Abstract
Background
Stress is causally associated with anxiety. While the underlying cellular mechanisms are not well understood, the basal forebrain cholinergic neurons (BFCNs) have been implicated in stress response. p75NTR is a pan-neurotrophin receptor expressed almost exclusively in BFCNs in adult brain. The present study investigates whether and how p75NTR, via regulation of the cholinergic system and hippocampal synaptic plasticity, influences stress-related behaviors.
Methods
We used a combination of slice electrophysiology, behavioral analyses, pharmacology, in vivo microdialysis and neuronal activity mapping to assess the role of p75NTR in mood and stress-related behaviors and its underlying cellular and molecular mechanisms.
Results
We show that acute stress enables hippocampal long-term depression (LTD) in adult wild-type mice, but not in mice lacking p75NTR. The p75NTR mutant mice also exhibit two distinct behavioral impairments: baseline anxiety-like behavior and a deficit in coping with and recovering from stressful situations. Blockade of stress-enabled LTD with a GluA2-derived peptide impaired stress recovery without affecting baseline anxiety. Pharmacological manipulations of cholinergic transmission mimicked the p75NTR perturbation in both baseline anxiety and responses to acute stress. Finally, we show evidence of misregulated cholinergic signaling in animals with p75NTR deletion.
Conclusions
Our results suggest that loss of p75NTR leads to changes in hippocampal cholinergic signaling, which may be involved in regulation of stress-enabled hippocampal LTD and in modulating behaviors related to stress and anxiety.
Keywords: hippocampus, neurotrophin, LTD, basal forebrain, p75NTR, anxiety
Introduction
Exposure to stressful life events is a risk factor for anxiety disorder development. Failure to regulate the acute stress response may lead to neurobiological changes leading to stress-related disorders(1), but the underlying mechanisms remain essentially unknown. One approach to studying pathogenesis is to examine physiological and behavioral responses to acute stress. Following stress exposure, the hypothalamic-pituitary-adrenal (HPA) axis is activated, resulting in rapid increases in glucocorticoid levels. By providing negative regulation over the HPA axis, the hippocampus is a key component of the stress-response system(2, 3).
Acute stress has significant effects on hippocampal synaptic plasticity. Unlike in juvenile hippocampus, long-term depression(LTD) is not easily induced with low-frequency stimulation(LFS) in adult(4,5). However, stress exposure allows for LTD induction in adult(5). In contrast to the well-studied roles of long-term potentiation(LTP), the functions of hippocampal LTD are less understood. LTD blockade in nucleus accumbens dampens amphetamine-induced behavioral sensitization(6), and genetic and pharmacological manipulations that impair hippocampal LTD are correlated with deficits in behavioral flexibility(7-9). Blocking LTD reverses the memory retrieval deficit observed following stress exposure(10). Thus, LTD may serve as a cellular mechanism ensuring proper behavioral response to changing environments.
The p75 neurotrophin receptor, encoded by Ngfr gene, binds all members of the neurotrophin family. In the uninjured adult brain p75NTR expression is primarily restricted to basal forebrain cholinergic neurons(BFCNs)(11-13), a subset of which project to hippocampus from medial septum. The relationship between p75NTR, the cholinergic system and hippocampal synaptic plasticity is not well understood. Moreover, how these systems intersect to regulate behavior is essentially unexplored. Hence, we designed studies to probe the role of p75NTR in hippocampal synaptic plasticity as related to anxiety and stress recovery. We show that stress-enabled hippocampal LTD is absent in adult p75NTR-/- animals, is regulated by cholinergic signaling and participates in the stress response. LTD blockade, with a membrane permeable peptide that prevents AMPA receptor endocytosis(6,14), also impairs stress coping. Collectively, our data suggest that stress-enabled hippocampal LTD may be important for stress recovery, and provide insight into the possible intersection of p75NTR signaling with the cholinergic system in regulating anxiety-like behavior and the acute stress response.
Materials and Methods
Subjects
p75NTR-/- animals were crossed >15X from mixed BALB/C-129 /SV(15) to C57Bl/6. 8-10wk, male p75NTR-/- and WT were housed in a 12/12hr cycle. Animals for peptide and pharmacology studies were 8-10wk old C57Bl/6J mice(Jackson, Bar Harbor, ME) and 20-24wk old CD-1 mice for social conflict. Procedures were conducted in accordance with NIH guidelines and approved by the NIMH Institutional Animal Care and Use Committee. Stressor: 30min elevated platform(1mX10cmX10cm) exposure. Corticosterone levels: Trunk blood was collected for radioimmunoassay(MP Biomedicals, Solon, OH). Behavioral Experiments: Behavioral experiments were carried out using standard protocols and analyzed by blinded experimenters. Behavior was tracked with automated behavioral scoring apparatus when possible with codes unbroken until after data generation. Detailed information in the Supplement. Social conflict experiments were carried out essentially as described(16). Elevated Plus Maze was carried out as previously reported(17,18). Behavior was scored with TopScanSuite(CleverSys Inc., Reston, VA). Light Dark Box: After 1hr habituation to the testing room, the 10min. test was scored using TopScanSuite. Open Field Test: Locomotion in an open field arena was recorded and analyzed with Ethovision(Noldus, Leesburg, VA). Novelty Induced Hypophagia: Animals were trained for 30min/d/3d to drink sweetened condensed milk. On d4 latency to drink was recorded in a novel environment. Stress Induced Hyperthermia: Electronic ID transponders were implanted under the skin 3d prior to experiment and baseline temperatures were recorded with a hand-held probe. Treatments: (-)and (+)Phenserine tartrate(0.5mg/kg, i.p, synthesized by Laboratory of Neurosciences, NIA), Tat-GluA23Y and scrambled-Tat peptide(both 5umol/kg for i.p injection and 30pmol/side for CA1 microinfusion, synthesized by AnaSpec, Fremont, CA), Scopolamine(0.5mg/kg, Research Biochemicals International, Natick, MA). In Vitro Slice Preparation and Electrophysiology: Detailed protocols in the Supplement. 350μm slices were placed in an ACSF-perfused recording chamber. For LTD 10uM bicuculline methiodide was added. Electrodes were positioned in CA1 and responses evoked by Schaffer collateral stimulation. Intensity was increased to determine maximal fEPSP slope and adjusted to yield 40-60% of max. Experiments with maximal fEPSPs < 0.5 mV or with substantial changes in fiber volley were rejected. LTP was induced by one 1s/100Hz stimulus train, and LTD by 900 pulses at 1Hz. Immunohistochemistry: Detailed protocols in the Supplement. Animals were perfused with paraformaldehyde and brains cryopreserved in sucrose. 50μm sections were used with standard protocols using an anti c-fos antibody(PC38, Calbiochem). Stereotaxic Surgeries and Microinjection: Detailed protocols in the Supplement. For CA1 infusions, a guide shaft was implanted above CA1(AP-2.92, ML ±3.37, DV -1.43), and for microdialysis studies, a guide shaft into CA3(AP -2.70, ML 3.25, DV-0.5). Peptide administration was controlled by an infusion pump(0.04μl/min, 0.5μl total volume). Injection cannula and microdialysis probe placement were inspected in Nissl-stained sections. Identified probe locations for animals used in assays are plotted in Figure S6A,B in the Supplement. Microdialysis and ACh assay: Detailed protocols in the Supplement. Microdialysis probes were inserted 12h prior to collection and perfused with ACSF. ACh in dialysates was determined by HPLC using a column(530×1 mm I.D)(MF-8904, BAS). A post-column IMER(50 × 1mm I.D.)(BAS MF-8903) was used to convert Ach to hydrogen peroxide, which was detected in a wired enzyme electrode(MF-2095 BAS), a carbon electrode set at +100mV vs Ag/AgCl was used. The mobile phase flow rate was 140μl/min and injection volume of 10μl yielded a detection limit of 15fmol/10uL. Choline and ACh were quantified using external standards. The assay was linear from 10 to 1000fmol. Quantification was performed with BAS ChromGraph software. Data are presented as percent of averaged WT baseline values.
Statistics
Student’s t-test and 1-way ANOVA with Newman-Keuls post hoc analysis were used. 2-way ANOVA were used for multiple comparisons when warranted. Data are presented as means +/-SEM. Statistical significance was set at P<0.05 and *, P<0.05, **, P<0.01 and ***, P<0.001 and analyzed using GraphPadPrism.
Results
Regulation of stress-enabled LTD
LTD is reliably induced by low-frequency stimulation(LFS) in slices from juvenile animals, but induction is difficult in adult(4). However, acute stress facilitates LTD induction in adult hippocampus(5,19-22). It is unclear whether juvenile LTD and stress-enabled LTD share similar underlying mechanisms. In mice with Ngfr deletion(p75NTR-/-), LFS-induced LTD is impaired in the juvenile(23, 24). In the developing hippocampus, p75NTR is widely expressed, including in hippocampal neurons(23), but in the uninjured adult brain, p75NTR expression is mainly restricted to basal forebrain cholinergic neurons (BFCNs)(12,13). This raises the question whether stress-enabled LTD is also impaired in adult p75NTR-/- hippocampus. Exposure to elevation stress allowed for robust LFS-induced LTD in wild-type (WT), but not p75NTR-/-(Fig.1A). The synaptic efficacy at 60min after LFS onset was 68±4% in WT, but 97±2% in p75NTR-/-. LFS failed to induce LTD in slices derived from WT adult animals under non-stressed conditions(data not shown).
Fig. 1.
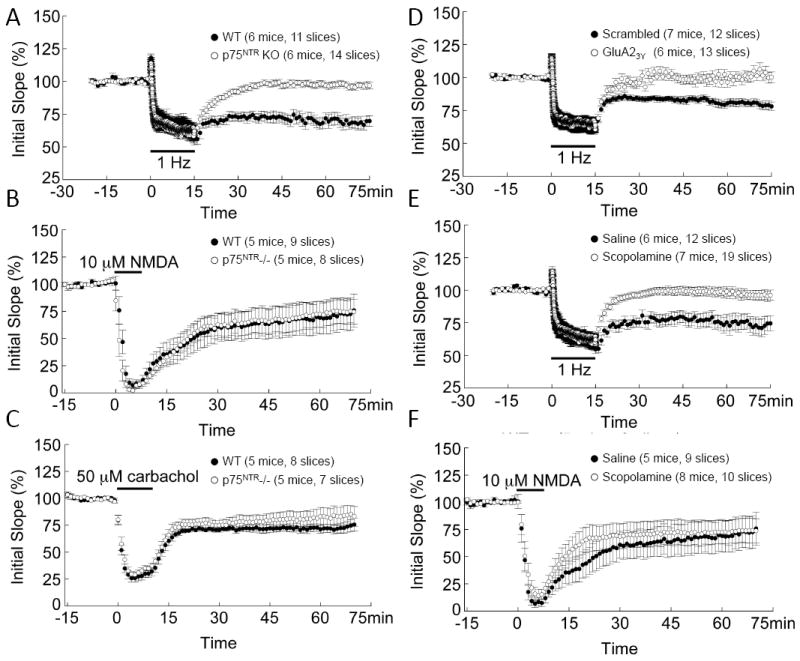
Regulation of stress-enabled hippocampal LTD. (A) Reliable LFS-induced, stress-enabled LTD was found in WT slices, but not p75NTR-/- slices (P<2×10-6). (B) LTD induced by NMDA (7min, 10μM) was similar between WT and p75NTR-/- mice (P>0.05). (C) LTD induced by carbachol (10min, 50μM) was similar between WT and p75NTR-/-(P>0.05). (D) Reliable stress-enabled LTD was induced in slices from scrambled peptide-injected, but not GluA23Y peptide(P<5×10-5). (E) Reliable stress-enabled LTD was induced in slices from saline injected mice, but not scopolamine injected mice (P<0.003). (F) NMDA-induced LTD was normal in slices from scopolamine injected mice. Note saline group is shared with control group in Fig. 1B.
Loss of p75NTR does not affect alternative forms of synaptic plasticity under baseline conditions or after stress in the adult. Ngfr deletion had no effect on basal synaptic transmission, as reflected by normal input-out(I/O) curves(Figure S1A in the Supplement). Paired-pulse facilitation(PPF) and response to high-frequency stimulation(HFS) were normal(Fig.S1B-C in the Supplement), suggesting that Ngfr deletion does not affect presynaptic transmitter release. As expected, long-term potentiation(LTP) was reduced after stress(Fig.S1D in the Supplement, LTPleft>LTPright (see also(5, 25). Differing from a previous report(26), we found no difference in LTP between WT and p75NTR-/- under stressed or non-stressed conditions(Fig.S1D in the Supplement).
Next, we examined other forms of LTD that are not influenced by stress exposure. NMDA perfusion led to a robust LTD lasting >60 min in both WT and p75NTR-/- (Fig.1B). Application of the muscarinic agonist carbachol induces an NMDA-independent form of LTD in adult hippocampus(27). Carbachol resulted in robust LTD in slices from both WT and p75NTR-/- (Fig.1C). These results suggest that the stress-enabled LTD seen in adult hippocampus is mechanistically different from other forms of LTD.
To complement our studies, we used a membrane permeable LTD-blocking peptide, Tat-GluA23Y, which specifically inhibits LTD without affecting LTP(6,10,28). We confirmed that administration of Tat-GluA23Y peptide(5μmol/kg, i.p.), but not a Tat-scrambled peptide, blocked stress-enabled LTD at CA1 synapses(Fig.1D). The GluA23y peptide had no effect on synaptic response to LFS(Fig.1D, black bar, EPSP during 1Hz stimulation), I/O curves, PPF or synaptic response to HFS(Fig.S2A-C in the Supplement). Thus, Tat-GluA23Y peptide selectively blocks expression of stress-enabled LTD without affecting basic synaptic properties.
Adult BFCNs project from medial septum to hippocampus. To determine whether alterations in cholinergic signaling modulate stress-enabled LTD, we examined LTD in the presence of the muscarinic antagonist scopolamine. Scopolamine(0.5mg/kg, i.p.) prevented stress-enabled LTD in WT animals(Fig.1E). The effect is specific to stress-enabled LTD as NMDA-induced LTD was unaffected(Fig.1F). Moreover, scopolamine did not affect LFS responses(Fig.1F, see response to 1Hz LFS), basal synaptic transmission(I-O curves, Fig.S2D in the Supplement), probability of transmitter release(PPF, Fig.S2E in the Supplement), or size of the ready-releasable pool of synaptic vesicles(Fig.S2F in the Supplement).
Stress reaction and recovery in mice lacking LTD
We next examined the role of stress-enabled LTD in the behavioral response to stress. Hyperthermia is a well-established read-out of the recovery from acute stress(29). The stress-induced hyperthermia(SIH) paradigm was used to examine the stress response in WT and p75NTR-/-. Body temperatures were measured every 5min during stress exposure and recovery. Baseline and stressed temperature profiles of WT and p75NTR-/- mice were similar(Fig.2A). However, p75NTR-/- mice did not return to baseline as quickly as WT(Fig.2A). These results raise the possibility that p75NTR, and possibly its effect on stress-enabled LTD, are necessary for stress recovery.
Fig.2.
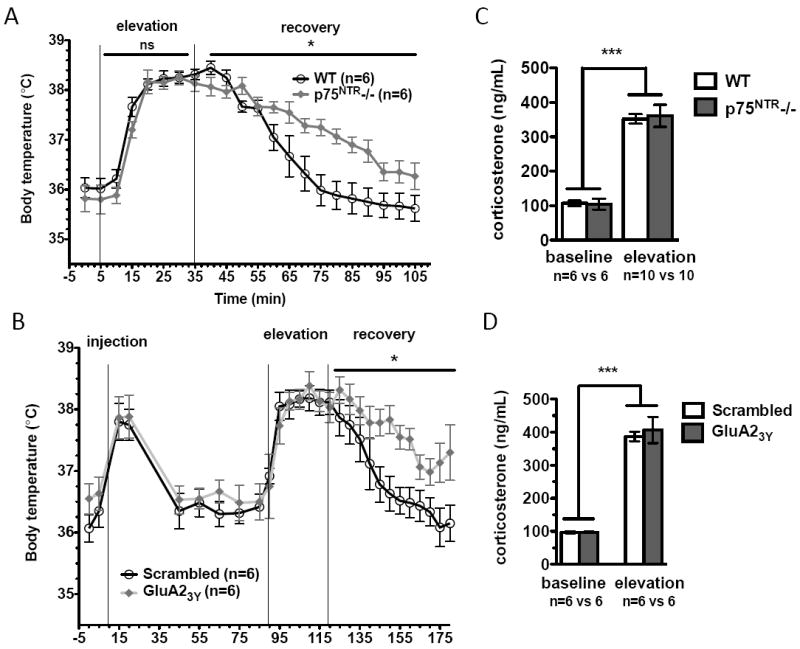
Inhibition of stress-enabled LTD impairs recovery from stress-induced hyperthermia. (A) No difference in baseline body temperatures was detected between WT and p75NTR-/- animals (P=0.3601). Both groups mount a normal stress response (time variation [P<0.0001], genotype variation [P=0.2326]), but p75NTR-/- recovery is impaired (time variation [P<0.0001], genotype variation [P=0.0203]). (B) No differences in baseline body temperatures detected between scrambled and GluA23Y-peptide injected mice (P=0.1314). Both groups mount a normal stress response (time variation [P<0.0001], genotype variation [P=0.8461]), but recovery of GluA23Y -injected mice is impaired (time variation [P<0.0001], genotype variation [P=0.0274]). (C) No difference in plasma corticosterone before and after exposure to elevation stress between WT and p75NTR-/- animals (P=<0.0001 for stress effect, but P=0.8911 for genotype). (D) No difference in plasma corticosterone levels before and after elevation stress in animals injected with the scrambled or the Tat-GluA23Y peptide (P<0.0001 for stress effect, but P=0.6454 for genotype).
To confirm that LTD is important for stress recovery, we analyzed animals administered either the Tat-GluA23Y or a Tat-scrambled peptide. i.p. injection itself is a mild stressor, and elicited a transient increase in body temperature that returned to baseline within 40min(Fig.2B). No difference was found in body temperature profiles between animals injected with scrambled versus Tat-GluA23Y peptide during stress exposure(Fig.2B). Similar to p75NTR-/-, animals injected with Tat-GluA23Y peptide, but not those with scrambled, displayed impaired capacity to recover from SIH(Fig.2B).
Glucocorticoid receptor activation participates in stress-enabled LTD induction(19,20). To rule out the possibility that a reduction in glucocorticoid activation contributes to the observed LTD deficits, we measured stress-induced changes in plasma corticosterone levels. As expected, acute stress elicited an increase in corticosterone(Fig.2C). Ngfr deletion had no effect on either basal levels or stress-induced increases in corticosterone(Fig.2C). Corticosterone levels were similar between animals injected with either scrambled or GluA23Y peptides both before and after stress, indicating that LTD deficits are not a result of impaired glucocorticoid signaling(Fig.2D).
Stress-induced neuronal activation in the absence of LTD
To better understand the circuits involved in the stress response, we analyzed neuronal activation using c-fos immunohistochemistry. Following elevation stress, labeled c-fos neurons were increased in CA1, the basolateral amygdala(BLA), medial pre-frontal cortex(mPFC), dentate gyrus, CA3 and para-ventricular nucleus of the hypothalamus(PVN) in both WT and p75NTR-/-(Fig.3A-C and Fig.S3A-C in the Supplement). There was no difference between genotypes in baseline density of c-fos+ cells in any area examined(Fig.3A-C, Fig.S3A-C,G in the Supplement). However, stress-induced neuronal activation was significantly decreased in p75NTR-/- compared to WT in hippocampal CA1, dentate gyrus and CA3(Fig.3A, Fig.S3A,B in the Supplement). In contrast, no difference was found in stress-induced neuronal activation between the stressed groups in BLA(Fig.3B), mPFC(Fig.3C) or PVN(Fig.S3C in the Supplement). As reported previously, we found an absence of c-fos immunolabeled cells in medial septum at baseline and no induction in response to stress(data not shown)(30). These results point to hippocampus as the major site where neuronal activation can be regulated by p75NTR in response to stress.
Fig.3.
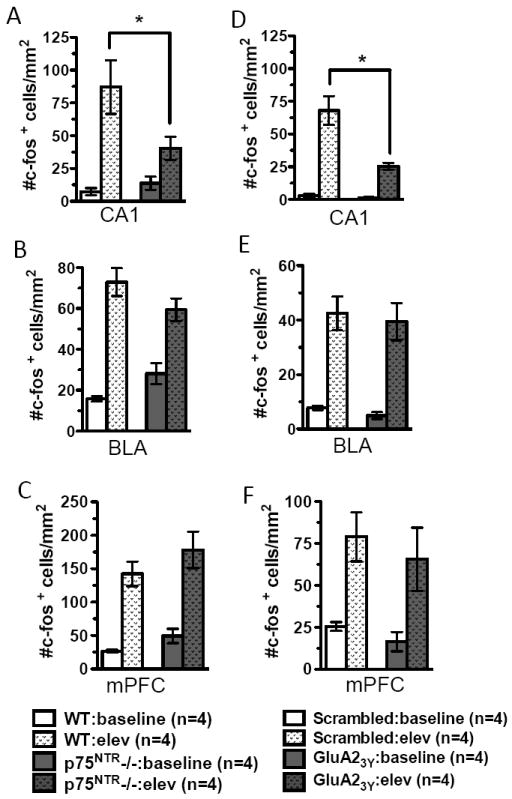
Effects of impaired stress-enabled LTD on neuronal activation patterns. (A) Compared to WT, p75NTR-/- show fewer c-fos+ cells in hippocampal CA1 following elevation (elev) stress. (B) p75NTR-/- and WT animals show similar numbers of c-fos+ cells in the basolateral amygdala (BLA) following stress. (C) p75NTR-/- and WT animals show comparable c-fos labeled cells in medial prefrontal cortex (mPFC) following stress. (D) Animals injected with GluA23Y peptide show significantly fewer c-fos labeled cells in CA1 following stress as compared to scrambled peptide. (E) Animals injected with the GluA23Y and scrambled peptides show comparable c-fos labeled cells in BLA following stress. (F) Animals injected with GluA23Y and scrambled peptides show comparable c-fos labeled cells in the mPFC following acute stress.
Since p75NTR regulates hippocampal stress-enabled LTD, we examined the effect of LTD blockade on neuronal activation. Baseline c-fos+ cell densities were similar in animals administered either scrambled or GluA23Y peptide(Fig.3D-F and Fig.S3D-F in the Supplement). Again, there was a dramatic increase in c-fos labeling following stress exposure, but injection of GluA23Y peptide did not alter the magnitude of increase in BLA(Fig.3E), mPFC(Fig.3F) or PVN(Fig.S3F in the Supplement). However, neuronal activation was significantly reduced in CA1 in animals administered GluA23Y peptide(Fig.3D). In contrast to our observations in p75NTR-/-, we saw no effect of GluA23Y on c-fos induction in the dentate and CA3 regions(Fig.S3D-E in the Supplement). Taken together, these results imply that p75NTR selectively controls CA1 neuronal activation in response to stress by regulating stress-enabled LTD.
Role of p75NTR and cholinergic transmission in baseline mood behaviors
The effects of p75NTR in stress-induced neuronal activation, LTD and stress recovery suggest a role in regulating stress-related mood behaviors. We first characterized innate or baseline depressive-like behaviors in p75NTR-/- mice using forced swim test(FST) and tail suspension test(TST)(3,31,32) as well as saccharin preference test (SPT)(3,32), and found no difference between p75NTR-/- and WT(Fig.S4A-C in the Supplement). Moreover, both genotypes showed the expected decrease in immobility after antidepressant administration(Fig.S4B in the Supplement). These results suggest that mutation of p75NTR does not lead to baseline depressive-like behaviors or affect the ability to respond to antidepressant treatment.
We used four tests to measure baseline anxiety-like behaviors. First, mice were tested in the elevated plus maze(EPM). Adult p75NTR-/- animals exhibited significant decreases in time spent in the open arms and in number of entries into the open arm as compared to WT animals(Fig.4A). Second, in the light-dark box(LDB), p75NTR-/- mice spent less than half the overall time and made less than half the number of entries into the light as compared to WT(Fig.4B). Third, in the open field test(OFT) p75NTR-/- mice spent significantly less time in the center zone as compared to WT(Fig.4C). This deficit could not be accounted for by differences in locomotion as mutant animals showed normal habituation(Fig.S4D in the Supplement), and similar total distance traveled(Fig.4C). Finally, in the novelty-induced hypophagia(NIH) test, animals display increased latency to approach a previously learned food reward when it is offered in a novel environment. The “anxiety index”, as reflected by the latency to approach in the novel environment, increased dramatically in p75NTR-/- mice, as compared to WT(Fig.5A). Our results, taken together with a previous report(33), suggest that p75NTR-/- animals exhibit baseline anxiety in the absence of stress.
Fig. 4.
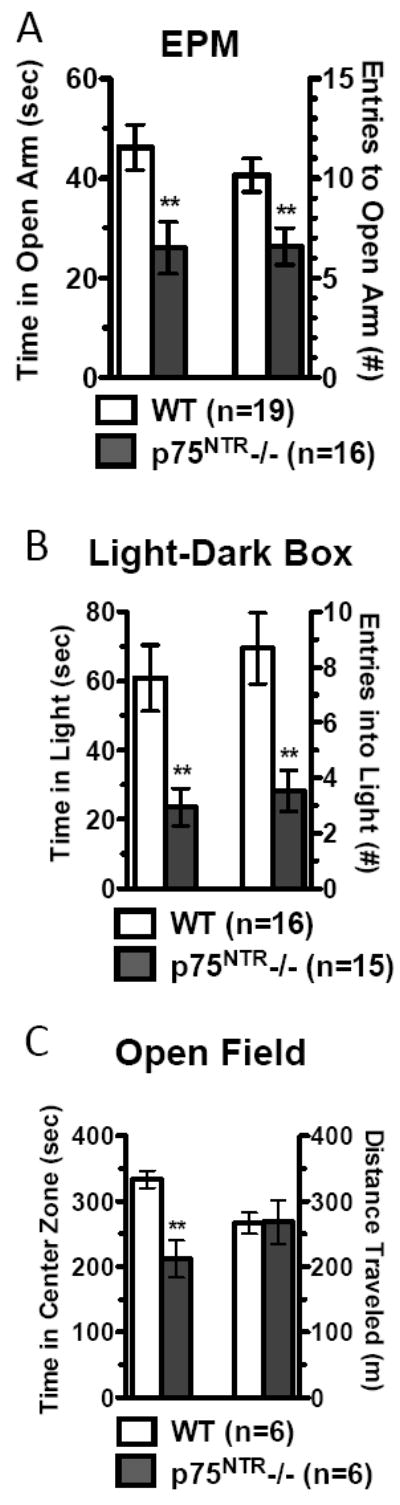
p75NTR deletion leads to baseline anxiety-like behavior. (A) In the elevated plus maze p75NTR-/- mice spent less time in the open arm (P=0.0062) and made fewer entries (P=0.0158). (B) In the light-dark box test p75NTR-/- mice spent less time in the light side (P=0.0024) and made fewer entries (P=0.0020). (C) In the open field test, p75NTR-/- mice show no difference in total locomotion (P=0.9496), but spend less time in center (P=0.0024).
Fig.5.
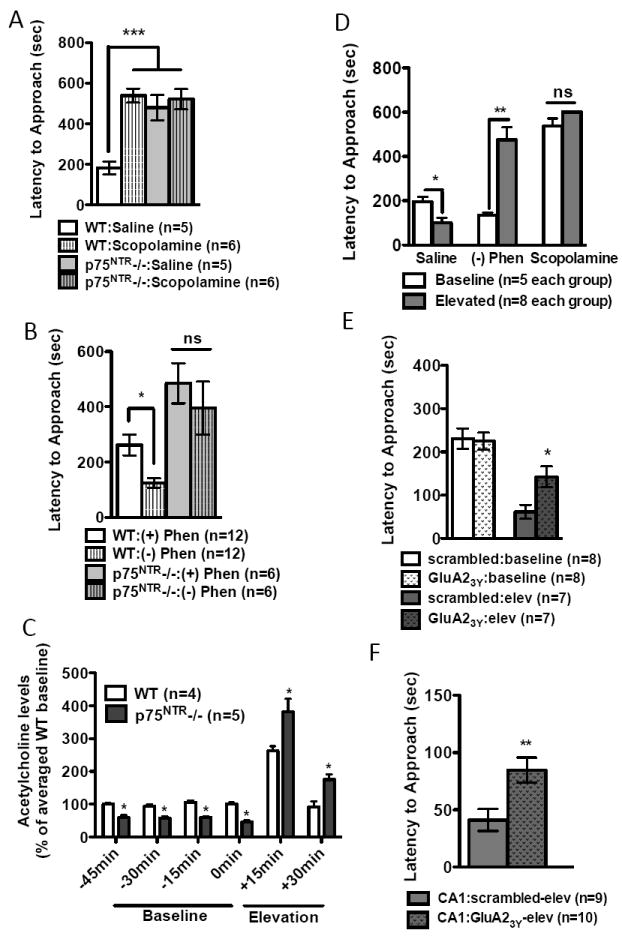
Cholinergic regulation of anxiety-like behavior. (A) Scopolamine increased novelty-induced hypophagia (NIH) in WT, but not in p75NTR-/-. (B) Compared to its inactive enantiomer ((+) phenserine), the acetylcholinesterase inhibitor (-) phenserine decreased NIH in WT animals, but had no effect in p75NTR-/-. (C) p75NTR deletion causes changes in extracellular ACh levels in hippocampal CA3. In vivo microdialysate measurements were obtained in 15 min increments, with four baseline measurements obtained before animals were exposed to 30 mins of elevation stress. Differences between genotypes were significant by student’s t-test at each individual point (-45min:P=0.0029, -30min:P=0.0034, -15min:P=0.0003, 0min:P=0.0001, 15min:P=0.0416 and +30min:P=0.0101). A highly significant interaction was found between enotype and time (P<0.001) (D) Effects of cholinergic signaling on post-stress anxiety levels. Elevation stress led to a significant decrease in saline treated animals (P=0.0013). Administration of (-)-phenserine leads to an increase in anxiety-like behavior following elevation stress (P=0.009). Elevation stress does not affect latency times in scopolamine treated animals (P=0.1034). (E) Elevation stress causes an acute decrease in NIH latency that can be regulated by LTD blockade. This effect was significantly blocked in animals that have been injected with GluA23Y peptide. (F) Intrahippocampal infusion of the GluA23Y peptide increases latency response times following stress exposure.
To determine how p75NTR regulates anxiety, we examined adult hippocampal neurogenesis in p75NTR-/-. In contrast with a previous report(33), we found no effect of p75NTR-/- on proliferation(Fig.S5A in the Supplement), short-term(Fig.S5B in the Supplement) or long-term survival(Fig.S5C in the Supplement). Differences in methodologies used to assess neurogenesis may explain the discrepancy. Regardless, loss of adult neurogenesis in the hippocampus does not appear capable of inducing either anxiety-like or depressive-like behaviors(16, 34, 35). Thus, we think it more likely that differences in anxiety-like behavior result from changes in hippocampal plasticity due to either developmental alterations in circuit formation or misregulated cholinergic innervation.
Given that in adult brain p75NTR is almost exclusively expressed in BFCNs, it was logical to examine whether manipulation of cholinergic transmission elicits effects on anxiety. Blockade of cholinergic transmission by intra-hippocampal injection of scopolamine results in anxiety-like behaviors in LDB(36, 37), and administration of acetylcholinesterase inhibitors(AChE-Is) is anxiolytic(38,39). For its convenience and sensitivity, we used NIH test to measure anxiety in all subsequent experiments. As expected, scopolamine(0.5mg/kg, i.p., 30min prior to testing) led to an increase in the anxiety index in WT, but failed to increase baseline anxiety in p75NTR-/- mice(Fig.5A). A straightforward interpretation is that p75NTR acts through cholinergic transmission to regulate anxiety behavior. However, we cannot rule out a ceiling effect of p75NTR deletion, making it impossible for scopolamine to further increase the anxiety index.
If inhibition of muscarinic transmission is anxiogenic, facilitating cholinergic transmission should be anxiolytic. We used (-)-phenserine, a non-competitive AChE-I that elicits a rapid and lasting increase in extracellular ACh(40,41). In WT, (-)-phenserine(0.5mg/kg, i.p.), but not its cholinergically inert enantiomer (+)-phenserine, resulted in decreased anxiety index(Fig.5B). Compared to WT, p75NTR-/- mice again showed an increase in baseline anxiety(Fig.5B). However, administration of(-)-phenserine in p75NTR-/- failed to elicit the expected anxiolytic response(Fig.5B). It is possible that Ngfr deletion leads to developmental defects in postsynaptic neurons innervated by cholinergic fibers, precluding the ability of transient ACh to rescue the phenotype.
Role of p75NTR, cholinergic transmission and LTD in stress-enable anxiety behaviors
To better understand how cholinergic transmission regulates anxiety and stress reponse, we analyzed extracellular ACh concentrations in hippocampal CA3 in WT and p75NTR-/- under baseline conditions and after stress exposure(Fig.5C). HPLC analysis yielded basal ACh levels of 7.4+/- 0.8 pmol/15uL, and as expected, we found that stress exposure increased ACh release in WT hippocampus ~250% over basal levels(Fig.5C). Compared to WT, baseline levels of ACh were decreased in p75NTR-/-, but increased when assayed after acute stress(Fig.5C). We next administered saline, (-)-phenserine or scopolamine and measured the anxiety index at baseline or directly after elevation stress. Three interesting phenomena were observed(Fig.5D). 1) In control conditions (saline), the anxiety index was significantly decreased directly following stress exposure. 2) Facilitating synaptic ACh ((-)-phenserine) during elevation stress elicited a detrimental effect, i.e. increased anxiety index, and 3) When cholinergic transmission was blocked (scopolamine), animals exhibited a high anxiety index even under baseline conditions, and elevation stress no longer induced a further increase in anxiety. Taken together, these results suggest that tight regulation of cholinergic signaling is critical during the stress response, and that either increased or decreased ACh levels can lead to improper behavioral responses.
The above experiment also unraveled a previously unappreciated fact: exposure to acute stress may lead to an active coping strategy – reducing the level of anxiety. To determine whether the anxiolytic effect observed after elevation stress is mediated via stress-enabled LTD, we systemically administered either scrambled or GluA23Y peptide and tested NIH. Indeed, blockade of LTD significantly attenuated the decrease in the anxiety index after stress, without affecting baseline levels(Fig.5E). Although our prior c-fos labeling studies suggested that systemic administration of GluA23Y peptide did not have widespread effects on neuronal activation, we ensured the specificity of this effect by performing intra-hippocampal infusion. Peptides were bilaterally infused into CA1, and 1h later animals were exposed to stress with behavior assayed directly following stress exposure. The results from the CA1 infusion experiments mirrored those obtained from systemic administration; animals administered GluA23Y peptide displayed a higher anxiety index after stress(Fig.5F). Thus, stress-enabled LTD in the hippocampus may mediate suppression of anxiety-like behavior in response to acute stress.
Decreased stress coping behavior during social conflict
To determine whether LTD might serve as a general mechanism for stress coping, we examined the ability of p75NTR-/- mice to effectively cope with a different type of stress: social conflict. Animals were exposed to an aggressor CD-1 mouse for 5 min/d, during which we scored time spent immobile and time spent exploring an aggressor. In the first 3d, WT and p75NTR-/- developed subordinate behavior(i.e. decreasing exploration and increasing immobility) at a similar rate. Starting on d4, the p75NTR-/- animals showed more pronounced subordinate behaviors than WT animals, and this trend continued to d8(Fig.6A-B). To ascertain whether p75NTR-/- phenotype resulted from blocking LTD induction, we performed similar experiments with animals administered scrambled and GluA23Y peptides. Indeed, there was an increase in immobility times(Fig.6C), and a decrease in exploration times in animals injected with GluA23Y peptide, but not those injected with the scrambled peptide(Fig.6D). Together, these results raise the possibility that stress-enabled LTD is an active coping mechanism, dampening anxiety-like behavior in the aftermath of acute stress, facilitating effective recovery, and promoting resiliency.
Fig.6.
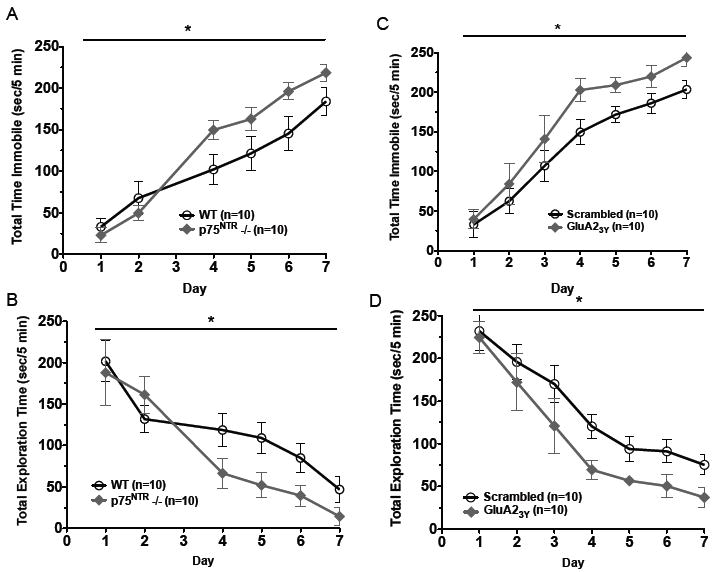
Maladaptive effects from deficits in acute stress coping mechanisms. (A) Compared to WT, p75NTR-/- animals spend more time immobile in a social conflict setting (P=0.0077). (B) Compared to WT, p75NTR-/- animals have decreased exploratory behavior in the presence of the aggressor (P=0.0189). (C) Compared to the scrambled peptide, animals administered the GluA23Y before daily social conflict encounters spend more time immobile (P=0.0106). (D) Compared to the scrambled peptide, animals administered the GluA23Y show decreased bouts of exploratory behavior in a social conflict setting (P=0.0236).
Discussion
Effective regulation of the acute response to stress is crucial to prevent development of maladaptive coping responses, as occurs in post-traumatic stress disorder. In adult animals, acute stress enables hippocampal LTD induction. Ngfr deletion eliminates this stress-enabled LTD. The p75NTR-/- mice exhibit two distinct behavioral deficits: anxiety behavior under baseline conditions and difficulties in recovering from stress. Using an LTD-blocking peptide, we demonstrate that stress-enabled LTD is important for stress recovery and coping with a social conflict situation, but not for baseline anxiety. Moreover, cholinergic transmission before and after acute stress is altered in p75NTR-/- mice, and pharmacological manipulations of cholinergic transmission mimicked the p75NTR perturbation in both baseline anxiety and acute stress response. These results reveal an important role for LTD in stress coping, and suggest a mechanistic link between p75NTR, cholinergic transmission and LTD in stress-induced anxiety.
The stress-enabled LTD deficit observed in adult p75NTR-/- appears mechanistically different from the LTD deficit in juvenile p75NTR-/-. First, in juvenile mice, p75NTR is expressed in dendritic spines of CA1 pyramidal neurons, and p75NTR interacts with PICK1, a postsynaptic density protein that binds NMDAR and regulates LTD(42). In the adult, p75NTR is only expressed in terminals of cholinergic projections, but not in CA1 neurons themselves(43). Second, NMDA-induced LTD is impaired in juvenile p75NTR-/- hippocampus, but we show that NMDA-induced LTD is normal in adult p75NTR-/-. Finally, although juvenile p75NTR-/- mice do not exhibit LFS-induced LTD, this deficit is not dependent on cholinergic transmission as is the case with stress-facilitated LTD in adult p75NTR-/- mice(23). Thus, it is possible that p75NTR regulates LFS-LTD indirectly in the adult via changes in cholinergic transmission, but directly in juvenile hippocampus by regulating NMDAR signaling in CA1 pyramidal cells.
Our studies confirm that acute stress leads to hippocampal c-fos induction. An interesting question is whether c-fos induction is upstream or downstream of hippocampal LTD. Both the LTD blocking peptide and Ngfr knockout impair c-fos induction in hippocampal CA1(Fig.3A and 3D). Moreover, scopolamine, which also prevents stress-enabled LTD in CA1(Fig.1E), has been shown to block stress-induced c-fos activation(44). Together, these results support the latter possibility: that stress-enabled LTD is required for CA1 c-fos induction. While we cannot rule out the contributions of LTD in other brain areas, our experiment using intra-CA1 injection of the GluA23Y peptide further support a role for stress-enabled LTD in stress coping.
Although Ngfr deletion selectively eliminates stress-enabled LTD without affecting other forms of hippocampal synaptic plasticity, p75NTR-/- mice exhibit behavioral deficits in both baseline conditions and after acute stress. A baseline anxiety phenotype in the p75NTR-/- animals was previously reported as an increase in the latency to feed in a novelty-suppressed feeding task(33). However, this report did not see an increase in anxiety behavior in the elevated plus maze or light-dark box. These differing results may be a result of technical differences in assay setup. For example, in the previous report both WT and p75NTR-/- animals spent only ~3% of their time in the open arm; assay conditions that would be excellent for detecting an increase in anxiolytic behavior, but difficult for discerning an increase in anxiety behavior. Unlike p75NTR mutation, blockade of stress-enabled LTD selectively impairs the response to stress, including recovery of body temperature after elevation stress, post-stress anxiety as well as coping with social conflict, without affecting baseline anxiety. Thus, p75NTR signaling seems to have differing roles before and after stress: to reduce baseline anxiety in an LTD-independent manner, and to enhance stress coping capability via stress-enabled LTD.
Given that p75NTR is almost exclusively expressed in BFCNs in the adult brain, it is not unexpected that its deletion would lead to alterations in cholinergic transmission. Using in vivo microdialysis, we have uncovered a complex misregulation in extracellular ACh levels in the p75NTR-/- hippocampus: a decrease in baseline conditions but an increase in response to acute stress. Consistent with this finding, we found that enhancement of cholinergic transmission under baseline conditions has an anxiolytic effect, but facilitation of cholinergic transmission during stress exposure impairs the recovery process, increasing the anxiety index. Thus, it appears that the baseline anxiety phenotypes in p75NTR-/- mice may be a result of decreased tonic cholinergic activation, while the stress-induced phenotypes arise from an alternative, LTD-dependent mechanism. Our studies add initial insight into the complex relationships between p75NTR, cholinergic signaling and hippocampal synaptic plasticity. Further studies will be required to more directly assess these interactions.
An unexpected finding is the marked decrease in anxiety-like behavior immediately following acute stress exposure. Inhibition of LTD, rather than directly causing an increase in anxiety-like behavior per se, actually prevents the decrease in post-stress anxiety. Future studies should address whether this robust post-stress decrease in the anxiety index is a functionally relevant component of the adaptive response to acute stress. In addition to its prominent role in memory formation and retrieval, the hippocampus is believed to regulate stress coping and resiliency(45,46). LTD induction following stress may affect the threshold for firing at the CA1-subiculum synapse and hence modulate output to brain areas involved in mediating stress-related behaviors including the prefrontal cortex, amygdala and hypothalamus. Hippocampal LTD could also directly participate in the response to trauma by dampening the consolidation of traumatic memories as its blockade can reverse the impairment of memory retrieval following acute stress(10). The present study is an excellent foundation for further exploration of how stress-enabled LTD regulates mood in response to acute stress.
Supplementary Material
Acknowledgments
This work was supported by NIMH Intramural Program. Technical support for RIA assays was performed by Diane Venable. Kathleen Cardinale contributed technical assistance with genotyping and immunohistochemistry experiments. KM and RJS are supported by NARSAD Young Investigator Awards.
Footnotes
Portions of this manuscript have been presented in abstract form at the 2007 American College of Neuropsychopharmacology Meeting, the 2007 Society of Biological Psychiatry Meeting, the 2009 Society for Neuroscience Meeting and the 2010 Collegium Internationale Neuro-Psychopharmacologicum Meeting.
Financial Disclosures KM, RJS, YL, DVJ, DP, JSG and NHG reported no biomedical financial interests or potential conflicts of interest. HKM and BL are now paid employees of Johnson & Johnson Pharmaceutical Research and GlaxoSmithKline, R&D China, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olff M, Langeland W, Gersons BP. The psychobiology of PTSD: coping with trauma. Psychoneuroendocrinology. 2005;30:974–982. doi: 10.1016/j.psyneuen.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological reviews. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JJ, Alger BE. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J Neurosci. 1995;15:1577–1586. doi: 10.1523/JNEUROSCI.15-02-01577.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 6.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science (New York, NY) 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, et al. Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- 9.Morice E, Billard JM, Denis C, Mathieu F, Betancur C, Epelbaum J, et al. Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology. 2007;32:2108–2116. doi: 10.1038/sj.npp.1301354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Progress in neurobiology. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 12.Buck CR, Martinez HJ, Chao MV, Black IB. Differential expression of the nerve growth factor receptor gene in multiple brain areas. Brain Res Dev Brain Res. 1988;44:259–268. doi: 10.1016/0165-3806(88)90224-6. [DOI] [PubMed] [Google Scholar]
- 13.Lu B, Buck CR, Dreyfus CF, Black IB. Expression of NGF and NGF receptor mRNAs in the developing brain: evidence for local delivery and action of NGF. Exp Neurol. 1989;104:191–199. doi: 10.1016/0014-4886(89)90029-0. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. The EMBO journal. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 16.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Current psychiatry reports. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 19.Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Holscher C, Anwyl R, Rowan MJ. Glucocorticoid receptor and protein/RNA synthesis-dependent mechanisms underlie the control of synaptic plasticity by stress. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature neuroscience. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- 24.Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M. The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci. 2005;25:9989–9999. doi: 10.1523/JNEUROSCI.2492-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behavioral and neural biology. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- 26.Barrett GL, Reid CA, Tsafoulis C, Zhu W, Williams DA, Paolini AG, et al. Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus. 2010;20:145–152. doi: 10.1002/hipo.20598. [DOI] [PubMed] [Google Scholar]
- 27.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox CJ, Russell K, Titterness AK, Wang YT, Christie BR. Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo. Hippocampus. 2007;17:600–605. doi: 10.1002/hipo.20302. [DOI] [PubMed] [Google Scholar]
- 29.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neuroscience and biobehavioral reviews. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.de Medeiros MA, Carlos Reis L, Eugenio Mello L. Stress-induced c-Fos expression is differentially modulated by dexamethasone, diazepam and imipramine. Neuropsychopharmacology. 2005;30:1246–1256. doi: 10.1038/sj.npp.1300694. [DOI] [PubMed] [Google Scholar]
- 31.Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 8. Chapter 8. 2001. p. 10A. [DOI] [PubMed] [Google Scholar]
- 32.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 33.Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. The European journal of neuroscience. 2008;28:883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- 34.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, NY) 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 36.Smythe JW, Bhatnagar S, Murphy D, Timothy C, Costall B. The effects of intrahippocampal scopolamine infusions on anxiety in rats as measured by the black-white box test. Brain research bulletin. 1998;45:89–93. doi: 10.1016/s0361-9230(97)00311-0. [DOI] [PubMed] [Google Scholar]
- 37.Smythe JW, Murphy D, Bhatnagar S, Timothy C, Costall B. Muscarinic antagonists are anxiogenic in rats tested in the black-white box. Pharmacology, biochemistry, and behavior. 1996;54:57–63. doi: 10.1016/0091-3057(95)02130-2. [DOI] [PubMed] [Google Scholar]
- 38.Sienkiewicz-Jarosz H, Czlonkowska AI, Siemiatkowski M, Maciejak P, Szyndler J, Plaznik A. The effects of physostigmine and cholinergic receptor ligands on novelty-induced neophobia. J Neural Transm. 2000;107:1403–1412. doi: 10.1007/s007020070004. [DOI] [PubMed] [Google Scholar]
- 39.Sienkiewicz-Jarosz H, Maciejak P, Krzascik P, Czlonkowska AI, Szyndler J, Bidzinski A, et al. The effects of central administration of physostigmine in two models of anxiety. Pharmacology, biochemistry, and behavior. 2003;75:491–496. doi: 10.1016/s0091-3057(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 40.Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Current Alzheimer research. 2005;2:281–290. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- 41.Klein J. Phenserine. Expert opinion on investigational drugs. 2007;16:1087–1097. doi: 10.1517/13543784.16.7.1087. [DOI] [PubMed] [Google Scholar]
- 42.Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greferath U, Bennie A, Kourakis A, Bartlett PF, Murphy M, Barrett GL. Enlarged cholinergic forebrain neurons and improved spatial learning in p75 knockout mice. The European journal of neuroscience. 2000;12:885–893. doi: 10.1046/j.1460-9568.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- 44.Wirtshafter D. Cholinergic involvement in the cortical and hippocampal Fos expression induced in the rat by placement in a novel environment. Brain research. 2005;1051:57–65. doi: 10.1016/j.brainres.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 45.McEwen BS, Sapolsky RM. Stress and cognitive function. Current opinion in neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 46.Sapolsky RM, Zola-Morgan S, Squire LR. Inhibition of glucocorticoid secretion by the hippocampal formation in the primate. J Neurosci. 1991;11:3695–3704. doi: 10.1523/JNEUROSCI.11-12-03695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


