Abstract
Pathological 43-kDa transactive responsive sequence DNA-binding protein (TDP-43) has been recognized as the major disease protein in amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration with ubiquitin positive, tau and α-synuclein negative inclusions (FTLD-U) and the transitional forms between these multisystem conditions. In order to develop TDP-43 into a successful ALS biomarker, the natural history of TDP-43 pathology needs to be characterized and the underlying pathophysiology established. Here we propose a spatial and temporal “two-axis” model of central nervous system vulnerability for TDP-43 linked degeneration and discuss recent studies on potential biomarkers related to pathological TDP-43 in the cerebrospinal fluid (CSF), blood, and skeletal muscle. The model includes the following “two arms”: First, a “motor neuron disease” or “spinal cord/brainstem to motor cortex” axis (with degeneration possibly ascending from the lower motor neurons to the upper motor neurons); and secondly, a “dementia” or “corticoid/allocortical to neocortex” axis (with a probable spread of TDP-43 linked degeneration from the mediotemporal lobe to wider mesocortical and neocortical brain areas). At the cellular level, there is a gradual disappearance of normal TDP-43 in the nucleus in combination with the formation of pathological aggregates in the cell body and cellular processes, which can also be used to identify the stage of the disease process. Moreover, TDP-43 lesions in subpial/subependymal or perivascular localizations have been noted in TDP-43 linked neurodegeneration, and this might account for increased CSF and blood TDP-43 levels through mechanisms that remain to be elucidated.
1. Introduction
With the advent of new technologies in medicine and related biomedical sciences, concepts of neurodegenerative diseases continue to be refined and descriptions of novel disease are being created. Based on advances over the past decades, we now know that most neurodegenerative conditions are characterized by protein misfolding and also may be linked to a number of other common features such as mutations in genes that encode major disease proteins of familial disorders and their sporadic counterparts; the presence of pathological protein aggregates; alterations in protein solubility and metabolic changes of corresponding disease proteins. The clinical phenomenology of these diseases is diverse and variably correlates with the topographical pattern of the central nervous system (CNS) pathology. Hence, overlapping CNS disease categories have been established based on clinical phenomenology and genetic findings, as well as pathological abnormalities including biochemical studies (Armstrong et al., 2005; Skovronsky et al., 2006). Indeed, major disease groups such as tauopathies or α-synucleinopathies have emerged during the last few decades. In 2006, pathological transactive response DNA-binding protein with a molecular weight of 43 kDa (TDP-43) was shown to be the major disease protein in amyotrophic lateral sclerosis (ALS) with or without dementia, and in frontotemporal lobar degeneration with ubiquitinated inclusions (previously designated FTLD-U, but now referred to as FTLD-TDP) with or without motor neuron disease (MND) (Arai et al., 2006; Neumann et al., 2006). The discovery of this new pathological protein lends support to the notion that FTLD-TDP and ALS are clinico-pathologically overlapping diseases and together they form a new class of neurodegenerative protein misfolding disorders termed TDP-43 proteinopathies (Geser et al., 2009b). However, a small subgroup of ALS and FTLD-U cases with neuronal inclusions composed of an ubiquitinated protein, negative for pathological TDP-43, tau, or α-synuclein, were shown to be characterized by the pathological aggregation of a protein called fused in liposarcoma (FUS), also referred to as translated in liposarcoma (TLS) (Baumer et al., 2010; Huang et al., 2010; Neumann et al., 2009; Roeber et al., 2008). There is a high degree of functional homology between FUS and TDP-43, as both are ubiquitously expressed DNA/RNA-binding proteins involved in multiple aspects of gene expression, transcription regulation, RNA splicing, RNA transport and translation (Lagier-Tourenne and Cleveland, 2009; Neumann et al., 2009; Cohen et al., 2011).
The potential use of TDP-43 as a “bio-marker” stems from the concept that pathophysiologic processes, which lead to structural and functional abnormalities, are accompanied by a variety of biological changes including alterations in TDP-43 expression and deposition, which might be measured. A validated and sensitive biomarker offers both significant diagnostic and clinical benefits, and also provides the potential to objectively assess of therapeutic interventions. It has been noted that the evolution of pathology throughout the nervous system in a range of different neurodegenerative disorders is not random but follows patterns, and consequently, grading or staging schemes have been developed to account for this temporal and spatial development of degeneration. Hence, a successful biomarker also might reflect the temporo – spatial disease progression of the underlying pathology.
ALS is a progressive, usually adult onset, neurodegenerative disorder with an annual incidence rate of 1.5 – 2.0 per 100.000, a prevalence of about 6 in 100.000, and a median survival from disease onset to death of around 2-5 years (Chio et al., 2009; Mitchell and Borasio, 2007). The 1998 El Escorial revised criteria for a diagnosis of ALS require the presence of the following: (1) evidence of lower motor neuron (LMN) degeneration by clinical, electrophysiological or neuropathologic examination, (2) evidence of upper motor neuron (UMN) degeneration by clinical examination, and (3) progressive spread of symptoms or signs within a region or to other regions, as determined by history or examination. Furthermore, exclusion criteria have been elaborated (i.e., the absence of the following: (1) electrophysiological and pathological evidence of other disease processes that might explain the signs of LMN and/or UMN degeneration, and (2) neuroimaging evidence of other disease processes, which might explain the observed clinical and electrophysiological signs (Brooks, 1994; Brooks et al., 2000). There is now extensive pathological, clinical, neuropsychological and neuroimaging evidence that ALS pathology extends well beyond the motor cortex which is referred to as ALS-Plus syndrome (Brooks, 1994; Brooks et al., 2000; Geser et al., 2010a). Cognitive and behavioural deficits are frequently seen and there is evidence of frontotemporal, cerebellar, corpus callosum, and basal ganglia involvement (Strong et al., 2009).
The findings of mutations in the gene encoding TDP-43 (TARDP) in cases of familial and rare sporadic ALS patients further corroborate the significance of pathological TPD-43 as being mechanistically implicated in the disease process (Neumann, 2009; Pesiridis et al., 2009; Banks et al., 2008; Mackenzie et al., 2010; Daoud et al., 2009; Gijselinck et al., 2007; Gitcho et al., 2008; Guerreiro et al., 2008; Kabashi et al., 2008; Kuhnlein et al., 2008; Rutherford et al., 2008; Sreedharan et al., 2008; Van Deerlin et al., 2008; Yokoseki et al., 2008; Kamada et al., 2009; Corrado et al., 2009; Lemmens et al., 2009; Del Bo et al., 2009; Pamphlett et al., 2009; Williams et al., 2008; Tamaoka et al., 2010; Nozaki et al., 2010). In fact, many of the TARDBP variants display autosomal-dominant inheritance in familial ALS patients, suggesting that they may be pathogenic mutations. FUS gene mutations have been mainly reported in familial and sporadic cases of ALS (including patients with additional non-MND features (Mackenzie et al., 2010; Vance et al., 2009; Kwiatkowski et al., 2009; Rademakers et al., 2009; Mackenzie et al., 2011; Robertson et al., 2011, Baumer et al., 2010; Huang et al., 2010; Yan et al., 2010; Broustal et al., 2010). Ataxin-2 is a polyglutamine (polyQ) protein encoded in the ataxin 2 gene (ATXN2) by trinucleotide repeats of CAG. ATXN2 is mutated in spinocerebellar ataxia type 2, and intermediate length or high polyQ expansions in this gene have been found to be significantly associated with sporadic and familial ALS (Elden et al., 2010; Daoud et al., 2011; Lee et al., 2011a; Lee et al., 2011b; Van Demme et al., 2011). A significant association of expanded ATXN2 repeats with the development of progressive supranuclear palsy was also observed (Ross et al., 2011). Furthermore, it has been shown that CAG repeat expansions in ATXN2 associated with ALS are CAA interrupted repeats (Yu et al., 2011). Mutations in other genes involved in the pathogenesis of ALS include superoxide dismutase 1 (SOD1) (Rosen et al., 1993), senataxin (SETX) (Chen et al., 2004), angiogenin (ANG) (Greenway et al., 2006), and optineurin (OPTN) (Maruyama et al., 2010). TARDBP mutations have rarely been reported in patients with either sporadic or familial FTLD-MND and FTLD-TDP , respectively (Borroni et al., 2009, Borroni et al., 2010; Benajiba et al., 2009, Chio et al., 2010; Banks et al., 2008; Gijselinck et al., 2007; Rollinson et al., 2007; Schumacher et al., 2007; Benajiba et al., 2009). Progranulin (GRN) gene mutations cause FTLD with TDP-43 pathology (Cairns et al., 2007; Baker et al., 2006; Cruts et al., 2006; Sleegers et al., 2008) with the clinical and molecular phenotype differing between cases with and without GRN gene abnormalities (Chen-Plotkin et al., 2008; Chen-Plotkin et al., 2011). GRN may also modify the course of disease in patients with ALS (Sleegers et al., 2008). Complex clinico-pathological syndromes associated with TDP-43 pathology have been described for the following genes: TARDBP, valosin containing protein (VCP, FTLD with Paget disease of the bone), and dynactin (DCTN1, Perry syndrome) genes (Kovacs et al., 2009; Neumann et al., 2007). Other genetic abnormalities might play a role as well. To give examples, there are FTD-MND families linked to chromosome 9p (Le Ber et al., 2009), and genome-wide association studies have revealed susceptibility loci on this chromosome (i.e., 9p21.2) or chromosome 19 (i.e., 19p13.3, UNC13A) (Van Es et al., 2009; Laaksovirta et al., 2010; Shatunov et al., 2010; Mackenzie et al., 2010). Furthermore, Rollinson and coworkers (2009) suggested that ubiquitin-associated protein 1 (encoded on chromosome 9p) is a genetic risk factor for subjects diagnosed with FTLD according to clinical diagnostic criteria by Neary and colleagues (1998). More recently, a genome-wide association study suggested that polymorphisms at locus 7p21, within the gene TMEM106B, are a strong risk factor for clinical FTLD (van der Zee et al., 2011) and FTLD with TDP-43 pathology (Van Deerlin et al., 2010). Although genome-wide association studies did not show analogous results for ALS (Dunckley et al., 2007; Chio et al., 2009), protective alleles at the TMEM106B locus have been demonstrated to correlate with better cognitive scores in ALS (Vass et al., 2011).
Early intervention with disease modifying therapies is likely to yield the most beneficial results for ALS and other neurodegenerative diseases. Cerebrospinal fluid (CSF) biomarkers are likely to be the most informative because the CSF is in closer contact with the CNS than other more accessible peripheral fluids such as plasma or urine, and the CSF is therefore most likely to reflect neurodegenerative processes underlying ALS through changes in one or more CSF analytes (Turner et al., 2009). Although earlier research on CSF, blood plasma, and various neuroimaging methods have proposed a number of candidate ALS biomarkers with only limited success, at present, there is no diagnostic test for ALS based on chemical biomarkers (for review see: Agosta et al., 2010; Bowser and Lacomis, 2009; Kolarcik and Bowser, 2006; Pradat and Dib, 2009; Strong, 2008; Sussmuth et al., 2008; Turner et al., 2009). Thus, the quest to identify informative CSF biomarkers for ALS, which would enable the early and reliable diagnosis of ALS, continues. For example, several potential CSF biomarkers identified for ALS include inflammatory proteins such as interferon gamma, granulocyte-macrophage colony stimulating factor, granulocyte colony stimulating factor, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 a/b, and interleukins 2, 6, 8, 10, 15 and 17 (Kuhle et al., 2009; Mitchell et al., 2009), axonal proteins like neurofilament light and heavy chains (Brettschneider et al., 2006; Zetterberg et al., 2007), growth factors like insulin-like growth factor-1, erythropoietin and vascular endothelial growth factor (Bilic et al., 2006; Brettschneider et al., 2007; Mitchell et al., 2009), and angiotensin II (Kawajiri et al., 2009). However, further studies of these analytes are needed to confirm and extend our understanding of their potential diagnostic value for distinguishing ALS subjects from normal controls as well as from subjects with other neurodegenerative diseases in order to determine if changes in their CSF levels are specific to ALS or if they also occur in other neurological conditions. Finally, proteomic studies have been conducted to identify new candidate ALS biomarkers and these strategies offer considerable promise (Brettschneider et al., 2008; Pasinetti et al., 2006; Ranganathan et al., 2005). However, since proteomic methods do not allow reliable quantification of analyte levels, they will require follow up studies using enzyme-linked immunosorbent assays or other complementary methods to establish an informative quantitative diagnostic assay for ALS .
As pathological TDP-43 has now been established as the defining feature of ALS, there is a pressing need to investigate the potential role of this protein as a new ALS biomarker. Despite recent studies on TDP-43 pathology in the CNS, there has not yet been a comprehensive review of the development of a temporo-topographical TDP-43 neurodegeneration model in ALS. In the following sections, studies outlining or mapping the pattern of degeneration in MND (with or without dementia) are discussed, followed by discussion of the role of TDP-43 in this degenerative process as well as the potential of TDP-43 to be used as a biomarker for ALS and FTLD-TDP.
2. Neuropathology and neurobiology
2. 1. CNS patterns of pathological TDP-43
Human post-mortem studies are cross-sectional by nature. Nevertheless, since topographical patterns of pathology with differences in relative severity can be described and correlated with clinical information, a model of vulnerability can be developed from post-mortem studies. Such a model might ultimately describe the temporal sequence of CNS degeneration including early, intermediate, and late stages of disease progression, with CNS areas that are most vulnerable to degeneration being first affected. In the advanced stages of the disease, signs of neurodegeneration such as neuronal loss and reactive gliosis, as well as cellular inclusion pathology are expected to increase. However, end stages might be associated with relatively little inclusion pathology due to the high level of neurodegeneration which involves the clearing of cell debris (including TDP-43 lesions released by dying cells) by macrophages (“burnt out cases”). In addition, pathologic proteins might change their cellular characteristics, i.e., their relative location in the cell, during the disease process, which can also be used to indicate the stage of the disease.
This approach had been successfully employed for many neurodegenerative diseases. In fact, for Alzheimer's disease (AD), cortical neurofibrillary pathology has been reported to start in the transentorhinal cortex, and to spread out towards the hippocampus and neocortex as reflected by the Braak neurofibrillary stages (Braak et al., 2006; Braak and Braak, 1991). It has been demonstrated in the past that occasionally subcortical nuclei show changes even earlier (Braak et al., 2006; Braak and Del Tredici, 2004). Recently, it has been shown in a study of individuals under thirty that neurofibrillary tangle formation does not begin in the cerebral cortex but, rather, in select subcortical nuclei, and it may start quite early, i.e., before puberty or in early young adulthood (Braak and Del Tredici, 2011). Amyloid-β (Aβ) deposition occurs early on in cortical brain regions with the later involvement of subcortical CNS areas (Thal et al., 2002; Thal et al., 2000). There is uncertainty as to which neocortical brain areas show the first Aβ plaques, but the temporal cortex seems to be affected according to most pathology studies. Furthermore, a three point staging system has been developed for agyrophilic grain disease defining early to intermediate changes occurring in temporal lobe structures, followed by the subsequent involvement of the insular and cingulate cortex (and other non-cortical areas such as the septal nuclei) (Saito et al., 2004). As for TDP-43 proteinopathies such as ALS and FTLD-TDP, no staging scheme denoting a temporal-spatial progression has been developed based on pathological TDP-43 linked neurodegeneration. A study proposed a four stage system both for tau positive and negative FTLD (Broe et al., 2003; Kril and Haliday, 2011). This is based on the distribution and severity of gross atrophy. Temporal lobe changes showed the most linear relationship with clinical indices of disease progression. The Lewy body diseases (i.e., Parkinson's disease, dementia with Lewy bodies and Parkinson's disease dementia) are characterized by neuronal α-synuclein deposits and are known to spread out from caudal parts of the neuraxis and olfactory system, afflicting the cortical system later in the disease course with anteromedial temporal mesocortex being involved prior to associational and primary cortical areas (Braak et al., 2003; Del Tredici et al., 2002). For multiple system atrophy (MSA), a disease with prominent α-synuclein positive oligodendroglial inclusion pathology, grading scales have been developed (Jellinger et al., 2005; Wenning et al., 2002). In fact, for striatonigral degeneration, which is the hallmark finding underlying the parkinsonian variant of MSA, a disease progression rising up the nigro-striatal system has been proposed (Wenning et al., 2002). The term “minimal change MSA” denotes an early disease stage with degeneration largely restricted to the substantia nigra (Wenning et al., 1994). For Huntington's disease, a disease progression scheme within the striatum has been described (Vonsattel et al., 1985). The understanding of the spatio-temporal involvement of different anatomic and functional systems represents the basis for understanding the pathophysiology and pathobiology of these diseases. Staging schemata for TDP-43 linked diseases have not yet been developed; although early data on whole brain pathology and topographical distribution with clinical correlations are now available and might serve as a basis for further studies. In fact, ALS with or without dementia has been split into two different patterns (i.e., type 1 and type 2) by means of an analysis of the distribution of neuronal TDP-43 pathology. When comparing these, type 2 is the more severe and FTLD-TDP -like “dementia type” that involves the hippocampal formation, frontotemporal neocortex, basalganglia and substantia nigra. Type 1 is the so-called “classical ALS type” showing TDP-43 pathology in the pyramidal system with the LMN being involved more frequently than the motor cortex. This classical ALS type is also characterized by TDP-43 lesions in non-motor nuclei of the midbrain (Nishihira et al., 2008). Similarly, in a study of ALS cases with long disease duration, TDP-43 linked neurodegeneration was mild overall, but present to a higher level in the lower as compared to the upper motor neuron (Nishihira et al., 2009a). However long-standing disease might be associated with widespread significant TDP-43 linked neurodegeneration that includes both the LMN and UMN (Nishihira et al., 2009b). A case study of an ALS patient with a TARDBP mutation showed greater motor neuron degeneration in the spinal cord compared with the upper motor neuron, and a comparative sample of 16 ALS cases without TARDBP mutations was described as showing similar neuropathological features (Pamphlett et al., 2010). Moreover, in a study focusing on the maturation process of neuronal cytoplasmic inclusion in ALS, it was shown that long inclusions called threads or “coarse skeins”, as well as their presumed precursors, which are shorter and thinner linear inclusions called linear wisps or “fine skeins” occurred at a similar frequency in lower and upper motor neurons. However, round inclusions, which are the presumed aggregates of dot-like inclusions, appeared to occur more frequently in the anterior horns of motor neurons than the motor cortex (Mori et al., 2008). FUS linked ALS has also been shown to be associated with somewhat less upper motor neuron as opposed to lower motor neuron inclusion pathology (Mackenzie et al., 2011). An earlier study using ubiquitin immunohistochemistry demonstrated that dense rounded or irregular ubiquitin-immunoreactive cytoplasmic inclusions (dense bodies) and loosely arranged bundles (“skeins”) of filamentous-appearing material were present in the anterior horns of all ALS cases examined (Leigh et al., 1991). However, inclusions in pyramidal neurons of the motor cortex were only present infrequently in a minority of cases. Earlier pathology studies have supported the notion of a primary LMN involvement with the subsequent onset of UMN degeneration. This early work is outlined in a review by Chou and Norris, which also elaborates the concept of a lower motor neuron disease spreading monosynaptically to UMN (Chou and Norris, 1993). However, based on clinical experience, a spectrum of MND has been defined that includes progressive muscular atrophy (PMA), LMN dominant MND, LMN/UMN combined MND, UMN dominant MND, and primary lateral sclerosis (PLS). Consistent with this clinical phenomenology, an alternate view has been proposed which favors the idea of heterogeneity of MND phenotypes in ALS with a highly variable site of disease onset (Ravits and La Spada, 2009). This concept was derived from the clinical observation that MND begins at a focal location and spreads out from this point through the anatomy of the UMN and LMN in a logical manner, which was used to infer the underlying neuropathology. Previous post-mortem studies of the clinically LMN disorder PMA has demonstrated LMN pathology with a significant number of individuals, but not all, also manifesting UMN pathology (see Ince et al., 2003; Tsuchiya et al., 2004, and references therein). We recently examined six patients with an isolated LMN disorder (Geser et al., 2011). Cases with a disease duration over 4 years were designated as PMA, and those with a more rapid course as MND/LMN. All patients showed significant TDP-43 linked degeneration of LMNs, and five cases showed a lesser degree of motor cortex degeneration. Additional brain areas were affected to varying degrees, ranging from predominantly brainstem pathology to significant involvement of the whole CNS including neocortical and limbic areas. These findings suggest that MND-related TDP-43 pathology might follow a sequentially additive pattern of development spreading from the spinal cord/brainstem into other parts of the CNS. This might reflect disease progression, thus representing the basis for the development of whole CNS pathological TDP-43 evolution schemata. For PLS, which is characterized clinically by upper but not lower motor neuron involvement (Hudson et al., 1993; Pringle et al., 1992; Tartaglia et al., 2007), post-mortem data on pathological TDP-43 are scarce (Dickson et al., 2007; Josephs and Dickson, 2007). In fact, the histological findings of two of the four published cases were similar (Josephs and Dickson, 2007). There was frontotemporal and hippocampal, but no LMN brainstem TDP-43 pathology; neurodegeneration in the motor cortex was severe in one case, and mild to moderate in the other. Since there is widespread cortical degeneration in these cases they have been referred to as FTLD-PLS. This suggests that the concept of “primary lateral sclerosis” should be seen, at least in part, in the context of a widespread frontotemporal degeneration that also involves primary cortical areas such as the motor cortex. Two other published PLS cases showed either no or low level TDP-43 pathology restricted to the hypoglossal nucleus in the brainstem (Dickson et al., 2007). Clinically, longstanding PLS may eventually develop into ALS (Bruyn et al., 1995). As to the question of differentiating between ALS and PLS, it was suggested that a patient presenting with spasticity who does not develop wasting within 3 years most likely has PLS (Tartaglia et al., 2007). However, in this study with clinical symptoms and signs being the main outcome measure, clinical diagnostic criteria based on definitions outlined earlier have been used for classifying patients into either ALS or PLS groups (Brooks et al., 2000; Pringle et al., 1992). Pathology data in the older literature utilizing ubiquitin immunohistochemistry in PLS cases, or in disorders that mimic PLS, showed that inclusion pathology is not restricted to the UMN; rather it is also present in LMN and/or cortical brain areas, which corroborates the idea of a clinico-pathological continuum between PLS and ALS (for a case report of PLS and a table displaying the previous literature see Tan et al, (2003). It is of note that the idea of a multisystem disease of ALS was underlined by TDP-43 studies which showed non-pyramidal TDP-43 lesions occurring in the brainstem or subcortical brain structures such as basal ganglia of ALS cases (Braak et al., 2010; Dickson et al., 2007; Geser et al., 2008a; Zhang et al., 2008).
Clues as to the concept of temporal and spatial evolution of TDP-43 pathology throughout the CNS might emerge from the study of a sufficient number of normal controls and diseases in which TDP-43 is present as an additional feature, “secondary” to other, disease defining, pathologies(Geser et al., 2009a; Kovacs et al., 2010). Indeed, the first TDP-43 studies on the brainstem and pyramidal system in both healthy controls and patients with a disease other than TDP-43 proteinopathies have become available which demonstrate, in part, various degrees of TDP-43 pathology. However, these need to be supplemented by the screening of larger cohorts in order to have reliable data on the topographical distribution and relative severity of changes which occur early in the disease course (Geser et al., 2010b; Geser et al., 2011; Mori et al., 2008). Furthermore, it has been reported that there are ubiquitinated inclusions that are negative for pathological TDP-43 in the medulla oblongata of control subjects and the striatum of normal controls and ALS patients with or without dementia (Davidson et al., 2009; Zhang et al., 2008). The significance of these findings needs to be further explored. As to a CAG repeat disorder associated with neurodegeneration and inclusion pathology in multiple CNS areas, i.e., spinocerebellar ataxia type 3 or Machado-Josephs disease (Yamada et al., 2008), it has been shown that TDP-43 pathology occurs rarely and is “almost entirely” restricted to the LMN (Tan et al., 2009). Besides the “spinal cord-brainstem-motor cortex” axis of the CNS, cortical structures in normal or disease controls have also been examined for the presence of pathological TDP-43. We have recently shown that significant TDP-43 pathology is present predominantly in mediotemporal lobe structures in about 30% of subjects with or without severe mental illness aged 65 years or older; in addition, increasing age was associated with a greater degree of TDP-43 pathology (Geser et al., 2010b). This allows for the differentiation of an earlier “limbic” type from a later “neocortical” type of TDP-43 pathology with the latter building upon the former. Several studies of AD have also reported that medial temporal lobe limbic structures including the hippocampus and amygdala are particularly vulnerable to TDP-43 pathology (Arai et al., 2009; Higashi et al., 2007b; Hu et al., 2008; Kadokura et al., 2009; Uryu et al., 2008). Furthermore, hippocampal sclerosis has been associated with a higher TDP-43 burden than AD, and two types of TDP-43 pathology distribution have been proposed (Amador-Ortiz et al., 2007). These include (1) a diffuse type of pathology with an involvement of the dentate fascia of the hippocampus, the entorhinal cortex, the occipitotemporal gyrus, as well as the inferior temporal gyrus, and (2) a limbic type of pathology, showing TDP-43 immunoreactivity limited to the dentate fascia and entorhinal cortex, with sparse or no involvement of the occipitotemporal gyrus. Diffuse neurofibrillary tangles with calcification, which is a rare presenile dementia, has been recently shown to be associated not only with a high burden of tau pathology, but also with limbic α-synuclein and limbic TDP-43 pathology in all of the examined cases, as well as with more widespread cortical α-synuclein and TDP-43 pathology in a significant number of patients (Habuchi et al., 2011). In a single case of familial British dementia, which is a rare autosomal disorder associated with a stop-codon mutation in the BRI gene as well as Abri plaques, cerebral amyloid angiopathy, and tau pathology, TDP-43 pathology occurs predominantly in medial temporal lobe structures (Schwab et al., 2009; Vidal et al., 1999). It has been previously reported that AD-like tau pathology in this disease is found primarily in the limbic system and brainstem (Holton et al., 2001). Furthermore, the pattern of Abri plaques is different from that seen in AD. In a study of MSA, which is an α-synucleinopathy, we recently showed that TDP-43 lesions were scarce overall which was probably due to the relatively young age at death (on average mid to late 60s) of the cohort studied (Geser et al., 2011). In the cases where TDP-43 pathology was present, it was predominantly located in mediotemporal structures (or the brainstem). Patients with Lewy body diseases (Arai et al., 2009; Higashi et al., 2007b; Nakashima-Yasuda et al., 2007; Yokota et al., 2010a) and cases with the tauopathies progressive supranuclear palsy and corticobasal degeneration (Uryu et al., 2008; Yokota et al., 2010b) showed varying levels of pathological TDP-43 in the amygdala or hippocampus/entorhinal cortex, whereas other brain areas were less consistently affected in these diseases. For cases with argyrophilic grain disease, concomitant TDP-43 pathology has been shown to be most intense in the amygdala and the adjacent entorhinal cortex (Fujishiro et al., 2009). Topographical data on FUS pathology in FUSopathies are incomplete because of the relative rarity of these cases; however, it has been demonstrated that in some cases of FTLD associated with pathological FUS, the hippocampus shows a somewhat higher degree of FUS pathology than the neocortex, while in other cases the level of FUS pathology is similar in both regions (Mackenzie et al., 2011; Neumann et al., 2009). Topographical data on pathological TDP-43 comparing different CNS regions in a variety of other diseases such as Parkinsonism–dementia complex and ALS of the Chamorro population on Guam, corticobasal syndrome with TDP-43 pathology, Huntington's disease, chronic traumatic encephalopathy, or some familial forms of FTD/FTD-MND/ALS with cognitive impairment are incomplete or inconclusive, though the dentate gyrus seems to be afflicted in particular in the Guaminan Parkinsonism-dementia complex (Geser et al., 2008b; Hasegawa et al., 2007; Miklossy et al., 2008; Schwab et al., 2008). In summary, these studies point to a progression of TDP-43 pathology which starts in mediotemporal lobe structures and spreads out to include higher order association cortices at a late stage in the disease development. The occipital or visual cortex seems to be affected at a very late stage (Geser et al., 2009b); other primary areas such as the auditory or motor cortex in FTLD need to be further examined, but the rarity of the FTLD-PLS cases and their association with widespread severe cortical TDP-43 pathology (see above) also suggests late stage involvement.
Hence, we here propose a “two axis” model of whole CNS vulnerability of susceptibility for TDP-43 pathology including the following “two arms” (see Figure): First, a “motor neuron disease” or “spinal cord/brainstem to motor cortex” axis, possibly with degeneration ascending from the LMN to the UMN; and secondly , a “dementia” or “corticoid/allocortical to neocortex” axis, probably with a spread of TDP-43 linked degeneration from the mediotemporal lobe to wider mesocortical and neocortical brain areas. By analogy, similar patterns of changes outlining disease progression have been proposed for other pathological proteins including the archi- and neocortex as reflected by the widely adopted Braak neurofibrillary stages for AD (Braak et al., 2006), and also a scheme for the brainstem and cortex for the Lewy body disorders (Braak et al., 2003; Del Tredici et al., 2002) that serves as a basis for the current consensus criteria for the diagnosis of dementia with Lewy bodies (McKeith et al., 2005). This cumulative evidence suggests that areas of the CNS might be differentially susceptible to pathways leading to neurodegeneration associated with a variety of different pathological proteins. Thus, there might be some region-specific, rather than disease-specific, mechanisms (Yokota et al., 2010a; Yokota et al., 2010b). It appears that there are as yet unidentified mutually augmenting interactions of pathological proteins which eventually lead to their aggregation. The synergistic interaction between tau, Aβ and α-synuclein as recently shown in an animal model might also apply to TDP-43 (Clinton et al., 2010).
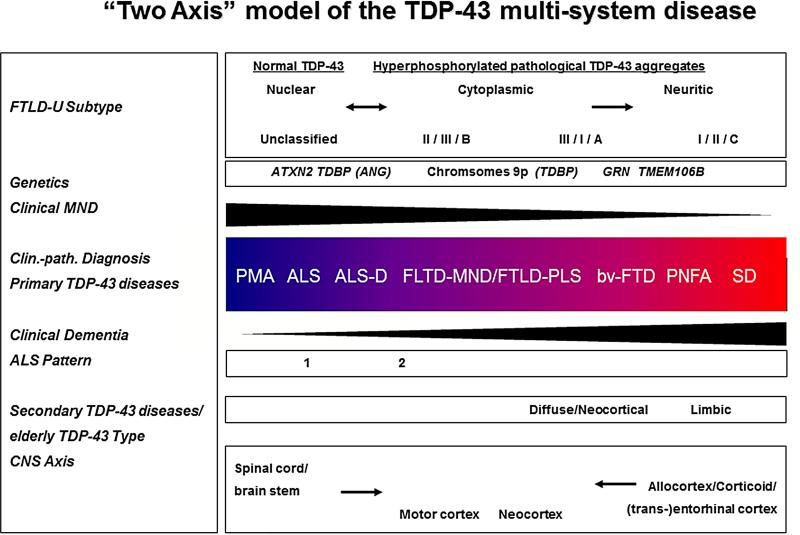
“Two axis” model of (TAR) DNA binding protein with a Mr of 43 (TDP-43) multisystem diseases.
Schematic illustration of a “two axis” concept of a clinico-pathological (clin.-path.) spectrum model of the TPD-43 diseases extending from PMA at one end to semantic dementia (SD) at the other, with the overlapping categories ALS, ALS with dementia (ALS-D), FTLD with MND/PLS, behavioral variant of frontotemporal dementia (bv-FTD), and progressive nonfluent aphasia (PNFA) being situated at intermediate positions between the two poles. The CNS “two axis” concept includes the following: First, a “motor neuron disease” or “spinal cord/brainstem to motor cortex” axis, with degeneration possibly ascending from the LMN to the UMN. Secondly, a “dementia” or “corticoid/allocortical to neocortex” axis, with a probable spread of TDP-43 linked degeneration from the mediotemporal lobe to wider mesocortical and neocortical brain areas. The pathophysiologic process might develop along either of these major arms, or along both in parallel, albeit with different relative severities and times of onset, respectively. Lower black arrows signify the topographical evolution of TDP-43 linked neurodegeneration with (1) an spinal/cord to motor cortex/neocortex ascending axis on the left and (2) and allocortical/corticoid/(trans-)entorhinal to neocortical arm on the right. Black arrowhead-like triangles denote clinical syndrome with motor neuron disease decreasing and dementia increasing from left to right. Color change in central box denotes increasing or decreasing spread and severity of TDP-43 pathology in the brain and spinal cord, as an approximate estimation. Specifically, blue represents predominant involvement of the spinal cord and red represents predominant involvement of cortical areas. Other subcortical brain areas are not explicitly represented in this color-coded diagram. “FTLD with ubiquitin positive, tau and α-synuclein negative inclusions (FTLD-U) subtype” as shown in the upper left corner refers to the classification of the cortical pattern of TDP-43 or ubiquitin positive inclusions according to Sampathu, Neumann and colleagues (2006)/Mackenzie and collaborators (2006)/harmonized classification system (Mackenzie et al., 2011). An “unclassified type”, which denotes the absence of TDP-43 lesions or the presence of a degree of TDP-43 pathology burden too low for subtyping according to Geser, Martinez-Lage et al., (2009), was added. The Sampathu/Neumann scheme, in brief, defines the following: subtype 1 is characterized by frequent long neuritic profiles; subtype 3 shows abundant small neuritic profiles and neuronal cytoplasmic inclusions and subtype 2 is characterized by neuronal cytoplasmic inclusions. Upper black arrows signify the hypothetical translocation of the normal nuclear localization of the protein under pathological conditions (“pathological TDP-43”) into the cytoplasm and eventually dystrophic cellular processes; the double headed arrow on the left denotes that there might be, at least, an impaired shuttling of TDP-43 between the cytoplasm and the nucleus. The ALS pattern refers to the two distributional types as described by Nishihara (2008) with “classical ALS”-like pyramidal and limited non-pyramidal TDP-43 pathology in type 1, while type 2 is the “ALS-FTLD”-like phenotype with significant cortical pathology. The “Secondary TDP-43 diseases/elderly TDP-43 Type” refers to significant limbic pathology, or in later disease stages, the addition of neocortical/diffuse pathology according to Amador-Ortiz and colleagues (2007) for the secondary TDP-43 disease AD, as well as Geser and colleagues (2010b) for a normal or mildly cognitively impaired elderly population. The row labeled “genetics” exemplifies genetic changes including mutations in the TDP-43 (TARDBP), progranulin (GRN) and angiogenin (ANG) genes, intermediate length or high polyglutamine expansions in the ataxin (ATXN2) gene, polymorphisms (TMEM106B), and linkage to chromosome 9p.
Modified with permission from: Geser F, Lee VM-Y, Trojanowski J. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: A spectrum of TDP-43 proteinopathies, Neuropathology, 2010; 30:103-112.
2.2 Cellular patterns of pathological TDP-43
As outlined above, a large numbers of studies suggest that ALS, FTLD-MND, and FTLD-TDP are different manifestations of a multiple-system TDP-43 proteinopathy linked to similar mechanisms of neurodegeneration (e.g., Dickson et al., 2007; Geser et al., 2009b). Nevertheless, different morphological categories of cortical degeneration have been delineated by using ubiquitin immunohistochemistry. This is based on the localization of inclusions within different parts of cells, as well as their relative distribution throughout the layers of the cortex. Hence, two analogous classifications schemes describing these morphological categories were developed separately by two groups - Samapthu/Neumann and colleagues, as well as Mackenzie and collaborators (Mackenzie et al., 2006; Sampathu et al., 2006). The scheme according to Sampathu and Neumann is described in the following, but it should be noted that the Sampathu/Neumann subtype 1, 2, and 3 correspond respectively to Mackenzie subtype 2, 3, and 1. A recent report has harmonized these discrepancies by introducing a new classification scheme of type A, B and C which equate to Sampathu/Neumann subtype 3, 2, and 1 (MacKenzie et al., 2011). Subtype 2 of the latter scheme is characterized by ubiquitin-positive pathology in both superficial and deep cortical layers with a predominance of cytoplasmic inclusions and only rarely neuritic inclusions. Subtype 3 shows a predominance of ubiquitin-positive pathology in superficial cortical layers with an abundance of cytoplasmic inclusions (often ring-shaped) and frequent short neuritic profiles. With the hypothesized continuation of TDP-43 displacement from the cytoplasm into cellular processes subtype 1 emerges which is characterized by a predominance of long neuritic profiles over cytoplasmic inclusions as well as a relative abundance of pathology in superficial cortical layers. Intranuclear inclusions occur variably, but have been reported to be associated with the morphological subtype characterized by both cytoplasmic and neuritic inclusion pathology which equates subtype 1 pathology as described by Mackenzie and colleagues, or subtype 3 according to the Sampathu/Neumann scheme (Mackenzie et al., 2006; Neumann, 2009). Taken together, the Sampathu/Neumann subtypes 2 and 3 feature more pathological ubiquitinated TDP-43 in the cytoplasm than subtype 1, where the pathology tends to predominate in the distal parts of the cells, i.e., cellular processes. Strikingly, the appearance of pathological TDP-43 aggregates in the cell is accompanied by a loss of normal “endogenous” TDP-43 staining in the cell nucleus, a phenomenon, which is often referred to as “nuclear clearing” (Neumann et al., 2006). A further distinct cytopathological profile consists of a cleared nucleus with diffuse or granular cytoplasmic TDP-43 staining (“pre-inclusions”) that may represent incipient TDP-43 inclusion formation and are not part of the morphological classification schemes used for FTLD-TDP (Giordana et al., 2009; Brandmeir et al., 2008; Mackenzie et al., 2006; Sampathu et al., 2006). Occasionally, single cells with nuclei devoid of TDP-43 staining are present together with a weak, diffuse, or virtually absent cytoplasmic TDP-43 immunoreactivity, and cell loss is not obvious in these cases (Geser et al., 2010b). This might support the idea of very early cellular pathology as TDP-43 begins to redistribute in the cytoplasm or in the early phase of impaired shuttling of TDP-43 between the nucleus and the cytoplasm prior to more severe later stages of neuronal loss and reactive tissue changes.
The translocation of TDP-43 from the nuclei to the cytoplasm and the cellular processes, or an impaired TDP-43 cytoplasm to nucleus shuttling process, ultimately lead to the sequestration of the protein into insoluble aggregates, and the mechanisms responsible for this redistribution of protein are still poorly understood. However, it is known that TDP-43 is a 414 amino acid nuclear protein encoded by the TARDBP gene on chromosome 1 (for review see (Buratti and Baralle, 2008; Cohen et al., 2011; Chen-Plotkin et al., 2010; Gendron et al., 2010; Mackenzie and Rademakers, 2008; Wang et al., 2008). It is a highly conserved protein ubiquitously expressed in many tissues including the CNS where it is present in neuronal and glial nuclei and to a lesser extent in the cytoplasm. The physiological functions of TDP-43 are diverse and incompletely characterized, but they likely involve the regulation of multiple biological processes through TDP-43 binding to DNA, RNA, or proteins (Geser et al., 2010a; Chohen et al., 2011). Under disease conditions, the profile of the TDP-43 proteinopathies has been shown in sporadic and familial FTLD-TDP and ALS tissue to comprise ubiquitination, variable hyperphosphorylation and N-terminal truncation of TDP-43. Sodium dodecyl sulfatepolyacrylamide gel electrophoresis of sarkosyl-insoluble extracts isolated from affected cortical regions showed disease-specific bands at ~45 kD, ~25 kD, as well as high molecular weight aggregate smears which were found in addition to the normal band at 43 kD (Geser et al., 2009a; Neumann et al., 2006). It was also shown that there is an enrichment of C-terminal fragments in TDP-43 inclusions in the cortex but not in the spinal cord of FTLD-TDP and ALS cases where rather the full length protein is present (Igaz et al., 2008). Experiments using phosphorylation specific anti-TDP-43 antibodies, which target multiple abnormally phosphorylated sites in carboxyl-terminal regions of deposited TDP-43 (Hasegawa et al., 2008), confirmed the findings that different TDP-43 species may form distinct inclusions in cortical versus spinal cord cells (Neumann et al., 2009; Geser et al., 2010). A variety of conditions that have been shown to be associated with phosphorylated TDP-43 pathology including sporadic and familial forms of the major TDP-43 diseases (FTLD-TDP and ALS) and various conditions with secondary TDP-43 pathology, such as AD or dementia with Lewy bodies (Hasegawa et al., 2008; Neumann et al., 2009; Arai et al., 2009). Accumulation of phosphorylated C-terminal fragments were readily seen in Western blots in affected cortical brain regions from patients with the “primary” (ALS and FTLD-TDP ) and “secondary” TDP-43 diseases. A small number of C-terminal fragments were detected suggesting that they may represent the same C-terminal fragment with different degrees of phosphorylation, or different C-terminal fragments with the same sites of phosphorylation, or a combination of both (Hasegawa et al., 2008; Neumann et al., 2009; Arai et al., 2009; Geser et al., 2010). The specific cleavage site of the pathological C-terminal fragment purified from FTLD-TDP brains was identified at Arg208, and it was demonstrated that the expression of this and other TDP-43 C-terminal fragments in cultured cells recapitulates key features of TDP-43 proteinopathy (Igaz et al., 2009). By altering endogenous TDP-43 nuclear trafficking using temperature sensitive cultured cells cytoplasmic punctuate TDP-43 aggregates formed and were associated with nuclear clearing (Winton et al., 2008a). Furthermore, TDP-43 aggregates may form when endogenous cytoplasmic TDP-43 is either restricted from entering the nucleus or prevented from exiting the nucleus in mutant cell cultures where nuclear export and nuclear localization mechanisms were defective. Mutant forms of TDP-43 also replicated the biochemical profile of pathological TDP-43 in FTLD-TDP /ALS. Thus, FTLD-TDP/ALS pathogenesis may be linked mechanistically to deleterious perturbations of nuclear trafficking and solubility of TDP-43 (Winton et al., 2008a). Additionally, the in vitro expression of A90V missense mutation, a benign TARDBP gene variant occurring in the nuclear localization signal sequence (Pesiridis et al., 2009), led to its sequestration with endogenous TDP-43 as insoluble cytoplasmic aggregates (Winton et al., 2008b).
These cell culture experiments are an important tool for investigating the cellular mechanisms and pathways that are involved in human neurodegenerative diseases and they also help to model the time course of the disease. For post-mortem studies, it is possible to indicate how far this process of TDP-43 translocation out of the nucleus had advanced at the time of death. Subtype 1 as described by Sampathu/Neumann scheme (Sampathu et al., 2006) with predominant dystrophic neuritic pathology shows the most “neocortical variant” degeneration; subtype 2 and subtype 3 which both have significant cytoplasmic TDP-43 pathology are similar to each other and are much closer to the motor neuron disease phenotype than subtype 1 (Geser et al., 2010a; Geser et al., 2009b). These patterns are also associated with different predominating clinical syndromes. Indeed, it has been reported that cases with predominantly neuronal intracytoplasmic inclusions correspond to FTLD-MND clinically, whereas cases with predominantly dystrophic neurites show semantic dementia, and when neuronal cytoplasmic inclusions and dystrophic neurites are coupled with neuronal intranuclear inclusions, progressive nonfluent aphasia is present – albeit not excluding overlap between these categorical patterns in single cases (Brandmeir et al., 2008; Higashi et al., 2007a; 2007b; Mackenzie et al., 2006; Ostberg and Bogdanovic; Pearson et al., 2011; Seelaar et al., 2007). Moreover, it has been shown that FTLD-TDP patients with numerous neuronal cytoplasmic inclusions, as occurs in FTLD-TDP subtype 2 or 3, have shorter survival times than those with subtype 1 (Grossman et al., 2007). It appears that the dis-localization of a nuclear protein in the cytoplasm or an impaired cytoplasm to nucleus shuttling of TDP-43 are early stages in a disease associated with short disease duration, as evidenced by the shorter survival or earlier disease onset of ALS or patients with MND combined with FTLD as compared with FTLD-TDP without MND or FTLD associated with tau pathology (Forman et al., 2006; Hodges et al., 2003; Hu et al., 2009; Josephs et al., 2005). Moreover, in elderly patients with or without severe mental illness cortical pathology appears to be in an intermediate stage and consistent with subtype 2 (Geser et al., 2010b). Similarly, in “classical” ALS cases frontotemporal pathology is often too mild for subtyping due to the subtle nature of early cortical changes (hence it is termed “unclassified type”). For ALS (with dementia) cases where the degree of cortical pathology can be classified, subtype 2 is a frequent finding (Geser et al., 2008a; Ishihara et al., 2010). This is consistent with the idea of early to intermediate cellular changes in MND (with dementia) showing a more rapid disease course than FTLD associated dementia (Geser et al., 2009b; Grossman et al., 2007; Mackenzie et al., 2006). Moreover, the vast majority of cases with “secondary” TDP-43 pathology have been reported to show a subtype 2 or 3 profile (Amador-Ortiz et al., 2007; Arai et al., 2009; Geser et al., 2008b; Habuchi et al.; Higashi et al., 2007b; Josephs et al., 2008; Kadokura et al., 2009; Nakashima-Yasuda et al., 2007; Uryu et al., 2008) according to the Sampathu/Neumann scheme (Sampathu et al., 2006) denoting stages in the process of cellular dis-localization of pathological TDP-43 similar to those found in ALS or ALS combined with FTD. Finally, consistent with clinico-pathological data (Geser et al., 2009b), it has been demonstrated by means of an ante-mortem magnetic resonance imaging approach (i.e., voxel-based morphometry that assesses regional grey matter atrophy) that FTLD-TDP subtypes have distinct clinical and neuroimaging features with subtype 3 showing subcortical (i.e., striatum and thalamus) in addition to cortical atrophy. The ALS-like “unclassified type” did not reveal areas of significant atrophy at the predetermined statistical significance threshold (Rohrer et al., 2010).
3. Biological markers of ALS based on pathological TDP-43
These early data using an ante-mortem approach to visualize ongoing degeneration in post-mortem confirmed TDP-43 proteinopathy cases lead us now to the search for in vivo TDP-43 related markers in CSF, blood or skeletal muscle. Recent studies reporting elevated TDP-43 plasma levels in clinically diagnosed FTLD (and AD) and increased CSF TDP-43 levels in FTLD-TDP and ALS patients as compared with controls are intriguing, as they might offer a diagnostic ante-mortem tool as well as a biomarker for interventional clinical trials, but they need to be verified in larger cohorts and, ideally, should be confirmed by post-mortem follow up studies (Foulds et al., 2008; Foulds et al., 2009; Kasai et al., 2009; Noto et al., 2011; Steinacker et al., 2008). In the following, these studies are discussed, and mechanistic hypotheses outlining how these changes might relate to CNS TDP-43 pathology are addressed.
3.1 CSF
A study of ALS patients (with or without cognitive dysfunction) and FTLD (with or without MND) did not find any evidence for pathologically altered TDP-43 in the CSF, such as specific C-terminal fragments or hyperphosphorylated full-length bands (Steinacker et al., 2008). However, relative quantification of 45-kDa bands revealed that patients with ALS and FTLD had higher TDP-43 levels than controls but with a prominent overlap of values. For the cases of ALS with cognitive dysfunction, TDP-43 levels were not significantly different from control cases. Although these data are promising, they need to be replicated in larger cohorts in order to increase statistical power. A similar study showed that ALS patients as a group had significantly higher levels of TDP-43 in their CSF than age-matched controls (Kasai et al., 2009). Patients examined within the first 10 months of disease onset showed significantly higher TDP-43 levels than those after this time point, a finding which raised the possibility that increased CSF TDP-43 might be used as an early disease stage marker of ALS. The morphologic and pathophysiologic correlates of this remain speculative. As mentioned earlier, end stages of neurodegeneration may be associated with the relative absence or scarcity of inclusion pathology, and, indeed, it has been reported that ALS cases with long disease duration show mild TDP-43 pathology in combination with moderate to severe neuronal loss in the spinal cord anterior horn (Nishihira et al., 2009a). It is conceivable that the level of TDP-43 in the CSF follows the same time course as its pathologic appearance in the CNS with more TDP-43 leaking out of the CNS in the early stages of disease. The recent report by Noto and colleagues that although ALS patients have higher TDP-43 CSF levels than various disease controls (including other neurodegenerative or neuroinflammatory diseases), short disease duration is associated with lower levels than cases with a longer history, needs to be further investigated in prospective studies (Noto et al., 2011). The same study showed that the sensitivity of the CSF TDP-43 enzyme-linked immunosorbent assay used was close to 60%, and the specificity 96%, indicating that this might be an useful confirmatory rather than screening test. The actual site of this pathological TDP-43 leaking out of the CNS remains hypothetical, and the pathogenetic mechanisms are elusive. We recently reported a predominance of TDP-43 pathology in subependymal or subpial regions in an age dependent manner (Geser et al., 2010b; Geser et al., 2011). The significance of this spatial association of TDP-43 pathology and the CSF system is unknown, and further studies are needed to define the relationship of TDP-43 lesions with brain surfaces. However, this might imply an association between mechanisms of TDP-43 with processes involving the ependymal lining and the CSF system. Mechanistic speculations might thus include a bidirectional interaction of the CNS and the CSF to be further defined.
3.2 Blood
We have also reported age dependent perivascular TDP-43 pathology in older subjects with and without severe mental illness. In fact, in a subset of elderly individuals there is a greater abundance of TDP-43 pathology around blood vessels (i.e., in the perivascular tissue), or directly associated with them. Moreover, most of these cases had a documented clinical history of cardiovascular problems or related pathology. Considering the high prevalence of chronic vascular changes in elderly individuals, there might not necessarily be a link between ischemia and pathological TDP-43 (Lee et al., 2008), but our recent findings suggest further study of this possibility (Geser et al., 2010b). Although it also remains to be established what the localization of TDP-43 pathology around or at blood vessels means in terms of a hypothesized “interaction” between blood and the brain, it has been suggested that increased TDP-43 plasma levels occur in FTLD-TDP and AD and may thereby act as a marker of TDP-43 pathology within the brain (Foulds et al., 2008). In a further study, in FTLD, but not AD, TDP-43 plasma levels correlated significantly with the pathology score when using a TDP-43 phosphorylation-dependent antibody (Foulds et al., 2009). Interestingly, a study using immunohistochmical and immunoelectron microscopic techniques reported a “TDP-43 microvasculopathy” in FTLD-TDP (and familial Lewy body disease) and suggested that abnormal TDP-43 fibrillary inclusions may occur in astrocytic end-feet, raising the possibility of an impairment in the integrity of the blood-brain barrier (Lin et al., 2009). It is of note that patients showing a clinical syndrome of FTLD (including FTLD-ALS) and FTLD-TDP or FTLD-tau at post-mortem examination can be potentially identified ante-mortem by assaying levels of specific, non-TDP-43 analytes that are well-known and readily measurable in the CSF (Hu et al., 2010). De Marco and colleagues recently suggested a different approach for a potential biomarker for ALS. The authors showed by means of western immunoblot analysis of circulating lymphomonocytes that TDP-43 accumulated in the cytoplasm with concomitant nuclear depletion in four ALS patients with TARDBP mutations and in about 50% of sporadic twelfe ALS patients compared to thirteen healthy controls (De Marco et al., 2011). Further, one clinically unaffected relative of a case with TARDBP mutation showed the same pathological pattern, whereas the two other unaffected relatives without the mutation did not.
Muscle
The skeletal muscle of sporadic ALS patients, another potential ante-mortem diagnostic approach, has been shown to be devoid of pathological TDP-43 both by immunohistochemistry and biochemistry (Hernandez Lain et al., 2010; Soraru et al., 2010). A case of FTLD-TDP with inclusion body myopathy and Paget disease of the bone, that is caused by VCP mutations and is also associated with TDP-43 pathology in the brain, has been reported to show cytoplasmic TDP-43 deposits in muscle fibres (Neumann et al., 2007; Olive et al., 2009). In terms of studies of ALS with known mutations, one group demonstrated that a patient with G287S TARDBP mutation had biochemical abnormalities for TDP-43 (and FUS), but showed no evidence of inclusion formation by immunohistochemistry (Hernandez Lain et al., 2010). The same group reported biochemical FUS, but no TDP-43 abnormalities in one out of four sporadic ALS cases. The significance of these findings needs to be further examined in larger patient cohorts.
Conclusion
ALS is a multisystem disease linked to pathological TDP-43 (i.e. TDP-43 proteinopathy) with a gradual disappearance of the normal nuclear localization of the protein and the emergence of cytoplasmic or neuritic pathologic hyperphosphorylated aggregates. Early CNS lesions sites include (1) the pyramidal system, in particular the lower motor neurons and (2) mediotemporal lobe structures. Detailed neuropathological staging schemata remain to be elaborated in the future. Furthermore, TDP-43 lesions in subpial/subependymal CNS or perivascular localizations have been noted in TDP-43 linked neurodegeneration, and might account, at least in part, for increased TDP-43 levels in the CSF or blood plasma through mechanisms that remain to be elucidated.
Research highlights.
To date, there are no validated biomarkers for ALS which would allow a precise staging of the disease in vivo and which would help to predict further course and response to therapy –
TDP-43 is found in the CNS of ALS patients
First studies searching for altered levels of TDP-43 in CNS and blood deliver promising results for TDP as a candidate biomarker in ALS
Further research is needed in order to confirm the diagnostic and prognostic value of CSF TDP-42 in ALS
Abbreviations
- ALS
amyotrophic lateral sclerosis
- TDP-43
43-kDa transactive responsive sequence DNA-binding protein
- FTLD
frontotemporal lobar degeneration
- FTLD-U
frontotemporal lobar degeneration with ubiquitin positive, tau and α-synuclein negative inclusions
- CSF
cerebrospinal fluid
- FUS
fused in liposarcoma
- LMN
lower motor neuron
- UMN
upper motor neuron
- AD
Alzheimer's disease
- MSA
multi system atrophy
- MND
motor neuron disease
- FTD
frontotemporal dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosta F, Chio A, Cosottini M, De Stefano N, Falini A, Mascalchi M, Rocca MA, Silani V, Tedeschi G, Filippi M. The Present and the Future of Neuroimaging in Amyotrophic Lateral Sclerosis. AJNR Am J Neuroradiol. doi: 10.3174/ajnr.A2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–36. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Lantos PL, Cairns NJ. Overlap between neurodegenerative disorders. Neuropathology. 2005;25:111–24. doi: 10.1111/j.1440-1789.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- Baumer D, Hilton D, Paine SM, Turner MR, Lowe J, Talbot K, Ansorge O. Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology. 75:611–8. doi: 10.1212/WNL.0b013e3181ed9cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic E, Bilic E, Rudan I, Kusec V, Zurak N, Delimar D, Zagar M. Comparison of the growth hormone, IGF-1 and insulin in cerebrospinal fluid and serum between patients with motor neuron disease and healthy controls. Eur J Neurol. 2006;13:1340–5. doi: 10.1111/j.1468-1331.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- Bowser R, Lacomis D. Applying proteomics to the diagnosis and treatment of ALS and related diseases. Muscle Nerve. 2009;40:753–62. doi: 10.1002/mus.21488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The pathological process underlying Alzheimer's disease in individuals under thirty. Acta Neuropathol. 2011;121:171–81. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Alzheimer's disease: intraneuronal alterations precede insoluble amyloid-beta formation. Neurobiol Aging. 2004;25:713–8. doi: 10.1016/j.neurobiolaging.2003.12.015. discussion 743-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Ludolph A, Thal DR, Del Tredici K. Amyotrophic lateral sclerosis: dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol. 120:67–74. doi: 10.1007/s00401-010-0683-0. [DOI] [PubMed] [Google Scholar]
- Brandmeir NJ, Geser F, Kwong LK, Zimmerman E, Qian J, Lee VM, Trojanowski JQ. Severe subcortical TDP-43 pathology in sporadic frontotemporal lobar degeneration with motor neuron disease. Acta Neuropathol. 2008;115:123–31. doi: 10.1007/s00401-007-0315-5. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Mogel H, Lehmensiek V, Ahlert T, Sussmuth S, Ludolph AC, Tumani H. Proteome analysis of cerebrospinal fluid in amyotrophic lateral sclerosis (ALS). Neurochem Res. 2008;33:2358–63. doi: 10.1007/s11064-008-9742-5. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Petzold A, Sussmuth SD, Ludolph AC, Tumani H. Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology. 2006;66:852–6. doi: 10.1212/01.wnl.0000203120.85850.54. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Widl K, Schattauer D, Ludolph AC, Tumani H. Cerebrospinal fluid erythropoietin (EPO) in amyotrophic lateral sclerosis. Neurosci Lett. 2007;416:257–60. doi: 10.1016/j.neulet.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–78. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol. 6:211–20. doi: 10.1038/nrneurol.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler. 2009;10:310–23. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SM, Norris FH. Amyotrophic lateral sclerosis: lower motor neuron disease spreading to upper motor neurons. Muscle Nerve. 1993;16:864–9. doi: 10.1002/mus.880160810. [DOI] [PubMed] [Google Scholar]
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 30:7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen TJ, Lee VM, Trojanowski JQ. TDP-43 functions and pathogenic mechanisms implicated in TDP-43 proteinopathies. Trends Mol Med. 2011 doi: 10.1016/j.molmed.2011.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson Y, Amin H, Kelley T, Shi J, Tian J, Kumaran R, Lashley T, Lees AJ, DuPlessis D, Neary D, Snowden J, Akiyama H, Arai T, Hasegawa M, Bandopadhyay R, Sikkink S, Pickering-Brown S, Mann DM. TDP-43 in ubiquitinated inclusions in the inferior olives in frontotemporal lobar degeneration and in other neurodegenerative diseases: a degenerative process distinct from normal ageing. Acta Neuropathol. 2009;118:359–69. doi: 10.1007/s00401-009-0526-z. [DOI] [PubMed] [Google Scholar]
- De Marco G, Lupino E, Calvo A, Moglia C, Buccinna B, Grifoni S, Ramondetti C, Lomartire A, Rinaudo MT, Piccinini M, Giordana MT, Chio A. Cytoplasmic accumulation of TDP-43 in circulating lymphomonocytes of ALS patients with and without TARDBP mutations. Acta Neuropathol. doi: 10.1007/s00401-010-0786-7. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–9. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, Chatterjee A, Hurtig HI, Karlawish JH, Rosen HJ, Van Deerlin V, Lee VM, Miller BL, Trojanowski JQ, Grossman M. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds P, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, Snowden JS, Allsop D, Mann DM. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer's disease and frontotemporal lobar degeneration. Acta Neuropathol. 2008;116:141–6. doi: 10.1007/s00401-008-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, Johnson N, Weintraub S, Akiyama H, Arai T, Hasegawa M, Bigio EH, Benson FE, Allsop D, Mann DM. Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol. 2009;118:647–58. doi: 10.1007/s00401-009-0594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol. 2009;117:151–8. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- Gendron TF, Josephs KA, Petrucelli L. Review: transactive response DNA-binding protein 43 (TDP-43): mechanisms of neurodegeneration. Neuropathol Appl Neurobiol. 36:97–112. doi: 10.1111/j.1365-2990.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008a;65:636–41. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 30:103–12. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Malunda JA, Hurtig HI, Duda JE, Wenning GK, Gilman S, Low PA, Lee VM, Trojanowski JQ. Subcortical TDP-43 pathology occurs infrequently in multiple system atrophy. Neuropathol Appl Neurobiol. doi: 10.1111/j.1365-2990.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Kwong LK, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the TDP-43 diseases. J Neurol. 2009a;256:1205–14. doi: 10.1007/s00415-009-5069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol. 2009b;66:180–9. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 67:1238–50. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Stein B, Partain M, Elman LB, McCluskey LF, Xie SX, Van Deerlin VM, Kwong LK, Lee VM, Trojanowski JQ. Motor neuron disease clinically limited to the lower motor neuron is a diffuse TDP-43 proteinopathy. Acta Neuropathol. doi: 10.1007/s00401-011-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VM, Trojanowski JQ. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008b;115:133–45. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- Grossman M, Wood EM, Moore P, Neumann M, Kwong L, Forman MS, Clark CM, McCluskey LF, Miller BL, Lee VM, Trojanowski JQ. TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol. 2007;64:1449–54. doi: 10.1001/archneur.64.10.1449. [DOI] [PubMed] [Google Scholar]
- Habuchi C, Iritani S, Sekiguchi H, Torii Y, Ishihara R, Arai T, Hasegawa M, Tsuchiya K, Akiyama H, Shibayama H, Ozaki N. Clinicopathological study of diffuse neurofibrillary tangles with calcification With special reference to TDP-43 proteinopathy and alpha-synucleinopathy. J Neurol Sci. doi: 10.1016/j.jns.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–94. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- Hernandez Lain A, Millecamps S, Dubourg O, Salachas F, Bruneteau G, Lacomblez L, Leguern E, Seilhean D, Duyckaerts C, Meininger V, Mallet J, Pradat PF. Abnormal TDP-43 and FUS proteins in muscles of sporadic IBM: similarities in a TARDBP-linked ALS patient. J Neurol Neurosurg Psychiatry. doi: 10.1136/jnnp.2010.208868. [DOI] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Appearance pattern of TDP-43 in Japanese frontotemporal lobar degeneration with ubiquitin-positive inclusions. Neurosci Lett. 2007a;419:213–8. doi: 10.1016/j.neulet.2007.04.051. [DOI] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res. 2007b;1184:284–94. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–54. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- Holton JL, Ghiso J, Lashley T, Rostagno A, Guerin CJ, Gibb G, Houlden H, Ayling H, Martinian L, Anderton BH, Wood NW, Vidal R, Plant G, Frangione B, Revesz T. Regional distribution of amyloid-Bri deposition and its association with neurofibrillary degeneration in familial British dementia. Am J Pathol. 2001;158:515–26. doi: 10.1016/S0002-9440(10)63993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Chen-Plotkin A, Grossman M, Arnold SE, Clark CM, Shaw LM, McCluskey L, Elman L, Hurtig HI, Siderowf A, Lee VM, Soares H, Trojanowski JQ. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology. doi: 10.1212/WNL.0b013e318200d78d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–20. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Seelaar H, Josephs KA, Knopman DS, Boeve BF, Sorenson EJ, McCluskey L, Elman L, Schelhaas HJ, Parisi JE, Kuesters B, Lee VM, Trojanowski JQ, Petersen RC, van Swieten JC, Grossman M. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Arch Neurol. 2009;66:1359–64. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Zhang J, Geser F, Trojanowski JQ, Strober JB, Dickson DW, Brown RH, Jr, Shapiro BE, Lomen-Hoerth C. Extensive FUS-Immunoreactive Pathology in Juvenile Amyotrophic Lateral Sclerosis with Basophilic Inclusions. Brain Pathol. doi: 10.1111/j.1750-3639.2010.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AJ, Kiernan JA, Munoz DG, Pringle CE, Brown WF, Ebers GC. Clinicopathological features of primary lateral sclerosis are different from amyotrophic lateral sclerosis. Brain Res Bull. 1993;30:359–64. doi: 10.1016/0361-9230(93)90265-d. [DOI] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM. Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies. J Biol Chem. 2009;284:8516–24. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince PG, Evans J, Knopp M, Forster G, Hamdalla HH, Wharton SB, Shaw PJ. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology. 2003;60:1252–8. doi: 10.1212/01.wnl.0000058901.75728.4e. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Ichikawa H, Suzuki Y, Shiota J, Nakano I, Kawamura M. Is lesion of Exner's area linked to progressive agraphia in amyotrophic lateral sclerosis with dementia? An autopsy case report. Behav Neurol. 23:153–8. doi: 10.3233/BEN-2010-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK. Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord. 2005;20(Suppl 12):S29–36. doi: 10.1002/mds.20537. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Frontotemporal lobar degeneration with upper motor neuron disease/ primary lateral sclerosis. Neurology. 2007;69:1800–1. doi: 10.1212/01.wnl.0000277270.99272.7e. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Knopman DS, Whitwell JL, Boeve BF, Parisi JE, Petersen RC, Dickson DW. Survival in two variants of tau-negative frontotemporal lobar degeneration: FTLD-U vs FTLD-MND. Neurology. 2005;65:645–7. doi: 10.1212/01.wnl.0000173178.67986.7f. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Jr., Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–7. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–73. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- Kasai T, Tokuda T, Ishigami N, Sasayama H, Foulds P, Mitchell DJ, Mann DM, Allsop D, Nakagawa M. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2009;117:55–62. doi: 10.1007/s00401-008-0456-1. [DOI] [PubMed] [Google Scholar]
- Kawajiri M, Mogi M, Higaki N, Tateishi T, Ohyagi Y, Horiuchi M, Miki T, Kira JI. Reduced angiotensin II levels in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2009;119:341–4. doi: 10.1111/j.1600-0404.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- Kolarcik C, Bowser R. Plasma and cerebrospinal fluid-based protein biomarkers for motor neuron disease. Mol Diagn Ther. 2006;10:281–92. doi: 10.1007/BF03256203. [DOI] [PubMed] [Google Scholar]
- Kovacs GG, Botond G, Budka H. Protein coding of neurodegenerative dementias: the neuropathological basis of biomarker diagnostics. Acta Neuropathol. 119:389–408. doi: 10.1007/s00401-010-0658-1. [DOI] [PubMed] [Google Scholar]
- Kuhle J, Lindberg RL, Regeniter A, Mehling M, Steck AJ, Kappos L, Czaplinski A. Increased levels of inflammatory chemokines in amyotrophic lateral sclerosis. Eur J Neurol. 2009;16:771–4. doi: 10.1111/j.1468-1331.2009.02560.x. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–4. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115:305–11. doi: 10.1007/s00401-007-0331-5. [DOI] [PubMed] [Google Scholar]
- Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE, Gallo JM, Weller RO, Anderton BH. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain. 1991;114(Pt 2):775–88. doi: 10.1093/brain/114.2.775. [DOI] [PubMed] [Google Scholar]
- Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol. 2009;68:1167–76. doi: 10.1097/NEN.0b013e3181baacec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Munoz DG, Kusaka H, Yokota O, Ishihara K, Roeber S, Kretzschmar HA, Cairns NJ, Neumann M. Distinct pathological subtypes of FTLD-FUS. Acta Neuropathol. doi: 10.1007/s00401-010-0764-0. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R. The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia. Curr Opin Neurol. 2008;21:693–700. doi: 10.1097/WCO.0b013e3283168d1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathologica. 2011 doi: 10.1007/s00401-011-0845-8. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Miklossy J, Steele JC, Yu S, McCall S, Sandberg G, McGeer EG, McGeer PL. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol. 2008;116:625–37. doi: 10.1007/s00401-008-0439-2. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369:2031–41. doi: 10.1016/S0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Freeman WM, Randazzo WT, Stephens HE, Beard JL, Simmons Z, Connor JR. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology. 2009;72:14–9. doi: 10.1212/01.wnl.0000333251.36681.a5. [DOI] [PubMed] [Google Scholar]
- Mori F, Tanji K, Zhang HX, Nishihira Y, Tan CF, Takahashi H, Wakabayashi K. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;116:193–203. doi: 10.1007/s00401-008-0396-9. [DOI] [PubMed] [Google Scholar]
- Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–9. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- Neumann M. Molecular neuropathology of TDP-43 proteinopathies. Int J Mol Sci. 2009;10:232–46. doi: 10.3390/ijms10010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–31. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nishihira Y, Tan CF, Hoshi Y, Iwanaga K, Yamada M, Kawachi I, Tsujihata M, Hozumi I, Morita T, Onodera O, Nishizawa M, Kakita A, Takahashi H. Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta Neuropathol. 2009a;117:45–53. doi: 10.1007/s00401-008-0443-6. [DOI] [PubMed] [Google Scholar]
- Nishihira Y, Tan CF, Onodera O, Toyoshima Y, Yamada M, Morita T, Nishizawa M, Kakita A, Takahashi H. Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathol. 2008;116:169–82. doi: 10.1007/s00401-008-0385-z. [DOI] [PubMed] [Google Scholar]
- Nishihira Y, Tan CF, Toyoshima Y, Yonemochi Y, Kondo H, Nakajima T, Takahashi H. Sporadic amyotrophic lateral sclerosis: Widespread multisystem degeneration with TDP-43 pathology in a patient after long-term survival on a respirator. Neuropathology. 2009b;29:689–96. doi: 10.1111/j.1440-1789.2008.00999.x. [DOI] [PubMed] [Google Scholar]
- Noto YI, Shibuya K, Sato Y, Kanai K, Misawa S, Sawai S, Mori M, Uchiyama T, Isose S, Nasu S, Sekiguchi Y, Fujimaki Y, Kasai T, Tokuda T, Nakagawa M, Kuwabara S. Elevated CSF TDP-43 levels in amyotrophic lateral sclerosis: Specificity, sensitivity, and a possible prognostic value. Amyotroph Lateral Scler. doi: 10.3109/17482968.2010.541263. [DOI] [PubMed] [Google Scholar]
- Ostberg P, Bogdanovic N. Semantic dementia with lower motor neuron disease showing FTLD-TDP type 3 pathology (sensu Mackenzie). Neuropathology. doi: 10.1111/j.1440-1789.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, Brown RH, Cudkowicz ME, Newhall K, Peskind E, Marcus S, Ho L. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66:1218–22. doi: 10.1212/01.wnl.0000203129.82104.07. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, Murray A, Hernandez D, Guerreiro R, Singleton AB, Neal J, Morris HR. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. doi: 10.1007/s00415-010-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesiridis GS, Lee VM, Trojanowski JQ. Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis. Hum Mol Genet. 2009;18:R156–62. doi: 10.1093/hmg/ddp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradat PF, Dib M. Biomarkers in amyotrophic lateral sclerosis: facts and future horizons. Mol Diagn Ther. 2009;13:115–25. doi: 10.1007/BF03256320. [DOI] [PubMed] [Google Scholar]
- Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis. Clinical features, neuropathology and diagnostic criteria. Brain. 1992;115(Pt 2):495–520. doi: 10.1093/brain/115.2.495. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Williams E, Ganchev P, Gopalakrishnan V, Lacomis D, Urbinelli L, Newhall K, Cudkowicz ME, Brown RH, Jr., Bowser R. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95:1461–71. doi: 10.1111/j.1471-4159.2005.03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–11. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol. 2008;116:147–57. doi: 10.1007/s00401-008-0395-x. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, Dearmond SJ, Miller BL, Seeley WW. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 75:2204–11. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–52. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C, Arai T, Hasegawa M, Akiyama H, Yu S, McGeer PL. TDP-43 pathology in familial British dementia. Acta Neuropathol. 2009;118:303–11. doi: 10.1007/s00401-009-0514-3. [DOI] [PubMed] [Google Scholar]
- Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–65. doi: 10.1097/NEN.0b013e31818e8951. [DOI] [PubMed] [Google Scholar]
- Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, de Rijik MC, Rizzu P, ten Brummelhuis M, van Doorn PA, Kamphorst W, Willemsen R, van Swieten JC. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–85. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol. 2006;1:151–70. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- Soraru G, Orsetti V, Buratti E, Baralle F, Cima V, Volpe M, D'Ascenzo C, Palmieri A, Koutsikos K, Pegoraro E, Angelini C. TDP-43 in skeletal muscle of patients affected with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 11:240–3. doi: 10.3109/17482960902810890. [DOI] [PubMed] [Google Scholar]
- Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, Pabst A, Uttner I, Tumani H, Lee VM, Trojanowski JQ, Kretzschmar HA, Ludolph A, Neumann M, Otto M. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. 2008;65:1481–7. doi: 10.1001/archneur.65.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ. The syndromes of frontotemporal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:323–38. doi: 10.1080/17482960802372371. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–46. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Sussmuth SD, Brettschneider J, Ludolph AC, Tumani H. Biochemical markers in CSF of ALS patients. Curr Med Chem. 2008;15:1788–801. doi: 10.2174/092986708785133031. [DOI] [PubMed] [Google Scholar]
- Tan CF, Kakita A, Piao YS, Kikugawa K, Endo K, Tanaka M, Okamoto K, Takahashi H. Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol. 2003;105:615–20. doi: 10.1007/s00401-003-0687-0. [DOI] [PubMed] [Google Scholar]
- Tan CF, Yamada M, Toyoshima Y, Yokoseki A, Miki Y, Hoshi Y, Kaneko H, Ikeuchi T, Onodera O, Kakita A, Takahashi H. Selective occurrence of TDP-43-immunoreactive inclusions in the lower motor neurons in Machado-Joseph disease. Acta Neuropathol. 2009;118:553–60. doi: 10.1007/s00401-009-0552-x. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Rowe A, Findlater K, Orange JB, Grace G, Strong MJ. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol. 2007;64:232–6. doi: 10.1001/archneur.64.2.232. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K, Braak E, Braak H. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–48. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Sano M, Shiotsu H, Akiyama H, Watabiki S, Taki K, Kondo H, Nakano I, Ikeda K. Sporadic amyotrophic lateral sclerosis of long duration mimicking spinal progressive muscular atrophy exists: additional autopsy case with a clinical course of 19 years. Neuropathology. 2004;24:228–35. doi: 10.1111/j.1440-1789.2004.00546.x. [DOI] [PubMed] [Google Scholar]
- Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–64. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–81. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–77. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wang IF, Wu LS, Shen CK. TDP-43: an emerging new player in neurodegenerative diseases. Trends Mol Med. 2008;14:479–85. doi: 10.1016/j.molmed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Quinn N, Magalhaes M, Mathias C, Daniel SE. “Minimal change” multiple system atrophy. Mov Disord. 1994;9:161–6. doi: 10.1002/mds.870090206. [DOI] [PubMed] [Google Scholar]
- Wenning GK, Seppi K, Tison F, Jellinger K. A novel grading scale for striatonigral degeneration (multiple system atrophy). J Neural Transm. 2002;109:307–20. doi: 10.1007/s007020200025. [DOI] [PubMed] [Google Scholar]
- Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008a;283:13302–9. doi: 10.1074/jbc.M800342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winton MJ, Van Deerlin VM, Kwong LK, Yuan W, Wood EM, Yu CE, Schellenberg GD, Rademakers R, Caselli R, Karydas A, Trojanowski JQ, Miller BL, Lee VM. A90V TDP-43 variant results in the aberrant localization of TDP-43 in vitro. FEBS Lett. 2008b;582:2252–6. doi: 10.1016/j.febslet.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Sato T, Tsuji S, Takahashi H. CAG repeat disorder models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:71–86. doi: 10.1007/s00401-007-0287-5. [DOI] [PubMed] [Google Scholar]
- Yokota O, Davidson Y, Arai T, Hasegawa M, Akiyama H, Ishizu H, Terada S, Sikkink S, Pickering-Brown S, Mann DM. Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol. doi: 10.1007/s00401-010-0731-9. [DOI] [PubMed] [Google Scholar]
- Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DM. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol. 120:55–66. doi: 10.1007/s00401-010-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen PM. Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. Eur J Neurol. 2007;14:1329–33. doi: 10.1111/j.1468-1331.2007.01972.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tan CF, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathol. 2008;115:115–22. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]