Summary of recent advances
Protozoan parasites cause tremendous human suffering worldwide, but strategies for therapeutic intervention are limited. Recent studies illustrate that the paradigm of microbes as social organisms can be brought to bear on questions about parasite biology, transmission and pathogenesis. This review discusses recent work demonstrating adaptation of social behaviors by parasitic protozoa that cause African sleeping sickness and malaria. The recognition of social behavior and cell-cell communication as a ubiquitous property of bacteria has transformed our view of microbiology, but protozoan parasites have not generally been considered in this context. Works discussed illustrate the potential for concepts of sociomicrobiology to provide insight into parasite biology and should stimulate new approaches for thinking about parasites and parasite-host interactions.
Keywords: Cell-cell communication, social behavior, Trypanosoma, Plasmodium
Introduction
Social behaviors are most widely recognized in the communication and cooperation observed in metazoans, ranging from navigation strategies and group hierarchies in insect communities to complex social networking in humans and other primates. However, communication and cooperation among individuals in a group also occurs at the cellular level, as illustrated in collective motility of migrating cells during wound healing, tissue morphogenesis and tumor metastases. Moreover, cell-cell communication and cooperative behavior is not restricted to higher animals and recent years have seen a surge in the study and understanding of social interactions and their underlying mechanisms in microbial systems.
Social interactions among microbes give rise to multicellular groups having emergent behaviors that are not possible in single cells [1-3] [4-8]. For example, quorum sensing enables synchronization of gene expression and cellular activities to allow a population to act as a group [1]. Surface-associated behaviors such as biofilm formation and swarming motility allow microbes to establish communities with enhanced protection against external agonists and promote colonization and penetration of biotic and abiotic surfaces [9-13]. Cell-cell signaling during sporulation in myxobacteria and slime molds directs group motility behaviors and developmental programs in which cellular differentiation gives rise to multicellular forms having distinct cell types with specialized functionalities, thereby enhancing survival through division of labor [14,15]. In extreme cases, multispecies biofilms and microbial mats constitute complex microbial ecosystems where numerous microbes communicate, cooperate and battle with each other [16]. Ultimately, the goal is to enhance survival and proliferation of the organism and when the microbe is a pathogen, this has dire consequences for the host [5,17,18].
In the bacterial world, cell-cell communication is the rule and considering social behavior as a ubiquitous property of bacteria has transformed our view and understanding of microbiology [1-3]. Social behaviors are also well-documented in eukaryotic microbes [19] [5,6,20]. However, despite the tremendous influence that the paradigm of “sociomicrobiology” has had on our understanding of microbiology, one group of microbes, the parasitic protozoa, seem to have been left without an invitation to the party. Studies of these organisms generally consider them as individual cells in suspension cultures or animal models of infection, while social interactions are largely unstudied.
Parasitic protozoa are etiologic agents of several major human maladies, including malaria, epidemic dysentery, Leishmaniasis and African sleeping sickness, that affect over half a billion people worldwide. Parasites also limit economic development in some of the poorest regions on the planet and are thus major contributors to the global human health and economic burden. Parasites have complex life cycles requiring transmission through multiple hosts, survival in diverse environments and a wide variety of cellular differentiation events. Hence, there are numerous facets of parasite biology that may benefit from, or may even depend upon, social interactions. In this review, we highlight recent work on social behavior in two well- studied parasites, Trypanosoma brucei that causes sleeping sickness and Plasmodium parasites that cause malaria. In addition to uncovering underappreciated aspects of parasite biology, these studies illustrate the potential for sociomicrobiology concepts to advance understanding of the biology, transmission and pathogenesis of parasitic protozoa.
Cell-cell signaling and cell density-dependent behavior
The protozoan parasite Trypanosoma brucei is the etiologic agent of African trypanosomiasis, which causes widespread mortality and morbidity of humans and livestock in sub-Saharan Africa. These parasites are transmitted to the bloodstream of a mammalian host through the bite of a tsetse fly vector. In the mammalian host, T. brucei must balance competing objectives of promoting parasite proliferation and limiting pathologic consequences to preserve the host as nutrient source (Figure 1). In addition, as a vector-borne pathogen, T. brucei must ready itself for survival in the tsetse vector and must maintain sufficient parasite density in the bloodstream to permit transmission during a tsetse blood meal [21,22]. Parasitemia is controlled in part via host immune defenses, but T. brucei is an expert at evading these defenses and thus benefits from differentiation of proliferating “slender” form parasites into growth-arrested “stumpy” forms [23-25]. Differentiation into non-dividing stumpy forms is irreversible in the bloodstream and premature commitment to this pathway would jeopardize maintenance of the infection [23,26]. Control is provided via a postulated quorum sensing-type system in which a soluble, parasite-derived “stumpy induction factor” (SIF) accumulates as parasite cell density increases and triggers parasite differentiation only after a sufficient parasitemia has been achieved [23,24]. The nature of SIF and the SIF signaling pathway are not known, but cyclic nucleotide signaling has been suggested to be involved [24,25]. Stumpy-form parasites are pre- adapted for survival in the tsetse midgut, while slender forms are not. Thus, SIF-dependent slender-to-stumpy differentiation limits maximum parasite density in the mammalian host and simultaneously modulates parasite preparation for survival in the next host, optimizing probability of transmission [22,23].
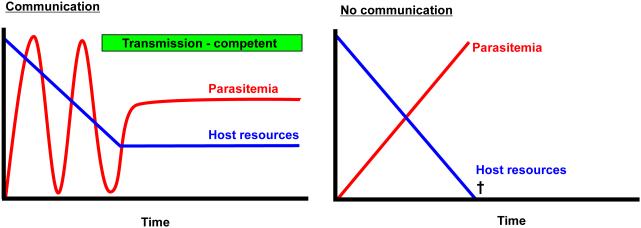
Figure 1. Cell-cell communication benefits T. brucei.
Parasite-parasite communication (chart on left) via cell density-dependent signaling controls T. brucei differentiation from proliferating forms that are adapted for survival in the bloodstream to growth-arrested, transmission competent forms that are adapted for survival in the tsetse vector. By linking differentiation to population density, the parasite avoids depletion of host nutrients and prevents premature commitment to a developmental form that is not optimized for survival in the mammalian host. Without density-dependent cell-cell communication (chart on right), continued parasite proliferation would deplete host resources and thus reduce chances for transmission.
Recent work has provided insight into slender-to-stumpy differentiation and its contribution to T. brucei disease progression and transmission. Previously, studies were limited by subjective parameters for distinguishing slender from stumpy-form parasites. MacGregor and colleagues [21] used a stumpy-specific marker, PAD [27] to conduct a quantitative analysis of trypanosome population dynamics during chronic infection in mice. They demonstrated that stumpy forms dominate the parasite population throughout late stages of infection. The quantitative nature of the approach enabled mathematical modeling, which provided overwhelming support for a quorum sensing mechanism. Moreover, the authors were able to make specific predictions for the cell types that produce SIF and define kinetic parameters for its production, activity and turnover. These data will facilitate efforts to identify the SIF molecule(s). Because SIF is produced only by a subset of cell types in the population, the system has the capacity to make qualitative as well as quantitative assessments of population dynamics. Interestingly, the findings also have implications for immune evasion strategies employed by T. brucei, because stumpy forms do not undergo antigenic variation [28]. Overall, the results emphasize the importance of parasite-parasite communication as a critical element in disease progression and transmission.
Another fascinating example of parasite surveillance of its own population during infection comes from studies of sex ratio adjustment in the malaria parasite Plasmodium chabaudi [29]. Malaria affects an estimated 247 million people worldwide [30]. Malaria parasites are transmitted through the bite of an Anopheles mosquito. In the transmission cycle, male and female gametocytes are produced in the mammalian bloodstream and taken up during a mosquito blood meal. Within the mosquito, gametocytes mature, then fuse and complete their life cycle in a series of steps that culminate in formation of infectious parasites in the mosquito salivary gland. The ratio of female to male gametocytes varies and is biased toward females. This sex ratio distribution contributes to parasite fitness and influences parasite evolution, but the factors controlling it are unknown. In multicellular animals, gamete sex ratio distribution is governed by rules of social evolution theory, which predict that sex ratios are dictated by population diversity [31,32]. In essence, at low population diversity, female gametes outnumber males and as population diversity increases, the ratio of females to males decreases. In an elegant series of experiments, Pollitt and colleagues tested this theory in mixed Plasmodium infections using different numbers of Plasmodium genotype variants [33]. They found that the parasites adjusted their sex ratio in response to the presence of unrelated genotypes in the parasite population. Their results indicate that not only can malaria parasites sense population density during an infection, but they can also sense diversity in the population and adjust their behavior in response. In addition to resolving a long-standing question about Plasmodium biology, the studies offered a test of one of the basic tenets of social evolutionary theory, thus emphasizing another aspect of the value in applying social biology concepts to parasite biology.
Life on a surface and social motility in T. brucei
Most microbes are associated with surfaces in their natural environments and engage in surface-induced social behaviors, such as biofilm formation and various forms of social motility [5,6,8,12,13]. These group activities facilitate surface colonization, defense and efficient use of nutrients [34] [11,13]. T. brucei is extracellular in both hosts and spends most of its lifecycle in direct contact with host tissue surfaces. Within the tsetse in particular, parasite movement across, and colonization of tissue surfaces are critical for development and transmission [35,36] [37]. Currently, T. brucei is studied almost exclusively in suspension cultures and little is known about how life on a surface influences parasite behavior.
With bacteria and fungi, cultivation on semisolid agarose matrices has proven valuable for studies of social behavior [12] [5,13]. Oberholzer, Lopez and colleagues thus employed semisolid agarose matrices to study surface behavior of procyclic-form (insect life cycle stage) T. brucei [38]. They discovered a novel group behavior, termed social motility, in which parasites assembled into multicellular communities with emergent properties that are not evident in single cells. Initially, parasites collect into small groups that move en masse across the agarose surface and grow larger through recruitment of other cells (Figure 2). At the periphery of the inoculation site, groups of parasites collect in nodes of high cell density and then advance outward, forming radial projections (Figure 3). The number and spacing of radial projections is generally consistent from one group to the next and patterns formed resemble those generated during surface colonization by swarming bacteria [12,13]. The events of T. brucei social motility occur in defined stages as summarized schematically in Figure 3A.
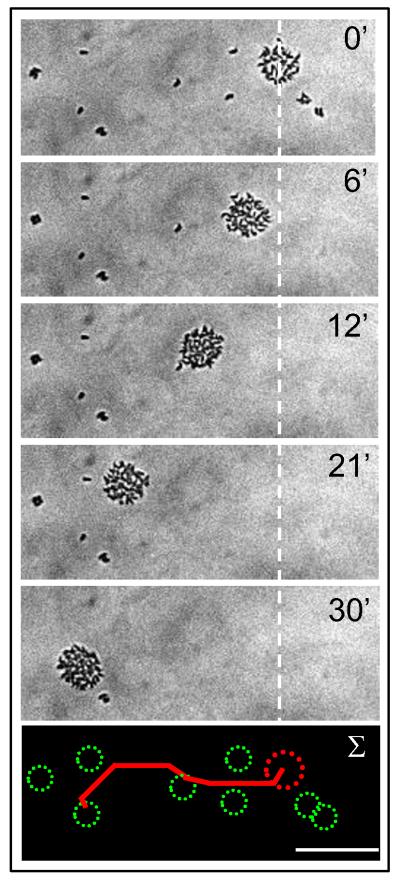
Figure 2. T. brucei cooperative motility on a surface.
In response to surface exposure, T. brucei cells assemble into small groups that migrate en masse across the surface and enlarge through recruitment of other cells. Panels are time-lapse images (see movie M1) showing movement of a group of parasites (top right of top panel) across the surface of a semisolid agarose plate, with dashed white line indicating starting position of the group. Bottom panel shows summary. Elapsed time is indicated in minutes. Scale bar is 100 um.
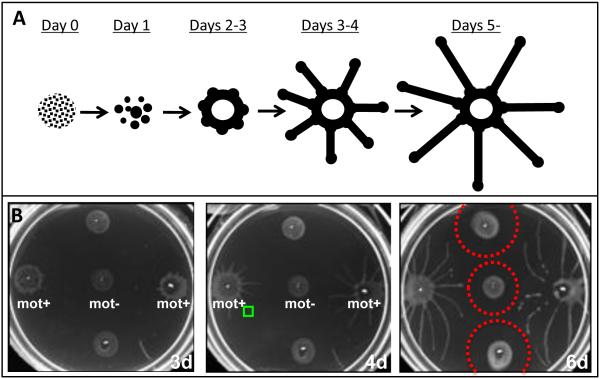
Figure 3. Social motility in T. brucei.
When cultivated on semi-solid surfaces, T. brucei engages in complex social interactions that culminate in the formation of characteristic colony patterns. (A) Schematic diagram of the main steps of social motility in T. brucei, with parasites represented in black. Initially, individual parasites (Day 0) form small groups (Day 1). These groups move en masse across the surface and grow through recruitment of additional parasites. Groups assemble at the periphery of the inoculation site, concentrating in nodes (Days 2-3). From these nodes, parasites advance outward, forming radial projections (Days 3-5) that are regularly-spaced and advance at the leading edge only (Days 5+). (B) Suspension cultures of wild type (mot +) or motility mutant (mot −) parasites were inoculated on semisolid agarose and imaged at 3, 4 or 6 days (3d, 4d, 6d) post inoculation. Social motility requires active parasite motility, as motility mutants (mot-) fail to undergo social motility. Individual cells in each projection are highly motile (see movie M2, corresponding to a region represented by the green box in panel B4d). Projections can sense neighboring cells and halt or redirect their movements to avoid contact, resulting a zone of avoidance (dotted red circles in panel B6d). Adapted from [38] with permission.
Several features of T. brucei social motility indicate cell-cell communication governs the behavior. First, coordination among individuals to enable group movement is striking, e.g. Figure 2 and movie M1, and in some cases, group movements occur only when other parasites are detected nearby, suggesting cell-cell communication within and between groups. Additionally, individual cells within each radial projection are highly motile (Movie M2) and can freely move out and back from lateral edges, yet the group advances only at its leading edge. This indicates that polarized migration of the group is governed by parasites ‘choosing’ to move in a specific direction and suggests that parasite-derived signals may govern spacing of adjacent projections. In support of this idea, radial projections continue to advance unless they encounter a separate group of parasites, in which case movement is halted or diverted to avoid contact (Figure 3B). Adjacent projections alter their course in parallel, indicating that signaling between groups controls group movement. The zone of avoidance is a direct function of parasite number, suggesting that a diffusible substance(s) is responsible, as has been reported for swarming motility in bacteria [39,40]. Overall, the work demonstrates the capacity of protozoan parasites to engage in group activities and reveals a level of complexity and cooperativity to trypanosome behavior that was not previously recognized. The findings also offer a convenient assay for studying environmental sensing in these organisms, which is an understudied problem.
Conflict, Competition and Cross-Kingdom Interactions
Wherever there is interaction among individuals, there is potential for conflict and competition. Bacteria engage in all manner of intercellular warfare and competition, ranging from growth inhibition and cytolysis of competing species, to bacterial cannibalism [1,4,8,39,41]. In an interesting case of sibling rivalry, neighboring colonies of Paenibacillus dendritiformis mutually inhibit each other’s growth through secreted signaling molecules while growth inhibition does not occur in a single colony [39]. The behavior bears strong resemblance to the avoidance behavior observed in T. brucei social motility (Fig. 3), suggesting that procyclic form trypanosomes produce secreted factors that affect neighboring cells. Another instance of parasite-parasite competition has been reported for mixed T. brucei infections in mice [42] in which mutual competitive suppression was observed between co-infecting T. brucei strains of varying virulence. The authors report that mutual suppression of parasite growth in the host is correlated with extended host survival, suggesting that the less virulent strain reduces the pathogenic impact of the more virulent strain. The extent of mixed infections for T. brucei in the field is not known, but for some parasites, such as Plasmodium, the majority of natural infections are expected to involve multiple strains [43].
It is taken as de-facto knowledge that host-parasite interactions influence infection outcome. However, parasites are not the only microbes present in their hosts. The influence that the microbial flora of the mammalian host or insect vector exerts on parasite biology, transmission and pathogenesis is mostly unknown. For evolutionary ecologists, the influence of an organism’s microbial flora on infection is well-known [44]. Recent work has demonstrated for both T. brucei and Plasmodium, that the presence or absence of specific bacterial symbionts in the insect vector is associated with refractoriness to parasite infection [45,46]. Thus, as is the case for bacterial pathogens [18], cross-kingdom social interactions exert significant influence on the biology of pathogenic protozoa.
Summary and Perspective
Protozoan parasites cause tremendous human suffering worldwide, but strategies for therapeutic intervention are limited. Recent studies illustrate that the paradigm of microbes as social organisms can be brought to bear on questions about parasite biology, transmission and pathogenesis. In addition to uncovering novel aspects of parasite biology, these studies suggest alternative strategies for therapeutic intervention may include targeting parasite-parasite communication. Experimentally tractable parasite systems also provide opportunities for empirically testing rules that govern social behavior.
Microbes derive a variety of benefits from social interactions and group behaviors (Figure 4). A focus of future efforts should be to determine which of these benefits apply in specific parasite systems. It will also be important to elucidate the underlying mechanisms. At a minimum, systems are required for production, perception and transduction of extracellular signals, whether diffusible or cell contact-mediated. Proteomic analyses of parasite surface proteins will facilitate efforts to define these systems [47] [48,49]. Exolipids are used as surfactants in bacterial surface motility [13] and parasites express abundant glycolipids and glycoproteins on their surface. Cyclic nucleotide signaling plays a major role in the regulation of social behaviors in other organisms [6,24,50] and has been implicated in T. brucei SIF signaling [24] and social motility (unpublished observation). Combined with other similarities discussed above, these observations indicate that mechanistic insights may come from comparing social behaviors in bacteria and parasitic protozoa.
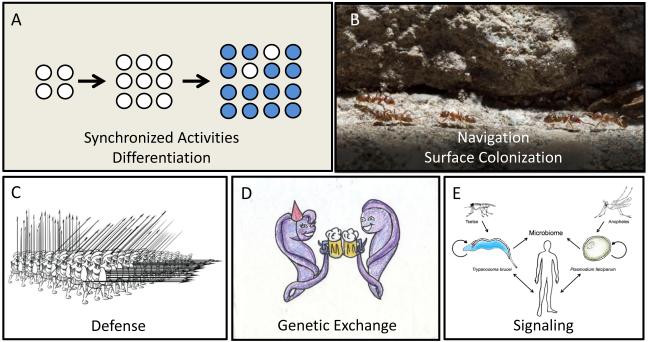
Figure 4. Benefits of social behavior.
(A) Cell density signaling mechanisms enable synchronization of cellular activities thus preserving group level behaviors (blue circles) for when they are most advantageous. Additionally, not all individuals are equally receptive to the signals thus allowing for differentiation within a population (white among blue circles). (B) Cell-cell communication and cooperative motility facilitate colonization of tissue surfaces and navigation through specific host compartments. (C) Group defensive strategies protect against environmental agonists. (D) Social interactions facilitate genetic exchange. (E) While the current review has primarily considered social behavior in the context of parasite-parasite signaling, cell-cell communication also occurs between the parasite and vector, host, and host microbiome, all of which will impact parasite transmission and pathogenesis. Studying these interactions is also expected to provide insight into the signal transduction pathways utilized by parasites.
Microbial social behavior was once considered to be a cottage industry of only a few species, but is now recognized to be ubiquitous among bacteria. Likewise, the few examples of social behavior in parasites discussed here may be just the tip of the iceberg and much more lies beneath the surface that is yet to be explored.
Supplementary Material
Movie M1. Communities move en masse to recruit parasites. Time-lapse microscopy was used to capture a community moving across the surface of the agarose to recruit other parasites. Images were taken once every minute for 30 minutes. The video is played at 60x speed.
Movie M2. Cells within projections are highly motile. Fluorescently-labeled parasites are shown actively moving within a migrating projection. Video was shot in real-time first under fluorescent conditions and then under bright-field.
Highlights.
Protozoan parasites cause tremendous human suffering worldwide, but strategies for therapeutic intervention are limited >Recent studies illustrate that the paradigm of microbes as social organisms can be brought to bear on questions about parasite biology, transmission and pathogenesis >This review discusses recent work demonstrating adaptation of social behaviors by parasitic protozoa that cause African sleeping sickness and malaria >The recognition of social behavior and cell-cell communication as a ubiquitous property of bacteria has transformed our view of microbiology, but protozoan parasites have not generally been considered in this context >Works discussed illustrate the potential for concepts of sociomicrobiology to provide insight into parasite biology and should stimulate new approaches for thinking about parasites and parasite-host interactions.
Acknowledgments
Funding for the work was provided by grants from the NIH-NIAID (AI052348), Burroughs Wellcome Fund and the Beckman Young Investigator program to KLH. MAL is the recipient of an NIH-NRSA (F31AI085961) fellowship. HTN is the recipient of an NIH-NRSA (GM007185) fellowship. MO is the recipient of a Swiss National Science Foundation Fellowship. We would also like to thank Eric Nguyen and Anthony Nguyen for their artistic contribution to Figure 4.
Footnotes
Ethics in Publishing: General Statement
The authors would like to declare no conflicts of interest regarding this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Annotations
- ••1.Bassler B, Losick R. Bacterially Speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. An excellent review that describes communication systems used by bacteria, including long and short range signaling systems, as well as examples of multicellular community development and fratricide.
- •2.Shapiro JA. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. This review discusses the concepts of social behavior in bacteria and the impact that considering bacteria as social organisms has had on our view of microbiology.
- •3.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. This review introduces the term “sociomicrobiology” and considers the impact of social interactions on microbes and microbial pathogenesis.
- 4.Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009;33:206–224. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- 5.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Shaulsky G, Kessin RH. The cold war of the social amoebae. Current Biology. 2007;17:R684–692. doi: 10.1016/j.cub.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velicer GJ, Vos M. Sociobiology of the myxobacteria. Annu Rev Microbiol. 2009;63:599–623. doi: 10.1146/annurev.micro.091208.073158. [DOI] [PubMed] [Google Scholar]
- 9.Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev. 2011 Jun 9; doi: 10.1111/j.1574-6976.2011.00286.x. doi: 10.1111/j.1574-6976.2011.00286.x Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- •11.Butler MT, Wang Q, Harshey RM. Cell density and mobility protect swarming bacteria against antibiotics. Proc Natl Acad Sci U S A. 2010;107:3776–3781. doi: 10.1073/pnas.0910934107. The authors show that swarming motility of bacteria on surfaces provides resistance to antibiotics.
- •12.Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. A thorough review of different types of surface motilities employed by various bacteria, including mechanisms, regulation and conditions that favor surface motility.
- 13.Verstraeten N, Braeken K, Debkumari B, Fauvart M, Fransaer J, Vermant J, Michiels J. Living on a surface: swarming and biofilm formation. Trends Microbiol. 2008;16:496–506. doi: 10.1016/j.tim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Firtel RA, Meili R. Dictyostelium: a model for regulated cell movement during morphogenesis. Curr Opin Genet Dev. 2000;10:421–427. doi: 10.1016/s0959-437x(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser D. Coupling cell movement to multicellular development in myxobacteria. Nat Rev Microbiol. 2003;1:45–54. doi: 10.1038/nrmicro733. [DOI] [PubMed] [Google Scholar]
- 16.Decho AW, Norman RS, Visscher PT. Quorum sensing in natural environments: emerging views from microbial mats. Trends Microbiol. 2009;18:73–80. doi: 10.1016/j.tim.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Fraser GM, Hughes C. Swarming motility. Curr Opin Microbiol. 1999;2:630–635. doi: 10.1016/s1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 18.Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol. 2009;12:192–198. doi: 10.1016/j.mib.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryot Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verstrepen KJ, Fink GR. Genetic and epigenetic mechanisms underlying cell-surface variability in protozoa and fungi. Annual Review of Genetics. 2009;43:1–24. doi: 10.1146/annurev-genet-102108-134156. [DOI] [PubMed] [Google Scholar]
- ••21.MacGregor P, Savill NJ, Hall D, Matthews KR. Transmission stages dominate trypanosome within-host dynamics during chronic infections. Cell Host Microbe. 2011;9:310–318. doi: 10.1016/j.chom.2011.03.013. The authors used a stumpy-specific protein, PAD1, as a molecular marker to enable quantitative analysis of stumpy formation during chronic infections of T. brucei. By using mathematical modeling, the authors describe the parameters controlling trypanosome within-host dynamics and provide support for a quorum-sensing-like mechanism in the slender-to-stumpy developmental transformation.
- •22.Pollitt LC, MacGregor P, Matthews K, Reece SE. Malaria and trypanosome transmission: different parasites, same rules? Trends Parasitol. 2011;27:197–203. doi: 10.1016/j.pt.2011.01.004. This review discusses evolutionary theory in the context of the trade-off between transmission and within-host survival in African trypanosomes and malaria parasites, with a focus on common concepts that apply to the two systems. Evolutionary ecology is used to predict results of within-host competition from mixed infections and the strategies employed by each parasite to maximize fitness and transmission potential.
- •23.Matthews KR. Controlling and coordinating development in vector-transmitted parasites. Science. 2011;331:1149–1153. doi: 10.1126/science.1198077. Vector-borne parasites such as the causative agents of malaria, African trypanosomiasis, Chagas disease, leishmaniasis, filariasis and schistomiasis, face numerous barriers to survival and transmission. This excellent review articulates the challenges faced by these parasites, both in their mammalian hosts and insect vectors, and reviews strategies and mechanisms the parasites employ to overcome these challenges. A common theme is sensing of the host environment and adapting parasite developmental cycles so as to enhance survival and optimize transmission efficiency.
- 24.Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- 25.Matthews KR. The developmental cell biology of Trypanosoma brucei. J Cell Sci. 2005;118:283–290. doi: 10.1242/jcs.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews KR, Ellis JR, Paterou A. Molecular regulation of the life cycle of African trypanosomes. Trends Parasitol. 2004;20:40–47. doi: 10.1016/j.pt.2003.10.016. [DOI] [PubMed] [Google Scholar]
- •27.Dean S, Marchetti R, Kirk K, Matthews KR. A surface transporter family conveys the trypanosome differentiation signal. Nature. 2009;459:213–217. doi: 10.1038/nature07997. Authors identify a surface protein in the carboxylate-transporter family, PAD (proteins associated with differentiation), that is necessary for citrate/cis-aconitate-induced stumpy differentiation of T. brucei. PAD is also the first stumpy-specific molecular marker identified for T. brucei.
- 28.Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- ••29.Reece SE, Drew DR, Gardner A. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453:609–614. doi: 10.1038/nature06954. Plasmodium chabaudi parasites were observed to alter their ratios of female versus male sexual stages based on the presence of competing genotypes in infected animals. The results indicate that the parasites not only sense their own population density, but also population diversity and adjust their behavior in response. The study also provided a test of evolutionary theory and provided insight into the long-standing question of how plasmodium parasites control sex ratios.
- 30.Garcia LS. Malaria. Clin Lab Med. 2010;30:93–129. doi: 10.1016/j.cll.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton WD. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 32.Reece SE, Shuker DM, Pen I, Duncan AB, Choudhary A, Batchelor CM, West SA. Kin discrimination and sex ratios in a parasitoid wasp. J Evol Biol. 2004;17:208–216. doi: 10.1046/j.1420-9101.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 33.Pollitt LC, Mideo N, Drew DR, Schneider P, Colegrave N, Reece SE. Competition and the evolution of reproductive restraint in malaria parasites. Am Nat. 2011;177:358–367. doi: 10.1086/658175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser D. Bacterial swarming: a re-examination of cell-movement patterns. Curr Biol. 2007;17:R561–570. doi: 10.1016/j.cub.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 35.Roditi I, Lehane MJ. Interactions between trypanosomes and tsetse flies. Curr Opin Microbiol. 2008;11:345–351. doi: 10.1016/j.mib.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Sharma R, Gluenz E, Peacock L, Gibson W, Gull K, Carrington M. The heart of darkness: growth and form of Trypanosoma brucei in the tsetse fly. Trends Parasitol. 2009;25:517–524. doi: 10.1016/j.pt.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickerman K, Tetley L, Hendry KA, Turner CM. Biology of African trypanosomes in the tsetse fly. Biol Cell. 1988;64:109–119. doi: 10.1016/0248-4900(88)90070-6. [DOI] [PubMed] [Google Scholar]
- ••38.Oberholzer M, Lopez MA, McLelland BT, Hill KL. Social motility in african trypanosomes. PLoS Pathog. 2010;6:e1000739. doi: 10.1371/journal.ppat.1000739. The authors report that T. brucei engages in social behavior when cultivated on semi-solid surfaces. The results indicate that the parasites sense, communicate and cooperate, demonstrating a level of complexity to parasite behavior that was not previously recognized. The authors also point out that social motility provides a convenient system for investigating environmental sensing in trypanosomes.
- •39.Be’er A, Zhang HP, Florin EL, Payne SM, Ben-Jacob E, Swinney HL. Deadly competition between sibling bacterial colonies. Proc Natl Acad Sci U S A. 2009;106:428–433. doi: 10.1073/pnas.0811816106. Paenibacillus dendritiformis secretes a deadly compound that mutually inhibits growth of sibling colonies grown next to each other. Authors find that there is a defined threshold and the secreted material is lethal only if it exceeds this threshold. Mathematical modeling was then used to illustrate this phenomenon and predict behaviors associated with a threshold of antibacterial compounds being produced.
- 40.Caiazza NC, Shanks RM, O’Toole GA. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol. 2005;187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berleman JE, Scott J, Chumley T, Kirby JR. Predataxis behavior in Myxococcus xanthus. Proc Natl Acad Sci U S A. 2008;105:17127–17132. doi: 10.1073/pnas.0804387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balmer O, Stearns SC, Schotzau A, Brun R. Intraspecific competition between co-infecting parasite strains enhances host survival in African trypanosomes. Ecology. 2009;90:3367–3378. doi: 10.1890/08-2291.1. [DOI] [PubMed] [Google Scholar]
- 43.Babiker HA, Ranford-Cartwright LC, Walliker D. Genetic structure and dynamics of Plasmodium falciparum infections in the Kilombero region of Tanzania. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):11–14. doi: 10.1016/s0035-9203(99)90321-8. [DOI] [PubMed] [Google Scholar]
- 44.Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–215. doi: 10.1126/science.1188235. [DOI] [PubMed] [Google Scholar]
- 45.Cirimotich CM, Dong Y, Clayton AM, Sandiford SL, Souza-Neto JA, Mulenga M, Dimopoulos G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science. 2011;332:855–858. doi: 10.1126/science.1201618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farikou O, Njiokou F, Mbida Mbida JA, Njitchouang GR, Djeunga HN, Asonganyi T, Simarro PP, Cuny G, Geiger A. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes--an epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect Genet Evol. 2010;10:115–121. doi: 10.1016/j.meegid.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 47.de Miguel N, Lustig G, Twu O, Chattopadhyay A, Wohlschlegel JA, Johnson PJ. Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics. 2010;9:1554–1566. doi: 10.1074/mcp.M000022-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •48.Oberholzer M, Langousis G, Nguyen HT, Saada EA, Shimogawa MM, Jonsson ZO, Nguyen SM, Wohlschlelgel JA, Hill KL. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol Cell Proteomics. 2011 June 19; doi: 10.1074/mcp.M111.010538. Epub ahead of print. The authors isolate intact flagella from T. brucei and use mass spectrometry to define independent flagellar membrane and matrix proteomes. Analysis if flagellar membrane proteome revealed a diverse and dynamic host-parasite interface that is enriched for proteins suited for parasite-parasite and host-parasite signaling.
- 49.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, Moch JK, Muster N, Sacci JB, Tabb DL, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 50.Jonas K, Melefors O, Romling U. Regulation of c-di-GMP metabolism in biofilms. Future Microbiol. 2009;4:341–358. doi: 10.2217/fmb.09.7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie M1. Communities move en masse to recruit parasites. Time-lapse microscopy was used to capture a community moving across the surface of the agarose to recruit other parasites. Images were taken once every minute for 30 minutes. The video is played at 60x speed.
Movie M2. Cells within projections are highly motile. Fluorescently-labeled parasites are shown actively moving within a migrating projection. Video was shot in real-time first under fluorescent conditions and then under bright-field.