Abstract
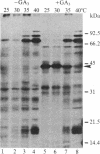
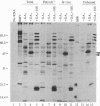
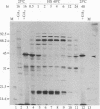
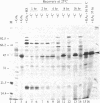
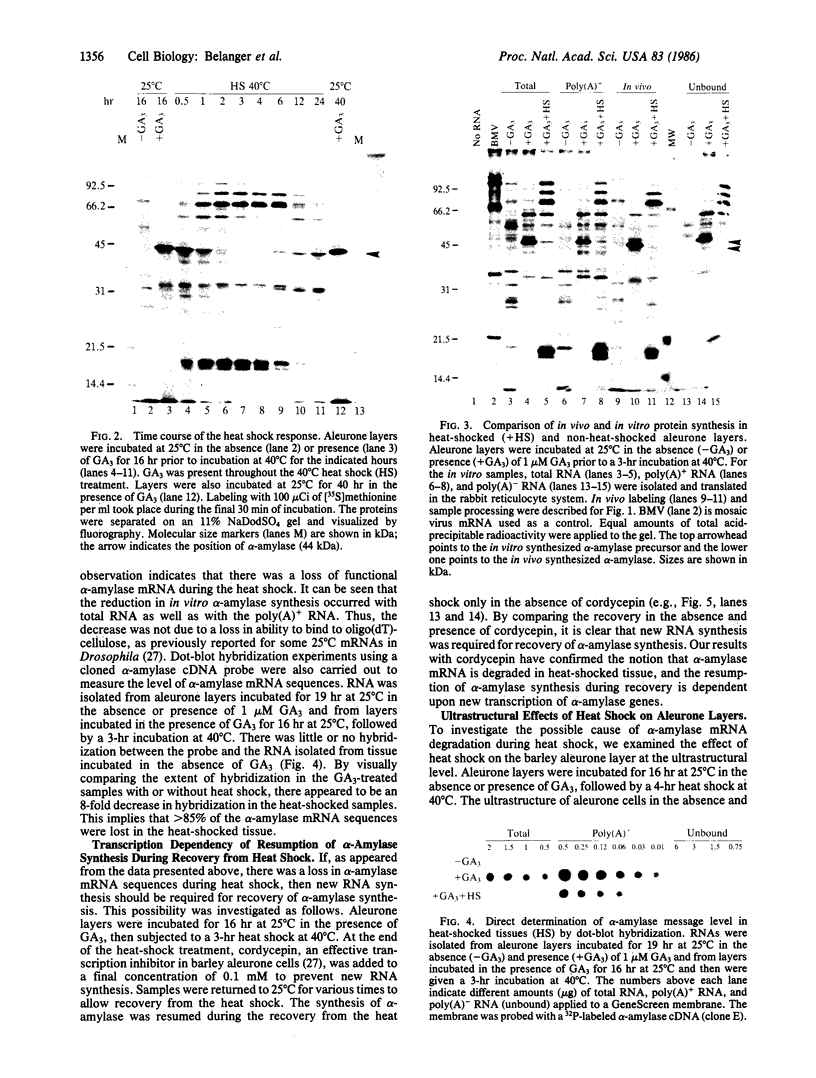
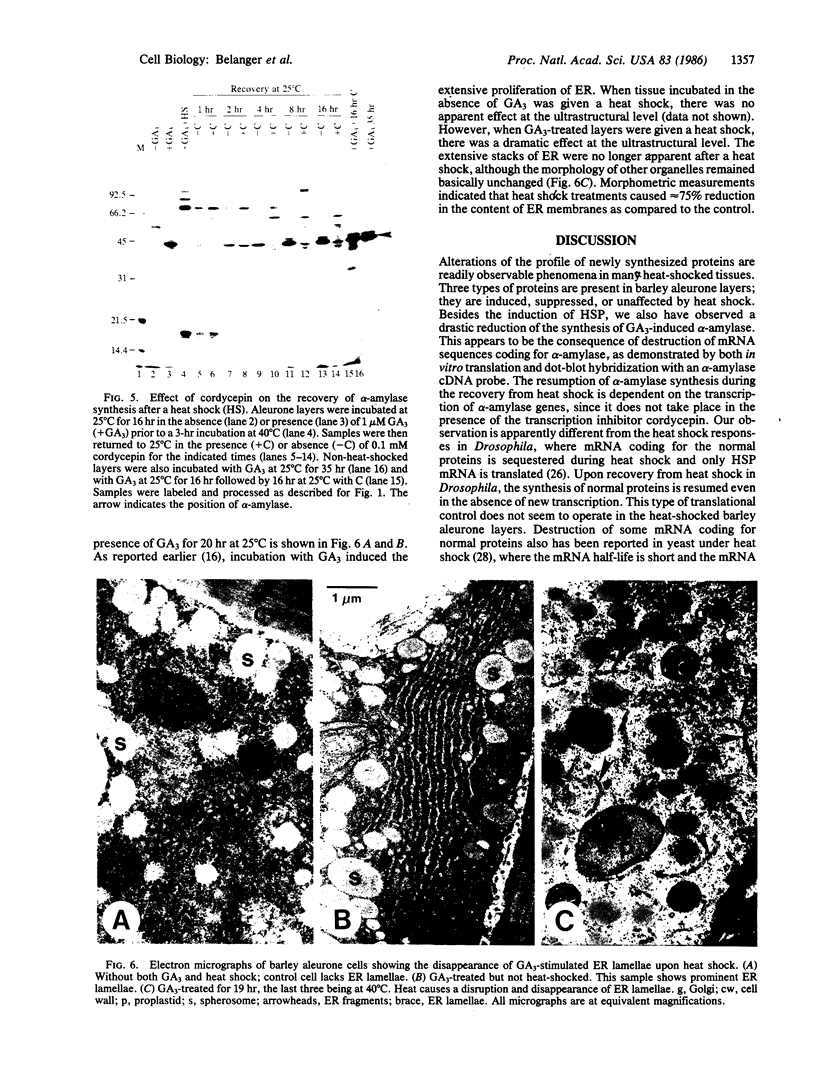
In response to a phytohormone, gibberellic acid, the aleurone layers of barley seeds synthesize and secrete alpha-amylases, which are coded by a set of stable mRNAs. When aleurone layers are subjected to heat shock treatment, the synthesis of alpha-amylase is suppressed while heat shock proteins are induced. The suppression of alpha-amylase synthesis is not the result of translational control as reported in several other systems. Rather, the sequences of alpha-amylase mRNA are rapidly degraded during heat shock as shown by in vitro translation and dot blot hybridization with a cDNA probe. Upon recovery from heat shock, the tissue resumes the synthesis of alpha-amylase in 2-4 hr. However, in the presence of a transcription inhibitor, cordycepin, the resumption of synthesis of alpha-amylase does not take place, indicating that new transcription of alpha-amylase genes is necessary for this recovery process. The degradation of alpha-amylase mRNAs correlates with the rapid destruction of endoplasmic reticulum as observed by electron microscopy, a phenomenon that has not been reported previously as a heat shock response. Since alpha-amylase mRNA is associated with the endoplasmic reticulum via membrane-bound polyribosomes, we suggest that the destruction of the endoplasmic reticulum during heat shock causes the destabilization and the eventual degradation of alpha-amylase mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Ubiquitin is a heat shock protein in chicken embryo fibroblasts. Mol Cell Biol. 1985 May;5(5):949–956. doi: 10.1128/mcb.5.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. A., Casson L. P., Goldberg A. L. Heat shock regulatory gene htpR influences rates of protein degradation and expression of the lon gene in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6647–6651. doi: 10.1073/pnas.81.21.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. T., Varner J. E. Hormonal control of messenger ribonucleic acid metabolism in barley aleurone layers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4783–4786. doi: 10.1073/pnas.71.12.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Shiu E. C., Hahn G. M. Similarities in cellular inactivation by hyperthermia or by ethanol. Radiat Res. 1980 May;82(2):257–268. [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Chandra G. R., Maxwell E. S. Hormonal control of alpha-amylase gene expression in barley. Studies using a cloned CDNA probe. J Biol Chem. 1983 Feb 25;258(4):2370–2375. [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Vierling E., Key J. L. Ribulose 1,5-Bisphosphate Carboxylase Synthesis during Heat Shock. Plant Physiol. 1985 May;78(1):155–162. doi: 10.1104/pp.78.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L., Ruddat M. Effect of gibberellic Acid and actinomycin d on the formation and distribution of rough endoplasmic reticulum in barley aleurone cells. Plant Physiol. 1973 Mar;51(3):549–558. doi: 10.1104/pp.51.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig S., Hensse S., Keitel C., Elsner C., Wittig B. Heat shock gene expression is regulated during teratocarcinoma cell differentiation and early embryonic development. Dev Biol. 1983 Apr;96(2):507–514. doi: 10.1016/0012-1606(83)90187-2. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P., Perlman A. J., Tata J. R. Transient paralysis by heat shock of hormonal regulation of gene expression. EMBO J. 1984 Dec 1;3(12):2763–2770. doi: 10.1002/j.1460-2075.1984.tb02207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. T., Wallner S. J. Heat stress responses in cultured plant cells : development and comparison of viability tests. Plant Physiol. 1983 Jul;72(3):817–820. doi: 10.1104/pp.72.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. M., Mascarenhas J. P. High temperature-induced thermotolerance in pollen tubes of tradescantia and heat-shock proteins. Plant Physiol. 1985 Aug;78(4):887–890. doi: 10.1104/pp.78.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]