Abstract
Neocortical circuits are most sensitive to sensory experience during a critical period of early development. Previous studies implicate that BDNF and GABAergic inhibition may control the timing of the critical period. By using an in vitro maturation model, we found that neurons at DIV (day in vitro) 7, around a period when functional synapses start to form and GABAergic inhibition emerges, displayed the most dynamic activation of ERK1/2 and CREB by exogenous BDNF. The BDNF-stimulated transcriptional up-regulation of CREB target genes was also the highest in DIV 7 neurons. The basal level of ERK1/2 and CREB activity, as well as the expression of CREB target genes, increased along with maturation, and neurons at DIV 13 and 22 displayed less dynamic responses to BDNF. Further, we found that the developmentally regulated GABAergic inhibition correlated with the decline of BDNF-mediated signaling during maturation. BDNF stimulation along with suppression of GABAergic inhibition enhanced the activation of ERK1/2-CREB signaling and gene transcription in mature neurons. Conversely, BDNF stimulation along with enhancement of GABAergic inhibition reduced the overall induction of intracellular signaling in younger neurons. We propose that the less dynamic molecular changes may play a certain role in the loss of plasticity during maturation.
Keywords: BDNF, GABAergic inhibition, maturation, plasticity, primary cortical neurons, TrkB
Introduction
Neural networks are being organized in a highly orderly manner during brain development. It has been implicated that brain-derived neurotrophic factor (BDNF) plays a critical role in neuronal growth, maturation, differentiation, and formation of appropriate synaptic connections (Cohen-Cory et al. 2010; Yoshii and Constantine-Paton 2010). Studies on synaptic plasticity show that long-term potentiation (LTP) and long-term depression (LTD), particularly in the cortex, are more dynamic during the “critical period” of early postnatal development, when immature circuits start to be organized into proper connections that mediate adult brain function (Morishita and Hensch 2008). Reductions in plasticity with maturation are found in the visual system and somatosensory cortex as well as in the auditory cortex. This is indicated by a decline in LTP after the critical period of (Crair and Malenka 1995; Lehmann and Lowel 2008; Hogsden and Dringenberg 2009). Furthermore, previous studies have also shown that the possibility of LTD expression elicited by low frequency stimulation is reduced in mature animals as compared with neonates (Dudek and Bear 1993). Supportively, numerous laboratories have failed to observe LTD in adult hippocampus (O’Dell and Kandel 1994). During aging, when memory function declines, hippocampal synapses also exhibit alterations in synaptic plasticity (Foster and Norris 1997). In particular, the threshold for LTP induction is increased (Rosenzweig et al. 1997), and the decay of LTP is accelerated in aged rats (Burke and Barnes 2006). Conversely, susceptibility to the induction of LTD is enhanced during aging (Norris et al. 1996; Foster and Norris 1997). Although brain maturation and aging lead to declines in certain aspects of plasticity, the molecular and cellular mechanisms are unclear. It have been known that BDNF participates in the regulation of LTP and LTD, suggesting a role of BDNF in learning and memory processes (Minichiello 2009). It is demonstrated that BDNF overexpression accelerates certain aspects of cortical maturation, and causes early termination of the critical period for ocular dominance plasticity (Huang et al. 1999). These data suggest the function of BDNF-mediated signaling in cortical plasticity during the critical period. Recent studies have implicated that the maturation of cortical GABAergic inhibition may affect the onset and termination of the critical period (Sale et al. 2010). However, it is unknown whether BDNF-mediated signaling declines during development, and whether the maturation of GABAergic function affects BDNF signaling.
The synaptic effects of BDNF-mediated signaling are mainly mediated by the TrkB receptor tyrosine kinase (Nagappan and Lu 2005). Binding of BDNF to TrkB triggers autophosphorylation of the tyrosine residue in its intracellular domain, and activates mitogen-activated protein kinase (MAPK) (Nagappan and Lu 2005). Activation of the ERK1/2 MAPK may influence gene expression through cAMP-response element binding (CREB)-mediated transcription. Consistent with the function of BDNF, the activation of ERK1/2 and CREB plays a key role in neuronal survival and synaptic plasticity. In this study, we used primary cortical cultures at different developmental ages, and examined the differences of these cultures in response to exogenous BDNF. We focused on BDNF-mediated signaling at three different levels (Yoshii and Constantine-Paton 2010). First, we examined the activation of the ERK1/2-CREB. Second, we examined CREB-regulated transcription of plasticity-related immediate early genes (IEGs), including Arc (activity-regulated cytoskeleton protein), c-fos, and Bdnf exon IV. Third, we examined the activity of cAMP-response element (CRE) and serum-response element (SRE), which are important cis-elements to control the promoter activity of Arc, c-fos, and Bdnf exon IV. We found that, comparing to DIV 3, 13 and 22 neurons, DIV 7 neurons displayed the most dynamic responses to exogenous BDNF. Along with in vitro maturation, the BDNF-mediated signaling became less dynamic in DIV 13 and 22 neurons. Further, we show that the dynamic range of neuronal responses can be expanded by BNDF stimulation paired with suppression of GABAergic inhibition in mature neurons.
Materials and methods
Primary neuronal culture and BDNF treatment
Primary cortical neurons were obtained from postnatal day 0 rat pups as described previously (Zhou et al. 2009). Briefly, after dissection, the dorsolateral cortices (containing visual cortex) were cut into small pieces, and digested with 10 units/ml papain (Worthington, Freehold, NJ) and 100 units/ml DNase I (Roche) in dissociation buffer (82 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2, 0.25 mM CaCl2, 20 mM glucose, 0.001% phenol red, 0.45 mg/ml cysteine, and 1.5 mM HEPES, pH 7.6) at 37°C for 30 min. The digestion was washed with dissociation buffer, and triturated with Neurobasal A (Invitrogen, Carlsbad, CA). Approximately 0.8 million cells were seeded in each well of the 12-well plate coated with poly-D-lysine (50 μg/ml, Sigma, St. Louis, MO). One hour after plating, Neurobasal A was replaced with growth media consisting of Neurobasal A, B27 supplement (Invitrogen), 100 units/ml penicillin, 0.1 mg/ml streptomycin, and 0.5 mM glutamine. One-third of the medium was replenished once every 3 days during the course of culturing. Cultures were pretreated with vehicle or various inhibitors such as K252a (200 nM, a TrkB inhibitor, from Sigma), TAT-PEP5 (1 μM, a p75 NTR inhibitor, from EMD), PTX (2 μM, a GABAA receptor antagonist, from Sigma), diazepam (2 μM, a GABAA receptor agonist, from Sigma), muscimol (5 μM, a GABAA receptor antagonist, from Sigma), or TTX (0.2 μM, a sodium channel blocker, from Tocris) before BDNF treatment. BDNF (purchased from R&D systems) was added directly into the medium. The neurons were kept in the 5% CO2 incubator at 37°C during the treatment.
Western blot
Following BDNF treatment, samples were harvested in 60 μl 1 × SDS loading buffer (10 mM Tris-HCl pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate, 0.01% bromophenol blue, and 5% β-mercaptoethanol), and boiled for 5 min. Lysates were separated by 10% SDS- PAGE, and transferred to nitrocellulose membranes. Antibodies against phospho-CREB at ser133 (p-CREB) (1:10000, Millipore) and phospho-ERK1/2 at Thr202/Tyr204 (p-ERK1/2) (1:2000, Cell Signaling) were used to detect the activated form of CREB and ERK1/2, respectively.
The relative expression level of NR2A, NR2B, CaMKIIα, TrkB, p75 NTR, and GAD1 at different ages was determined by Western blot. Anti-NR2A, anti-NR2B, anti-TrkB, anti-p75 NTR, and anti-GAD1 antibodies were purchased from Cell signaling. Anti-CaMKIIα antibody was from Millipore. Cultures at different development stages were harvested in H buffer (50 mM β-glycerophosphate, 1.5 mM EGTA, 0.1 mM Na3VO4, 1 mM DTT, and protease inhibitor cocktail from Roche). Five-microliter of the sample was used to determine the protein concentration by using the Bio-Rad protein assay kit; the remaining was mixed with an equal volume of 2 × SDS loading buffer, boiled for 5 min, and used for Western blot analysis. The primary antibody incubation was overnight at 4°C, and the secondary antibody (HRP-conjugated, 1:5000, Pierce) incubation was 1 hr at room temperature. Signal was detected with the ECL system (SuperSignal® West Pico, Pierce, Rockford, IL). The signal intensity was normalized to the level of β-actin (1:10000, Sigma). Several exposure times were used to obtain signals in the linear range. The signals were quantified using Scion Image software (Scion Corp. Frederick, Maryland).
Reverse transcription (RT) and real-time quantitative polymerase chain reaction (Q-PCR)
Total RNA was extracted from neurons after BDNF treatment by the TRIzol method (Invitrogen). 0.5 μg of total RNA was used for reverse transcription (RT) using SuperScript III kit (Invitrogen). The expression level of Arc, c-fos, and Bdnf exon IV was determined by real-time Q-PCR using the Bio-Rad Syber Green System. The primers used for Arc are: AGACACAGCAG ATCCAGCTG (forward) and TGGCTTGTCTTC ACCTTCAG (reverse). The primers used for c-fos are: AGCCTTTCCTACTACCATTCC (forward), and ATTCCGGCACTTGGCTGCAG (reverse). The primers used for Bdnf exon IV are: AGCCTTTCCTACTACCATTCC (forward), and ATTCCGGCACTTGGCTGCAG (reverse). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalization purpose. The primers for GAPDH are: TCCATGACAACTTTGGCATTGTGG (forward) and GTTGCTGTTGAAGTCGCAGGAGAC (reverse). Annealing and melting temperature was 55°C and 94°C for all genes, respectively. The cycle number of 40 was used to obtain the complete amplification curves. The concentration of cDNA was adjusted so that the threshold cycle was between 20 and 30 (data not shown). Each RT Q-PCR sample was analyzed in triplicate by the IQ5 software (Bio-Rad). The mRNA level was normalized to GAPDH and calculated with the 2−ΔΔCt method.
Neuronal transfection and luciferase activity assay
Cultured neurons were transfected at DIV 1, 5, 11, and 20 by using Lipofectamine™ 2000 (Invitrogen). Because neurons at DIV 20 could not tolerate the cytotoxicity of Lipofectamine™ 2000, we applied NMDA receptor antagonist MK801 (10 μM) for 10 min before transfection to protect neurons from cytotoxicity-induced cell death. Following manufacturer’s instruction, 0.2 μg luciferase reporter plasmid and 0.3 μg Renilla-luc plasmid were mixed with Lipofectamine™ 2000, and added to each well (24-well cell culture cluster, Corning, NY) that contained about 0.4 million neurons. Two days after transfection, neurons were treated with BDNF (10 ng/ml) for 6 hours. Cells were lysed, and luciferase activity of the lysate was analyzed with Dual-Glo Luciferase Assay (Promega) according to the manufacturer’s instruction. The luciferase activity was measured by a Veritas Microplate Luminometer. The CRE-Luc reporter plasmid was from Dr. Daniel Storm (University of Washington). The SRE-Luc reporter plasmid was kindly provided by Dr. Michal Hetman (University of Louisville). Renilla Luciferase reporter construct and TK-pRL (Renilla-luciferase) were from Dr. Richard Miksicek (Michigan State University). To label neurons with green fluorescence, neurons were transfected with EGFP expression plasmid (1 μg for each well of the 12-well cell culture cluster) by Lipofectamine™ 2000. After 48 hrs, the transfected neurons were observed with a Nikon fluorescence microscope.
Statistics
All quantification data was presented as average ± SEM. Data were analyzed by Student’s t-test for comparisons between two groups or one-way analysis of variance (ANOVA) for comparisons among multiple groups. All significant differences by ANOVA analysis passed post hoc (SNK) analysis. Different letters in the graphs represent different SNK mean groupings. Difference was considered significant when the p value is less than 0.05.
Results
Developmental changes in synaptic protein expression and morphology in primary cortical neurons
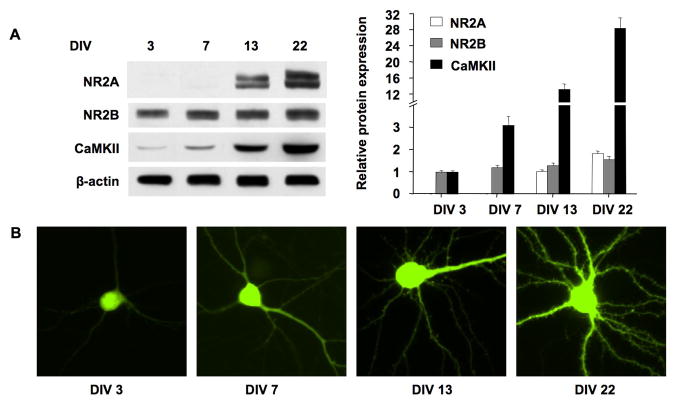
Previous studies have shown that dissociated neurons undergo dynamic developmental changes, which may recapitulate some aspects of in vivo development, such as synapse formation and maturation, as well as establishment of functional synaptic connections (Ichikawa et al. 1993; Papa et al. 1995; Lesuisse and Martin 2002). Because different culture conditions and supplement may affect the developmental process in vitro, we further confirmed these developmental changes in our experimental setup. It has been known that NMDA receptors and Ca2+/calmodulin-dependent kinase II (CaMKII) are highly concentrated in the postsynaptic density (PSD) (Kennedy 1993). The expression of NR2A- and NR2B-containing NMDA receptors shows a well-characterized developmental switch both in vivo and in primary cortical culture (Sheng et al. 1994; Li et al. 1998). NR2B subunit is abundant during early development, and NR2A level increases progressively with development. Therefore, we first examined the expression of NR2A, NR2B, and CaMKIIα at 4 different ages: DIV 3, 7, 13, and 22. As shown in Fig. 1A, the expression of NR2A was undetectable at DIV 3 and DIV 7, but progressively increased at DIV 13 and 22. The expression of NR2B displayed a slight increase with development. The expression of CaMKIIα progressively increased from DIV 3 to DIV 22.
Figure 1.
Expression profile of NR2A, NR2B, and CaMKIIα, and morphological changes during in vitro maturation. (A) Cultures at DIV 3, 7, 13, and 22 were harvested. 30 μg of total protein was separated by SDS-PAGE. The expression level of NR2A, NR2B, and CaMKIIα was examined by Western blot. Experiments were carried out in triplicate. Left panel: representative Western blots. β-actin was used as an internal control; Right panel: quantification for the relative expression level of the indicated proteins. Since NR2A expression was undetectable at DIV 3 and DIV 7, the comparison was only made for DIV 13 and DIV 22 neurons. (B) Neurons at DIV 1, 5, 11, and 20 were transfected with EGFP expressing plasmids using Lipofectamine™ 2000. 48 hrs later, the EGFP-expressing neurons were observed with fluorescence microscope. Representative EGFP-labeled neurons are shown.
To visualize the morphological changes during development, we transfected neurons with EGFP at DIV 1, 5, 11, and 20, and observed the morphology of EGFP-labeled neurons 48 hours after transfection. As shown in Fig. 1B, the cell body size and dendritic arborization of neurons gradually increased with development. There was no visible dendritic spine at DIV 3 and DIV 7. The dendritic spine was observed at DIV 13, and abundant at DIV 22. These results are consistent with previous reports (Ichikawa et al. 1993; Papa et al. 1995; Li et al. 1998; Lesuisse and Martin 2002), and indicate that our cultured neurons do show developmental changes similarly to those observed in vivo.
Dose- and time-dependent effects of exogenous BDNF application on CREB activation and Arc transcription
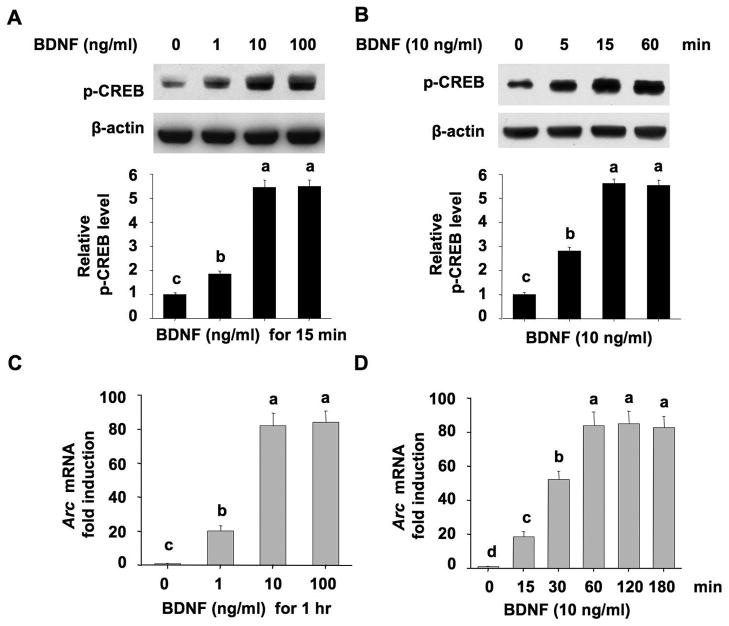
Here, we focused on BDNF-induced activation of CREB (as measured by the phosphorylation at Ser133) and up-regulation of a CREB target gene Arc, both of which are implicated in BDNF-mediated plasticity and neural development. First, we treated DIV 7 cultures with different concentrations of BDNF at 1, 10, and 100 ng/ml. Then, we examined phosphorylation of CREB at Ser133 15 min after BDNF treatment, and the transcription of Arc gene 60 min after BDNF treatment. BDNF at 1 ng/ml led to significant activation of CREB and up-regulation of Arc gene expression (Fig. 2A and 2C, the induction was 1.9-fold for p-CREB and 20.3-fold for Arc when compared to the untreated neurons). At 10 ng/ml, BDNF stimulated much more phosphorylation of CREB and the expression of Arc gene (Fig. 2A and 2C, 5.4- and 82.1-fold induction for p-CREB and Arc, respectively). BDNF at 100 ng/ml did not stimulate more induction than 10 ng/ml. Next, we treated cultures with BDNF (10 ng/ml) for different lengths of time. The BDNF-stimulated CREB phosphorylation peaked at 15 min (5.6-fold induction), and maintained for at least one hour (Fig. 2B). At 15 min, the expression of Arc gene was markedly increased (18.7-fold up-regulation), reached the peak value (84.1-fold) at 60 min after BDNF treatment, and was not further increased thereafter (Fig. 2D). Based on these results, we chose 10 ng/ml BDNF to stimulate the neurons. The level of protein phosphorylation and gene expression was determined 15 min and 60 min after BDNF stimulation, respectively, for the later experiments.
Figure 2.
Up-regulation of CREB phosphorylation and transcription of Arc mRNA by exogenous BDNF. (A) Induction of CREB phosphorylation at Ser133 by different concentrations of BDNF. Neurons at DIV 7 were treated with 1, 10, or 100 ng/ml BDNF for 15 min, and neuronal samples were examined for the level of p-CREB by Western blot. (B) Time course of BDNF-induced up-regulation of p-CREB at Ser133. Neurons at DIV 7 were treated with BDNF (10 ng/ml) for 5, 15, or 60 min, and harvested for the measurement of p-CREB at Ser133. (C) Up-regulation of Arc mRNA by different concentrations of BDNF. Neurons at DIV 7 were treated with 1, 10, or 100 ng/ml BDNF for 1 hour, and then total RNA was extracted for reverse transcription followed by real-time Q-PCR analysis by using Arc and GAPDH primers. (D) Time course of BDNF-induced up-regulation of Arc mRNA expression. Neurons at DIV 7 were treated with BDNF (10 ng/ml) for 15, 30, 60, 120, or 180 min, and then harvested for RT-PCR measurement of Arc mRNA level. One-way ANOVA followed by post-hoc (SNK) analysis was used for statistic determination. Different letters in these graphs indicate distinct SNK mean groupings (a > b > c > d).
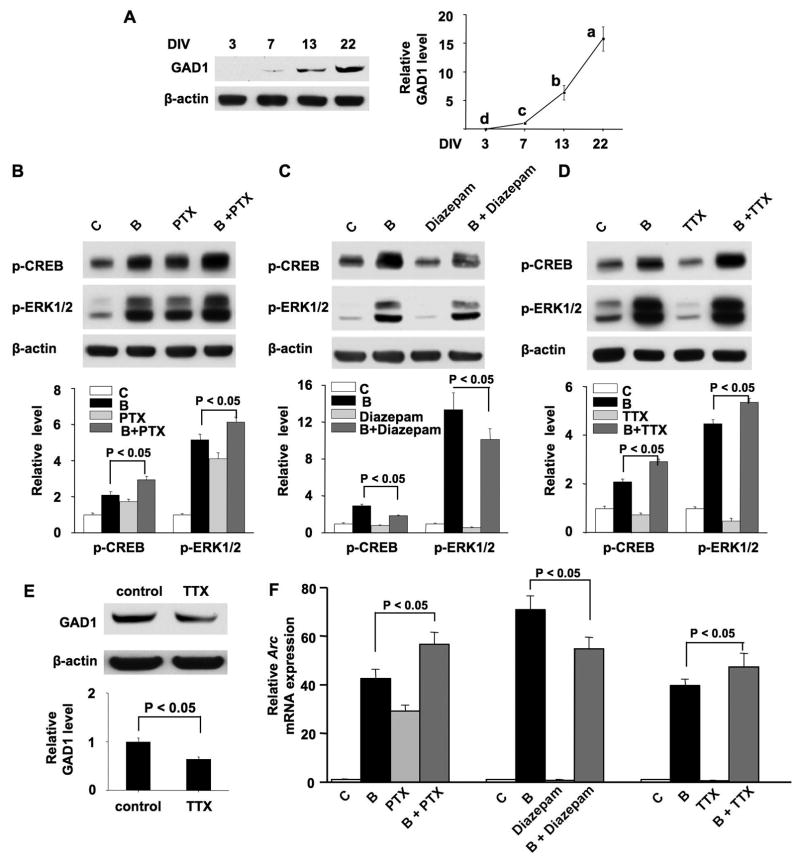
Figure 5.
Manipulation of GABAergic inhibition and electric activity alters the dynamic range of the plasticity-related intracellular signaling. (A) Samples were collected from cortical cultures at DIV 3, 7, 13, and 22. Thirty micrograms of total protein were used to determine the level of GAD1 by Western blot analysis using anti-GAD1 antibody. Experiments were carried out in triplicate. The representative Western blots are shown in the left panel. The level of β-actin was used for normalization purpose. One-way analysis of variance (ANOVA) revealed that GAD1 expression is statistically different (p < 0.001) in neurons at different DIV. Different letters indicate distinct SNK mean groupings (a > b > c > d). (B) and (D) cultures at DIV 13 were pretreated with PTX (2 μM) or TTX (0.2 μM) for 12 hrs, and then stimulated with BDNF (10 ng/ml) for 15 min. The levels of p-CREB and p-ERK1/2 were examined. (C) Cultures at DIV 7 were pretreated with diazepam (2 μM) or vehicle for 48 hrs, and then stimulated with BDNF at DIV 9 for 15 min. Samples were then harvested, and the levels of p-CREB and p-ERK1/2 were examined. (E) Cultures at DIV 13 were treated with TTX (0.2 μM) for 12 hrs, and then GAD1 expression was measured. In B, C, D, and E, the upper panels are representative Western blot results. The lower panels are quantifications of Western blot signal (normalized to β-actin). (F) Cultures were pretreated with PTX or Diazepam or TTX as described in B, C and D, and then stimulated with BDNF for 1 hr. Relative expression of Arc (normalized to GAPDH) was determined by real-time RT-PCR. C: control; B: BDNF; PTX: picrotoxin; B + PTX: pretreatment with PTX followed by BDNF stimulation; B + diazepam: pretreatment with diazepam followed by BDNF stimulation; B + TTX: pretreatment with TTX followed by BDNF stimulation.
Developmental changes of BDNF-mediated signaling in cultured cortical neurons
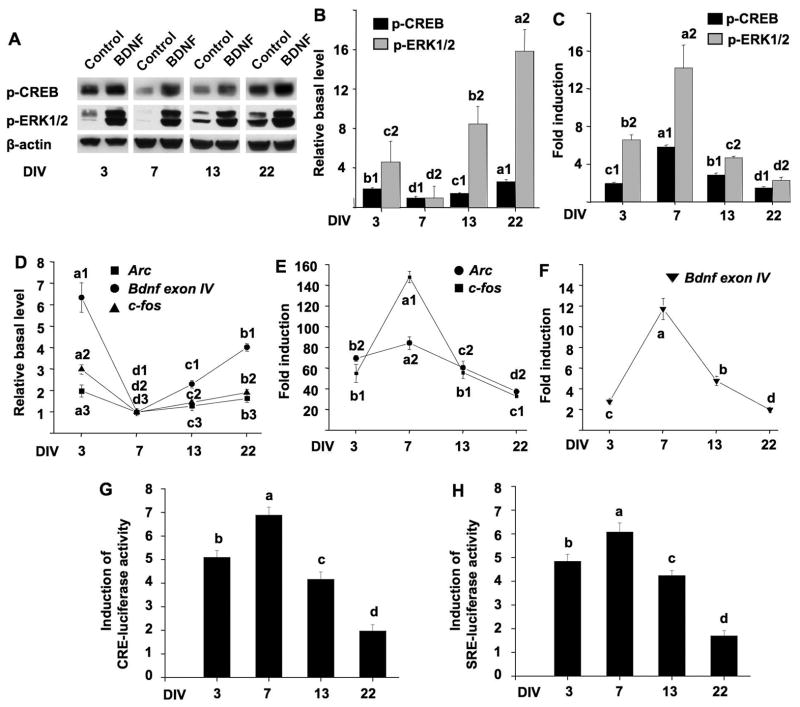
To determine the responses of cultured neurons to exogenous BDNF at different developmental stages, we examined BDNF-mediated intracellular signaling in DIV 3, 7, 13, and 22 neurons. BDNF binds to its receptor TrkB and activates ERK1/2 and CREB. Because the phosphorylation of ERK1/2 and CREB correlates with their activity, we used Western blot to detect the level of p-ERK1/2 and p-CREB. As shown in Fig. 3A and 3B, the basal levels of p-CREB and p-ERK1/2 in underwent a developmental changes. They were at their lowest level at DIV 7, and gradually increased at DIV 13 and 33. DIV 3 neurons displayed higher basal level of p-CREB and p-ERK1/2, which may be associated with the adaptation of cultures to the new environment (i.e. the culturing plate). Although BDNF (at 10 ng/ml) induced up-regulation of p-CREB and p-ERK1/2 at all developmental stages, DIV 7 neurons showed the most dynamic changes (5.8-fold induction for p-CREB; 14.1-fold induction for p-ERK1/2) (Fig. 3A and 3C). The relative changes of p-CREB and p-ERK1/2 in response to BDNF began to decline in DIV 13 neurons (2.8-fold for p-CREB; 4.6-fold for p-ERK1/2), and further decreased in DIV 22 neurons (1.4-fold for p-CREB; 2.2-fold for p-ERK1/2) (Fig. 3A and 3C). Interestingly, BDNF caused a higher induction of ERK1/2 phosphorylation (6.5-fold induction) in DIV 3 neurons than DIV 13 and 22 neurons.
Figure 3.
Developmental changes of BDNF signaling in cultured cortical neurons. Cultures at DIV 3, 7, 13, and 22 were treated with BDNF (10 ng/mL). Samples for Western blot analysis and RT-PCR analysis were collected 15 min and 60 min after BDNF stimulation, respectively. (A) Representative Western blots for p-ERK1/2 and p-CREB are shown. β-actin was used as an internal control to show equal protein loading. (B) Change of the basal levels of p-CREB and p-ERK1/2. Phosphorylation level of CREB and ERK1/2 in the absence of BDNF stimulation was measured at four different DIVs by Western blotting. Relative basal levels are plotted after setting the values of DIV 7 neuron as 1. (C) Quantification of BDNF-induced p-ERK1/2 and p-CREB up-regulation. The relative level in untreated neurons was set as 1. (D) Change of the basal levels of Arc, c-fos and Bdnf exon IV mRNA in the absence of BDNF stimulation. The relative expression levels were determined by quantitative RT-PCR, and plotted after setting the values of DIV 7 neurons as 1. (E) and (F) Real-time RT-PCR results to show BDNF-induced up-regulation of Arc, c-fos, and Bdnf exon IV expression throughout in vitro development. GAPDH was used as an internal control. (G) and (H) Cultures at DIV 1, 5, 11, and 20 were transfected with CRE-luc (or SRE-luc) and Renilla-luc. Forty-eight hours after transfection, neurons were treated with BDNF (10 ng/ml) for 6 hrs, and luciferase activity was analyzed. Fold induction of CRE- (G) and SRE-mediated (H) luciferase up-regulation at four different developmental stages is shown. All experiments were carried out in triplicate except for the luciferase experiments (quadruplicate). One-way analysis of variance (ANOVA) revealed that the effect of developmental stages was statistically significant (p < 0.0001) in all graphs. Different letters in these graphs indicate distinct SNK mean groupings (a > b > c > d); different numbers indicate different measurements.
The BDNF-induced ERK1/2-CREB activation may lead to genomic changes through gene transcription (Bramham et al. 2010; Sheng and Greenberg 1990; West et al. 2001). Here, we selected three CREB target genes: Arc, c-fos and Bdnf. They have been suggested as functional molecules involved in BDNF-mediated synaptic plasticity and neuronal modifications (Cohen and Greenberg 2008). The transcription of Bdnf is controlled by multiple promoters, and the activity-dependent the transcription of exon IV-containing mRNA in the cortex is mediated through promoter IV (Greer and Greenberg 2008; Cohen and Greenberg 2008). Therefore, we measured BDNF-activated Bdnf exon IV mRNA in this study. Consistent with the developmental profile of p-CREB and p-ERK1/2, the mRNAs of Arc, c-fos, and Bdnf exon IV in un-stimulated naïve neurons were at their lowest level at DIV 7, and increased at DIV 13 and 22. The expression of these CREB target genes was also higher at DIV 3 than DIV 7 (Fig. 3D). Mirroring the effects of maturation on BDNF-induced ERK1/2-CREB activation, one-way ANOVA analysis showed that there was a remarkable effect of development on BDNF-induced transcriptional up-regulation (p < 0.001, Fig. 3E and 3F). Comparing to controls, BDNF induced the most dramatic changes at DIV 7. For Arc, c-fos, and Bdnf exon IV, the induction was 84.5-, 148.4-, and 11.7-fold, respectively. As neurons matured, the induction was reduced to 60.1-, 56.1-, and 4.8-fold at DIV13, and further decreased to 37.6-, 33-, and 1.99-fold at DIV 22 (Fig. 3E and 3F). Interestingly, at DIV 3, the up-regulation of Arc and c-fos displayed a relative higher induction at 69.7- and 55.2-fold, respectively (Fig. 3E).
Because CRE (cAMP-responsive element) is identified in the promoters of these CREB target genes, and the promoters of Arc and c-fos also include serum response element (SRE) (Bramham et al. 2010; Sheng and Greenberg 1990; West et al. 2001), we further measured CRE- and SRE-mediated transcription in response to BDNF (10 ng/ml) at different developmental stages. We transfected DIV 1, 5, 11, and 20 neurons with CRE-luc or SRE-luc reporter construct. Forty-eight hours after the transfection, we stimulated neurons with BDNF (10 ng/ml) and measured luciferase activity. Consistent with what was observed for the transcription of Arc, c-fos, and Bdnf exon IV, we found that DIV 7 neurons showed the highest induction of CRE- and SRE-mediated transcription after BNDF stimulation (Fig. 3G and 3H, the induction was 6.9- and 6.1-fold for CRE and SRE, respectively). As neurons developed, the induction gradually decreased at DIV 13 and 22 (Fig. 3G and 3H). In DIV 3 neurons, BDNF stimulated a higher induction of CRE- and SRE-luciferase activity (Fig. 3G and 3H, the induction was 5.1- and 4.2-fold for CRE and SRE, respectively) than in DIV 13 and DIV 22 neurons. Because younger neurons always displayed higher transfection efficiency than older neurons, it was not feasible to determine how maturation affects the basal activity of CRE and SRE in naïve neurons.
Taken together, neurons at DIV 7 displayed the highest sensitivity to exogenous BDNF. The sensitivity progressively reduced along with neuronal maturation. The decreased sensitivity could be partially due to the increased basal level of the signaling molecules in mature neurons, leading to less dynamic range for BDNF-mediated induction.
Developmental expression of TrkB in cultured cortical neurons
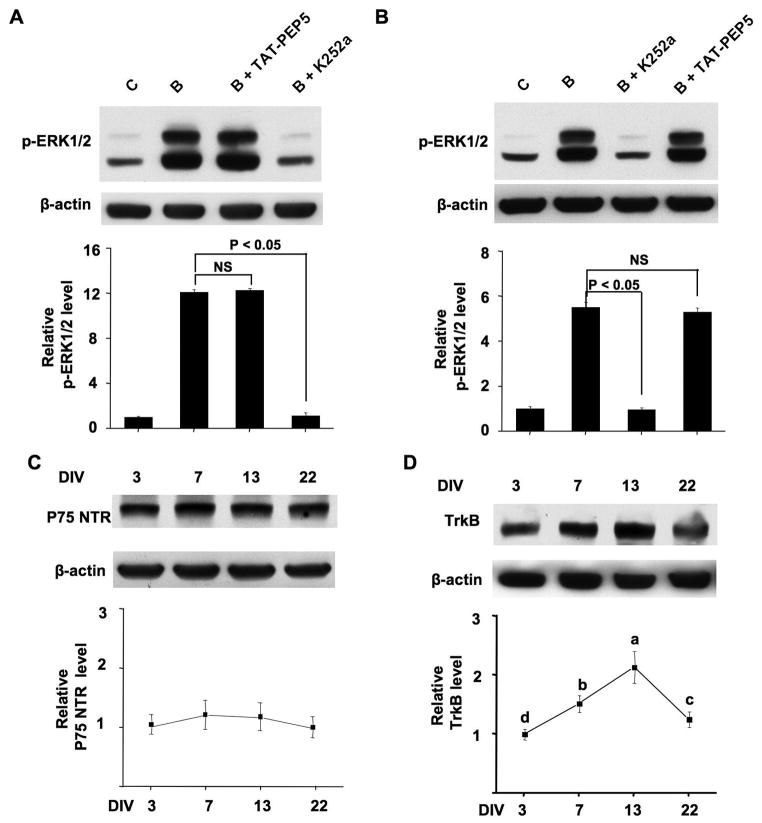
BDNF may exert its cellular functions through two separate receptors, the TrkB receptor tyrosine kinase and p75 NTR (Lu et al. 2005). The fully processed mature BDNF interacts preferentially with TrkB to activate its intrinsic tyrosine kinase activity. In contrast, the precursor BDNF (pro-BDNF) binds p75 NTR with high affinity. Thus, we examined the function of these two receptors in BDNF-stimulated intracellular signaling. We pretreated DIV 7 (Fig. 4A) and 13 (Fig. 4B) neurons with a TrkB inhibitor K252a (200 nM) or a cell-permeable p75 NTR inhibitor TAT-PEP5 (1 μM) for 30 min before BDNF treatment. We found that K252a, but not TAT-PEP5, blocked BDNF-induced phosphorylation of ERK1/2 (Fig. 4A and 4B) and CREB (data not shown). These results indicate that the BDNF responses are mainly mediated by TrkB receptor, but not p75 NTR. Further, the expression of p75 NTR was relatively constant during in vitro maturation (Fig. 4C). Next, we examined the level of TrkB expression at four different developmental stages. As shown in Fig. 4D, there was significant effects of development on the expression of TrkB (p < 0.001, one-way ANOVA). TrkB expression was relatively low at DIV 3, gradually increased, reached the highest level at DIV 13, and then declined at DIV 22. These data are consistent with a previous in vivo study (Fryer et al. 1996), showing that the expression of TrkB is developmentally regulated. However, the expression profile of TrkB did not correlate with the developmental profile of neuronal response to exogenous BDNF. Therefore, our results suggest that the decline of BDNF-mediated induction of signaling changes following maturation may be not attributed to the developmental changes of TrkB, but rather be associated with other developmentally regulated intrinsic cellular properties.
Figure 4.
Expression of TrkB and p75 NTR in cortical neurons at different DIV. (A) BDNF responses are mediated by TrkB but not p75 NTR. Cultures at DIV 7 (A) or DIV 13 (B) were pretreated with K252a (200nM) or TAT-PEP5 (1 μM) for 30 min before BDNF treatment, then p-ERK1/2 was examined by Western blot (n = 3 for all groups). (C) and (D) Equal amount of protein (60 μg) from neurons at DIV 3, 7, 13, and 22 was separated by SDS-PAGE. The expression level of p75 NTR (C) and TrkB (D) was determined by Western blot (n=3 for each group). The upper panels are representative Western blot results. β-actin was used as an internal control. The lower panels show quantification for the relative expression of p75 NTR and TrkB after normalization to β-actin. One-way analysis of variance (ANOVA) revealed that TrkB expression is statistically different (p < 0.001) in neurons at different DIV. Different letters indicate distinct SNK mean groupings (a > b > c > d). C: control; B: BDNF; B + TAT-PEP5: pretreatment with TAT-PEP5 followed by BDNF stimulation; B + K252a: pretreatment with K252a followed by BDNF stimulation.
The decline of BDNF-induced signaling is associated with the increase of GABAergic inhibition
Previous studies have shown that GABAergic inhibition increases with development and plays an important role in cortical plasticity (Sale et al. 2010). Thus, we wondered whether reduction of BDNF-induced signaling induction in mature neurons is associated with increase of GABAergic inhibition. First, we examined the expression of GABA synthesis enzyme glutamic acid decarboxylase 67 (GAD1, also called GAD67) at different developmental stages. As shown in Fig. 5A, the expression of GAD1 was undetectable at DIV 3, exhibited a low level at DIV 7, further increased at DIV 13, and reached a much higher level at DIV 22. This result is consistent with the previous in vivo data (Kiser et al. 1998; Huang et al. 1999), suggesting stronger GABAergic inhibition in more mature neurons. Because significant GAD1 expression was observed at DIV 13, we next used a GABAA antagonist picrotoxin (PTX, 2μM) to reduce GABAergic transmission. Neurons at DIV 13 were pre-treated with PTX for 12 hours (a longer pretreatment was avoided, because it caused a change in culture condition by affecting the pH value of the medium), and then stimulated by BDNF for 15 min. Suppression of GABAergic inhibition by PTX caused significant up-regulation of p-CREB, p-ERK1/2, and Arc transcription (Fig. 5B and 5F). Compared to neurons treated with BDNF, neurons co-treated with PTX and BDNF showed a significantly higher induction (Fig. 5B and 5F). Although the sensitivity to BDNF was not increased in neurons pretreated with PTX, these data suggest that the dynamic range of plasticity-related intracellular signaling could be expanded in mature neurons by GABAergic disinhibition along with BDNF stimulation.
Next, we examined how precocious increase in GABAA receptor signaling accelerates the decline of neuronal responses. Previous studies (Ganguly et al. 2001) and our result on the developmentally regulated GAD1 expression show that GABAergic inhibition emerges at around DIV 7. To increase GABAergic transmission, we treated DIV 7 neurons with diazepam, an agonist for the GABAA receptor benzodiazepine site. Diazepam (2 μM) was applied for 48 hrs (total of two applications, once every 24 hrs), at the end of which there was no sign of cell death. Compared to non-treated neurons, BDNF induced a significantly less activation of CREB and ERK1/2 phosphorylation, as well as Arc transcription in neurons pre-treated with diazepam (Fig. 5C and 5F). Similar results were obtained with another GABAA agonist muscimol (5 μM) (data not shown). We noticed that diazepam alone caused mild reduction of p-CREB, p-ERK1/2, and Arc mRNA expression (the change was 0.84-, 0.56-and 0.76-fold, respectively; p < 0.05, Student’s t-test when compared to the non-pretreated control) (Fig. 5C, 5F). These findings are consistent with the PTX data: manipulation of GABAergic inhibition does not necessarily alter the neuronal sensitivity to BDNF, but has impact on the overall dynamic range and induction of BDNF-mediated signaling. In the case of diazepam, when GABAergic transmission was enhanced in relatively younger neurons (from DIV 7 to 9), the dynamic range of plasticity-related intracellular signaling could be compressed.
Previous reports have shown that loss of visual input (sensory deprivation) in an eye by lid suture, as well as enucleation or intracortical TTX injection, leads to the reduction of GABA production and GABAA receptor-mediated neuronal inhibition (Benson et al. 1994; Hendry et al. 1994; He et al. 2006). Such manipulations have been shown to enhance the visual stimuli-induced responses in the deprived-eye column within the visual cortex. TTX injection in vivo silences neuronal activity by blocking action potential, and subsequently leads to deprivation of inputs. Thus, we decided to examine how TTX affects BDNF-mediated signaling in cultured neurons. Chronic application of TTX (0.2 μM) for 12 hrs did not result in any significant neuronal death at DIV 13, but a longer incubation (such as 24 hrs) triggered a mild neuronal death (data not shown). After treating DIV 13 neurons with TTX for 12 hrs, the level of p-CREB and p-ERK1/2 (Fig. 5D), as well as Arc mRNA expression (Fig. 5F) was slightly decreased (the decrease was 0.73-, 0.52- and 0.65-fold, respectively; p < 0.05, Student’s t-test when comparing to neurons not treated with TTX). However, the BDNF-stimulated activation of these molecular events was significantly enhanced when comparing to neurons not pre-treated with TTX (Fig. 5D and 5F). Consistent with the in vivo study (Benson et al. 1994), TTX treatment suppressed the expression level of GAD1 by 1.5-fold (Fig. 5E). Therefore, attenuation of GABAergic inhibition by blocking electrical activity also expands the dynamic range of intracellular signaling in mature neurons. Concurrent with the decrease in GAD1, TTX suppressed the basal signaling. Therefore, blocking electric activity in mature DIV 13 neurons also enhanced sensitivity to exogenous BDNF. These data, along with the effects of PTX and diazepam, further support that the reduction of dynamic range of the intracellular signaling during maturation is related to the emergence and establishment of GABAergic inhibition.
Discussion
It is known that the postnatal brain development in rodents undergoes a critical period of maturation within the first few weeks after birth. During the critical period, neural circuits exhibit an increased sensitivity to external stimuli and sensory experience (Spolidoro et al. 2009). It has been accepted that the high levels of plasticity within the critical period is important to support proper neuronal development. Interestingly, as the brain matures, the activity-dependent responses decline and loss of certain aspects of synaptic plasticity is apparent. However, the molecular and cellular mechanisms are not well known. BDNF plays important roles in the development of synaptic connectivity, and regulates the time course of the critical period in the CNS (Berardi et al. 2000). Such function is associated with the level of BDNF expression during development, which increases gradually from embryonic day 15 to the second postnatal week (Kaisho et al. 1991; Ivanova and Beyer 2001). Because primary cortical culture shows similar time course of synapse development to that of cortex in vivo (Blue and Parnavelas 1983; Ichikawa et al. 1993), we used it as an in vitro model to show that BDNF-mediated intracellular signaling may be most dynamic around the critical period, and decline during maturation.
We found that DIV 7 neurons displayed the most dynamic responses to exogenous BDNF, as implicated by the highest induction of ERK1/2-CREB activation and gene transcription. Despite the significant difference between in vitro and in vivo neural development, DIV 7 neurons show numerous features that are noticeable in the critical period. First, there is low level of synaptic functional proteins, such as synaptophysin (Lesuisse and Martin 2002), CaMKIIα, and NR2A (Fig. 1A). However, there is emergence of functional synapses (Dichter 1978; Ichikawa et al. 1993) and GABAergic inhibition at DIV 7 (Ganguly et al. 2001; Soriano et al. 2008). The dramatic increase of GAD1 from DIV 7 to 22 is reflected in vivo during the period of eye opening (Blue and Parnavelas 1983). Importantly, the emergence of GABAergic inhibition regulates the onset and duration of the critical period (Jiang et al. 2005). Consistently, we observed that BDNF-induced up-regulation of CRE activity is maximal at DIV 7, and gradually declined along with maturation. This is in line with the previous report that monocular deprivation up-regulates CRE-mediated transcription in the visual cortex at the beginning of critical period, and this up-regulation is less after the end of the period (Pham et al. 1999). Although cultured neurons do not have proper network organization and receive no sensory input, they do show a most sensitive development window to BDNF stimulation. It is interesting that the basal level of ERK1/2-CREB phosphorylation and expression of CREB target genes is at the lowest in DIV 7 neurons. Such cellular and genetic programming may allow more room for up-regulation, and make DIV 7 neurons more sensitive to external stimuli.
Along with maturation, neurons at DIV 13 and 22 displayed more mature synapses and more elaborate dendritic arborization (Ichikawa et al. 1993; Papa et al. 1995). Neurons at these developmental stages also showed increased expression in synaptic proteins such as NR2A, CaMKIIα, and GAD1, indicating molecular aspects of maturation. Compared to DIV 7, the basal level of p-ERK1/2, p-CREB, and mRNA of CREB target genes was significantly increased in neurons at DIV 13 and 22. The higher basal level of the intracellular signaling may serve an intrinsic molecular constraint, leaving less room for the induction of BDNF-mediated signaling in mature neurons. Evidently, DIV 13 and 22 neurons showed progressively less induction in response to BDNF. Although the “absolute value” of p-ERK1/2 and p-CREB in BDNF-stimulated neurons was comparable between DIV 13/22 and DIV 7, the dynamic range of induction (i.e. the fold changes before and after BDNF stimulation) is compressed in mature neurons, reflecting reduction of “molecular plasticity”. The compressed and less dynamic induction range in mature neurons is in good accordance with the extensive in vivo evidence that the mature brain is far less responsive to external stimuli than the younger and developing brain (Hensch 2005). It is important to point out that, although DIV 3 neurons displayed higher basal level of Arc and c-fos mRNA than DIV 13/22 neurons, they showed more dynamic BDNF-stimulated induction. Therefore, the basal level of signaling molecules may not be the only factor to control the dynamic range of neuronal response.
Another possible mechanism underlying the maturation-associated decline in molecular responses may be linked to the developmental increases of GABAergic inhibition, as suggested by in vivo studies on age-dependent decline in cortical plasticity (Sale et al. 2010). Alteration of GABAergic inhibition levels by genetic, pharmacological or environmental manipulation may lead to precocious onset or delay of critical period (Sale et al. 2010; Jiang et al. 2005). The number of GABAergic synapses as well as evoked GABAergic responses exhibits a dramatically increase from a very low level at birth to the adult level after a few weeks of postnatal development (e.g. at the end of critical period) (Huang et al. 1999; Jiang et al. 2005). In correlation, the activity-dependent neuronal modification displays high sensitivity to sensory experience during the critical period, then becomes less sensitive in adulthood. Our results show that certain intracellular signaling molecules become less sensitive and less modifiable during in vitro maturation. Several lines of evidence support the functional relevance of GABAergic inhibition on the decline of BDNF-mediated signaling. First, the expression level of GAD1 was low in DIV 7 neurons, which displayed the highest molecular sensitivity to BDNF. The higher expression level of GAD1 in DIV 13 and 22 neurons is associated with less dynamic changes in plasticity-related signaling. This is in agreement with a previous study showing that the changes in the rate of GABAergic maturation influence cortical plasticity during the critical period (Jiang et al. 2005). Second, reduction of GABAergic inhibition by chronic treatment with antidepressant fluoxetine (Maya Vetencourt et al. 2008), GABAergic antagonist, or exposure to enriched environment (Harauzov et al. 2010; Sale et al. 2007), results in restoration of plasticity in adult brain. It is unclear how dampening GABAergic inhibition can restore certain degree of plasticity in adult cortex. Our in vitro study suggests that suppression GABAergic function by PTX does not restore sensitivity to BDNF in mature neurons. Rather, suppression of GABAergic inhibition had additive effects on BDNF-mediated signaling. The dynamic and modifiable range of certain intracellular signaling was expanded when BDNF stimulation is paired with PTX pre-treatment. Therefore, GABAergic disinhibition helped to restore the dynamic range of molecular modification in mature neurons. On the other hand, our data show that enhancing GABAergic inhibition in DIV 7 neurons compressed the overall induction range without significantly affecting sensitivity to BDNF. Interestingly, these results are consistent with the previous in vivo studies showing that acceleration of GABAergic maturation by overexpressing BDNF or enrichment advances the onset of critical period environment (Huang et al. 1999; Cancedda et al. 2004).
Previous in vivo studies implicated that lowering neuronal activity, such as visual deprivation with dark rearing, helps to maintain visual cortex in an immature state and attenuates the developmental decline in cortical plasticity (Gianfranceschi et al. 2003). The visual responsiveness of deprived neurons is increased following monocular deprivation (MD) (Turrigiano and Nelson 2004). Further, visual deprivation in adulthood reactivates juvenile-like ocular dominance plasticity (He et al. 2006). Although it has been demonstrated that visual deprivation leads to reduction of GABAergic inhibition and GABAA receptor expression (Hendry et al. 1994; Turrigiano and Nelson 2004), it is not know whether suppression of neuronal activity and GABAergic inhibition share common mechanisms. Here, we found that blocking electrical activity by TTX did cause reduction in GAD1 expression. In contrast to the effects of PTX, TTX treatment decreased the basal signaling level. Consequently, TTX-treated neurons showed increased sensitivity to external stimulus (i.e. exogenous BDNF), and the dynamic range of signaling induction was also expanded. These results further suggest the functional relevance of GABAergic inhibition, but also reveal different mechanism for the effects of neuronal activity suppression.
At DIV 3, although we observed some neurites, previous morphological studies indicate that synapse barely forms at this stage (Dichter 1978; Ichikawa et al. 1993). Additionally, the expression of functional synaptic proteins, such as synaptophysin (Lesuisse and Martin 2002), NR2A, CaMKIIα, and GAD1 is either low or undetectable. However, neuron at this stage displayed a relatively stronger response to BDNF than DIV 13 and DIV 22 neurons. Although the increase in p-CREB and expression of Bdnf exon IV at DIV 3 was lower compared to DIV 13, but it was still higher than that at DIV 22. Because the synaptic transmission is minimal at DIV 3, the responses to BDNF may be mainly related to neural growth or adaptation to the culturing condition. Alternatively, such response may be required for the preparation of the later dynamic circuit development and plastic changes.
Acknowledgments
This study was supported by NIH grants to HW (MH076906) and HX (DK066110-01).
Abbreviations
- Arc
activity-regulated cytoskeleton protein
- BDNF
brain-derived neurotrophic factor
- CRE
cAMP-response element
- CREB
cAMP response element-binding protein
- DIV
day in vitro
- EGFP
enhanced green fluorescent protein
- ERK1/2
extracellular signal-regulated kinase 1/2
- LTD
long-term depression
- LTP
long-term potentiation
- PCR
Polymerase Chain Reaction
- SRE
serum response element
Footnotes
The authors declare no conflict of interest.
References
- Benson DL, Huntsman MM, Jones EG. Activity-dependent changes in GAD and preprotachykinin mRNAs in visual cortex of adult monkeys. Cereb Cortex. 1994;4:40–51. doi: 10.1093/cercor/4.1.40. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol. 1983;12:697–712. doi: 10.1007/BF01181531. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Cancedda L, Putignano E, Sale A, Viegi A, Berardi N, Maffei L. Acceleration of visual system development by environmental enrichment. J Neurosci. 2004;24:4840–4848. doi: 10.1523/JNEUROSCI.0845-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Dichter MA. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978;149:279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Huntsman MM, Vinuela A, Mohler H, de Blas AL, Jones EG. GABAA receptor subunit immunoreactivity in primate visual cortex: distribution in macaques and humans and regulation by visual input in adulthood. J Neurosci. 1994;14:2383–2401. doi: 10.1523/JNEUROSCI.14-04-02383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hogsden JL, Dringenberg HC. Decline of long-term potentiation (LTP) in the rat auditory cortex in vivo during postnatal life: involvement of NR2B subunits. Brain Res. 2009;1283:25–33. doi: 10.1016/j.brainres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ichikawa M, Muramoto K, Kobayashi K, Kawahara M, Kuroda Y. Formation and maturation of synapses in primary cultures of rat cerebral cortical cells: an electron microscopic study. Neurosci Res. 1993;16:95–103. doi: 10.1016/0168-0102(93)90076-3. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett. 2001;307:21–24. doi: 10.1016/s0304-3940(01)01905-x. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Brain Res Rev. 2005;50:126–133. doi: 10.1016/j.brainresrev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kaisho Y, Shintani A, Ono Y, Kato K, Igarashi K. Regional expression of the nerve growth factor gene family in rat brain during development. Biochem Biophys Res Commun. 1991;174:379–385. doi: 10.1016/0006-291x(91)90531-b. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3:732–737. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- Kiser PJ, Cooper NG, Mower GD. Expression of two forms of glutamic acid decarboxylase (GAD67 and GAD65) during postnatal development of rat somatosensory barrel cortex. J Comp Neurol. 1998;402:62–74. [PubMed] [Google Scholar]
- Lehmann K, Lowel S. Age-dependent ocular dominance plasticity in adult mice. PLoS One. 2008;3:e3120. doi: 10.1371/journal.pone.0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuisse C, Martin LJ. Long-term culture of mouse cortical neurons as a model for neuronal development, aging, and death. J Neurobiol. 2002;51:9–23. doi: 10.1002/neu.10037. [DOI] [PubMed] [Google Scholar]
- Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Nagappan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TA, Impey S, Storm DR, Stryker MP. CRE-mediated gene transcription in neocortical neuronal plasticity during the developmental critical period. Neuron. 1999;22:63–72. doi: 10.1016/s0896-6273(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Sale A, Berardi N, Spolidoro M, Baroncelli L, Maffei L. GABAergic inhibition in visual cortical plasticity. Front Cell Neurosci. 2010;4:10. doi: 10.3389/fncel.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Soriano J, Rodriguez Martinez M, Tlusty T, Moses E. Development of input connections in neural cultures. Proc Natl Acad Sci U S A. 2008;105:13758–13763. doi: 10.1073/pnas.0707492105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro M, Sale A, Berardi N, Maffei L. Plasticity in the adult brain: lessons from the visual system. Exp Brain Res. 2009;192:335–341. doi: 10.1007/s00221-008-1509-3. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Moon C, Zheng F, Luo Y, Soellner D, Nunez JL, Wang H. N-methyl-D-aspartate-stimulated ERK1/2 signaling and the transcriptional up-regulation of plasticity-related genes are developmentally regulated following in vitro neuronal maturation. J Neurosci Res. 2009;87:2632–2644. doi: 10.1002/jnr.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]