Abstract
Deficits in dopamine D2/D3 receptor (D2R/D3R) binding availability using PET imaging have been reported in obese humans and rodents. Similar deficits have been reported in cocaine-addicts and cocaine-exposed primates. We found that D2R/D3R binding availability negatively correlated with measures of body weight at the time of scan (ventral striatum), at 1 (ventral striatum) and 2 months (dorsal and ventral striatum) post scan in rats. Cocaine preference was negatively correlated with D2R/D3R binding availability 2 months (ventral striatum) post scan. Our findings suggest that inherent deficits in striatal D2R/D3R signaling are related to obesity and drug addiction susceptibility and that ventral and dorsal striatum serve dissociable roles in maintaining weight gain and cocaine preference. Measuring D2R/D3R binding availability provides a way for assessing susceptibility to weight gain and cocaine abuse in rodents and given the translational nature of PET imaging, potentially primates and humans.
1. Introduction
Positron emission tomography (PET) imaging provides a non-invasive way of assessing brain function. Through the development of small animal PET scanners (i.e. μPET), the benefits of this technology have been extended to the study of rodents. Using PET and μPET imaging, we and others have previously reported deficits in dopamine D2/D3 receptor (D2R/D3R) binding availability in obese and drug-addicted humans (Wang et al., 2004), cocaine-exposed primates (Nader et al., 2008) and genetically obese rats (Thanos et al., 2008). A fundamental question that has emerged from these studies is whether such deficits are attributed to chronic drug exposure in drug addiction and chronic overeating in obesity, or whether the specific individuals and animals are genetically vulnerable to such deficits. If the answer to this question is the latter, then the concept of using D2R/D3R binding availability as a predictive biomarker for assessing vulnerability to drug abuse and obesity becomes evident. In this paper, we attempted to answer this question by measuring D2R/D3R binding availability using μPET in 10 non-obese and drug-naïve male adult Sprague-Dawley rats and then correlating these measures with body weight at the time of the scan and at 1 and 2 months post scan and cocaine conditioned place preference (CPP) 2 months after scanning. We hypothesized that D2R/D3R binding availability would negatively correlate with future measures of body weight and cocaine preference and thus could serve as a predictive non-invasive measure of weight gain and cocaine abuse propensity.
2. Methods
2.1 Animals
Rats between 12-14 weeks of age (n=10) were obtained from Taconic (Taconic, NY) and housed on a reverse 12 hour light/dark cycle with lights off at 7 am. Rats were fed a standard (Purina) laboratory rat chow. Food intake was monitored daily at approximately 1500h and all rats were weighed every other day immediately after food monitoring. All experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, 1996) and Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
2.2 In-vivo [11C]raclopride D2R μPET
μPET assessment of D2R in these rats was performed using a μPET R4 Scanner (CTI Concorde Microsystems, Knoxville, TN). Each rat was briefly anesthetized with isoflurane (~2-3 min) and its lateral tail vein was catheterized. Once awake, each rat was injected intravenously with [11C]raclopride (547 ± 72 μCi); specific activity, 10.4 ± 4.3 mCi/nmol). Injected volumes were approximately 400μl. 30 minutes later each rat was anesthetized with isoflurane and placed in a stereotaxic head holder (David Kopf Instruments; CA, USA) in a prone position on the bed of the scanner and scanning commenced. Total acquisition time was 30 min (17 frames: 6 frames, 10 s; 3 frames, 20 s; 8 frames, 60 s; 4 frames, 300 s) and data were acquired in fully 3-dimensional mode with maximum axial acceptance angle (+−28 degrees). Images were reconstructed using the optimization algorithm maximum a posteriori (MAP) with 30 iterations and a smoothing value of 0.01 mm. Qualitative and quantitative assessment of μPET images was performed as described previously using the PMOD software environment (PMOD Technologies, Zurich Switzerland) (Schiffer et al., 2009). Briefly, regions of interest (ROIs) were delineated on an magnetic resonance imaging (MRI) rat brain template set to Paxinos and Watson stereotaxic coordinates (Schweinhardt et al., 2003) to encompass the dorsal striatum (ML: +/−2.5 mm, AP: 0.5 mm, DV: −4.5 mm), ventral striatum (ML:+/−2.0 mm AP: 1.5 mm DV: −7.0 mm), and cerebellum (ML:0.0 mm, AP: −12.6 mm, DV: −3.5 mm) (all coordinates from bregma: ML (mediolateral), AP (anteroposterior), DV (dorsoventral). Then all μPET images were coregistered and spatially normalized to the combined MRI-ROI template using the Fusion module in PMOD as previously described (Thanos et al., 2008). This led to coregistered μPET-MRI images with a voxel size of 0.2 mm isotropic. ROI values in KBq/cc were extracted from receptor rich (dorsal and ventral striatum) and receptor poor (cerebellum) regions and converted to binding ratios (BR) using the following formula BR = (receptor rich-receptor poor) /receptor poor).
2.3 Cocaine Conditioned Place Preference (CPP)
CPP was assessed two months after μPET scanning. The CPP apparatus (Coulbourn Instruments) contained three compartments and has been previously described (Thanos et al., 2009). The CPP procedure was modified from (Thanos et al., 2009) and consisted of the following four phases: i) habituation, (ii) pretest, (iii) conditioning, and (iv) test. The habituation phase was administered on the first day. This consisted of transporting the animals to the room where the CPP apparatus was located. 30 minutes later the animals were transported back to the animal facility. On the second day, rats were transported to the CPP procedure room and placed in the middle chamber of the apparatus and were allowed 10 minute access to all chambers. Time spent in each chamber was recorded. For the next 8 days rats were injected with either cocaine (10 mg/kg (IP)) (Sigma-Aldrich (St. Louis, MO) or saline on alternate days. Using preference measurements from the pretest phase, rats were administered cocaine in the non-preferred chamber as observed during pretest. Rats were only allowed access to one of the two chambers each day during the conditioning phase. Daily sessions during this phase lasted for 30 minutes. The CPP test phase was conducted on day 11 with procedures identical to the pretest phase. The cocaine preference score was calculated as the percent change in time spent in the cocaine-paired chamber on test day vs. that on pretest day.
2.4 Statistical Analysis
Regression analysis was used to evaluate significant effects between each variable (body weight, CPP and binding availability). All regressions were fitted with a linear 1st degree polynomial equation and significance level was set to α = 0.05. Regression coefficients and analysis of variance were used to measure the regression strength between each pair of variables. All data passed normality (Shapiro-Wilk) and constant variance tests (α = 0.05). Statistical procedures were undertaken using Sigmastat 3.5 software (Systat Inc., San Jose, CA) and graphs were plotted using Sigmaplot 10.0 software (Systat Inc., San Jose, CA).
3. Results
3.1 D2R/D3R Binding Availability vs. Body Weight and Weight Gain
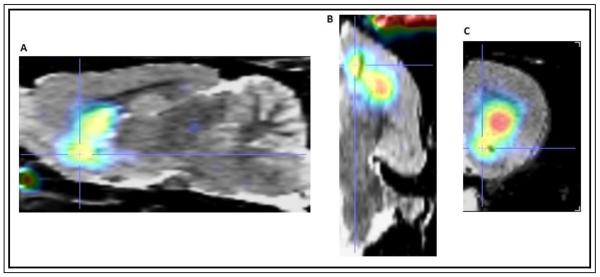
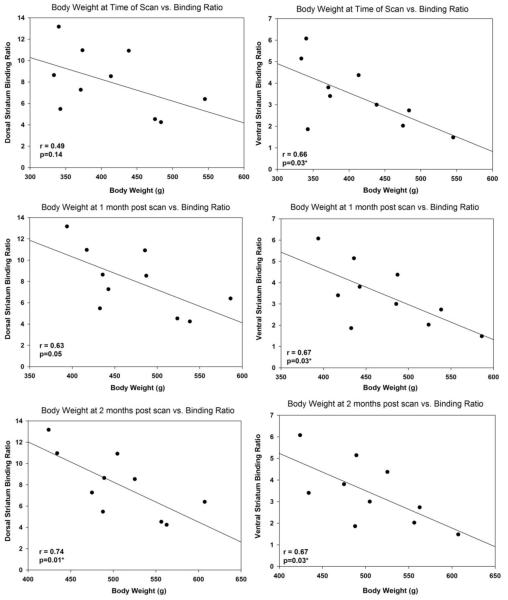
A representative image of D2R/D3R binding in the dorsal and ventral striatum is illustrated in Figure 1. All body weight measures were obtained prior to cocaine administration. In order to examine whether D2R/D3R binding availability can serve as a predictor for sensitivity to weight gain in non-obese animals, we compared D2R/D3R binding availability to body weight levels at the time of scan, and at 1 and 2 months after the scan using linear regression analysis. We also compared binding availability to weight gain at 1 and 2 months post scan. We found a significant negative correlation between basal D2R/D3R binding availability in the ventral striatum and body weight at the time of scan [F (1,9)=7.28; p = 0.03; r = 0.66] and 1 month post scan [F (1,9)=6.35; p = 0.03; r = 0.67]. At 2 months post scan, D2R/D3R binding availability and body weight were negatively correlated in both the dorsal [F (1,9)=8.84; p = 0.01; r = 0.74] and ventral striatum [F (1,9)=6.49; p = 0.03; r = 0.67] (Figure 2). With respect to weight gain, the only significant regression was observed at 1 month post scan where binding availability and weight gain were positively correlated [F (1,9)=6.37; p = 0.03; r = 0.66] (Figure 3). Similar regressions were conducted using food intake and binding availability values but no significant effects were found.
Figure 1.
Representative μPET image coregistered to the Schweinhardt MRI atlas (Schweinhardt et al., 2003) showing distinct uptake in dorsal and ventral striatum in (A) sagital, (B) horizontal, and (C) coronal planes.
Stereotaxic coordinates (in mm from bregma) were as follows according to the Paxinos and Watson atlas: Dorsal striatum: ML: +/−2.5 AP: 0.5 DV: −4.5 Venteal striatum: ML:+/−2.0 AP: 1.5 DV: −7.0 Cerebellum: ML:0.0 AP: −12.6 DV: −3.5
Figure 2.
Linear regression analysis comparing D2R/D3R binding availability (binding ratio) and body weight in the dorsal and ventral striatum at the time of scan, and at 1 and 2 months after scanning. At the time of scan, the regression was significant only in the ventral striatum (r = 0.66 p=0.03). At 1 month, the regression was significant at the ventral striatum (p=0.67; p=0.03) and trend toward significance was observed in the dorsal striatum (r = 0.63; p=0.05). At 2 months, the regression was significant in both the dorsal and ventral striatum (r=0.74; p=0.01) and (r=0.67; p=0.03).
Figure 3.
Linear regression analysis comparing D2R/D3R binding availability (binding ratio) and weight gain in the dorsal and ventral striatum at 1 and 2 months after scanning. The only significant effect was at 1 month, where the regression was significant at the ventral striatum (p=0.66; p=0.03).
3.2 D2R/D3R Binding Availability vs. Cocaine Preference
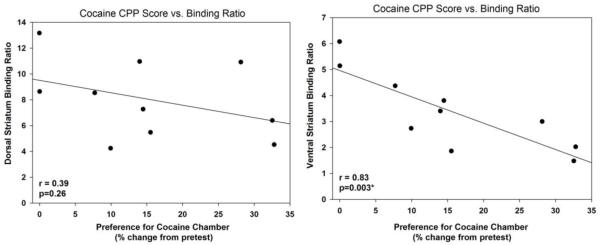
In order to examine whether D2R/D3R binding availability can serve as a predictor for cocaine preference, we compared D2R/D3R binding availability to the cocaine CPP preference score using linear regression analysis. We found a significant negative correlation between D2R/D3R binding availability and cocaine preference in the ventral striatum [F (1,9)=17.53; p = 0.003; r = 0.83] but not in the dorsal striatum [F (1,9)=1.44; p = 0.26; r = 0.39] (Figure 4).
Figure 4.
After assessing body weight, rats were exposed to a cocaine conditioned place preference experiment. Linear regressions analysis showed that D2R/D3R binding availability significantly correlated with the CPP score in the ventral (r=0.83; p=0.003) but not the dorsal striatum.
4. Discussion
We found that rats with the lowest body weight at the time of the scan showed the highest binding availability in the ventral striatum and vice versa (Figure 2). Body weight was also assessed at 1 and 2 months post scan and at all three times the correlation remained significant, and interestingly did not change (Figure 2). Additionally, at 2 months post scan, we observed a similar significant and negative correlation between body weight and binding in the dorsal striatum. We also observed a significant positive correlation between weight gain and binding availability in the ventral striatum at 1 month post scan but not at 2 months (Figure 3). No effects were found between binding availability in the dorsal striatum at 1 and 2 months post scan. Based on the prior findings of low D2R/D2R binding availability in obese individuals (Wang et al., 2004) and rats (Thanos et al., 2008), as well as the negative relationship between current and future body weight and D2R/D3R binding in non-obese rats shown in this paper, one would expect a negative relationship between weight gain and D2R/D3R binding availability (greater individual weight gain (hence greater individual body weight) would correlate with low D2R/D3R binding and vice-versa). However, the present results do not support this association: rats with high D2R/D3R binding availability in the ventral striatum gained more weight than rats with low D2R/D3R binding availability; but they weighed less. One interpretation may be that rats with lower weight (high D2R/D3R binding) are simply more likely to gain more weight than rats with greater body weight (low D2R/D3R binding) as a function of normal development. Indeed, growth charts from laboratory animal vendors show that at around the age the rats were scanned in our experiment (12-14 weeks), weight gain for Sprague Dawley rats slows down and begins to stabilize. It is possible that rats with low D2R/D3R binding may be characterized by accelerated weight gain earlier in life (before 12 weeks of age) than rats with high D2R/D3R binding. Therefore these rats would be characterized by greater body weight. Perhaps this is why we only see a significant effect at 1 and not 2 months post scan. Since we did not find significant correlations between D2R/D3R binding and food intake (at scan and 1 and 2 months post scan) these results suggest that abnormal D2R/D3R signaling observed in obese humans and rats in striatum may be due to metabolic issues more so than behavior (it was initially postulated that low D2R/D3R binding was a result of repeated dopamine increases in response to repeated feeding). Future studies are aimed at further investigating a role for striatal dopamine in metabolism.
After the 2 month body weight measurements, we evaluated CPP to cocaine in these same rats and found a significant and strong negative correlation between D2R/D3R binding availability in the ventral striatum measured 2 months prior to the CPP experiment and cocaine preference (Figure 4).
These findings suggest that while both the dorsal and ventral striatum may be involved in body weight regulation, it is the ventral striatum that plays a primary role in mediating susceptibility to cocaine abuse. These results further suggest the potential for using D2R/D3R binding availability to predict future body weight and susceptibility to cocaine abuse in rodents. Indeed, it was recently reported that D2R/D3R binding availability with μPET predicted cocaine self-administration behavior in rats with genetic predisposition to a form of impulsivity (Dalley et al., 2007). Furthermore, impulsivity and compulsivity have been shown to predict the development of addictive behavior (Belin et al., 2008). Here, using μPET, we extend these findings to a general laboratory rodent model, the outbred Sprague-Dawley rat, and show that individual differences in D2R/D3R binding have a predictive value for both body weight gain and psychostimulant abuse liability. Using such a measurement, one can classify rodents into those that express susceptibility to increased body weight and drug abuse and those that do not. One can then identify specific genes and genetic polymorphisms that may be differentially expressed in these animals before and after the body weight changes and drug exposure. One can also use D2R/D3R binding availability as a screening method to minimize variability in behavioral experiments by taking into account the variability in levels of D2R/D3R binding availability, as has been recently suggested for functional magnetic resonance imaging experiments (Mohr and Nagel, 2010). Findings from such studies can lead to the faster development of new treatments for obesity as well as drug addiction. Finally, since PET imaging using [11C]raclopride is a non-invasive research paradigm that is routinely employed in clinical research, this approach may have the potential to be translated to humans in order to determine both individual susceptibility to weight gain as well as potential for cocaine abuse. Indeed, this has recently been demonstrated using another imaging modality, functional magnetic resonance imaging, where striatal response predicted subsequent weight gain in humans (Stice et al., 2010).
Recent studies have suggested dissociable roles for the ventral and dorsal striatum in reinforcement learning as “critic” and “actor” respectively (O’Doherty et al., 2004). These studies argue that the ventral striatum is involved in learning to predict rewards while the dorsal striatum maintains the behavior necessary to guide future decisions (Kahnt et al., 2009). While preliminary, our findings support a functional dissociation between the dorsal and ventral striatum and extend the functional significance of these dissociable roles to encompass metabolic homeostatic mechanisms, like body weight regulation independent of feeding (we did not find significant correlations between binding availability and food intake). This notion arises from our findings that D2R/D3R binding availability in the ventral striatum correlated with body weight levels throughout the 2 month period following the scan (interestingly the correlation and significance values remained unchanged at each time point), while binding availability in the dorsal striatum showed no significant correlation at the time of scan, but a trend towards significance at 1 month, followed by a significant correlation at 2 months. These observations may suggest that D2R-mediated mechanisms in the ventral striatum are involved in predicting a set level of body weight, while complementary mechanisms in the dorsal striatum guide the necessary actions for ensuring that body weight is set to the levels predicted by the ventral striatum, perhaps via increases and decreases in energy expenditure (i.e. changes in metabolism, motor activity, etc.). A similar function of D2R/D3R may be involved in cocaine seeking behaviors since binding availability was found to predict future cocaine preference. However, unlike body weight measures, we did not assess cocaine preference longitudinally and therefore we do not know whether dorsal striatal binding availability may have correlated with cocaine preference at latter time-points, like it did for body weight. Nevertheless, this is an interesting idea and requires further substantiation.
In this study, we present evidence that D2R/D3R binding availability can be used to predict future body weight levels and cocaine preference in rats. We also show that individual differences in binding availability reflect individual differences in body weight and cocaine preference and vice-versa. Finally, our results suggest dissociable roles for the ventral and dorsal striatum in respectively “setting” and “maintaining” processes related to the regulation of body weight and cocaine preference.
Highlights.
D2R/D3R binding availability negatively correlates with future body weight
D2R/D3R binding availability negatively correlates with future cocaine preference
PET imaging with [11C]raclopride can be used to predict future body weight
PET imaging with [11C]raclopride can be used to predict future cocaine preference
ACKNOWLEDGMENTS
This work was supported by the NIAAA (AA 11034 & AA07574, AA07611) and a graduate teaching fellowship from SBU to MM. We thank Millard C. Jayne and Colleen Shea for PET tracer synthesis and scheduling as well as Brenda Anderson and John Robinson for helpful comments on the manuscript as part of the dissertation of MM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci. 2009;21:1332–1345. doi: 10.1162/jocn.2009.21092. [DOI] [PubMed] [Google Scholar]
- Mohr PN, Nagel IE. Variability in brain activity as an individual difference measure in neuroscience? J Neurosci. 2010;30:7755–7757. doi: 10.1523/JNEUROSCI.1560-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3223–3232. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences. N.R.C. Commission on Life Sciences. Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. National Academy Press; washington D.C.: 1996. [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Liebling CN, Reiszel C, Hooker JM, Brodie JD, Dewey SL. Cue-induced dopamine release predicts cocaine preference: positron emission tomography studies in freely moving rodents. J Neurosci. 2009;29:6176–6185. doi: 10.1523/JNEUROSCI.5221-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods. 2003;129:105–113. doi: 10.1016/s0165-0270(03)00192-4. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30:13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos P, Bermeo C, Rubinstein M, Suchland K, Wang G, Grandy D, Volkow N. Conditioned place preference and locomotor activity in response to methylphenidate, amphetamine and cocaine in mice lacking dopamine D4 receptors. J Psychopharmacol. 2009 doi: 10.1177/0269881109102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]