sulfate itself were found to be ototoxic, it is suggested that future therapeutic efforts may benefit from a combination of pharmacological treatments which seek to not only limit the uptake of cisplatin into cochlear cells but also increase its efflux.
HIGHLIGHTS.
Cisplatin ototoxicity was examined in neonatal rat cochlear organotypic cultures
Greater hair cell loss after low vs high (50 vs 1000 μM) concentration of cisplatin
Spiral ganglion neurons destroyed after low but not high concentration of cisplatin
Limited intracellular accumulation of cisplatin with high concentration exposure
Copper sulfate and cimetidine attenuated cisplatin-induced hair cell loss
Acknowledgments
This research was supported in part by National Institutes of Health grant 5R01DC006630-05. GMS is supported by R21CA127061, a departmental challenge grant from Research to Prevent Blindness and U54CA143876 from the National Cancer Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Our special thanks to Dhruba J Barali for providing us the Alexa Flur 488-labeled cisplatin, and to Donald E. Coling for preparing the negative control Alexa probe and providing helpful comments during manuscript preparation. We thank Weidong Qi, Yong Fu, Yongqi Li, Lei Wei and Raquel Lima for technical support.
ABBREVIATIONS
- ANF
auditory nerve fiber
- ATP
adenosine triphosphate
- ATP7A
copper-transporting ATPase
- ATP7B
copper-transporting ATPase
- BC
basilar cell layer
- Ctr1
copper transporter 1
- IC
intermediate cell layer
- IHC
inner hair cell
- MC
marginal cell layer
- OCT2
organic cation transporter 2
- OHC
outer hair cell
- PBS
phosphate buffered saline
- PC
pillar cell
- SGN
spiral ganglion neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Dose-dependent effect of 8-day cisplatin administration upon the morphology of the albino guinea pig cochlea. Hear Res. 2000;144:135–146. doi: 10.1016/s0378-5955(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Heeg MJ, Braunschweiger PG, Xie W, Wang PG. A carbohydrate-line cisplatin analogue having antitumor activity. Angewandte Chemie International Edition. 1999;38:1768–1769. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1768::AID-ANIE1768>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–1484. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kaminsky C, am Zehnhoff-Dinnesen A, Schinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29:355–360. doi: 10.1097/MPH.0b013e318059c220. [DOI] [PubMed] [Google Scholar]
- Di Nicolantonio F, Neale M, Onadim Z, Hungerford JL, Kingston JL, Cree IA. The chemosensitivity profile of retinoblastoma. Recent Results Cancer Res. 2003;161:73–80. doi: 10.1007/978-3-642-19022-3_7. [DOI] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002;164:115–126. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Ding D, He J, Salvi R, Coling D. Extracellular copper modulates cisplatin ototoxicity. Abstr Assoc Res Otolaryngol. 2009a;121:42. [Google Scholar]
- Ding D, He J, Yu D, Jiang H, Salvi R. Surprise- Extremely high doses of cisplatin do not destroy hair cells in rat cochlear cultures. Abst Assoc Res Otolaryngology. 2009b;119:41. [Google Scholar]
- Ding D, Qi W, Zhang M, Wang P, Jiang H, Salvi R. Cisplatin and its ototoxicity. Chinese Journal of Otology. 2008;6:125–133. [Google Scholar]
- Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–894. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–14302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimitsuki T, Nakagawa T, Hisashi K, Komune S, Komiyama S. Cisplatin blocks mechano-electric transducer current in chick cochlear hair cells. Hear Res. 1993;71:64–68. doi: 10.1016/0378-5955(93)90021-r. [DOI] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75:324–330. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Salvemini D, Salvi RJ. M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol Appl Pharmacol. 2003;186:46–54. doi: 10.1016/s0041-008x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–186. doi: 10.1620/tjem.219.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei R. Role of copper transporters in the uptake and efflux of platinum containing drugs. Cancer Lett. 2006;234:34–39. doi: 10.1016/j.canlet.2005.07.046. [DOI] [PubMed] [Google Scholar]
- Salvi R, He J, Ding D, Coling D. Cimetidine, a copper transporter inhibitor, reduces cisplatin ototoxicity in vitro. Abstr Assoc Res Otolaryngol. 2009;756:256. [Google Scholar]
- Skinner R. Best practice in assessing ototoxicity in children with cancer. Eur J Cancer. 2004;40:2352–2354. doi: 10.1016/j.ejca.2004.08.002. [DOI] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Smoorenburg GF. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hear Res. 2004;197:44–54. doi: 10.1016/j.heares.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience. 2010;168:288–299. doi: 10.1016/j.neuroscience.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


