SUMMARY
The shelterin complex plays both positive and negative roles in telomerase regulation. While shelterin prevents the checkpoint kinases ATM and ATR from fully activating DNA damage responses at telomeres, those kinases are also required for telomere maintenance. In fission yeast, cells lacking both Tel1 (ATM ortholog) and Rad3 (ATR ortholog) fail to recruit telomerase to telomeres, and survive by circularizing chromosomes. However, the critical telomere substrate(s) of Tel1ATM/Rad3ATR remained unknown. Here, we show that Tel1ATM/Rad3ATR-dependent phosphorylation of the shelterin subunit Ccq1 on Thr93 is essential for telomerase association with telomeres. In addition, we show that the telomerase subunit Est1 interacts directly with the phosphorylated Thr93 of Ccq1 to ensure telomere maintenance. The shelterin subunits Taz1, Rap1 and Poz1 (previously established inhibitors of telomerase) were also found to negatively regulate Ccq1 phosphorylation. These findings establish Tel1ATM/Rad3ATR-dependent Ccq1 Thr93 phosphorylation as a critical regulator of telomere maintenance in fission yeast.
INTRODUCTION
Stable maintenance of telomeres is critical to preserve genomic integrity, and telomere dysfunction has been linked to tumor formation and pre-mature aging in humans1. The GT-rich telomeric repeats are bound by the six-protein “shelterin” complex (TRF1, TRF2, RAP1, TIN2, TPP1 and POT1) and are extended by telomerase in humans2. In fission yeast Schizosaccharomyces pombe, a conserved shelterin complex, composed of Taz1 (TRF1/TRF2 ortholog), Rap1, Poz1 (possible analog of TIN2), Tpz1 (TPP1 ortholog) and Pot1, was recently identified3. The fission yeast shelterin complex additionally includes Ccq1, which is required to prevent checkpoint activation and to recruit telomerase to telomeres3-5.
While the shelterin complex is necessary to prevent the DNA damage checkpoint kinases ATM and ATR from fully activating DNA damage responses at telomeres6, these kinases are recruited to telomeres during S/G2-phases7,8 and play essential roles in telomere maintenance9. In fission yeast, simultaneous deletion of Tel1 (ATM ortholog) and Rad3 (ATR ortholog) leads to complete loss of telomeres and chromosome circularization10. By chromatin immunoprecipitation (ChIP) assays, we have previously determined that tel1Δ rad3Δ cells fail to recruit telomerase and also show reduced Ccq1 association with telomeres11. However, how Tel1ATM and Rad3ATR kinases promote telomerase recruitment remained unclear.
Here, we show that Tel1ATM/Rad3ATR-dependent phosphorylation of Ccq1 Thr93 is essential for telomerase association with telomeres. In addition, we show that the 14-3-3-like domain of the telomerase regulatory subunit Est112,13 specifically recognizes and binds to the phosphorylated Thr93 of Ccq1 to promote association of telomerase with telomeres. Phosphorylation of Ccq1 is negatively regulated by the telomerase inhibitors Taz1, Rap1 and Poz13,14-16, and telomere elongation and increased telomerase association with telomeres found in rap1Δ cells is dependent on Ccq1 Thr93 phosphorylation. On the other hand, Ccq1 Thr93 phosphorylation is increased as telomeres shorten in telomerase mutant cells. Taken together, we thus establish Tel1ATM/Rad3ATR-dependent Ccq1–Est1 interaction as a critical regulatory mechanism that ensures stable maintenance of telomeres in fission yeast cells.
RESULTS
Est1 interacts directly with shelterin subunit Ccq1
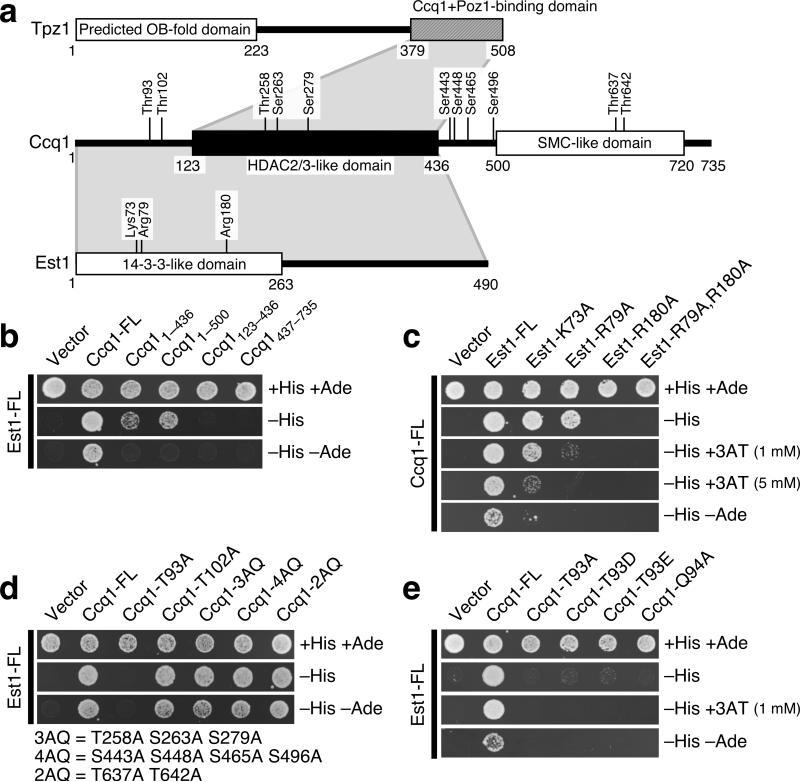
To better understand how localization of telomerase at telomeres is regulated in fission yeast, we performed pairwise yeast two-hybrid assays between telomerase (catalytic subunit Trt1TERT and regulatory subunit Est1) and shelterin complex subunits (Pot1, Tpz1, Poz1 and Ccq1). While we confirmed the previously identified Ccq1–Tpz1 interaction3 (Supplementary Fig. 1a), we also found that Est1 and Ccq1 interact with one another (Fig. 1). Bioinformatics analysis predicted that the central region of Ccq1 might form a structure similar to the Class II histone deacetylase (HDAC) complex subunits 2 and 3, while the C-terminal domain is likely to form a coiled-coil structure related to SMC (structural maintenance of chromosomes). Through truncation analysis of Ccq1, we determined that amino acids 123–436 of Ccq1 are sufficient for Ccq1–Tpz1 interaction (Supplementary Fig. 1a), while amino acids 1–436 of Ccq1 are required for Ccq1–Est1 interaction (Fig. 1b).
Figure 1.
Ccq1 interacts with both Tpz1 and Est1. (a) Schematic representation of Tpz1, Ccq1 and Est1. Conserved motifs and functional domains3,4,12,13 are indicated. For Ccq1, putative consensus Tel1ATM/Rad3ATR phosphorylation sites (TQ or SQ) are indicated. For Est1, amino acid residues within the 14-3-3-like domain predicted to be important for phosphopeptide binding13 are marked. Gray shaded areas indicate regions required for protein-protein interactions, determined by yeast two-hybrid assays. (b-e) Determination of regions and amino acid residues critical for the Ccq1-Est1 interaction by yeast two-hybrid assays. Wild-type full-length proteins are denoted as “FL”. Truncation constructs are indicated as subscripts denoting amino acid residue numbers. Ccq1 mutants carrying multiple alanine mutations at SQ/TQ sites were abbreviated as 3AQ, 4AQ or 2AQ as indicated.
Within fission yeast Est1, the only region that shows significant homology to other Est1 homologs is localized within its N-terminus12. Crystal structure of the equivalent region from mammalian EST1C/SMG7 protein suggested that this region (amino acids 1–263) of fission yeast Est1 might fold into a domain that resembles a 14-3-3 phosphopeptide binding protein13. Based on sequence alignments of Est1/SMG homologs17-19, we identified Lys73 or Arg79 and Arg180 of fission yeast Est1 as conserved residues that are most likely equivalent to Lys66 and Arg163 in EST1C/SMG7, amino acid residues critical for phospho-serine binding13 (Supplementary Fig. 2, 3a). Previous studies have established that EST1A/SMG6 (but not EST1C/SMG7) associates with the mammalian telomerase complex17-19, and thus likely to represent an ortholog of Est1 from fission and budding yeasts.
Intriguingly, mutational analysis of fission yeast Est1 revealed that Est1-R180A and Est1-R79A,R180A mutants completely lose their ability to interact with Ccq1 in yeast two-hybrid assays, while K73A and R79A mutants show reduced Est1–Ccq1 interaction, with R79A having a stronger effect (Fig. 1c). These results suggested that the ability of fission yeast Est1 to recognize phosphorylated amino acid residue(s) within Ccq1 might be important for mediating Ccq1–Est1 interaction.
14-3-3-like domain mutations of Est1 cause telomere loss
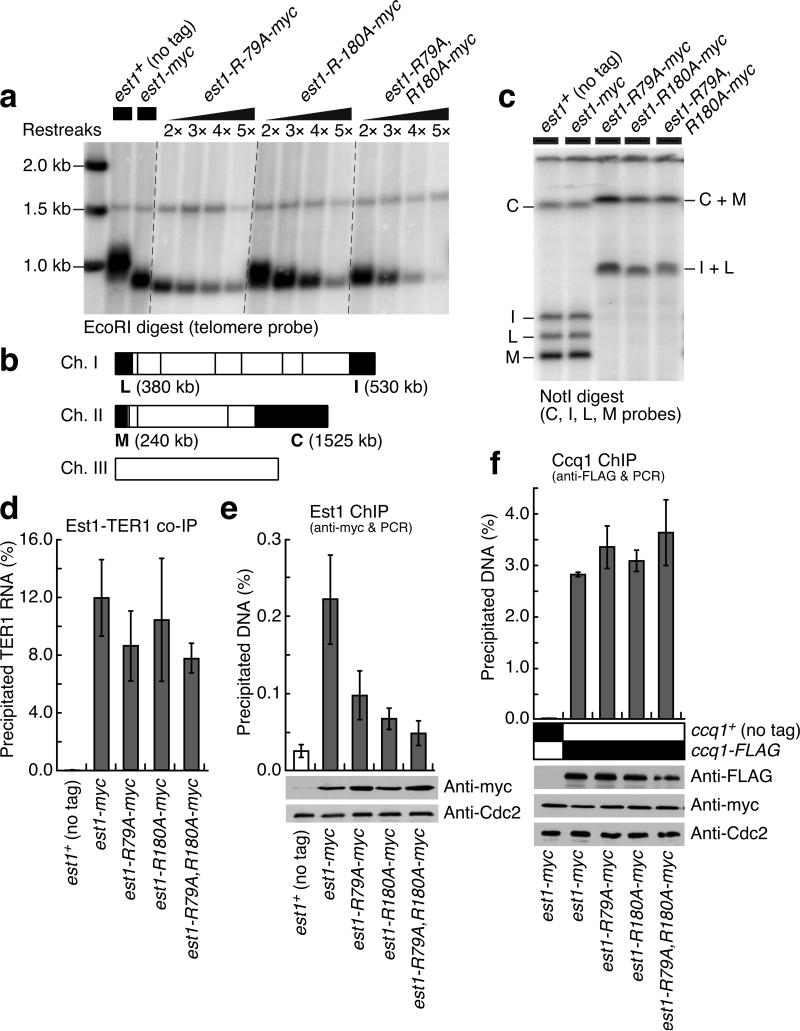
Upon integration of est1-R79A, est1-R180A or est1-R79A,R180A alleles at the est1+ locus, these mutant fission yeast cells showed progressive telomere shortening during repeated restreaking on agar plates (Fig. 2a). Moreover, an increasing number of cells showed a highly elongated morphology (indicative of checkpoint activation) and slow growth rate (data not shown), until they eventually recovered and generated “survivor” cells carrying circular chromosomes (Fig. 2b,c and Supplementary Fig. 4), much like trt1Δ, est1Δ, ccq1Δ or tel1Δ rad3Δ survivor cells5,10,12,20. We confirmed that these mutations do not affect Est1 stability or the interaction between Est1 and telomerase RNA (TER1) (Fig. 2d,e). Quantitative ChIP assays of early generation strains revealed that Est1-R79A, Est1-R180A or Est1-R79A,R180A proteins show substantial reduction in telomere association compared to wild-type Est1 (Fig. 2e). The observed reduction in telomere association might reflect combined effects of the est1 mutations on telomerase recruitment, processivity and/or retention. Among the three mutants, Est1-R79A showed a milder effect on telomere association, consistent with the observation that est1-R79A cells required more extensive restreaking on agar plates than est1-R180A or est1-R79A,R180A cells before chromosomes ultimately circularized (Fig. 2a and data not shown). By contrast, the 14-3-3-like domain mutations in Est1 did not affect association of Ccq1 with telomeres (Fig. 2f).
Figure 2.
The phosphopeptide-binding motif of Est1 is important for telomere maintenance. (a) Southern blot analysis of telomeres from successive restreaks. While addition of a 13myc-tag to Est1 resulted in slightly shorter but stable telomeres, mutations within the 14-3-3-like domain caused loss of telomeres for both tagged and untagged mutant alleles (Supplementary Fig. 4). (b) NotI restriction map of fission yeast chromosomes. The telomeric fragments (C, I, L and M) are marked. (c) The telomeric NotI-fragments from extensively restreaked cells, analyzed by pulsed-field gel electrophoresis. (d) Binding of Est1 to TER1 is not affected by est1 mutations, as monitored by co-IP experiments. Plots show mean values plus/minus one s.d. for three independent experiments. (e-f) Recruitment of Est1 mutants (e) and Ccq1 in est1 mutant backgrounds (f) to telomeres was monitored by ChIP assays. Assays were performed using early generation cell cultures, and the presence of telomeres was confirmed by both Southern blot and qPCR assays against TAS regions, which are lost upon chromosome circularization11,20 (data not shown). Protein expression levels were monitored by Western blot using the indicated antibodies. Cdc2 served as loading control. Plots show mean values plus/minus one s.d. for three (e) or two (f) independent experiments.
Telomere maintenance requires Ccq1 Thr93 phosphorylation
We found that both telomerase (Trt1TERT) association with telomeres (Fig. 3a) and Ccq1–telomerase (TER1) interaction (Supplementary Fig. 5a) are significantly increased in the absence of the telomerase inhibitors Poz1, Rap1 or Taz13,14,15,20, and that Ccq1 correspondingly exhibits enhanced Tel1ATM/Rad3ATR-dependent hyper-phosphorylation in poz1Δ, rap1Δ or taz1Δ cells (Fig. 3b,c and Supplementary Fig 5b,f). Ccq1 was also found to be essential for telomerase association with telomeres in both rap1+ and rap1Δ cells5,11 (Fig. 3a). Based on these observations and our previous findings that Tel1ATM and Rad3ATR kinases play essential but redundant role(s) in telomerase association with telomeres11, we hypothesized that Poz1, Rap1 and Taz1 act as inhibitors of telomerase action by limiting Tel1ATM/Rad3ATR-dependent phosphorylation of Ccq1. In addition, we hypothesized that binding of Tel1ATM/Rad3ATR target phosphorylation site(s) within Ccq1 by the 14-3-3-like domain of Est1 might play a critical role in association of telomerase with telomeres.
Figure 3.
Ccq1 Thr93 is essential for telomere maintenance. (a) Removal of telomerase inhibitors (Poz1, Rap1 and Taz1) results in increased association of telomerase (Trt1TERT) with telomeres, as monitored by dot-blot ChIP assays (top panel). Plots show mean values plus/minus one s.d. for four independent experiments. Trt1 and Cdc2 expression levels were monitored by Western blot (bottom panel). (b-c) Western blot analysis of Ccq1. Asterisk (*) marks λ-phosphatase sensitive slow mobility band of Ccq1. Early generation tel1Δ rad3Δ strains were generated using the Rad3-plasmid loss system11. (d) Southern blot analysis of telomeres during successive restreaks. (e) The telomeric NotI-fragments from extensively restreaked cells, analyzed by pulsed-field gel electrophoresis. (f) Quantitative real-time PCR ChIP analysis of Est1, Trt1 and Ccq1. ChIP assays of ccq1-T93A cells were performed using early generation cell cultures. Protein expression levels were monitored by Western blot. Plots show mean values plus/minus one s.d. for three (Est1 and Trt1) or two (Ccq1) independent experiments.
Accordingly, we mutated all eleven SQ/TQ sites (preferred phosphorylation sites for Rad3ATR/Tel1ATM kinases21) found in Ccq1 to AQ, to identify Tel1ATM/Rad3ATR-dependent phosphorylation site(s) within Ccq1 that might play critical roles in promoting Ccq1–Est1 interaction and telomere maintenance (Fig. 1a and Supplementary Fig. 3b). Experiments indicated that only ccq1-T93A affected the Ccq1–Est1 yeast two-hybrid interaction and caused progressive telomere shortening in fission yeast cells (Fig. 1d, 3d and Supplementary Fig. 5c). Unlike ccq1Δ cells, which immediately activate a Chk1-dependent DNA damage checkpoint response and exhibit cell elongation3,5, ccq1-T93A cells initially grew robustly and showed no obvious cell elongation (data not shown). However, much like telomerase mutant cells, later generations of ccq1-T93A cells became highly elongated as telomeres shortened, and eventually generated survivor cells with circular chromosomes upon successive restreaking on agar plates (Fig. 3e). Mutations of Thr93 to the phosphomimetic amino acid residues aspartic acid (D) or glutamic acid (E), and Gln94 to alanine caused identical telomere phenotypes as the T93A mutation (Fig. 1e, 3d,e), suggesting that phosphorylation at Thr93 as well as Tel1ATM/Rad3ATR consensus are required for Ccq1 function at telomeres. We further determined by ChIP assays that fission yeast cells carrying the ccq1-T93A allele failed to localize telomerase (Trt1TERT and Est1) to telomeres (Fig. 3f). By contrast, the T93A mutation did not affect association of Ccq1 with telomeres (Fig. 3f), Ccq1–Tpz1 interaction3 (Supplementary Fig. 1), SHREC (Snf2/Hdac-containing Repressor Complex)-dependent formation of heterochromatin at telomeres22 (Supplementary Fig. S6a), or interaction between the SHREC subunit Clr3 and Ccq13,22 (Supplementary Fig. 6b).
Thr93 phosphorylation regulates Est1-Ccq1 interaction
Western blot analysis of Ccq1 indicated that sites other than Thr93 must also be phosphorylated by Tel1ATM/Rad3ATR, since the λ-phosphatase sensitive slow mobility band seen on SDS PAGE could still be detected in ccq1-T93A rap1Δ cells (Supplementary Fig. 5d). However, since only ccq1-T93A affected the Ccq1-Est1 interaction in yeast two-hybrid assays and the ability of fission yeast cells to stably maintain telomeres (Fig. 1d and Supplementary Fig. 5c), other SQ/TQ sites do not appear to contribute significantly to telomerase function. Based on average terminal telomere length and Ccq1 mobility shift (Supplementary Fig. 7), we also concluded that Rad3ATR serves as the primary kinase responsible for Ccq1 hyper-phosphorylation in rap1Δ cells, while Tel1ATM is responsible for residual Ccq1 hyper-phosphorylation observed in rad3Δ rap1Δ cells. Furthermore, other checkpoint sensor proteins (Rad1 and Rad17) were found to be dispensable for Ccq1 hyper-phosphorylation in rap1Δ cells (Supplementary Fig. 7b). Interestingly, we also observed that cells carrying shorter telomeres (ccq1-T93A, est1Δ, and trt1Δ strains) exhibit hyper-phosphorylation of Ccq1 (Fig. 4b,c and Supplementary Fig. 5e,f), suggesting that shorter telomeres, which contain fewer Taz1 binding sites than longer telomeres14,23, are less efficient in preventing Tel1ATM/Rad3ATR-dependent hyper-phosphorylation of Ccq1. In addition, since Ccq1 is not hyper-phosphorylated after ionizing radiation treatment (Supplementary Fig. 8a), we concluded that Ccq1 is phosphorylated specifically in response to perturbations of the telomere status.
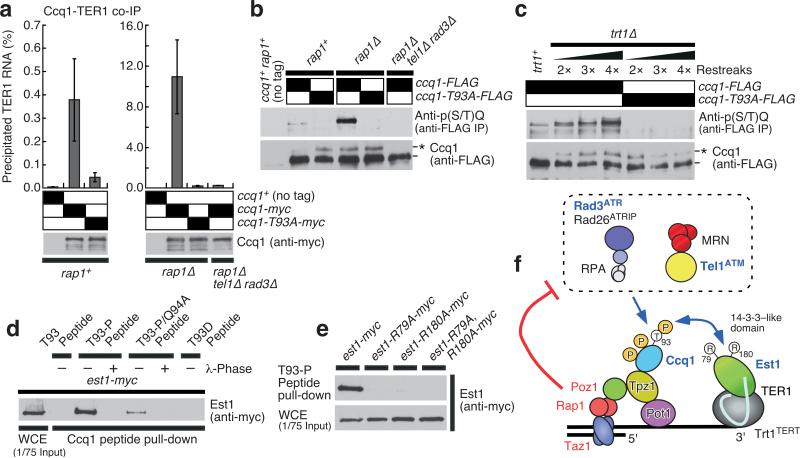
Figure 4.
Ccq1–Est1 interaction is dependent on phosphorylated Ccq1 Thr93. (a) Binding of Ccq1 to telomerase RNA TER1 was monitored, and expression of Ccq1 was examined by anti-myc Western blot. Plots show mean values plus/minus one s.d. for at least three independent experiments. (b-c) Western blot of immuno-purified (anti-FLAG) Ccq1 using anti-phospho-(S/T)Q antibody (top panels), and of whole cell extracts (WCE) using anti-FLAG antibody (bottom panels). (d) Est1 specifically recognizes phosphorylated Thr93 of Ccq1. Extracts of wild-type Est1-myc strains were incubated with Streptavidin-beads bound to either T93 (non-phosphorylated), T93-P (phosphorylated), T93-P/Q94A or T93D peptides. For T93-P and T93-P/Q94A, peptides were also treated with λ-phosphatase (+) prior to incubation with extracts. Peptide bound Est1 was analyzed by Western blot analysis. (e) Est1 binding to phosphorylated Thr93 of Ccq1 is dependent on its 14-3-3-like phosphopeptide binding motif. Extracts of wild-type and 14-3-3-like domain mutant est1 strains were incubated with the T93-P peptide bound to Streptavidin-beads. (f) A model of telomere length regulation in fission yeast.
By utilizing a phospho-(S/T)Q site-specific antibody which specifically recognized the region surrounding phosphorylated Thr93 (Fig. 4b and Supplementary Fig. 8b,c), we further confirmed that Thr93 of Ccq1 shows increased Tel1ATM/Rad3ATR-dependent phosphorylation in poz1Δ, rap1Δ and taz1Δ cells (Fig. 4b and Supplementary Fig. 5b). The level of Thr93 phosphorylation also progressively increased in trt1Δ cells, as telomeres gradually shorten (Fig. 4c and data not shown). Consistent with the notion that Ccq1–telomerase interaction is regulated by Tel1ATM/Rad3ATR-dependent phosphorylation of Ccq1 Thr93, we observed that Ccq1–TER1 interaction is significantly reduced in both ccq1-T93A and rap1Δ ccq1-T93A cells, and that enhanced Ccq1–TER1 interaction in rap1Δ cells was dependent on Tel1ATM and Rad3ATR kinases (Fig. 4a). Moreover, Southern blot analysis found that introduction of the ccq1-T93A allele leads to reversal of the telomerase- and Tel1ATM/Rad3ATR-dependent telomere elongation observed in rap1Δ cells11,24 (Supplementary Fig. 7c), consistent with the notion that Ccq1 Thr93 phosphorylation works downstream of telomerase inhibitors (Taz1, Rap1 and Poz1) and Tel1ATM/Rad3ATR to promote telomere extension by telomerase (Supplementary Fig. 7d). As taz1Δ cells accumulate more Rad26ATRIP (Rad3ATR regulatory subunit) at telomeres than taz1+ cells25, Taz1 (and most likely Rap1 and Poz1) may prevent hyper-phosphorylation of Ccq1 by limiting recruitment of the Rad3ATR-Rad26ATRIP complex to telomeres.
To directly test if Est1 binds to the region surrounding phosphorylated Thr93 of Ccq1, we synthesized short peptides representing amino acids 86–100 of Ccq1 with (T93-P) or without (T93) phosphorylated Thr93. In addition, we synthesized a T93-P/Q94A peptide, which incorporates phosphorylated Thr93 followed by a Q94A mutation that eliminates the preferred ATM/ATR phosphorylation site, and a T93D peptide, which incorporates a phosphomimetic mutation. These peptides were immobilized on magnetic beads and incubated with cell extracts from fission yeast expressing either wild-type or various 14-3-3-like domain mutant alleles of Est1-myc, and the peptide-bound Est1 was subsequently detected by Western blots. We found that only the T93-P peptide (but not T93 peptide or λ-phosphatase-treated T93-P peptide) interacted with Est1, and this interaction was abolished by the 14-3-3-like domain mutations in Est1 (Fig. 4d,e). Furthermore, the T93D peptide failed to interact with Est1, consistent with the failure of ccq-T93D and ccq1-T93E mutant alleles to maintain telomeres in fission yeast and to support Est1–Ccq1 interaction in yeast two-hybrid assays (Fig. 1e and 3d,e). Interestingly, the T93-P/Q94A peptide, while not as robust as the T93-P peptide, retained some Thr93 phosphorylation-dependent interaction with Est1 (Fig. 4d), supporting the notion that the most critical determinant responsible for the Est1–Ccq1 interaction is the phosphorylated Thr93 of Ccq1.
DISCUSSION
The current study provides major mechanistic insights into how the DNA damage checkpoint kinases Tel1ATM and Rad3ATR collaborate with the shelterin complex to maintain telomeres in fission yeast. As summarized in Fig. 4f, we identified Ccq1 as a critical telomere-bound Tel1ATM/Rad3ATR substrate required for telomere maintenance. Previously identified inhibitors of telomerase (Taz1, Rap1 and Poz1)3,14,15 were found to negatively regulate the phosphorylation of Ccq1, limit Ccq1–telomerase interaction, and limit association of telomerase with telomeres. Since the amount of telomere-bound Taz1, Rap1 and Poz1 would be reduced at shorter telomeres3,14,23, they may become less efficient in the inhibition of Tel1ATM/Rad3ATR-dependent phosphorylation of Ccq1 at shorter telomeres. The 14-3-3-like domain of Est1 could then recognize Ccq1 phosphorylated on Thr93 and promote preferential association of telomerase at shorter telomeres, although preferential binding of telomerase to shorter telomeres has thus far only been established for budding yeast Saccharomyces cerevisiae26-28. In fact, it should be emphasized that we have not yet directly demonstrated that telomere-bound Ccq1 is preferentially phosphorylated in cells carrying shorter telomeres.
In addition, it should be noted that tel1Δ rad3Δ cells show a more severe telomere dysfunction than telomerase mutant cells29, as they are also defective in telomere protection11. Thus, further investigations are clearly needed to understand the telomerase-independent role(s) of Tel1ATM and Rad3ATR in telomere maintenance. It is likely that phosphorylation of target protein(s) other than Ccq1 may also contribute to Tel1ATM/Rad3ATR-dependent protection of telomeres.
A study in S. cerevisiae has previously suggested that Tel1ATM/Mec1ATR-dependent phosphorylation of Cdc13 may promote the interaction between Est1 and Cdc13 to recruit telomerase30. However, more recent evidence indicated that Cdc13–Est1 interaction is unlikely to be regulated by Tel1ATM/Mec1ATR-dependent phosphorylation of Cdc1331. Furthermore, the 14-3-3-like domain of budding yeast Est1 appears to have lost the ability to bind phosphorylated amino acid residues, since amino acid residues critical for phospho-Ser/Thr binding are not conserved13 (Supplementary Fig. 2). Therefore, budding yeast cells, which have lost the shelterin complex during evolution2, appear to have evolved an alternative mechanism to recruit telomerase.
In contrast, the 14-3-3-like domains of mammalian Est1/SMG are predicted to recognize and bind phospho-amino acid residues13. Moreover, TRF1 and TRF2 (Taz1 orthologs) have been shown to inhibit telomere elongation and activation of ATM and ATR2,6,32,33, and POT1, TPP1 and TIN2 have been implicated in telomerase recruitment34-36 in mammalian cells. Therefore, the 14-3-3-like domain of mammalian Est1/SMG might also promote localization of telomerase by recognizing ATM/ATR-dependent phosphorylation site(s) within subunits of the shelterin complex or an unidentified Ccq1 homolog if such protein exists in mammalian cells.
Supplementary Material
ACKNOWLEDGEMENTS
We thank F. Ishikawa, J. P. Cooper, M. R. Flory, V. A. Zakian, A. M. Carr and P. Russell for sharing published strains and plasmids, L. Khair for her initial efforts to generate yeast strains and reagents used in this study, and P. Baumann (Stowers Institute) for generously sharing his unpublished anti-Ccq1 antibody. We also thank F. Ishikawa for communicating their unpublished results. J.K. was supported in part by the Federal Work-Study program. This work was supported by NIH grant GM078253 (T.M.N.).
METHODS
Yeast strains and plasmids
Fission yeast and budding yeast strains used in this study were constructed and cultured using standard methods37-40. Fission yeast strain genotypes are listed in Supplementary Table 1. Additional details on construction or sources of various deletion and tagged fission yeast strains were previously reported8,11,41,42. Plasmids used in this study are listed in Supplementary Table 2. Various point mutation and truncation constructs were generated with Phusion (NEB) or QuikChange Lightning (Agilent) site-directed mutagenesis kits.
Yeast two-hybrid assay
Yeast two hybrid assays were performed by mating S. cerevisiae MATa (Y2HGold: MATa trp1-901 leu2-3,-112 ura3-52 his3-200 LYS2::GAL1(UAS)-GAL1(TATA)-HIS3 GAL2(UAS)-GAL2(TATA)-ADE2 gal4Δ gal80Δ URA3::MEL1(UAS)-MEL1(TATA)-AUR1-C MEL1) strains harboring GAL4-DBD (DNA-binding domain) plasmids with MATΔ (Y187: MATΔ trp1-901 leu2-3,-112 ura3-52 his3-200 ade2-101 gal4Δ gal80Δ met-URA3::GAL1(UAS)-GAL1(TATA)-LacZ MEL1) strains harboring GAL4-AD (activation domain) plasmids, as described in the MATCHMAKER system manual (Clontech). Positive two-hybrid interactions were identified by spotting mated cells onto SD–HTL (–His) or SD–HTLA (–His –Ade) plates. To increase selection stringency, 1 or 5 mM 3-Amino-1,2,4-triazole (3AT) was added to SD–HTL plates.
Pulsed-field gel electrophoresis and Southern blot analysis
Pulsed-field gel electrophoresis of NotI-digested chromosomal DNA was performed as previously described29. For telomere length analysis by Southern blot, EcoRI-digested genomic DNA was separated on 1% agarose gel and probed with a telomeric DNA probe as previously described29.
Co-immunoprecipitation and Western blot analysis
Co-IP experiments (Est1–TER1, Ccq1–TER1 and Ccq1–Tpz1) were performed as previously described11. To detect the hyper-phosphorylated form of Ccq1, 7% (w/v) SDS PAGE gels (200:1 Acrylamide:Bis-acrylamide) were used to separate proteins from whole cell extracts (WCEs) or immunoprecipitations. Western blot analysis was performed using monoclonal anti-FLAG (M2-F1804, Sigma), monoclonal anti-myc (9B11, Cell Signaling), monoclonal anti-Cdc2 (y100.4, Abcam), or polyclonal anti-Ccq1 (a gift from P. Baumann, The Stowers Institute) antibodies as primary antibodies. For Western blot analysis using an anti-phospho-(S/T)Q antibody (Phospho-(Ser/Thr) ATM/ATR substrate antibody, 2851, Cell Signaling), Ccq1 was first immuno-purified from 20 mg of WCE using anti-FLAG (M2-F1804) antibody. Either HRP-conjugated (goat) anti-mouse (Pierce, 31430) or HRP-conjugated (goat) anti-rabbit (Pierce, 31460) was used as the secondary antibody.
Characterization of phospho-(S/T)Q antibody
Based on the manufacturer's datasheet, the Phospho-(Ser/Thr) ATM/ATR substrate antibody (2851, Cell Signaling) specifically recognizes peptides and proteins that contain phospho-Ser/Thr preceded by Leu (L) or similar hydrophobic amino acids at the –1 position and followed by Gln (Q) at the +1 position. Prior to our Ccq1 experiments, we tested this antibody in fission yeast against the checkpoint kinase Chk1, which is phosphorylated by Rad3ATR kinase at Ser345 (LS345Q) upon treatment with ionizing radiation (IR)43 (Supplementary Fig. 8d). Cultures of myc-tagged Chk1 were mock-treated or treated with IR (100 Gy). Chk1-myc was then immuno-purified (anti-myc) from cell extracts, and analyzed by Western blot analysis with the anti-phospho-(S/T)Q antibody.
We also tested the antibody in an ELISA assay using the T93 and T93-P peptides as described below. A streptavidin-coated plate (Millipore #20-183) was coated with 100 μl of 0.2 μM peptide in blocking buffer (1×PBST [0.15 M NaCl, 1.5 mM KH2PO4, 10 mM Na2HPO4, 3 mM KCl, 0.05% (v/v) Tween-20, pH 7.4] plus 1% (w/v) BSA) for 30 min at 25 °C, washed 3× with 1×PBST, and incubated with the anti-phospho-(S/T)Q antibody diluted at 1:1000 in blocking buffer for 30 min shaking at 25 °C. After 3× additional washes with 1×PBST, the plate was incubated with HRP-conjugated (goat) anti-rabbit antibody (1:3000 in blocking buffer) for 30 min at 25 °C. After 3× washes with 1×PBST and a final wash with water, we added 100 μl of the substrate (TMB/E solution, Millipore #ES022) for 5 min. The reaction was stopped by addition of 100 μl 0.3 M sulfuric acid, and the absorbance was recorded at 450 nm. For noise control, we performed a reaction without the anti-phospho-(S/T)Q antibody, but otherwise treated as described.
ChIP assay
Cells were processed for ChIP using monoclonal anti-myc (9B11) or anti-FLAG (M2-F1804) antibodies, and analyzed with quantitative real-time PCR11 or dot-blot with a telomeric DNA probe41 as previously described. In both cases, ChIP sample values were normalized to Input samples and plotted as % precipitated DNA. For cells carrying highly elongated telomeres, it was necessary to carry out ChIP assays based on dot-blot, since primers used in real-time PCR became too far away from chromosome ends.
Ccq1 T93-P peptide binding assay
Ccq1 peptides were chemically synthesized in vitro with short wild-type or mutant amino acid sequences corresponding to the Ccq1 amino acid residues 86-100 (HSENDFL-T93-QEVDEFP) and attached to Biotin via a 4×Gly linker at their N-terminus. The T93 and T93-P peptides carry unphosphorylated or phosphorylated Thr93, respectively. The T93-P/Q94A peptide carries a phosphorylated Thr93, followed by a Q94A mutation. The T93D peptide carries a T93D mutation. These peptides were immobilized on Streptavidin-conjugated Dynabeads (Invitrogen), and either left untreated or treated with λ protein phosphatase (NEB) for 20 min at 30 °C. Peptide binding experiments were performed using cell extracts prepared from fission yeast strains carrying wild-type or 14-3-3-like domain mutations of Est1. Bound proteins were isolated by incubating the mixture for 2 h at 4 °C in lysis buffer [50 mM Tris pH 8.0, 150 mM NaCl, 10% (v/v) glycerol, 5 mM EDTA, 0.5% (v/v) NP40, 50 mM NaF, 1 mM DTT, 1 mM PMSF, 0.2 mM APMSF, 1 mM Na3VO4, and “complete” proteinase inhibitor cocktail (Roche)], washed in lysis buffer, and boiled in 2xSDS sample buffer [100 mM Tris-HCl, pH 6.8, 20% (v/v) glycerol, 4% (w/v) SDS, 10% (v/v) β-mercaptoethanol, and 0.004% (w/v) bromophenol blue]. Est1 bound to Ccq1 peptides was subsequently detected by immunoblotting with monoclonal anti-myc antibody (9B11).
Footnotes
AUTHOR CONTRIBUTIONS
B.A.M. designed, performed and analyzed most of the experiments in this study, and wrote the paper. Y.-T.C. performed ChIP experiments in Fig. 3a, and initially observed Ccq1 hyper-phosphorylation. J.K. assisted B.A.M. in construction of various yeast two-hybrid plasmids. T.M.N. conceived the study, designed and performed experiments, analyzed data, and wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Lansdorp PM. Telomeres and disease. EMBO J. 2009;28:2532–40. doi: 10.1038/emboj.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 3.Miyoshi T, Kanoh J, Saito M, Ishikawa F. Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science. 2008;320:1341–4. doi: 10.1126/science.1154819. [DOI] [PubMed] [Google Scholar]
- 4.Flory MR, Carson AR, Muller EG, Aebersold R. An SMC-domain protein in fission yeast links telomeres to the meiotic centrosome. Mol Cell. 2004;16:619–30. doi: 10.1016/j.molcel.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Tomita K, Cooper JP. Fission yeast Ccq1 is telomerase recruiter and local checkpoint controller. Genes Dev. 2008;22:3461–74. doi: 10.1101/gad.498608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–71. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- 7.Verdun RE, Karlseder J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell. 2006;127:709–20. doi: 10.1016/j.cell.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Moser BA, et al. Differential arrival of leading and lagging strand DNA polymerases at fission yeast telomeres. EMBO J. 2009;28:810–20. doi: 10.1038/emboj.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabourin M, Zakian VA. ATM-like kinases and regulation of telomerase: lessons from yeast and mammals. Trends Cell Biol. 2008;18:337–46. doi: 10.1016/j.tcb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito T, Matsuura A, Ishikawa F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet. 1998;20:203–6. doi: 10.1038/2517. [DOI] [PubMed] [Google Scholar]
- 11.Moser BA, Subramanian L, Khair L, Chang YT, Nakamura TM. Fission yeast Tel1ATM and Rad3ATR promote telomere protection and telomerase recruitment. PLoS Genet. 2009;5:e1000622. doi: 10.1371/journal.pgen.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beernink HT, Miller K, Deshpande A, Bucher P, Cooper JP. Telomere maintenance in fission yeast requires an Est1 ortholog. Curr Biol. 2003;13:575–80. doi: 10.1016/s0960-9822(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 13.Fukuhara N, et al. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol Cell. 2005;17:537–47. doi: 10.1016/j.molcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JP, Nimmo ER, Allshire RC, Cech TR. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature. 1997;385:744–7. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- 15.Kanoh J, Ishikawa F. spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol. 2001;11:1624–30. doi: 10.1016/s0960-9822(01)00503-6. [DOI] [PubMed] [Google Scholar]
- 16.Chikashige Y, Hiraoka Y. Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol. 2001;11:1618–23. doi: 10.1016/s0960-9822(01)00457-2. [DOI] [PubMed] [Google Scholar]
- 17.Snow BE, et al. Functional conservation of the telomerase protein Est1p in humans. Curr Biol. 2003;13:698–704. doi: 10.1016/s0960-9822(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 18.Reichenbach P, et al. A human homolog of yeast Est1 associates with telomerase and uncaps chromosome ends when overexpressed. Curr Biol. 2003;13:568–74. doi: 10.1016/s0960-9822(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 19.Redon S, Reichenbach P, Lingner J. Protein RNA and protein protein interactions mediate association of human EST1A/SMG6 with telomerase. Nucleic Acids Res. 2007;35:7011–22. doi: 10.1093/nar/gkm724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura TM, Cooper JP, Cech TR. Two modes of survival of fission yeast without telomerase. Science. 1998;282:493–6. doi: 10.1126/science.282.5388.493. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama T, et al. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell. 2007;128:491–504. doi: 10.1016/j.cell.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Tomaska L, Willcox S, Slezakova J, Nosek J, Griffith JD. Taz1 binding to a fission yeast model telomere: formation of telomeric loops and higher order structures. J Biol Chem. 2004;279:50764–72. doi: 10.1074/jbc.M409790200. [DOI] [PubMed] [Google Scholar]
- 24.Miller KM, Rog O, Cooper JP. Semi-conservative DNA replication through telomeres requires Taz1. Nature. 2006;440:824–8. doi: 10.1038/nature04638. [DOI] [PubMed] [Google Scholar]
- 25.Carneiro T, et al. Telomeres avoid end detection by severing the checkpoint signal transduction pathway. Nature. 2010;467:228–32. doi: 10.1038/nature09353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi A, Shore D. Increased association of telomerase with short telomeres in yeast. Genes Dev. 2007;21:1726–30. doi: 10.1101/gad.438907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hector RE, et al. Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol Cell. 2007;27:851–8. doi: 10.1016/j.molcel.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Sabourin M, Tuzon CT, Zakian VA. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol Cell. 2007;27:550–61. doi: 10.1016/j.molcel.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura TM, Moser BA, Russell P. Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics. 2002;161:1437–52. doi: 10.1093/genetics/161.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng SF, Lin JJ, Teng SC. The telomerase-recruitment domain of the telomere binding protein Cdc13 is regulated by Mec1p/Tel1p-dependent phosphorylation. Nucleic Acids Res. 2006;34:6327–36. doi: 10.1093/nar/gkl786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao H, et al. Telomerase Recruitment in Saccharomyces cerevisiae is not dependent on Tel1-mediated Phosphorylation of Cdc13. Genetics. 2010;186:1147–59. doi: 10.1534/genetics.110.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sfeir A, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009;138:90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–75. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin H, et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature. 2007;445:559–62. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 35.Abreu E, et al. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol. 2010;30:2971–82. doi: 10.1128/MCB.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tejera AM, et al. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell. 2010;18:775–89. doi: 10.1016/j.devcel.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfa C, Fantes P, Hyams J, McLoed M, Warbrick E. Experiments with Fission Yeast. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- 38.Amberg DC, Burke DJ, Strathern JN. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2005. [Google Scholar]
- 39.Bähler J, et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–51. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 40.Krawchuk MD, Wahls WP. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast. 1999;15:1419–27. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1419::AID-YEA466>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khair L, Subramanian L, Moser BA, Nakamura TM. Roles of heterochromatin and telomere proteins in regulation of fission yeast telomere recombination and telomerase recruitment. J Biol Chem. 2009;285:5327–37. doi: 10.1074/jbc.M109.078840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nimmo ER, Pidoux AL, Perry PE, Allshire RC. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature. 1998;392:825–8. doi: 10.1038/33941. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Girona A, et al. Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc Natl Acad Sci U S A. 2001;98:11289–94. doi: 10.1073/pnas.191557598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.