Abstract
Tamoxifen has been used extensively in the treatment of breast cancer and other neoplasms. In addition to its well-known action on estrogen receptors it is also known to acutely block chloride channels that participate in cell volume regulation. Tamoxifen’s role in preventing cochlear outer hair cell (OHC) swelling in vitro suggested that OHC swelling noted following noise exposure could potentially be a therapeutic target for Tamoxifen in its role as a chloride channel blocker to help prevent noise induced hearing loss. To investigate this possiblity, the effects of exposure to Tamoxifen on physiologic measures of cochlear function in the presence and absence of subsequent noise exposure were studied. Male Mongolian gerbils (2–4 months old) were randomly assigned to different groups. Tamoxifen at ~10 mg/kg was administered to one of the groups. Five hours later they were exposed to a one-third octave band of noise centered at 8 kHz in a sound isolation chamber for 30 minutes at 108dB SPL. Compound action potential (CAP) thresholds and distortion product otoacoustic emission (DPOAE) levels were measured 30–35 days following noise exposure. Tamoxifen administration did not produce any changes in CAP thresholds and DPOAE levels when administered by itself in the absence of noise. Tamoxifen causes a significant increase in CAP thresholds from 8–15 kHz following noise exposure compared to CAP thresholds in animals exposed to noise alone. No significant differences were seen in the DPOAE levels the f2 = 8–15 kHz frequency range where maximum noise-induced increases in CAP thresholds were seen. Contrary to our original expectation, it is concluded that Tamoxifen potentiates the degree of damage to the cochlea resulting from noise exposure.
Keywords: ototoxicity, Tamoxifen, compound action potential, distortion product otoacoustic emissions, Noise induced hearing loss
1. Introduction
Ototoxicity is defined as damage to the inner ear following treatment with chemical substances (Chiodo & Alberti, 1994). A definitive link between the drug and hearing loss may be lacking from absence of systematic controlled studies on the effects of treatment on hearing loss (Mattsson, 2000). Hearing may be affected by direct damage to sensory epithelia, or indirect effects on inner ear homeostasis by noxious agents (Aran, 1995). An additive ototoxic effect when administered in the presence of loud noise is noted with aminoglycosides and Cisplatin (Marques et al., 1975 ; Boettcher et al., 1987). The causal link between exposure to a drug and hearing loss becomes less obvious when hearing is affected only in the presence of subsequent exposure to noise. As a result, these drugs are often incompletely characterized. Damage to hearing by some of these agents is irreversible and therefore has important economic and psychosocial costs to the individual and society.
Tamoxifen has been used extensively for the past 25 years for its antiestrogenic actions in the treatment of breast cancer and other neoplasms and is now also used as a chemopreventive for breast cancer in high-risk pre- and postmenopausal women (Jordan, 2001).
It is a widely used drug whose effects on different organ systems merit closer attention. This study provides evidence that Tamoxifen potentiates the damaging effects of noise on the inner ear but does not appear ototoxic by itself. Far from a study of the ototoxicity of Tamoxifen, this study was initially motivated by our finding that Tamoxifen appeared to prevent OHC swelling and delay cell death in cochlear explants (Siegel et al., 2001; Hudspeth, 2007, private communication), possibly by its lesser-known but widely studied action of acutely and powerfully blocking CLC-3 and/or other chloride channels that participate in cell volume regulation (Duan, et al., 1997; 2011; Sardini, et al., 2003; Abdullaev, et al., 2006). The rapid swelling and rupture of OHC seen in vitro is remarkably similar to the time course of similar changes seen in whole animals immediately following exposure to moderate to intense noise (Spoendlin, 1971; Bohne, 1976; Bohne, et al., 2007). We therefore considered the possibility that acutely administered Tamoxifen might protect against noise induced hearing loss by reducing OHC swelling and death. Accordingly we used physiological measures to test the hypothesis that Tamoxifen is protective against noise-induced permanent hearing loss. Contrary to this initial hypothesis, we found that rather than being protective, Tamoxifen appears to potentiate the harmful effects of noise exposure.
2. Materials and methods
2.1 Tamoxifen administration
This study used 43 adult male Mongolian gerbils aged 2 to 4 months and weighing 50–65g. Our initial hypothesis was based on the chloride channel blocking property of Tamoxifen and not on its antiestrogenic action. But if systemic application in vivo was effective in blocking chloride channels, it very likely also blocks ER, due to their relatively high binding affinity for Tamoxifen. Our first step therefore was to investigate whether Tamoxifen had any effect at all on hearing thresholds following noise exposure. If such an effect were to be demonstrated, additional experiments would be necessary to separate the possible mechanisms of action. To simplify interpretation, we chose to use males only. Our rationale was that using females would have added variability due to different levels of circulating estrogen and ER during the estrous cycle between sexes as well as possible sex-related differences in the action of Tamoxifen. Both factors might have otherwise compromised our main effect of interest: Tamoxifen’s blocking of chloride channels.
Only animals that clearly demonstrated a classical positive Preyers reflex indicating normal hearing were included in the studies. All procedures were approved by the Northwestern University Institutional Animal Care and Use Committee. Studies were rendered blind to the investigator by placing 1ml aliquots of sesame oil vehicle (V) into small vials and adding 7.9*10−4 grams of Tamoxifen (Tamoxifen Citrate, Sigma Laboratories, St.Louis, MO) to half of the vials (V + T) such that the dose was approximately 10 mg/kg body weight. The vials were encoded such that the investigator could not know whether a particular animal was receiving Tamoxifen or the vehicle alone. The 1ml of vehicle alone or vehicle +Tamoxifen was injected intraperitonealy in each animal. Tamoxifen administered in a similar dose and route was used to reach >10µM concentration in live animal studies of Tamoxifen’s effects in the hippocampus (Silva et al., 2000; Veliskova et al., 2000). Five hours after administering the experimental aliquot the animals were noise exposed, as peak levels of Tamoxifen are reached 3–6 hrs after oral administration of Tamoxifen at 200 mg/Kg in ovariectomized rats and mature mice (Robinson et al., 1991). As this was an initial exploratory study to evaluate the presence or absence of Tamoxifen’s effect on physiologic measures of cochlear function, quantitative blood and CSF levels of Tamoxifen after 3–6 hrs of its administration were not measured. Tamoxifen is known to readily cross the blood brain barrier (Yuan et al., 1995; Shughrue et al., 1997) and has been shown to affect hippocampal neurons at a similar dose in earlier studies (Silva et al., 2000; Veliskova et al., 2000). Another group (Q), (n=6) of normal gerbils (No manipulation), not exposed to noise or injected with either vehicle or Tamoxifen, served as a control against which to assess the effects of Tamoxifen and noise exposure on physiological measures examined in the experiments. As no injection was performed in these specific animals, the experimenter knew that they were controls.
2.2 Noise exposure
Gerbils were exposed to a narrow band of noise within a single-walled sound-isolation chamber (Industrial Acoustics, Bronx, NY, USA). The noise was generated using an analog noise source and filtered with a Bruel & Kiær (B & K) one-third octave band filter centered at 8 kHz. The noise was digitized at 44.1 KHz and saved as a .wav file. Out of band components were further reduced using digital filtering (Goldwave Inc, St. John’s, NFLD, Canada). A power amplifier (Symetrix A220, Mountlake Terrace, WA, USA) was used to amplify the noise which was delivered via an overhead speaker in the sound-isolation chamber at 108dB SPL. Noise levels were measured using an Etymotic Research ER10B+ microphone and a true RMS digital voltmeter at several positions in the animal cage and averaged. The noise exposure lasted 30 minutes. The noise exposures consistently resulted in elevated neural thresholds and reduced otoacoustic emission levels at least one month later, consistent with permanent damage to the hair cells and possibly to the sensory neurons as well. The term permanent threshold shift (PTS) has been used in instances when, post-exposure, hearing thresholds have stabilized, but are poorer than pre-exposure values (Morest et al., 1998, Ou et al., 2000). Animals that had been injected with Tamoxifen and those in the control condition were prepared for data collection 30–35 days following noise exposure.
2.3 Surgical Procedure
The animals were anesthetized with sodium pentobarbital (80 mg/kg), intraperitonealy before surgery. To maintain a surgical level of anesthesia throughout the measurement, supplemental injections were given approximately every hour at 1/3 the dose for induction. The body temperature, measured with a rectal probe, was maintained at 36–38°C during the experiment using a heating pad, to minimize cooling of the cochlea. A ventrolateral surgical approach was used to expose the bulla and gain access to the round window to measure CAP. After making a small hole in the bulla, a ball recording electrode, fashioned from Teflon-insulated silver wire (0.0055 in O.D.) was positioned on the round window. The bulla opening was not sealed.
2.4 Stimulus Delivery and Recording System
Stimuli generated by two high-frequency drivers built for Compound Action potential (CAP) and Distortion Product Otoacoustic Emission (DPOAE) measurements (Radioshack super tweeters #40-1310B) were fed through the Etymotic Research ER-10B+ otoacoustic emission probe with separate tubes for two-tone measurements, such that the stimuli were mixed passively in the ear canal. After surgical removal of the pinna, the emission probe was coupled to the bony external meatus through a 6 mm plastic speculum with a 2mm opening diameter. This enabled a good coupling of the probe to the ear canal and allowed the emission probe to be placed near the tympanic membrane. Sound sources were calibrated in a coupler constructed using a gerbil bony ear canal terminated with a B & K 4138 1/8” microphone in the plane of the eardrum. This method avoids unrealistic sound-pressure measurements provided by couplers of inappropriate size (Dallos et al., 1969, Pearce et al., 2001). Emission data were collected with the EMAV software (Neely & Liu, 1993) using a CARD DELUXE (Digital Audio Labs, Chanhassen, MN, USA) sound card at a 96 kHz sample rate. The calibration procedure controlled the stimulus sound pressure levels at the eardrum.
2.5 CAP
The stimuli used to evoke the CAP were tone bursts. These bursts were 10ms in duration and had a 1ms rise/fall time. The stimulus presentation rate was 21/s. Responses measured on the round window, were amplified (10 X) by a differential amplifier (DAM 50; World Precision Instruments, Sarasota, Fl, USA) and band-pass filtered between 300 and 3000 Hz. CAP thresholds were measured using custom software written in Visual Basic that used a Creative Labs Audigy sound card.. CAP waveforms were plotted after averaging responses to 64 tone bursts. A visual detection criterion was used to determine the smallest N1 amplitude of CAP waveform that could be readily distinguished from the noise floor. Thresholds were measured at 14 frequencies (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25 and 30 kHz).
2.6 DPOAE
The DPOAE of interest in our experiment was the cubic distortion tone, 2f1-f2. The DPOAE level across the frequency range f2=1 to 40 kHz was measured. Primary tones were presented at a fixed ratio, f2/f1 = 1.2. The levels of the stimulus tones were L1 = 65 dB SPL and L2 = 50 dB SPL. To relate DPOAE levels and CAP thresholds, the measured DPOAE levels for stimulus conditions with f2 falling into 1/3rd octave bands centered on the same frequencies as the CAP thresholds were measured. There were typically 2–3 stimulus conditions in each 1/3rd octave band.
2.7 Data analysis
The maximum effect of noise exposure is shifted from one-half to one octave toward higher frequencies, depending on the species (Mc Fadden, 1985; Schmiedt, 1986; Cody & Johnstone, 1991). Noise induced effects on CAP thresholds and DPOAE therefore were expected to be maximal one-half octave to one octave above the 8 kHz center frequency of the noise used for the exposure. In regions of the cochlea not stimulated excessively by the narrow band noise, little damage was expected. The thresholds were therefore compared within three frequency ranges 8–15 kHz; the frequency range where the maximum effects were observed and in the two ranges 1–7 kHz and 20–30 kHz, outside the frequency range of greatest expected effects of noise exposure. These ranges could be considered internal controls for the effect of Tamoxifen in the presence and absence of noise damage to the cochlea in the same animal. Independent samples t-tests using separate variance was used to evaluate differences between groups. A p-value of < 0.05 was considered significant.
3. Results
As an aid to the reader we have used the following notation for various combinations of Tamoxifen (T), vehicle (V), noise (N) and No manipulation in the quiet condition (Q). For example, the group of animals receiving Tamoxifen followed by noise exposure is labeled (T + N).
3.1 Test of hypothesis: Tamoxifen protects against noise induced damage to the cochlea
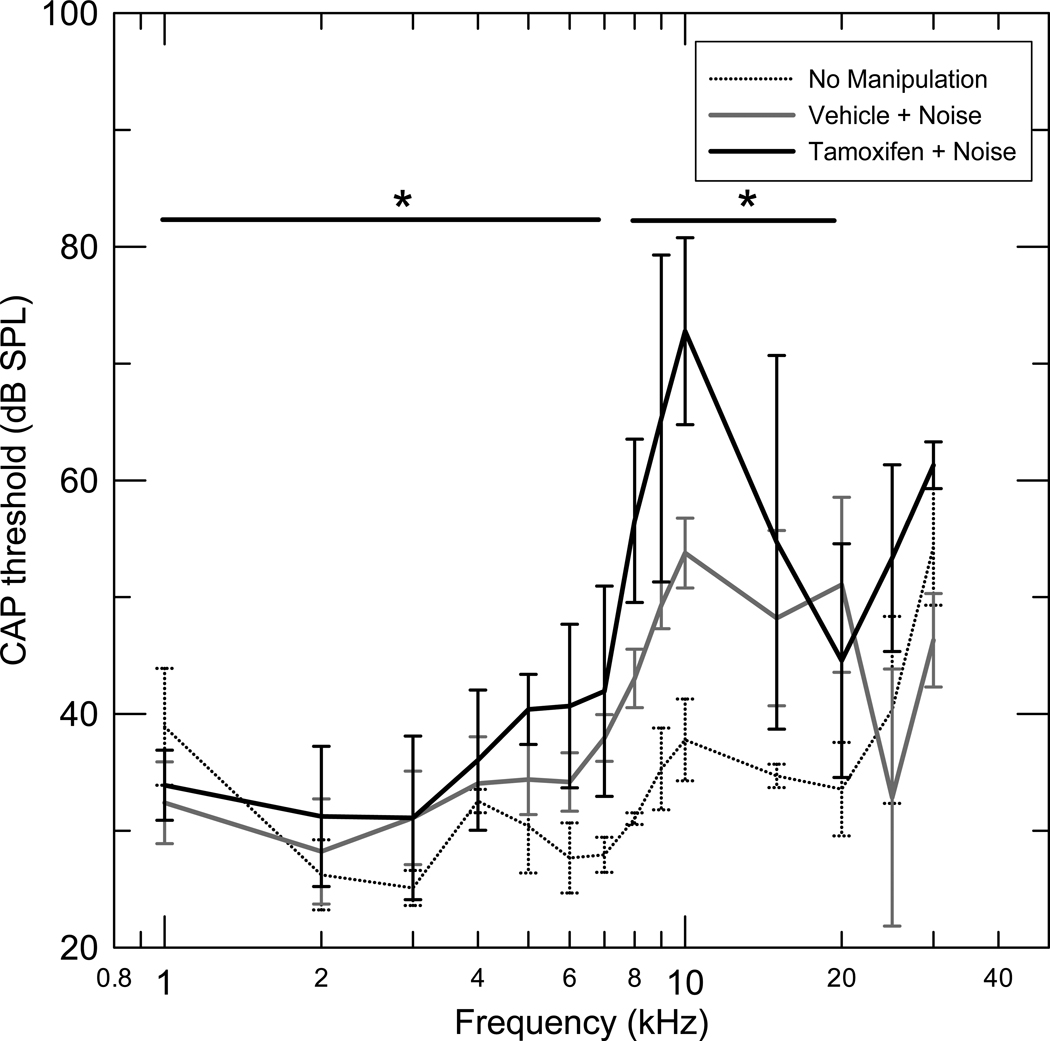
CAP thresholds following noise exposure in the range of 8–15 kHz showed significant increase in the Tamoxifen injected group (T+N), (n=7) compared to the vehicle injected (V+N) group, (n=6) (Fig 1, Table 1 for details). Thresholds in the 1–7 kHz frequency range were also significantly higher in the (T+N) compared to the (V+N) group (Table 1). The increase in thresholds in the 1–7 kHz range was significantly smaller compared to the increase in thresholds in the 8–15 kHz range of maximum expected noise induced threshold elevation (p< 0.01)(Fig 1).
Figure 1.
Mean compound action potentials with standard deviations for three groups. Tamoxifen + Noise (T+N) (n=7), Vehicle + Noise (V+N) (n=6) and No manipulation (Q) (n=6). The noise regimen was a one-third octave band of noise centered at 8 kHz for 30 minutes at 108 dB SPL. The thresholds were measured one month after exposure. The CAP thresholds in the range of 8–15 kHz (p< 0.03) and in the 1–7 kHz range (p<0.02) showed a significant increase in the Tamoxifen injected group (T+N) compared to the vehicle injected (V+N) group.
Table 1.
Independent samples t test results in CAP threshold levels
| 1–7 kHz | 8–15kHz | 25–30kHz | |
|---|---|---|---|
| (T+N) vs (V+N) n=7 vs n=6 |
p ≤ 0.02* | p ≤ 0.03* | p ≤ 0.11 |
| (Q) vs (V+N) n=6 vs n=6 |
p ≤ 0.08 | p≤0.001* | p ≤ 0.84 |
Significant
The CAP thresholds of a third group of 6 animals with no injection and no noise exposure (Q) (Fig 1) was compared to the (V+N) group. The (V+N) group had significantly elevated mean CAP thresholds compared to the (Q) group in the 8–20 kHz frequency range, while the mean thresholds between the above two groups were not significantly different in the frequency ranges 25–30 kHz, and 1–7 kHz (Fig 1 and Table 1).
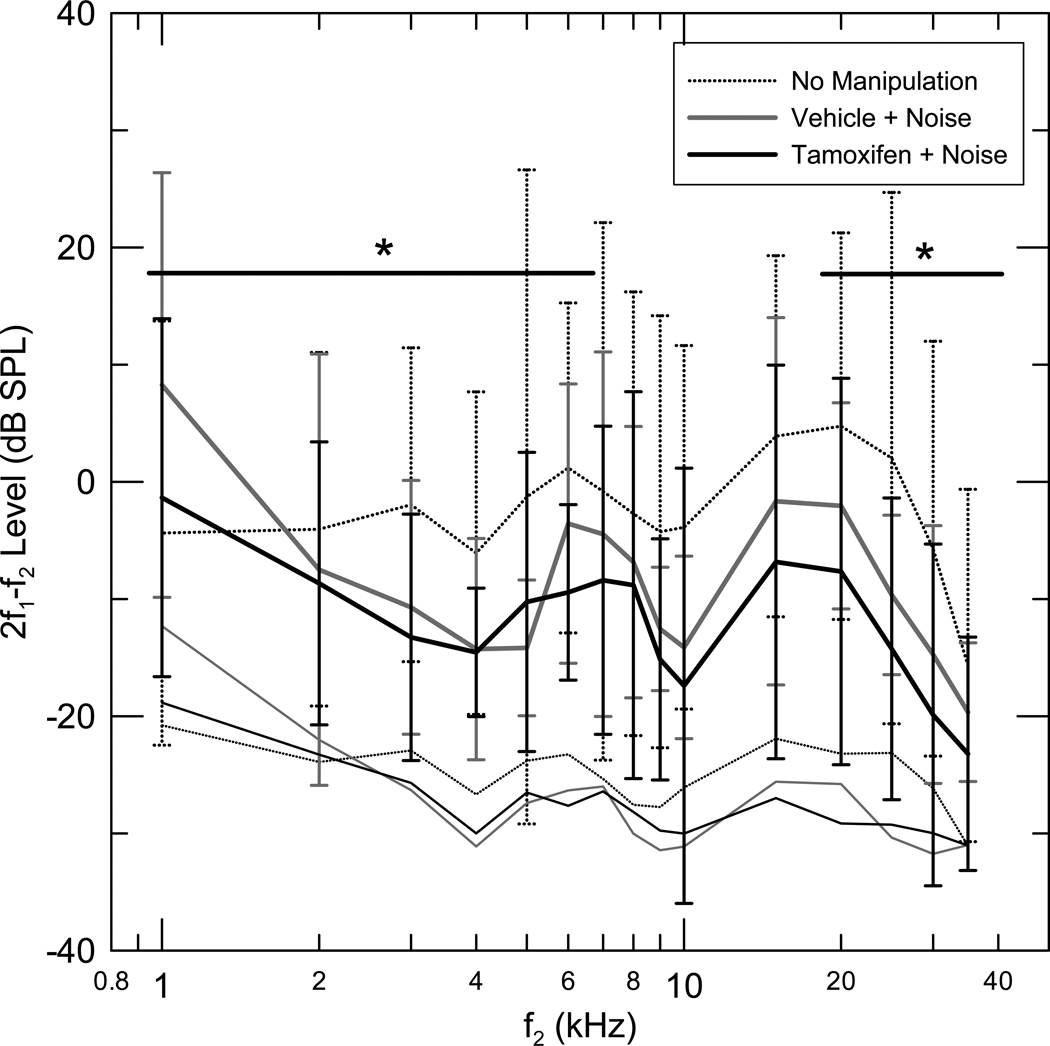
DPOAE levels for stimulus frequencies near the band of frequencies with greatest CAP threshold shifts (f2= 8–20 kHz) were reduced in the (V+N) group, relative to the (Q) group, (Fig 2, Table 2 for details). The mean DPOAE level of the (V+N) group was also significantly reduced from the (Q) group for f2 in the 25–30 KHz frequency range (Table 2).
Figure 2.
Mean one-third octave DPOAE level as a function of f2 center frequency for three groups, Tamoxifen + Noise (T+N) (n=7), Vehicle + Noise (V+N) (n=6), No manipulation (Q) (n=6). The DPOAE levels for each gerbil were calculated by averaging the DPOAE amplitude in the 1/3rd octave band around the f2 center frequencies. The mean DPOAE level corresponding to f2 center frequency was calculated in each group. Standard deviations and the mean 1/3rd octave noise floor are also depicted. No significant difference was seen in the DPOAE levels between the (V+N) and (T+N) groups in the (f2=8–20 kHz) frequency range (see Table 2, for further results).
Table 2.
Independent samples t test results in DPOAE levels
| 1–7 kHz | 8–20kHz | 25–30kHz | |
|---|---|---|---|
| (Q) vs (V+N) n=6 vs n=6 |
p ≤ 0.303 | p ≤ 0.003* | p ≤ 0.004* |
| (T+N) vs (V+N) n=7 vs n=6 |
p ≤ 0.004* | p ≤ 0.06 | p ≤ 0.08 |
| (T+N) vs (T+Q) n=7 vs n=12 |
p ≤ 0.001* | p ≤ 0.001* | p ≤ 0.03* |
Significant
No significant difference was seen in the mean DPOAE levels between the (V+N) and (T+N) groups in the (f2=8–20 kHz) frequency range (see Fig 2 and Table 2). The mean DPOAE level of the (T+N) group was significantly lower than the (V+N) group for the f2= 1–7 kHz frequency range. The DPOAE level of the (T+N) group was not significantly different from the (V+N) group in the 25–30 KHz frequency range (Table 2).
The hypothesis that Tamoxifen is protective against noise-induced damage to the cochlea is clearly not supported by the above results. In fact, the evidence establishes that Tamoxifen potentiates noise damage.
3.2 Effect of Tamoxifen in the absence of noise exposure
The previous results do not allow us to separate the individual effects of noise and Tamoxifen on cochlear damage. We therefore examined in a control experiment whether Tamoxifen causes an increase in hearing thresholds and decrease in otoacoustic emissions levels when administered in the absence of noise exposure (Quiet). Two groups of animals were compared, one that received Tamoxifen (T+Q) and one that received vehicle alone (V+Q) (n=12 for each group). Physiological measurements were made 30–35 days after the injections.
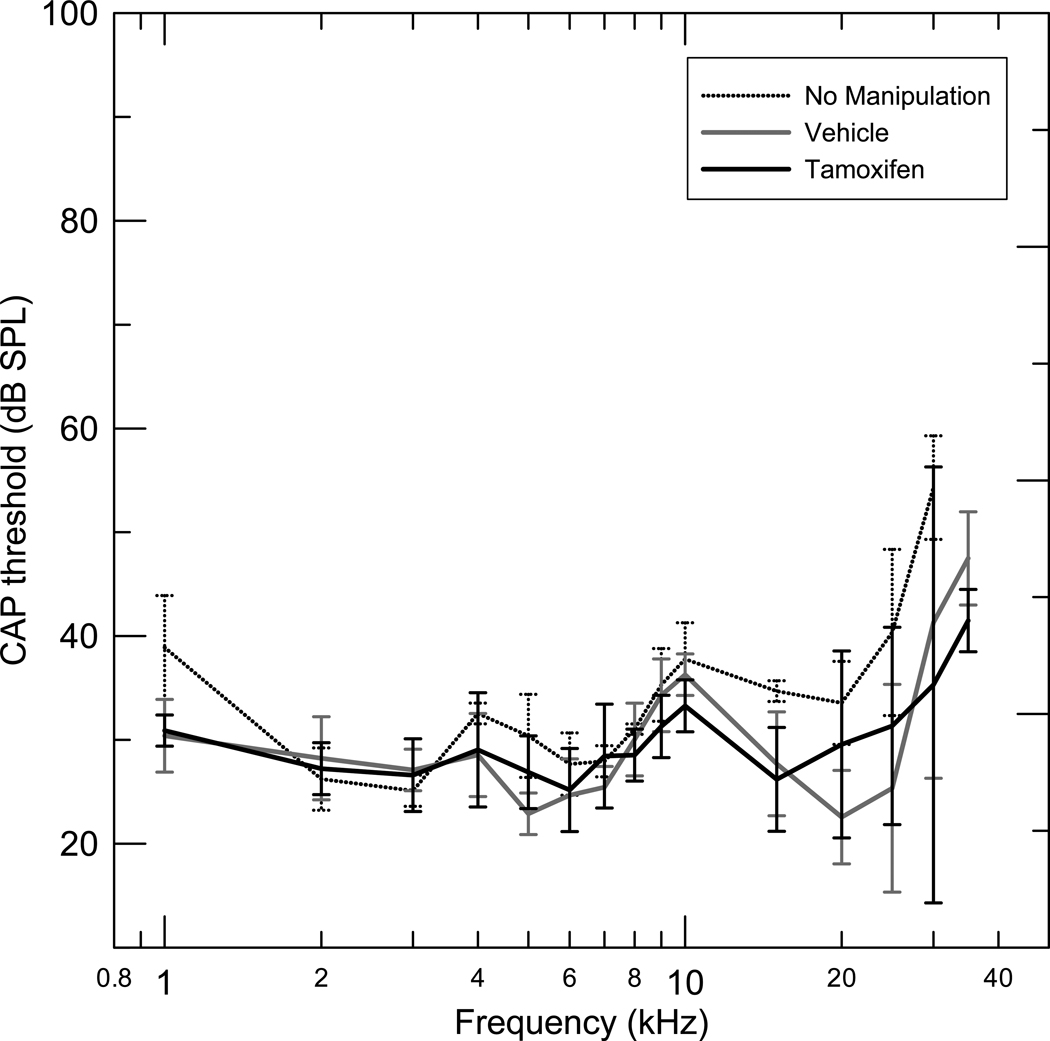
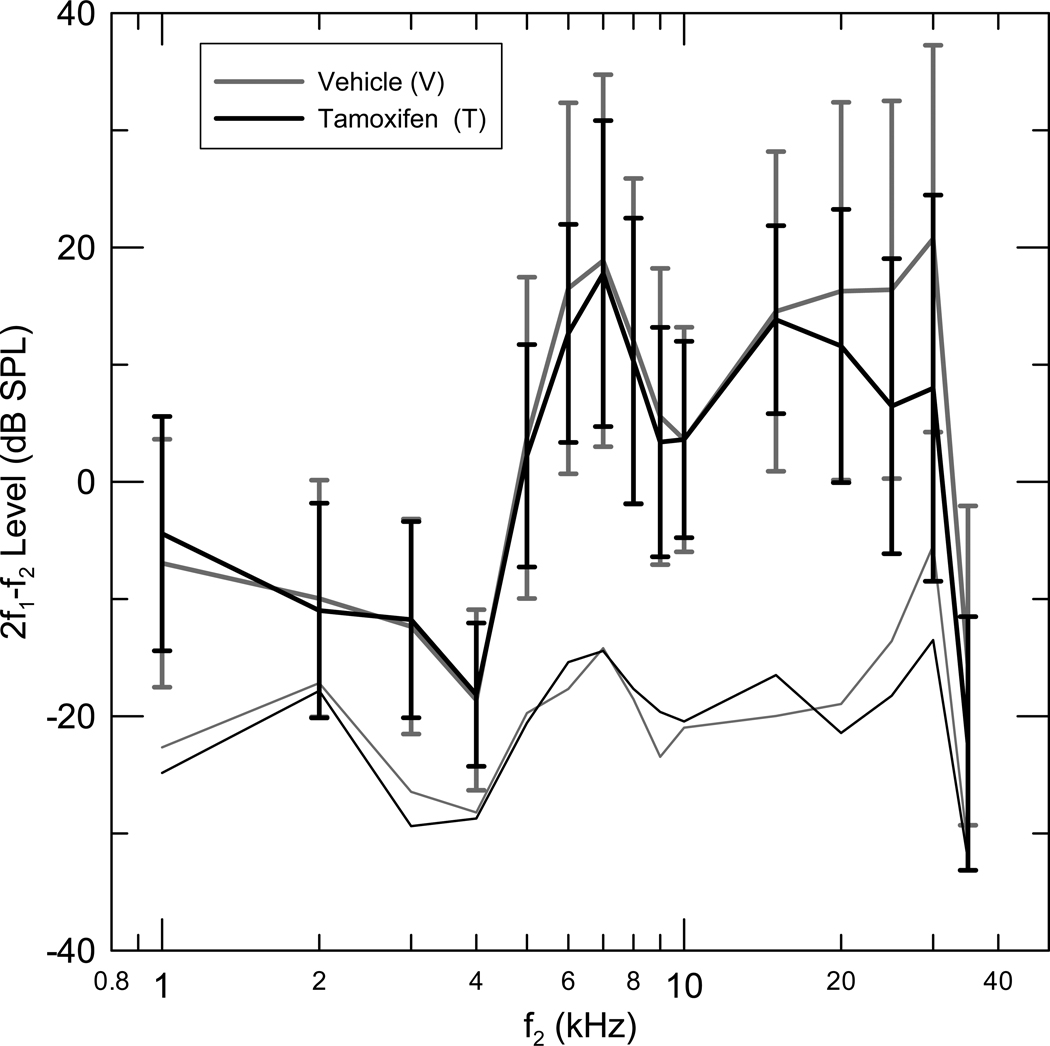
The (T+Q) group did not have significantly different mean CAP thresholds compared to the (V+Q) group in any of the three frequency ranges used for all comparisons (Fig 3). Mean (T+Q) group and (V+Q) group DPOAE levels were also not significantly different in any of the three frequency ranges analyzed (Fig 4)
Figure 3.
Mean compound action potentials with standard deviations for three groups, Tamoxifen in Quiet (T+Q) (n=12), Vehicle in Quiet (V+Q) (n=12) and No manipulation (Q) (n=6). The thresholds were measured one month after Tamoxifen and Vehicle administration. The (T+Q) group did not have significantly different mean CAP thresholds compared to the (V+Q) group in the 1–40 kHz frequency range, (p < 0.961).
Figure 4.
Mean one third octave DPOAE level as a function of f2 center frequency for two groups, Tamoxifen in Quiet (T+Q) (n=12), Vehicle in Quiet (V+Q) (n=12). The DPOAE levels for each gerbil were calculated by averaging the DPOAE amplitude in the 1/3rd octave band around the f2 center frequencies. The mean DPOAE level corresponding to f2 center frequency was calculated in each group. Standard deviations and the mean 1/3rd octave noise floor are also depicted. No significant difference was seen in the DPOAE levels between the (V+Q) and (T+Q) groups in the entire frequency range measured.
A secondary analysis was to determine if there was a difference in the effects of Tamoxifen on CAP thresholds and DPOAE levels in the (T+Q) and (T+N) conditions. The CAP thresholds of the (T+N) group were noted to be elevated in the frequencies of maximum expected noise effects, 8–15 KHz, compared to the (T+Q) group (p < 0.0001). The CAP thresholds in the 1–7 kHz frequency range were also significantly higher in the (T+N) group compared to the (T+Q) group (p < 0.0001). There was no significant difference in the CAP thresholds in the 20–35 kHz frequency range between the two groups (p < 0.41). DPOAE levels for the (T+N) group were reduced in all three frequency ranges examined compared to the (T+Q) group (Table 2). Our results therefore do not support the hypothesis that Tamoxifen is ototoxic when administered in the absence of noise.
4. Discussion
Our study establishes that Tamoxifen potentiates the degree of damage to the cochlea from noise exposure. We also show that Tamoxifen does not produce any changes in CAP thresholds and DPOAE levels when administered alone in Mongolian gerbils.
We found CAP threshold elevations in the (T+N) group were significantly different from the (V+N) group in the lower frequency range (1–7 kHz). But no significant differences in CAP thresholds were noted between these two groups in the highest frequency range (20–35 kHz). We interpret the latter result with caution, as thresholds in the base of the cochlea are especially vulnerable to cochlear cooling (Ohlemiller & Siegel, 1992) and damage from opening up the bulla (Overstreet et al., 2003). These effects could confound the CAP threshold measurements at higher frequencies, even though care was taken to minimize these effects. As a result, a strong case for the interpretation that Tamoxifen affects the apex of the cochlea more than the base cannot be made. Furthermore, CAP thresholds were significantly increased in the frequency ranges where the effects of the narrow-band noise were not expected in the (T+N) group, (1–7 KHz) compared to the thresholds in the (T+Q) group. These results together suggest Tamoxifen administration followed by noise exposure appears to increase CAP thresholds even in the regions of the cochlea not likely to have been damaged by the narrow band noise.
The 2f1-f2 DPOAE levels were lower in the (T+N) group compared to the (V+N) group in the 1–7kHz range of frequencies where effects of noise were not expected, but not significantly different between the two groups in the f2 = 8–15 kHz range where maximum threshold differences between these two groups were noted in the CAP measurements (Fig 2 and Table 2). Furthermore DPOAE levels were reduced in the (T+N) group across all frequencies examined compared to the (T+Q) group (Table 2). Together, these results suggest: a) One month after noise exposure, 2f1-f2 DPOAE levels for stimulus frequencies near the center frequency of noise exposure and at frequencies one-half to one octave higher are decreased; b) Tamoxifen administration followed by noise exposure appears to decrease DPOAE generation even in the regions of the cochlea not likely to have been damaged by the narrow band noise.
These results clearly do not support our initial hypothesis that Tamoxifen protects against noise-induced damage to the cochlea. If Tamoxifen’s action in preventing OHC swelling is mediated by blocking chloride channels in vitro, then it is not immediately apparent why it was not protective in vivo during histologically similar OHC swelling after noise exposure. Perhaps the more complex ionic environment of the cochlea in vivo makes a ready extrapolation of the effect noted in vitro difficult. Additional experiments would be needed to answer these new questions.
The ototoxic effect of Tamoxifen we noted in combination with noise is similar to the action of drugs such as aminoglycosides and Cisplatin (Boettcher et al., 1987), which have an additive action on hearing loss with noise exposure. Classical ototoxic drugs have been shown to be more damaging to the high frequency regions of the cochlea, similar regional variation of vulnerability of the cochlea to Tamoxifen is not clear from our study. Noise exposure, by decreasing blood flow (Thorne & Nuttall, 1987) and decreasing oxygenation to the organ of Corti (Thorne & Nuttall, 1989) increases the metabolic demand across the entire cochlea. Our evidence of increased CAP thresholds at all frequencies, along with the absence of an effect on CAP thresholds when Tamoxifen is administered in the absence of noise exposure, raises the possibility that Tamoxifen has an additive toxic effect in the presence of noise exposure across the cochlea. This could be accounted by an added increased metabolic demand and increased vulnerability to mitochondrial bioenergetic toxicity of Tamoxifen (Custodio et al., 1998; Cruz Silva et al., 2000; Mandlekar & Kong, 2001; Cardoso et al., 2001) in the face of noise.
It is also possible that the ototoxicity of Tamoxifen’s role is mediated by its effect on estrogen receptors (ER). ERs role in the auditory system has been noted in both male and female rodents (Charitidi et al., 2009). Understanding the role of ER in the auditory system is thought to be important for medical uses, including improving hormone replacement therapy against hearing loss especially in Turners syndrome, aging and following chemotherapy (Stenberg et al., 2001; Kilicdag et al., 2004; Jenkins et al., 2009). Loss of hearing in estrogen receptor ER-β knock-out mice indicates that ER-β is important in maintaining hearing (Simonoska et al., 2009). In the cochlea there is evidence for ER-α expressing cells in the spiral ganglion (Stenberg et al., 2001), and ER-β expressing cells nuclei of the inner and outer hair cells (Meltser et al., 2008) and stria vascularis (Stenberg et al., 2001). Some have also reported co-localization of these receptors in all four structures above (Motohashi et al., 2010). ER-β agonists have been shown to protect the auditory system from temporary threshold shift from acoustic trauma in young male and female ER knockout mice and provide partial protection from ER-α agonists (Meltser et al., 2008). Tamoxifen’s anti estrogenic effect is primarily mediated by its action on the estrogen receptor ER-β. It is therefore possible that Tamoxifen could have potentiated noise induced hearing loss by blocking estrogen’s effects through ER in the inner ear. In addition, long term blocking of ER by Tamoxifen has been shown to decrease auditory function in young adult mice (Thompson et al, 2006) raising the possibility that potentiation of noise damage by Tamoxifen could be contributed to by diminished protective effects of circulating estrogen. Future studies including histology and immunohistochemistry will be needed to extend our present results to evaluate if the ototoxic effects described are related or not to antiestrogenic effects of Tamoxifen and to better understand the loci and mechanisms of damage from Tamoxifen (including damage to outer hair cells, inner hair cells, stria and spiral ganglion neurons) as the present study was initially undertaken with a different hypothesis in mind.
In adult humans both ER-α and –β are present in the adult inner ear (Stenberg et al., 2001). A reduction in hearing sensitivity due to a decrease in circulating estradiol levels has also been noted (Strachan, 1996). A study with 143 women concluded that hormone replacement therapy may have a protective effect against hearing impairment in postmenopausal women (Hederstierna et al., 2007). Chemotherapy agents in the treatment of breast cancer and adjuvant endocrine therapies, including selective estrogen receptor modulators like Tamoxifen maintain a menopause like state with lower estrogen levels. A pilot study of eight women on chemotherapy for breast cancer including Tamoxifen also suggested hearing loss being present in this small group after treatment (Jenkins et al., 2009). More recently, doses of up to 250 mg/m2 Tamoxifen with oral loading doses of 680 mg/m2 have been used in Phase I and Phase II clinical trials in cancer therapeutics (Trump et al., 1992; McClay et al., 2001; Perez et al., 2003). It is therefore possible that Tamoxifen could have role in influencing hearing loss in humans in some specific contexts.
5. Conclusions
Our result that Tamoxifen potentiates the deleterious effects of noise exposure on physiological measures of cochlear function in animals could provide future insights useful in a clinical setting. Tamoxifen when administered in combination with other drugs that have an additive ototoxic effect with noise exposure (eg: Cisplatin) should be of concern and warrants further study in the clinical population.
Highlights.
Tamoxifen is a commonly used chemotherapeutic agent against breast cancer.
We report the ototoxic effect of Tamoxifen on measures of cochlear function.
Tamoxifen potentiates hearing loss resulting from noise exposure among Mongolian gerbils.
No hearing loss was noted when Tamoxifen was administered alone.
Acknowledgements
This work was completed as a thesis by the first author submitted in partial fulfillment of the Doctor of Philosophy degree from the Department of Communication Sciences and Disorders at Northwestern University. We thank the dissertation committee members Mario Ruggero and Peter Dallos for their invaluable insights into this work. This research was supported by NIH grant R01 DC03416 awarded to the second author.
Abbreviations
- OHC
Outer hair cell
- CAP
Compound action potential
- DPOAE
Distortion product otoacoustic emission
- PTS
Permanent threshold shift
- ER
Estrogen receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
The authors have no actual or potential conflict of interest to declare. All authors have approved the final article towards publication.
Contributor Information
Jagan A Pillai, Department of Communication Sciences and Disorders, 2240 Campus Drive, Northwestern University, Evanston, IL 60208, USA.
Jonathan H Siegel, Northwestern University, Department of Communication Sciences and Disorders, 2240 Campus Drive, Evanston, IL 60208, USA, j-siegel@northwestern.edu.
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J. Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran JM. Current perspectives on inner ear toxicity. Otolaryngol. Head. Neck. Surg. 1995;112:133–144. doi: 10.1016/S0194-59989570313-6. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Henderson D, Gratton MA, Danielson RW, Byrne CD. Synergistic interactions of noise and other ototraumatic agents. Ear. Hear. 1987;8:192–212. doi: 10.1097/00003446-198708000-00003. [DOI] [PubMed] [Google Scholar]
- Bohne BA. Mechanisms of noise damage in the inner ear. In: Henderson D, Hamernik RP, Dosanjh DS, Mills JH, editors. Effects of Noise on Hearing. New York: Raven Press; 1976. pp. 41–68. [Google Scholar]
- Bohne BA, Harding GW, Lee SC. Death pathways in noise damaged outer hair cells. Hear. Res. 2007;223:61–70. doi: 10.1016/j.heares.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Cardoso CM, Custodio JB, Almeida LM, Moreno AJ. Mechanisms of the deleterious effects of tamoxifen on mitochondrial respiration rate and phosphorylation efficiency. Toxicol. Appl. Pharmacol. 2001;176:145–152. doi: 10.1006/taap.2001.9265. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Tahera Y, Canlon B. Functional responses of estrogen receptors in the male and female auditory system. Hear. Res. 2009;252:71–78. doi: 10.1016/j.heares.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Chiodo AA, Alberti PW. Experimental, clinical and preventive aspects of ototoxicity. Eur. Arch. Otorhinolaryngol. 1994;251:375–392. doi: 10.1007/BF00181963. [DOI] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Acoustic trauma: single neuron basis for the "half-octave shift". J. Acoust. Soc Am. 1981;70:707–711. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- Cruz Silva MM, Madeira VM, Almeida LM, Custodio JB. Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim. Biophys. Acta. 2000;1464:49–61. doi: 10.1016/s0005-2736(99)00237-0. [DOI] [PubMed] [Google Scholar]
- Custodio JB, Moreno AJ, Wallace KB. Tamoxifen inhibits induction of the mitochondrial permeability transition by Ca2+ and inorganic phosphate. Toxicol. Appl. Pharmacol. 1998;152:10–17. doi: 10.1006/taap.1998.8510. [DOI] [PubMed] [Google Scholar]
- Dallos P, Schoeny ZG, Worthington DW, Cheatham MA. Some problems in the measurement of cochlear distortion. J. Acoust. Soc. Am. 1969;46:356–361. doi: 10.1121/1.1911697. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Duan DD. The ClC-3 chloride channels in cardiovascular disease. Acta. Pharmacol. Sin. 2011;32:675–684. doi: 10.1038/aps.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta. Otolaryngol. 2007;127:149–155. doi: 10.1080/00016480600794446. [DOI] [PubMed] [Google Scholar]
- Jenkins V, Low R, Mitra S. Hearing sensitivity in women following chemotherapy treatment for breast cancer: results from a pilot study. Breast. 2009;18:279–283. doi: 10.1016/j.breast.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Jordan VC. Chemoprevention with antiestrogens: the beginning of the end for breast cancer. Daniel G. Miller Lecture. Ann. N Y. Acad. Sci. 2001;952:60–72. doi: 10.1111/j.1749-6632.2001.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erkan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. Am. J. Obstet. Gynecol. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–477. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- Marques DM, Clark CS, Hawkins JE., Jr Potentiation of ototoxic injury by noise and ototoxic antibiotic in guinea pigs. J. Acoust. Soc. Am. 1975;57 S1(Abstract). [Google Scholar]
- Mattsson JL. Ototoxicity: an argument for evaluation of the cochlea in safety testing in animals. Toxicol. Pathol. 2000;28:137–141. doi: 10.1177/019262330002800117. [DOI] [PubMed] [Google Scholar]
- McClay EF, McClay MT, Monroe L, Jones JA, Winski PJ. A phase II study of high dose tamoxifen and weekly cisplatin in patients with metastatic melanoma. Melanoma. Res. 2001;11:309–313. doi: 10.1097/00008390-200106000-00014. [DOI] [PubMed] [Google Scholar]
- McFadden . In: The curious half octave shift: Evidence for a basalward migration of the traveling wave envelope with increasing intensity. In basic and applied aspects of noise induced hearing loss. Salvi RJ, Henderson D, Hamernik RP, Colletti V, editors. NATO ASI Series; 1985. [Google Scholar]
- Meltser I, Tahera Y, Simpson E, Hultcrantz M, Charitidi K, Gustafsson JA, Canlon B. Estrogen receptor beta protects against acoustic trauma in mice. J. Clin. Invest. 2008;118:1563–1570. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK, Kim J, Potashner SJ, Bohne BA. Long-term degeneration in the cochlear nerve and cochlear nucleus of the adult chinchilla following acoustic overstimulation. Microsc. Res. Tech. 1998;41:205–216. doi: 10.1002/(SICI)1097-0029(19980501)41:3<205::AID-JEMT4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Motohashi R, Takumida M, Shimizu A, Konomi U, Fujita K, Hirakawa K, Suzuki M, Anniko M. Effects of age and sex on the expression of estrogen receptor alpha and beta in the mouse inner ear. Acta. Otolaryngol. 130:204–214. doi: 10.3109/00016480903016570. [DOI] [PubMed] [Google Scholar]
- Neely ST, Liu Z. Tech Memo No. 17. Omaha: Boys Town National Research Hospital; 1993. EMAV: Otoacoustic emission averager,”. [Google Scholar]
- Ohlemiller KK, Siegel JH. The effects of moderate cooling on gross cochlear potentials in the gerbil: basal and apical differences. Hear. Res. 1992;63:79–89. doi: 10.1016/0378-5955(92)90076-y. [DOI] [PubMed] [Google Scholar]
- Ou HC, Bohne BA, Harding GW. Noise damage in the C57BL/CBA mouse cochlea. Hear. Res. 2000;145:111–122. doi: 10.1016/s0378-5955(00)00081-2. [DOI] [PubMed] [Google Scholar]
- Overstreet EH, 3rd, Richter CP, Temchin AN, Cheatham MA, Ruggero MA. High-frequency sensitivity of the mature gerbil cochlea and its development. Audiol. Neurootol. 2003;8:19–27. doi: 10.1159/000067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce M, Richter CP, Cheatham MA. A reconsideration of sound calibration in the mouse. J. Neurosci. Methods. 2001;106:57–67. doi: 10.1016/s0165-0270(01)00329-6. [DOI] [PubMed] [Google Scholar]
- Perez EA, Gandara DR, Edelman MJ, O'Donnell R, Lauder IJ, DeGregorio M. Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer. Invest. 2003;21:1–6. doi: 10.1081/cnv-120016397. [DOI] [PubMed] [Google Scholar]
- Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug. Metab. Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim. Biophys. Acta. 2003;1618:153–162. doi: 10.1016/j.bbamem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Acoustic distortion in the ear canal. I. Cubic difference tones: effects of acute noise injury. J. Acoust. Soc. Am. 1986;79:1481–1490. doi: 10.1121/1.393675. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology. 1997;138:5476–5484. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Sikka R, Zeddies DG, Gong Q. Intact explanted adult gerbil cochleae maintained at body temperature. Assoc. Res. Otolaryngol. 2001 Abs #21966. (Abstract) [Google Scholar]
- Silva I, Mello LE, Freymuller E, Haidar MA, Baracat EC. Estrogen, progestogen and tamoxifen increase synaptic density of the hippocampus of ovariectomized rats. Neurosci. Lett. 2000;291:183–186. doi: 10.1016/s0304-3940(00)01410-5. [DOI] [PubMed] [Google Scholar]
- Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, Gustafsson JA, Hultcrantz M. Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J. Endocrinol. 2009;201:397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta. Otolaryngol. 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Stenberg AE, Wang H, Fish J, 3rd, Schrott-Fischer A, Sahlin L, Hultcrantz M. Estrogen receptors in the normal adult and developing human inner ear and in Turner's syndrome. Hear. Res. 2001;157:87–92. doi: 10.1016/s0378-5955(01)00280-5. [DOI] [PubMed] [Google Scholar]
- Strachan D. Sudden sensorineural deafness and hormone replacement therapy. J. Laryngol. Otol. 1996;110:1148–1150. doi: 10.1017/s0022215100135984. [DOI] [PubMed] [Google Scholar]
- Thompson SK, Zhu X, Frisina RD. Estrogen blockade reduces auditory feedback in CBA mice. Otolaryngol. Head. Neck. Surg. 2006;135:100–105. doi: 10.1016/j.otohns.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Nuttall AL. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear. Res. 1987;27:1–10. doi: 10.1016/0378-5955(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Nuttall AL. Alterations in oxygenation of cochlear endolymph during loud sound exposure. Acta. Otolaryngol. 1989;107:71–79. doi: 10.3109/00016488909127481. [DOI] [PubMed] [Google Scholar]
- Trump DL, Smith DC, Ellis PG, Rogers MP, Schold SC, Winer EP, Panella TJ, Jordan VC, Fine RL. High-dose oral tamoxifen, a potential multidrug-resistance-reversal agent: phase I trial in combination with vinblastine. J. Natl. Cancer. Inst. 1992;84:1811–1816. doi: 10.1093/jnci/84.23.1811. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41 Suppl 6:S30–S35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Yuan H, Bowlby DA, Brown TJ, Hochberg RB, MacLusky NJ. Distribution of occupied and unoccupied estrogen receptors in the rat brain: effects of physiological gonadal steroid exposure. Endocrinology. 1995;136:96–105. doi: 10.1210/endo.136.1.7828562. [DOI] [PubMed] [Google Scholar]