Abstract
Overdose of acetaminophen (APAP), the active ingredient of Tylenol, is the leading cause of drug-induced acute liver failure in the US. As such, it is necessary to develop novel strategies to prevent or manage APAP toxicity. In this report, we revealed a novel function of the liver X receptor (LXR) in preventing APAP-induced hepatotoxicity. Activation of LXR in transgenic mice or by an LXR agonist conferred resistance to the hepatotoxicity of APAP, whereas the effect of LXR agonist on APAP toxicity was abolished in LXR deficient mice. The increased APAP resistance in LXR transgenic mice was associated with increased APAP clearance, increased APAP sulfation, and decreased formation of toxic APAP metabolites. The hepatoprotective effect of LXR may have resulted from the induction of anti-toxic Phase II conjugating enzymes such as Gst and Sult2a1, as well as suppression of pro-toxic Phase I P450 enzymes, such as Cyp3a11 and Cyp2e1. Promoter analysis suggested the mouse Gst isoforms as novel transcriptional targets of LXR. The suppression of Cyp3a11 may be accounted by the inhibitory effect of LXR on PXR-responsive transactivation of Cyp3a11. The protective effect of LXR in preventing APAP toxicity is opposite to the sensitizing effect of pregnane X receptor (PXR), constitutive androstane receptor (CAR), and retinoid X receptor α (RXRα). We conclude that LXR represents a potential therapeutic target for the prevention and treatment of Tylenol toxicity.
Keywords: nuclear receptor, gene regulation, transgenic mice, acetaminophen, xenobiotic detoxification
Introduction
Overdose of the analgesic and antipyretic acetaminophen (APAP) is the leading cause of drug-induced acute liver failure (1). APAP usually is well tolerated at recommended therapeutic doses and the majority of APAP is rapidly metabolized by the Phase II conjugating enzymes UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT) in the liver to nontoxic compounds (2), which is followed by renal and biliary excretion. Another metabolic pathway is bioactivation by Phase I cytochrome P450 enzymes to the highly reactive intermediate metabolite N-acetyl-p-benzoquinone-imine (NAPQI) (3). NAPQI has a short half-life under normal conditions and is eliminated by conjugation with glutathione (GSH), a reaction carried out by glutathione S-transferase (GST), and then further metabolized to a mercapturic acid and excreted into the urine (4). In the event of APAP overdose, the glucuronidation and sulfation pathways become saturated, and increasing amounts of APAP undergo P450-mediated formation of NAPQI, as well as depletion of GSH (5). Accumulated NAPQI then binds to cellular macromolecules, leading to structural and metabolic disarray of the cells (6). Furthermore, depletion of intracellular GSH renders the hepatocytes highly susceptible to oxidative stress and apoptosis.
CYP1A2, CYP2E1 and CYP3A are the most active P450s that convert APAP to NAPQI (7). Treatment with Cyp1a2 inducers increased APAP hepatotoxicity in rodents (8). Cyp2e1 was found to activate APAP to NAPQI. The implication of Cyp2e1 in APAP toxicity is consistent with the observation that alcohol sensitized rats to fatal APAP hepatotoxicity by inducing Cyp2e1 (9). In contrast, inhibition of Cyp2e1 by propylene glycol prevented APAP hepatotoxicity in mice. Cyp2e1 null mice were markedly resistant to APAP-induced lethality (10), and double null mice lacking both Cyp1a2 and Cyp2e1 were largely resistant to APAP toxicity (11). Inducers of CYP3A potentiated, whereas inhibitors of CYP3A prevented, APAP toxicity (12, 13). For these reasons, it was proposed that inhibitors of P450 enzymes may be of therapeutic value for the treatment of APAP hepatotoxicity (14).
At subtoxic doses, NAPQI is inactivated by GST-mediated GSH conjugation, leading to the conversions of NAPQI to APAP cysteine and mercapturate conjugates (4). Treatment of rodents with Oltipraz, a GST inducer, was linked to chemopreventive effects against APAP toxicity (15). Among GST isozymes, GST Pi was thought to be particularly important to detoxify NAPQI based on in vitro conjugation assays (16). However, mice deficient of Gstπ showed a surprisingly increased resistance to APAP hepatotoxicity (17), indicating that Gstπ may not contribute to the formation of GSH conjugates of NAPQI in vivo and could enhance APAP toxicity by accelerating the depletion of GSH. These data suggest that suppression of Gstπ and/or induction of other Gst enzymes may protect mice from APAP-induced hepatotoxicity.
The liver X receptors LXRα and LXRβ were isolated as sterol sensors (18, 19). Subsequent characterization revealed that LXRs have diverse physiological functions, ranging from cholesterol (18) and lipid metabolism (20) to anti-inflammation (21), hepatobiliary diseases (22, 23), and steroid hormone homeostasis (24, 25). We have previously reported that the expression of APAP-detoxifying Sult2a1/2a9 was positively regulated, whereas the expression of pro-toxic Cyp3a11 was reduced in LXR-activated mice (22). We thus hypothesized that LXR may affect APAP toxicity by regulating the APAP-metabolizing enzymes.
In this study, we showed that activation of LXR relieved APAP-induced hepatotoxicity. The benefits of LXR in preventing APAP toxicity may have resulted from a pattern of metabolic gene regulation that favored a decreased exposure of the host to the parent APAP as well as the toxic APAP metabolites.
Materials and Methods
Animals
The creation of Fabp-VP-LXRα (22) transgenic (Tg) mice and PXR−/− mice (26) was described. The LXRα and β double knockout (LXR DKO) mice (27) were kindly provided by Dr. David Mangelsdorf. The use of mice in this study has complied with all relevant federal guidelines and institutional policies.
APAP Dosing and Hepatotoxicity Analysis
Mice were fasted overnight before being given an oral administration of APAP (freshly prepared in 0.5% methyl cellulose) at 200 mg/kg. When necessary, wild-type (Wt), LXR DKO and PXR−/− mice were dosed with the LXR agonist TO1317 (10 mg/kg, i.p.) or vehicle for one week prior to APAP treatment. Mice were sacrificed 24 h after APAP administration and blood and liver tissues were harvested for histology by H&E staining and biochemical analysis by ANTECH Diagnostic (Lake Success, NY).
Northern Blot and Real-Time RT-PCR Analyses
Total RNA was isolated using the TRIZOL Reagent. Northern hybridization was carried out as described (25). In real-time RT-PCR analysis, RT was performed with the random hexamer primers and the Superscript RT III enzyme. SYBR Green-based real-time PCR was performed with the ABI 7300 Real-Time PCR System. Data were normalized against the control of cyclophilin signals. The sequences of real-time PCR primers are listed in Supplementary Table 1.
APAP Pharmacokinetic and Metabolic Analyses
Measurement of GST Activity
The total GST activity was measured using CDNB as a substrate as described (28). Briefly, GSH (1 mM final) was added to GST (0.5–5μg enzyme) in 1 ml of 100 mM potassium phosphate buffer (pH 6.5). After pre-incubation for 3 min, CDNB (1 mM final) was added, and the activity was measured using a spectrophotometer at 25 °C. The increase of absorbance resulting from conjugation of dinitrophenyl with glutathione was recorded at 340 nm every 15 s for 3 min.
Cloning of Mouse Gst Promoters and Luciferase Reporter Gene Assay
The 2.2-kb mouse Gstμ1 promoter was cloned by PCR using the following primer pair: Gstμ1-p5: 5′-ATCGACGCGTCCTCCAGACCCCAGCTAACTGTG-3′, and Gstμ1-p3: 5′-ACCGCTCGAGCTGTGGTCTTCTCAAACTGGCTTCAG-3′. The PCR products were digested with MluI and XhoI, and cloned into the same enzyme digested pGL3-Basic vector from Promega. The 1.9-kb mouse Gstπ1 promoter was cloned by PCR using the following primer pair: Gstπ1-pF: 5′-ACGGGGTACCACCCCAACTCTCTCATACACAC-3′, and Gstπ1-pR: 5′-ACCGCTCGAGTAGACAGAGGGGTACTCAGAGTG-3′. The PCR products were digested with Asp718 and XhoI, and cloned into the same enzyme digested pGL3-Basic vector. To generate the tk-Gstμ1/DR4 reporter, two copies of the sequence 5′-TGTCCTaggaTGAGATggccTCGGCT-3′, or its mutant variant 5′-GGGCCCaggaAGATTTggccTCGGCTT-3′, were synthesized and cloned into the tk-Luc reporter gene. The tk-Cyp3a11 report gene contains three copies of the DR-3 type PXRE (TGAACTataCGAACT) found in the mouse Cyp3a11 gene promoter. HepG2 cells were transfected on 48-well plates as described (29). When applicable, transfected cells were treated with drugs for 24 h before harvesting for luciferase and β-gal assays. The transfection efficiency was normalized against the β-gal activity.
Statistical Analysis
Results are expressed as means ± SD. Statistical analysis was performed using the unpaired student’s t test for the comparison between two groups.
Results
Activation of LXR in transgenic (Tg) mice conferred resistance to APAP hepatotoxicity
We have previously reported the creation of Tg mice that express the activated LXRα (Fabp-VP-LXRα) in the liver (Fig. 1A, and ref 22). Created by fusing the viral protein (VP) 16 activation domain of the herpes simplex virus to the amino-terminal of LXRα, VP-LXRα shares the same DNA binding specificity as its wild type counterpart and constitutively activates LXR target gene expression (22). Gene expression analysis revealed that activation of LXR in the liver regulated the expression of multiple drug-metabolizing enzymes and transporters (22), which prompted us to examine whether Tg mice had an altered sensitivity to APAP. In vehicle-treated mice, no significant alteration in the liver histology was found in Tg mice when compared to Wt mice (Fig. 1B). Following APAP treatment, the Wt liver showed expected typical necrotic liver damage. In contrast, Tg mice showed little signs of liver damage (Fig. 1C), suggesting that this genetic activation of LXR conferred resistance to APAP hepatotoxicity.
Fig. 1. Activation of LXR in transgenic mice conferred resistance to APAP hepatotoxicity.
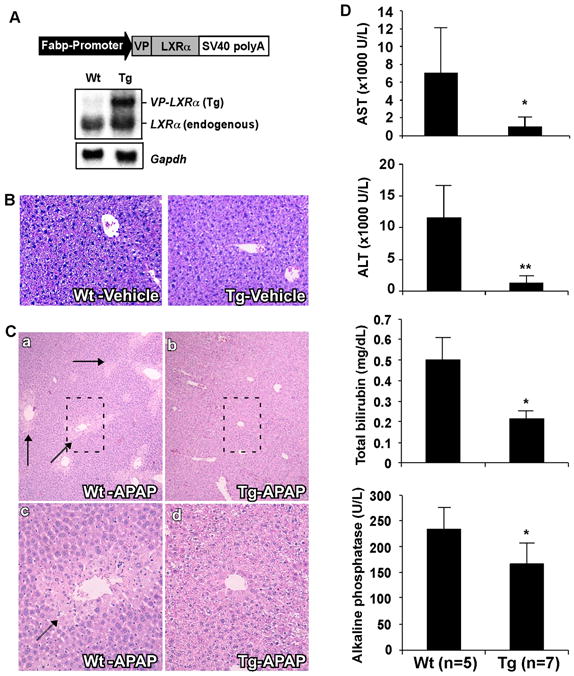
(A) Schematic representation of the Fabp-VP-LXRα transgene and confirmation of transgene expression by Northern blot analysis. Membrane was probed with a LXRα cDNA probe. Each lane represents RNA samples pooled from five mice of the same genotype. Fabp, rat fatty acid binding protein; SV40, SV40 poly(A) sequences; Tg, transgenic; VP, viral protein 16; Wt, wild type. (B) Representative H&E staining on liver paraffin sections of the vehicle-treated mice. (C) Representative H&E staining on liver paraffin sections of APAP-treated mice. APAP-induced centrilobular necrosis is indicated by arrows. C-c and C-d are higher magnifications of the framed fields in C-a and C-b, respectively. (D) Serum levels of AST, ALT, total bilirubin, and alkaline phosphatase in mice treated with APAP. *, P<0.05; **, P<0.01.
Consistent with their reduced histological liver damage, APAP-treated Tg mice showed improved serum chemistry compared to their Wt counterparts. These included lower serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities, total bilirubin, and alkaline phosphatase (Fig. 1D). The vehicle-treated Tg mice showed little signs of hepatotoxicity as judged by serum chemistry (Supplementary Fig. 1).
Treatment with the LXR agonist TO1317 relieved mice from APAP toxicity in an LXR-dependent manner
The hepatoprotective effect of the LXRα transgene prompted us to determine whether a pharmacological activation of LXR will have a similar effect in preventing APAP toxicity. Indeed, the TO1317-treated Wt C57BL/6J mice showed less histological liver damage (Fig. 2A) and lower serum level of AST and ALT (Fig. 2B) compared to their vehicle-treated counterparts.
Fig. 2. Treatment with the LXR agonist TO1317 relieved mice from APAP toxicity in an LXR-dependent manner.
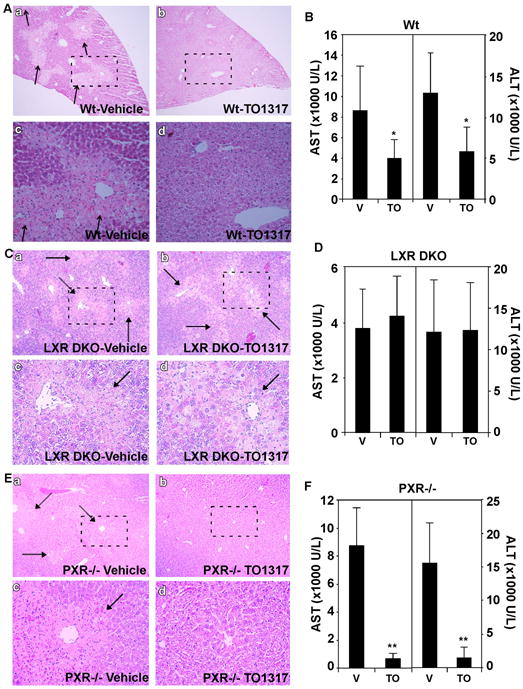
(A and B) Wt mice were pretreated with vehicle or TO1317 before receiving a gavage of APAP (200 mg/kg). Mice were sacrificed 24 h after drug administration and analyzed for histology (A) and serum chemistry (B). A-c and A-d are higher magnifications of the framed fields of A-a and A-b, respectively. (C and D) The same experiments as in (A and B), except that LXR α and β double knockout (LXR DKO) mice were used. B-c and B-d are higher magnifications of the framed fields of B-a and B-b, respectively. (E and F) The same experiments as in (A and B), except that PXR−/− mice were used. C-c and C-d are higher magnifications of the framed fields of C-a and C-b, respectively. APAP-induced centrilobular necrosis in (A, C, and E) is indicated by arrows. *, P<0.05; **, P<0.01. Each group contains 5–7 mice.
We then used LXR DKO mice to determine whether the hepatoprotective effect of TO1317 was LXR-dependent. The protective effect of TO1317 was abolished in LXR DKO mice, as indicated by the lack of relief in histological liver damage (Fig. 2C) as well as the serum levels of AST and ALT (Fig. 2D) in APAP- and TO1317-treated LXR DKO mice. These results demonstrated that the APAP protective effect of TO1317 required LXRs.
Because TO1317 was also reported to activate PXR (30), we then used PXR−/− mice to determine whether the hepatoprotective effect of TO1317 required PXR. In this experiment, PXR−/− mice were treated with APAP in the presence or absence of TO1317. The hepatic necrosis (Fig. 2E) and serum levels of AST and ALT (Fig. 2F) were both decreased in TO1317-treated PXR−/− mice compared to their vehicle-treated counterparts, suggesting that the protective effect of TO1317 was independent of PXR.
Activation of LXR in transgenic mice increased APAP clearance and decreased the formation of toxic APAP metabolites
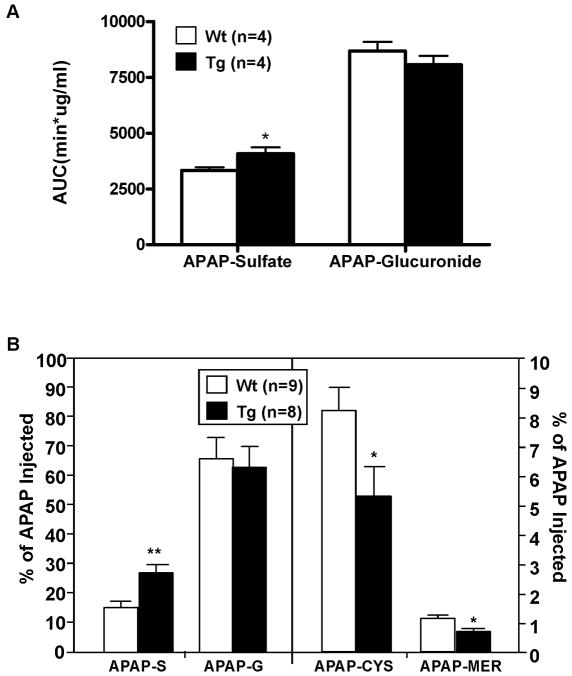
The resistance to APAP toxicity in Tg mice suggested that activation of LXR may promote APAP clearance and/or inhibit the formation of toxicity-indicating metabolites. To test this hypothesis, we examined the in vivo metabolism of APAP. Mice were given a single i.p. injection of APAP and collected for blood or urine. The pharmacokinetic estimations for the serum level of APAP, and APAP-sulfate and APAP-glucuronide are summarized in Table 1 and Fig. 3A, respectively. The decrease in area under curve (AUC), increase in clearance (CL), and decrease in half-life (T1/2) of parent APAP in Tg mice (Table 1) suggested that activation of LXR reduced the animal’s total exposure to the parent drug, which was associated with an increased production of APAP-sulfate (Fig. 3A). The glucuronide metabolite of APAP was unchanged. When the urinary levels of APAP metabolites were measured, we found that the level of APAP-sulfate was increased, whereas the level of APAP-glucuronide was unchanged in Tg mice (Fig. 3B). The urinary concentrations of APAP-cysteine and APAP-mercapulate, two APAP metabolites that indicate the formation of toxic metabolites, were decreased in Tg mice (Fig. 3B).
Table 1.
Pharmacokinetic estimation for the serum level of parent APAP
| Parameters | Values (Mean ± SD) | |
|---|---|---|
| Wt | Tg | |
| AUC (min*ug/ml) | 5121.3 ± 748.8 | 3250.9 ± 786.0** |
| Cls (ml/min) | 0.28 ± 0.04 | 0.47 ± 0.12* |
| t1/2 (min) | 109.2 ± 27.8 | 58.9 ± 17.9* |
, P<0.05,
, P<0.01. N=4 for each group.
Fig. 3. Activation of LXR in transgenic mice increased the serum and urinary levels of APAP-sulfate (APAP-S) and decreased formation of APAP-cysteine (APAP-CYS) and APAP-mercapulate (APAP-MER).
(A) The AUC of serum APAP-Sulfate, but not APAP-Glucuronide, was increased in Tg mice. (B) The urinary levels of APAP-G and APAP-S (left panel), and APAP-CYS and APAP-MER (right panel). Mice received a single i.p. dose of APAP (50 mg/kg) before 24-h urine collection. *, P<0.05; **, P<0.01.
Regulation of APAP metabolizing enzymes by LXR
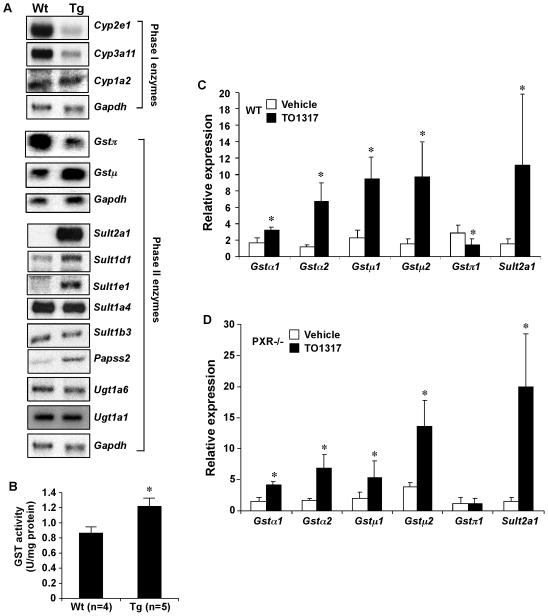
To understand the mechanism by which activation of LXR relieved APAP toxicity, we measured the mRNA expression of major APAP-metabolizing enzymes in Wt and Tg mice. As shown in Fig. 4A, among Phase I enzymes known to facilitate the formation of toxic APAP metabolites, the expression of Cyp3a11 and 2e1 was reduced, whereas the expression of Cyp1a2 remained largely unchanged in Tg mice as determined by Northern blot analysis. The same pattern of P450 regulation was confirmed by real-time PCR analysis (Supplementary Fig. 2). The inhibition of Cyp3a11 and 2e1 was consistent with the decreased formation of toxic APAP metabolites in Tg mice.
Fig. 4. Regulation of APAP metabolizing enzymes by LXR.
(A) The hepatic expression of Phase I and Phase II enzyme genes was measured by Northern blot analysis. Each lane represents RNA samples pooled from five mice. (B) Measurement of total GST activity. (C and D) The expression of Gst and Sult2a1 in the liver of vehicle- or TO1317-treated Wt (C) and PXR−/− (D) mice was measured by real-time PCR analysis. *, P<0.05; **, P<0.01.
Among Phase II enzymes, the expression of Gstπ and Gstμ was decreased and increased, respectively (Fig. 4A). The expression of Sult2a1 was induced as expected (26). Among other Sult enzymes, the expression of Sult1d1 and Sult1e1 was also induced, whereas the expression of Sult1a4 and 1b3 was unchanged. Papss2, the primary hepatic enzyme that catalyzes the formation of the sulfonyl group donor PAPS, was also induced (Fig. 4A). The expression of Ugt1a1 and 1a6 was unaffected (Fig. 4A), consistent with the observation that the APAP-glucuronide level was unchanged in Tg mice. Consistent with the pattern of Gst and Sult gene regulation, the liver extract of Tg mice showed increased enzymatic activities of Gst (Fig. 4B) and Sult (data not shown and ref #22).
The regulation of Gst and Sult2a1 was confirmed in Wt mice treated with TO1317. As shown in Fig. 4C, the expression of Gst α1, α2, μ1 and μ2 and Sult2a1 was increased, whereas the expression of Gstπ was decreased in TO1317-treated Wt mice. The TO1317 effect on the expression of Gst and Sult2a1 was independent of PXR, because a similar pattern of gene regulation was observed in TO1317-treated PXR−/− mice (Fig. 4D).
The mouse Gst gene promoters are likely transcriptional targets of LXR
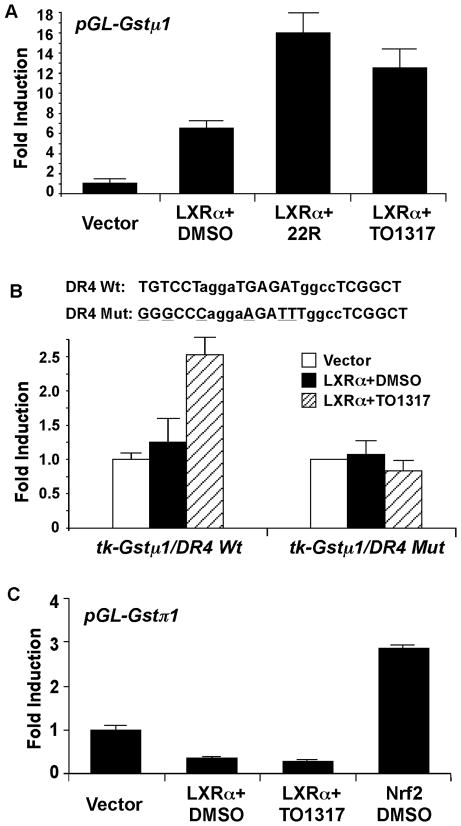
To understand the mechanism by which LXR regulate Gst, we cloned the mouse Gstμ1 and Gstπ1 gene promoters and characterized their regulation by LXR. As shown in Fig. 5A, the 2.2-kb Gstμ1 promoter report gene pGL-Gstμ1 was activated by the co-transfection of LXRα, and this activation was enhanced by the addition of LXR agonist 22(R)-hydroxycholesterol or GW3965. Inspection of the Gstμ1 gene promoter revealed several putative DR-4 type LXR response elements. A synthetic reporter tk-Gstμ1/DR4 that contained two copies of two overlapping DR-4 sites were activated by the co-transfected LXRα in a ligand-dependent manner, whereas the transactivation was abolished when both DR-4s were mutated (Fig. 5B). In contrast, the 1.9-kb Gstπ1 promoter report gene pGL-Gstπ1 was suppressed by the co-transfection of LXRα (Fig. 5C). The same Gstπ1 reporter gene was activated by the co-transfection of Nrf2, a known positive regulator of Gstπ (31).
Fig. 5. The mouse Gst gene promoters are likely transcriptional targets of LXR.
(A) The mouse Gstμ1 promoter reporter gene (pGL-Gstμ1) was transfected into HepG2 cells together with the indicated empty vector or LXRα expression vector. Transfected cells were treated with 22(R)-hydroxycholesterol (22R) or TO1317 (10 μM each) for 24 h before luciferase assay. The transfection efficiency was normalized against the β-gal activity from the co-transfected CMX-β gal vector. Results shown are fold induction over vector control and represent the averages and standard deviation from triplicate assays. (B) Same transfections in (A) were performed except that the synthetic tk-Gstμ1/DR4 reporter gene and its mutant variant were used. The reporter genes contained two copies of the two overlapping DR4s whose sequences are labeled. The mutant sequences are also labeled with the mutated nucleotides underlined. (C) The mouse Gstπ1 promoter reporter gene (pGL-Gstπ1) was transfected into HepG2 cells together with the indicated empty vector, LXRα expression vector, or Nrf1 expression vector. Transfected cells were treated with indicated drugs for 24 h before luciferase assay.
LXRα inhibited the transcriptional activity of PXR
To understand the mechanism by which LXR suppressed Cyp3a11 gene expression, we used transient transfection and reporter gene assay to determine whether LXRα can inhibit the transcriptional activity of PXR, the primary transcriptional regulator of Cyp3a11 (26). As shown in Fig. 6, co-transfection of LXRα inhibited the PXR ligand pregnenolone-16α-carbonitrile (PCN) induced activity of PXR on tk-Cyp3a11, a reporter gene that contains the PXR response element found in the Cyp3a11 gene promoter. LXRα alone had little effect on the reporter gene activity regardless of the treatment of GW3965. These results provided a plausible mechanism by which LXR suppressed the expression of Cyp3a11.
Fig. 6. LXRα inhibited the transcriptional activity of PXR.
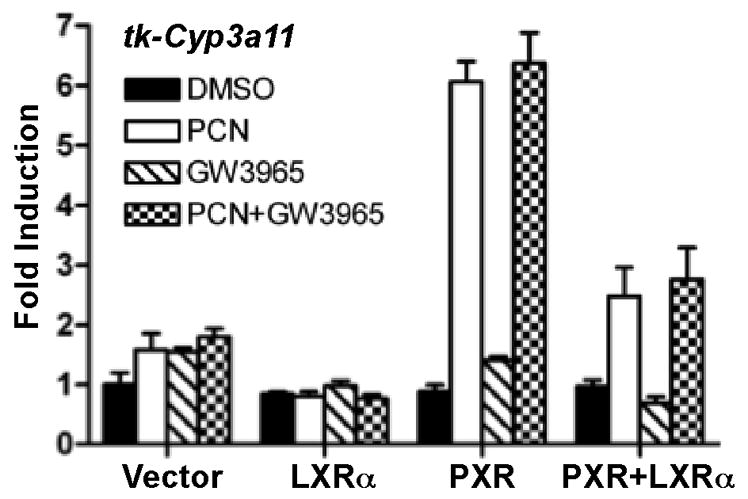
The Cyp3a11 reporter gene (tk-Cyp3a11) was transfected into HepG2 cells together with PXR and/or LXRα expression vectors. Transfected cells were treated with PCN or GW3965 (10 μM each) for 24 h before luciferase assay. The transfection efficiency was normalized against the β-gal activity from the co-transfected CMX-β gal vector.
Discussion
Mounting evidence has suggested that several liver-enriched nuclear receptors, including CAR (32), PXR (12, 33), RXRα (34) and FXR (35), play pivotal roles in APAP metabolism and toxicity. The nuclear receptor effects on APAP toxicity and their proposed mechanisms are summarized in Table 2. Activation of CAR or PXR was shown to heighten APAP hepatotoxicity. Treatment of mice with the CAR activator phenobarbital induced the expression of Cyp1a2 and Cyp3a11 and resulted in increased sensitivity to APAP (32). In contrast, administration of androstanol, a CAR antagonist, blocked hepatotoxicity in Wt mice. CAR−/− mice were resistant to APAP toxicity (32). Pre-treatment with PCN, a potent PXR agonist, enhanced APAP hepatotoxicity in Wt but not in PXR−/− mice (33). The heightened sensitivity in PCN-treated Wt mice was reasoned to be due to the induction of Cyp3a enzymes. In contrast, following PCN treatment, PXR−/− mice had lower Cyp3a11 expression, decreased NAPQI formation, and increased maintenance of hepatic GSH content (33). Using mice humanized for both PXR and CYP3A4, Cheng and colleagues showed that rifampicin-activated hPXR and CYP3A4 induction enhanced APAP toxicity (12), suggesting the human relevance of this effect. Loss of RXRα, the shared heterodimerization partner of CAR and PXR, protected mice from APAP toxicity primarily by regulating the expression of Gst enzymes (34).
Table 2.
Summary of nuclear receptor effects on APAP toxicity
| Effect on APAP toxicity when activated | Proposed mechanisms | References | |
|---|---|---|---|
| CAR | Sensitization | (1) Induction of Phase I enzymes Cyp1a2 and Cyp3a11, which can convert APAP to cytotoxic NAPQI; (2) Induction of Phase II enzyme Gstπ, which may deplete GSH used for NAPQI detoxification. | 32 |
| PXR | Sensitization | (1) Induction of Cyp3a and Cyp1a2; (2) Enhanced depletion of GSH. | 12, 33 |
| RXRα | Sensitization | Regulation of Gst expression and APAP-GSH conjugation. | 34 |
| FXR | Protection | (1) Induction of the Gstα class; (2) Induction of Gclm and Gpx1 that are involved in GSH homeostasis. | 35 |
| LXR | Protection | (1) Suppression of Cyp3a11 and Cyp2e1; (2) Induction of the detoxifying Phase II enzyme Sult2a1; (3) Isoform-specific Gst regulations that include induction of Gstα and Gstμ, and suppression of Gstπ. (4) Increased APAP clearance and decreased the formation of toxic APAP metabolites. | This study |
Our current results showed that unlike CAR and PXR, activation of LXR was beneficial in relieving APAP hepatotoxicity. The hepatoprotective effect of LXR may have resulted from the combined suppression of pro-toxic P450s and induction of anti-toxic Phase II enzymes Gst and Sult. The suppression of Cyp3a11 by LXR was opposite to the induction of the same enzyme in CAR/PXR-activated mice (32, 33). The induction of Cyp1a2, observed in CAR/PXR-activated mice (32, 33), was absent in LXR Tg mice. The suppression of Cyp3a by LXR was previously reported (22), which was proposed to be due to the cross-suppression of CAR by LXR (36). We now provide evidence suggesting that LXR may also suppress Cyp3a11 by antagonizing the positive regulation of Cyp3a11 by PXR. The suppression of Cyp2e1 by LXR has not been reported. Cyp2e1 is better known for its post-transcriptional regulation. LXR has recently been shown to regulate the E3 ubiquitin ligase inducible degrader of the LDLR (Idol) (37). It remains to be determined whether LXR can regulate the expression or activity of Cyp2e1 through a post-transcriptional mechanism.
Among the LXR responsive Phase II enzymes, the activation of Sult2a1 gene expression and lack of Ugt1a1 regulation by LXR have been reported (22). The isoform-specific regulation of Gst was intriguing. We reasoned the combined induction of Gstα and Gstμ classes and suppression of Gstπ may have contributed to the hepatoprotective role of LXR. The suppression of Gstπ in LXR-activated mice was consistent with the previous report that mice that lacked Gstπ were resistant to APAP hepatotoxicity (17). In contrast, an induction of Gstπ in CAR-activated mice was associated with the sensitizing effect (32). Our promoter analysis suggested that Gstμ1 and Gstπ1 gene promoters were positively and negatively regulated by LXR, respectively. The induction of Gstα and Gstμ isoforms was reminiscent of the effect of FXR, whose activation has recently been linked to protection against APAP-induced hepatic toxicity (35).
In summary, the current study demonstrated that LXR may represent a potential therapeutic target for the prevention and treatment of APAP overdoses via induction of APAP-detoxifying/clearance enzymes and suppression of pro-toxic P450 enzymes.
Supplementary Material
Supplementary Figure 1. Unchallenged VP-LXRα transgenic mice did not show liver toxicity.
Supplementary Figure 2. Regulation of APAP metabolizing P450 enzymes in VP-LXRα transgenic mice.
Supplementary Table 1. List of real-time PCR primers.
Acknowledgments
We thank Dr. David Mangelsdorf for LXR DKO mice and Dr. Song Li for synthesizing TO1317 for us.
Financial support: This work was supported in part by NIH grants DK083952 and ES019629 (to W.X.) and NSFC grant No. 31071027 (to Y.Z.).
Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- APAP-CYS
APAP-cysteine
- APAP-MER
APAP-mercapulate
- APAP-S
APAP-sulfate
- AST
aspartate aminotransferase
- Fabp
fatty acid binding protein
- Gst
glutathione S-transferase
- LXR
liver X receptor
- NAPQI
N-acetyl-p-benzoquinone-imine
- Sult
sulfotransferase
- Ugt
UDP-glucuronosyltransferase
References
- 1.Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol. 2008;24:287–297. doi: 10.1097/MOG.0b013e3282f9764b. [DOI] [PubMed] [Google Scholar]
- 2.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–1506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 3.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckett GJ, Chapman BJ, Dyson EH, Hayes JD. Plasma glutathione S-transferase measurements after paracetamol overdose: evidence for early hepatocellular damage. Gut. 1985;26:26–31. doi: 10.1136/gut.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potter WZ, Thorgeirsson SS, Jollow DJ, Mitchell JR. Acetaminophen-induced hepatic necrosis. V. Correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology. 1974;12:129–143. doi: 10.1159/000136531. [DOI] [PubMed] [Google Scholar]
- 6.Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- 7.Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 8.Kalhorn TF, Lee CA, Slattery JT, Nelson SD. Effect of methylxanthines on acetaminophen hepatotoxicity in various induction states. J Pharmacol Exp Ther. 1990;252:112–116. [PubMed] [Google Scholar]
- 9.McClain CJ, Kromhout JP, Peterson FJ, Holtzman JL. Potentiation of acetaminophen hepatotoxicity by alcohol. JAMA. 1980;244:251–253. [PubMed] [Google Scholar]
- 10.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 11.Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, et al. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 1998;152:193–199. doi: 10.1006/taap.1998.8501. [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, Ma X, Krausz KW, Idle JR, Gonzalez FJ. Rifampicin-activated human PXR and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab Dispos. 2009;37:1611–1621. doi: 10.1124/dmd.109.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostrubsky VE, Szakacs JG, Jeffery EH, Woods SG, Bement WJ, Wrighton SA, Sinclair PR, et al. Protection of ethanol-mediated acetaminophen hepatotoxicity by triacetyloleandomycin, a specific inhibitor of CYP3A. Ann Clin Lab Sci. 1997;27:57–62. [PubMed] [Google Scholar]
- 14.Walubo A, Barr S, Abraham AM, Coetsee C. The role of cytochrome-P450 inhibitors in the prevention of hepatotoxicity after paracetamol overdose in rats. Hum Exp Toxicol. 2004;23:49–54. doi: 10.1191/0960327104ht415oa. [DOI] [PubMed] [Google Scholar]
- 15.Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- 16.Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 1988;264:253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- 17.Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A. 2000;97:12741–12745. doi: 10.1073/pnas.220176997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repa JJ, Mangelsdorf DJ. The liver X receptor gene team: potential new players in atherosclerosis. Nat Med. 2002;8:1243–1248. doi: 10.1038/nm1102-1243. [DOI] [PubMed] [Google Scholar]
- 19.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 20.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uppal H, Saini SP, Moschetta A, Mu Y, Zhou J, Gong H, Zhai Y, et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology. 2007;45:422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- 23.Uppal H, Zhai Y, Gangopadhyay A, Khadem S, Ren S, Moser JA, Xie W. Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology. 2008;47:1331–1342. doi: 10.1002/hep.22175. [DOI] [PubMed] [Google Scholar]
- 24.Cummins CL, Volle DH, Zhang Y, McDonald JG, Sion B, Lefrancois-Martinez AM, Caira F, et al. Liver X receptors regulate adrenal cholesterol balance. J Clin Invest. 2006;116:1902–1912. doi: 10.1172/JCI28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, et al. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol. 2007;21:1781–1790. doi: 10.1210/me.2007-0187. [DOI] [PubMed] [Google Scholar]
- 26.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 27.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 28.Gong H, Singh SV, Singh SP, Mu Y, Lee JH, Saini SP, Toma D, et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol Endocrinol. 2006;20:279–290. doi: 10.1210/me.2005-0205. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Cheng Q, Ou Z, Lee JH, Xu M, Kochhar U, Ren S, et al. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinol. 2010;151:5721–5729. doi: 10.1210/en.2010-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenoy SD, Spencer TA, Mercer-Haines NA, Alipour M, Gargano MD, Runge-Morris M, Kocarek TA. CYP3A induction by liver x receptor ligands in primary cultured rat and mouse hepatocytes is mediated by the pregnane X receptor. Drug Metab Dispos. 2004;32:66–71. doi: 10.1124/dmd.32.1.66. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda H, Serria MS, Kakizaki I, Hatayama I, Satoh K, Tsuchida S, Muramatsu M, et al. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2(NF-E2-related factor 2) and androgen. Biochem J. 2002;364(Pt 2):563–570. doi: 10.1042/BJ20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Huang W, Chua SS, Wei P, Moore DD. Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science. 2002;298:422–424. doi: 10.1126/science.1073502. [DOI] [PubMed] [Google Scholar]
- 33.Guo GL, Moffit JS, Nicol CJ, Ward JM, Aleksunes LA, Slitt AL, Kliewer SA, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci. 2004;82:374–380. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 34.Dai G, Chou N, He L, Gyamfi MA, Mendy AJ, Slitt AL, Klaassen CD, et al. Retinoid X receptor alpha Regulates the expression of glutathione s-transferase genes and modulates acetaminophen-glutathione conjugation in mouse liver. Mol Pharmacol. 2005;68:1590–1596. doi: 10.1124/mol.105.013680. [DOI] [PubMed] [Google Scholar]
- 35.Lee FY, de Aguiar Vallim TQ, Chong HK, Zhang Y, Liu Y, Jones SA, Osborne TF, et al. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Y, Wada T, Zhang B, Khadem S, Ren S, Kuruba R, Li S, et al. A functional crosstalk between LXRa and CAR links lipogenesis and xenobiotic responses. Mol Pharmacol. 2010;78:666–674. doi: 10.1124/mol.110.064618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Unchallenged VP-LXRα transgenic mice did not show liver toxicity.
Supplementary Figure 2. Regulation of APAP metabolizing P450 enzymes in VP-LXRα transgenic mice.
Supplementary Table 1. List of real-time PCR primers.