Abstract
A marked increase in concomitant autoimmune diseases has previously been noted in patients with myasthenia gravis (MG). We show that these diseases occur both before and after the onset of MG and that the process is not influenced by thymectomy.
IgA deficiency (IgAD), which is strongly associated with the same HLA haplotype as early onset MG, has recently been suggested to be an autoimmune disease. However, there was no increase in the prevalence of IgAD in a large cohort of Swedish MG patients.
Keywords: myasthenia gravis, IgA deficiency, concomitant autoimmunity
1. Introduction
Myasthenia gravis (MG) is an antibody mediated autoimmune disorder, characterized by auto-antibodies against the nicotinic acetylcholine receptor (AChR-abs), situated on the muscle end-plate, that impair transmission of nerve impulses to the muscle. MG occurs in approximately 1 in 10,000 individuals in European populations and has a concordance rate of 30–40% in monozygotic twins (Lisak 1994), indicating a strong genetic component. Several genetic loci have previously been shown to be associated with MG, including PTPN22, IL-1, TNF-α, and the MHC, in particular the B8, DR3 haplotype in early onset MG and the B7, DR2 haplotype in late onset MG (Giraud et al. 2008).
MG often occurs concomitantly with other autoimmune disorders, including rheumatoid arthritis (RA) (Christensen et al. 1995), Type I diabetes (T1D) (Toth et al. 2006), thyroid disorders (Graves’ disease) (Christensen et al. 1995);(Sahay et al. 1965);(Kanazawa et al.2007);(Monden et al. 1986);(Marinó et al. 1997) and systemic lupus erythematosus (SLE) (Mevorach et al. 1995a);(Omar et al. 2010);(Hrycek 2009);(Vaiopoulos et al. 1994). In a long term study in Denmark, autoimmune disorders were concomitant with MG in 14% of cases, the most common being RA and thyroid disorders (Christensen et al. 1995). SLE has been reported in over 50 MG cases in the literature (Bhinder et al. 2006). Similar genetic associations, overlapping with other autoimmune conditions, including the HLA-B8, DR3 haplotype and the R620W variant of PTPN22, suggest that common mechanisms may exist which predispose MG patients for additional autoimmune disorders. However, it cannot be excluded that immunosuppressive treatment in MG, including thymectomy, disturbs the balance of immunity, leading to additional disorder(s) (Zonana et al. 2002).
Selective IgA deficiency (IgAD) is defined as serum IgA levels less than 0.07 g/l with normal levels of IgM and IgG. Its prevalence of 1 in 600 in European populations makes it the most common form of primary immunodeficiency (Hammarström & Smith 2007). A high rate of familial incidence and a high relative risk for siblings indicate a strong genetic predisposition. The extended HLA A1, B8, DR3, DQ2 haplotype has been shown to be strongly associated with IgAD, occurring in 45% of patients in Sweden (Mohammadi et al. 2008). This haplotype has also been shown to be associated with several autoimmune diseases, including T1D (Smith et al. 1978), celiac disease (CD) (Congia et al. 1994), SLE (Skarsvåg et al. 1992) and Graves’ disease (Farid et al. 1976), and an overrepresentation of IgAD among patients with these disorders has previously been observed (Ammann & Hong 1971);(Ryser et al. 1988);(Jorgensen et al. 2009). Recently, it has even been suggested that IgAD might itself be autoimmune in nature (Ferreira et al. 2010).
Although MG is associated with the B8, DR3, DQ2 haplotype, previous studies, in small sized materials (<110 patients), have not shown an increase in the prevalence of IgAD in MG patients (Liblau et al. 1992); (Lisak & Zweiman 1976);(Bramis et al. 1976);(Wetherall et al. 1976). The single large scale study published to date screened 333 patients, and found only one to be IgAD (Liblau et al. 1992). However, another study did observe a decrease in serum IgA in 13 of 51 MG patients as compared to 4 of 51 controls (Behan et al. 1976).
In view of the inconclusive data obtained so far, we sought to determine if an increased incidence of IgAD occurs in MG by testing our large cohort of Swedish patients for serum levels of IgA. We furthermore attempted to determine if HLA allele contribution could explain the overlap with IgAD, or lack thereof, by determining HLA alleles in our MG and IgAD patients.
2. Materials and Methods
Patients and controls
Five hundred and forty-seven Swedish Caucasian MG patients were included in the study. The diagnosis of myasthenia gravis was made as described previously (Drachman 1994) and clinical information was documented by the primary physician. Information on concomitant autoimmune disorders was also collected.
Five hundred and thirty-three Swedish IgAD patients were also included in the study. These consist of patients who were referred from their primary physician after infectious symptoms and diagnosis of IgA deficiency by the stated criterion (serum IgA<0.07 g/l measured by nephelometry), and blood donors routinely screened for IgAD.
Ethical permission was obtained from the Regional Ethical Review Board for use of the patient materials.
Anonymized control data for HLA alleles was obtained from the Swedish volunteer bone marrow registry (www.tobiasregistret.se). The data consists of 40,789 participants, HLA typed for HLA-B (n=40,789), HLA-DR (n=23,609) and HLA-DQ (n=1193). HLA-A data has not been collected in this database, and therefore, the frequency of HLA-A alleles in Sweden from a previous publication using 252 unrelated Swedish individuals was used (Engelmark et al. 2004).
HLA typing
HLA typing of patients for A, B, DR and DQ was obtained through the following methods. First, as part of the IMAGEN project (Rioux et al. 2009), most MG samples were typed at 1288 SNPs, 1230 of which were tag SNPs across the MHC (Rioux et al. 2009). Using this information, two digit imputations of HLA-A, B, DR and DQ were obtained using a previously validated method shown to have a 95% accuracy in imputing 4 digit types in European populations (de Bakker et al. 2006);(Leslie et al. 2008). In order to improve the accuracy further, alleles with mismatches between imputation and previous typing via PCR/serology, such as HLA-A3101, B3501, B4002, DR401, DR403 DR701, and DR1401 (Rioux et al. 2009), were subject to PCR-SSP in order to determine the correct alleles.
PCR-SSP was used to genotype samples at the HLA -A, B, DR and/or DQ loci (Olerup et al. 1993). The kits used in this study included the HLA-A low resolution (26F, 62G), the HLA-B low resolution (90F, 63G), the HLA-DR low resolution (40E, 83F), the HLA-DQ low resolution (41E, 91F) and the HLA- DQ-DR SSP Combi Tray (M84) from OlerupSSP AB, Saltsjöbaden, Sweden.
IgA measurement
Serum levels of IgG, IgA and IgM were measured by nephelometry at the Karolinska University Hospital Clinical Chemistry Laboratory. Samples with an IgA concentration below 0.07 g/l with normal levels of IgG and IgM were considered to be IgAD.
Statistical analysis
The Chi square test was used to compare the allele frequencies of HLA alleles between MG/IgAD patients and controls. For these measurements, a p-value below 0.05 was considered to indicate statistical significance. Estimates of HLA haplotype structure were constructed using PHASE 2.1.1 (Stephens et al. 2001).
Patient subgrouping
Subgrouping of MG patients was based on clinical criteria where patients who had an early onset of the disease (age of onset < 40 years; EOMG) were separated from those who had late onset of the disease (age of onset > 50 years; LOMG). Some MG patients (n=60) were negative for anti-AChR antibodies, a large proportion of which were EOMG patients (n=35). Due to a North-South European gradient present in anti-MuSK positivity (Vincent et al. 2008), very few anti-AChR ab negative patients were positive for anti- MuSK (4 of 18 tested), and therefore a subgroup based on anti-MuSK was not analyzed. Furthermore, it has been suggested that a majority of such seronegative patients are positive for low affinity anti-AChR antibodies (Leite et al. 2008) therefore, these patients were included in the MG cohort. Patients with thymoma and those with purely ocular symptoms were examined separately with regard to presence of IgAD.
SNP analysis
In order to visualize potential differences between MG patients, IgAD patients and controls, a map was constructed using genotyping data for 1719 SNPs across the MHC from upstream of HLA-A to downstream of HLA-DP. These data consisted of 1116 SNPs extracted from an Illumina custom array panel described previously (de Bakker et al. 2006), which were merged with 897 SNPs across the same region typed using the Illumina HumanHap300 (317K) genotyping BeadChip. Swedish MG patients, homozygous for the HLA B8, DR3, DQ2 haplotype (n=5) and with normal IgA levels, were subsequently compared to population based controls (n=6) homozygous for this haplotype with normal IgA levels and IgAD patients (n=10) to determine if associated regions differ between the affected individuals. The SNP map was previously investigated in B8, DR3, DQ2 homozygous IgAD patients (n=18) and controls (n=9) (Mohammadi et al. 2010).
3. Results
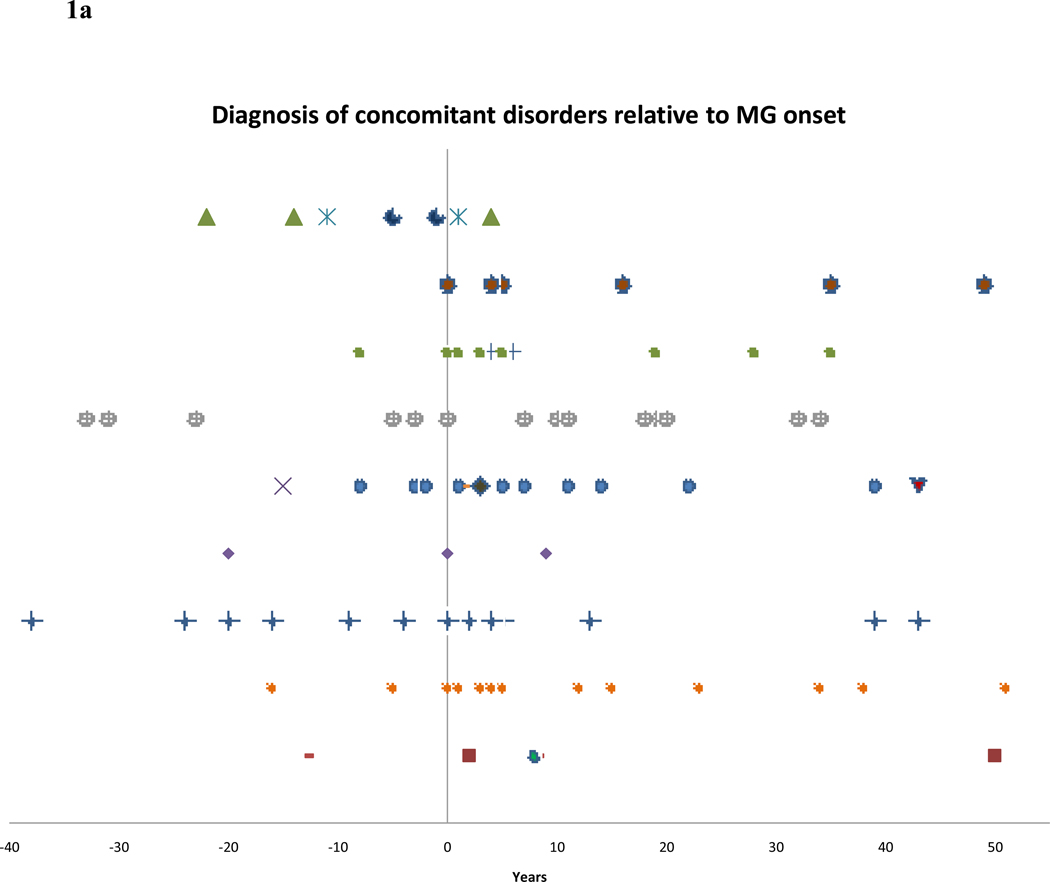
MG patients in our cohort displayed concomitant autoimmune disorders at a rate greater than that of the general population. Of the 547 MG patients, autoimmunity was diagnosed in 87 (15.9%). Figure 1a illustrates the incidence of concomitant disorders relative to the onset of MG in years. Several patients had more than one additional autoimmune disorder, including one patient with both RA and Crohn’s disease, one with IgAD, polymyositis, Sjögren’s syndrome and SLE, one with thyroiditis and MS one with both psoriasis and polyserositis, two with both RA and thyroiditis and one with both thyroidism and polymyositis. Patients with documented onset before/after MG where the exact time was not available were denoted +/−5 years for graphical representation. Included is one patient who developed SLE, polymyositis and Sjögren’s syndrome after MG.
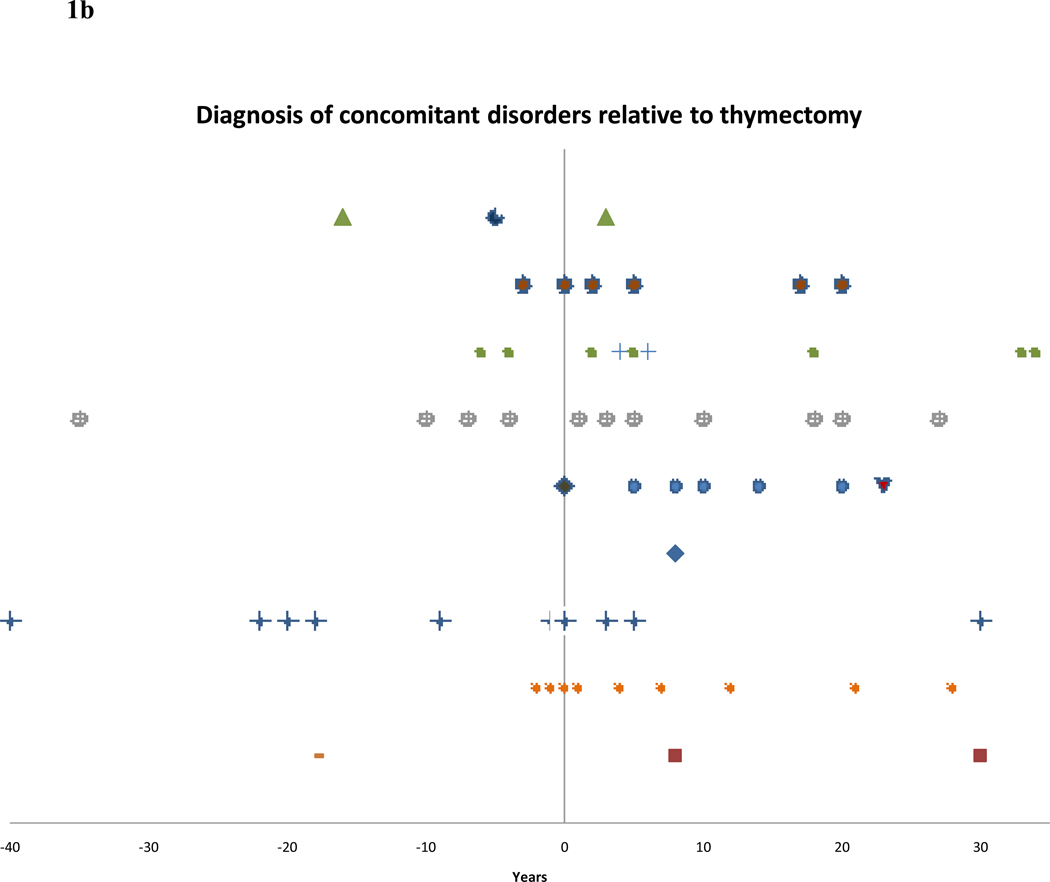
Figure 1.
a – Diagnosis of various autoimmune disorders in years relative to MG onset.
b – Diagnosis of various autoimmune disorders in years relative to thymectomy.
Figure 1b presents a similar view of the time of onset of additional disorders in thymectomized patients relative to surgery. Included are two patients who developed one disorder both before and after thymectomy (Figure 1b). Of the 87 patients with concomitant autoimmunity, 60 were thymectomized and 27 were not, compared with 263 thymectomized and 193 not thymectomized in the MG cohort without an additional autoimmune disorder (p=0.049). EOMG was slightly overrepresented in the patients with concomitant autoimmunity (57.5%) compared with patients without additional disorder(s) (48.2%) (p=0.11). Similarly, thymoma was present in 13 of the former patients (14.9%) compared with 46 patients without additional autoimmunity (10.2%) (p=0.20).
HLA imputation was conducted on a total of 428 MG patients, with the vast majority being previously typed using either PCR or serological testing. Alleles which have previously been shown to display frequent mismatches were then retyped by PCR-SSP, which was conducted on HLA-A alleles in 35 patients, HLA-B alleles in 133 patients, HLA-DR alleles in 88 patients and HLA-DQ alleles in 5 patients. In addition, full HLA typing was conducted using PCR-SSP on 105 patients for whom imputation information was not available. Some patients (n=24) could not be typed due to poor DNA quality or a lack of DNA. Thirty-eight patients for whom serum IgA levels were available, but not DNA for tissue typing, were included in the study. In total, complete HLA-A, B, DR and DQ information on 509 patients was included.
Of the entire MG cohort, 482 patients were tested for IgA serum levels, with missing measurements due to patient death, lack of serum or relocation. The overlap of patients with complete HLA information and IgA levels was 437.
The background rate of IgAD in Sweden was confirmed through empirical data obtained through routine measurement of IgA serum levels from blood donors conducted throughout various blood centers in Sweden over many years (Bachmann 1965). Of the 28,413 samples measured, 47 were found to be IgA deficient, representing 1 in every 604 individuals screened.
Two of the MG patients measured were found to be IgAD, which is not statistically different from the background in Sweden (p=0.19). IgAD was also associated to neither patients with thymoma (p=0.74), nor those with purely ocular symptoms (p=0.78) Both patients were heterozygous for the B8, DR3, DQ2 haplotype and both were anti-AChR antibody positive with thymic hyperplasia and generalized MG. Age of onset was 29 years in the first patient, and 26 years in the second patient who also had concomitant SLE, i.e. both fulfilled the criteria for EOMG. The complementary haplotype was B39, DR13, DQ3 in one patient and B51, DR11, DQ2 in the other.
The results of HLA typing at the A, B, DR, and DQ loci are presented in Table 1 as allele frequencies. Since HLA associations are known to vary in MG according to age of onset (Compston et al. 1980), we examined the EOMG patients (n=246) separately.
Table 1.
Summary results of HLA typing in MG/EOMG patients compared with IgAD patients and controls
| CONTROLS | MG | IGAD | EOMG | CONTROLS | MG | IGAD | EOMG | CONTROLS | MG | IGAD | EOMG | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 94.4% | 100.0% | 100.0% | 100.0% | B | 99.4% | 99.8% | 98.8% | 99.4% | DR | 100.0% | 100.0% | 100.0% | 100.0% |
| 1 | 11.5% | 20.7% | 26.7% | 26.9% | 7 | 15.2% | 13.9% | 7.8% | 8.5% | 1 | 12.3% | 11.0% | 14.4% | 9.3% |
| 2 | 37.5% | 30.9% | 28.4% | 28.3% | 8 | 11.7% | 18.4% | 28.2% | 28.5% | 3 | 11.3% | 16.8% | 29.5% | 26.2% |
| 3 | 16.1% | 14.2% | 10.4% | 10.2% | 13 | 1.6% | 1.6% | 2.6% | 0.8% | 4 | 19.5% | 19.4% | 11.8% | 17.3% |
| 11 | 4.8% | 6.0% | 5.1% | 5.0% | 14 | 0.6% | 1.9% | 5.3% | 1.4% | 7 | 8.5% | 6.1% | 14.0% | 2.8% |
| 23 | 2.2% | 1.1% | 1.2% | 15 | 11.4% | 8.5% | 3.8% | 7.5% | 8 | 5.0% | 4.4% | 1.9% | 3.5% | |
| 24 | 7.5% | 7.9% | 9.1% | 9.5% | 18 | 4.0% | 4.0% | 2.3% | 4.1% | 9 | 1.7% | 1.5% | 1.2% | 1.4% |
| 25 | 2.0% | 2.3% | 1.9% | 1.9% | 27 | 6.4% | 5.2% | 3.4% | 4.7% | 10 | 0.9% | 0.5% | 1.2% | 0.4% |
| 26 | 2.6% | 2.8% | 2.5% | 2.6% | 35 | 7.5% | 7.8% | 6.7% | 6.3% | 11 | 5.8% | 7.0% | 3.8% | 7.5% |
| 29 | 2.6% | 1.4% | 1.5% | 1.4% | 37 | 1.6% | 1.7% | 0.7% | 1.8% | 12 | 2.4% | 1.4% | 2.3% | 1.6% |
| 30 | 0.6% | 0.4% | 0.5% | 38 | 0.7% | 1.3% | 1.1% | 1.4% | 13 | 14.4% | 10.6% | 13.3% | 12.2% | |
| 31 | 2.6% | 3.4% | 4.7% | 4.5% | 39 | 1.7% | 1.5% | 1.3% | 1.8% | 14 | 2.5% | 4.0% | 2.8% | 4.1% |
| 32 | 2.4% | 2.7% | 3.2% | 3.1% | 40 | 9.7% | 10.0% | 9.8% | 10.2% | 15 | 15.0% | 15.2% | 2.8% | 11.4% |
| 33 | 0.4% | 0.7% | 0.8% | 0.7% | 41 | 0.6% | 0.7% | 0.8% | 0.6% | 16 | 0.7% | 2.2% | 0.8% | 2.2% |
| 34 | 0.0% | 0.0% | 0.0% | 44 | 12.5% | 9.8% | 12.8% | 8.5% | CONTROLS | MG | IGAD | EOMG | ||
| 36 | 0.0% | 0.0% | 0.0% | 47 | 0.5% | 0.1% | 0.8% | 0.2% | DQ | 100.0% | 100.0% | 100.0% | 100.0% | |
| 43 | 0.0% | 0.0% | 0.0% | 49 | 0.7% | 1.3% | 0.6% | 1.6% | 2 | 21.6% | 22.5% | 41.9% | 29.9% | |
| 66 | 0.0% | 0.0% | 0.0% | 50 | 0.4% | 1.4% | 0.7% | 1.0% | 3 | 32.9% | 30.6% | 22.3% | 28.9% | |
| 68 | 4.4% | 4.3% | 4.2% | 4.0% | 51 | 4.7% | 4.7% | 4.9% | 5.3% | 4 | 3.6% | 4.5% | 1.7% | 3.7% |
| 69 | 0.0% | 0.0% | 0.0% | 52 | 0.4% | 1.2% | 0.4% | 1.2% | 5 | 15.8% | 17.6% | 18.9% | 15.4% | |
| 74 | 0.0% | 0.0% | 0.0% | 55 | 1.0% | 0.9% | 0.8% | 1.0% | 6 | 30.6% | 24.9% | 15.2% | 22.2% | |
| 80 | 0.0% | 0.0% | 0.0% | 56 | 0.8% | 0.7% | 1.0% | 0.6% | ||||||
| 57 | 2.7% | 1.9% | 3.2% | 1.6% | ||||||||||
| 58 | 0.2% | 1.0% | 0.5% | 0.6% | ||||||||||
In order to measure the effect of haplotypes, data from the Tobias registry was used, for which complete B, DR and DQ information is available (n=1192). It should be noted that the presence of B8 and DR3, DQ2 is not absolutely indicative of a complete haplotype since recombined alleles may be inherited from each parent. We tested this by imputing haplotypes using PHASE 2.1.1, which has shown to have superior HLA haplotype reconstruction than the Expectation-Maximization algorithm (Bettencourt et al. 2008);(Castelli et al. 2010). The frequency of imputed B8, DR3, DQ2 haplotypes was 14.1% in MG, 24.7% in EOMG, 25.3% in IgAD, and 9.7% in a control population (n=672) previously typed (Ferreira et al. n.d.).
Under the assumption that all individuals with one B8, DR3 and DQ2 contain this haplotype, the frequency of the halotype is 9.8% in controls, 25.5% in IgAD patients (p=1.5×10−33), 15.1% in the entire MG group (p=8.1×10−6) and 23.8% in EOMG patients (p=1.9×10−16). These values correspond nearly identically to the imputed number of B8, DR3, DQ2 haplotype frequency, indicating that B8-DR3,DQ2 recombinants occurring together is rare. The haplotype was, however, not increased in the 87 MG patients with concomitant autoimmune disorders (13.0%, p=0.22) as compared with MG patients without concomitant autoimmunity. There was a slight, statistically non-significant difference in the frequency of A1 occurring together with B8, DR3, DQ2 between MG and IgAD (62.6% and 70.0%, respectively) (p=0.20).
Using the imputed haplotypes, the squared correlation coefficient (R2) between B8 and DR3 is 0.51 in controls, and 0.59 in all MG patients. This increases to 0.66 in EOMG and 0.69 in IgAD, due to the strong linkage disequilibrium (LD) within the haplotype. Nevertheless, in non-DR3 individuals, B8 is independently associated to MG (p=2.7×10−2), primarily due to the effect in EOMG (p=1.2×10−4), whereas no association is observed in IgAD.
We subsequently examined complementary haplotypes to the B8, DR3, DQ2 haplotype in heterozygotes in MG and EOMG patients with additional autoimmune diseases. In MG and in particular EOMG, B8 may occur in the absence of the complete haplotype with DR3-DQ2. In patients with MG and concomitant autoimmunity, an increase in DR1-DQ5 (33.3% compared with 8.9% of controls; p=2.5×10−3) occurs, although it should be noted that the number of patients with this haplotype is low (5 out of 15). This haplotype does, however, not occur in the two MG patients with IgAD (DR13, DQ3 and DR11, DQ3).
The SNP map for MG patients (n=5), controls (n=6) and IgAD patients (n=10) homozygous for the B8, DR3, DQ2 haplotype did not display any consistent differences (Supplementary table 2), with all individuals being homozygous through the region with some slight heterogeneity.
4 Discussion
The observation that concomitant autoimmune disorders are increased in myasthenia gravis patients was a partial basis for the original hypothesis that MG is autoimmune in nature (Simpson 1960). Despite many reports of related disorders in MG patients, a comprehensive overview of the topic has not been undertaken, nor have previous studies fully addressed whether IgAD is more common in patients with MG, since the relatively low number of patients examined is insufficient, given the low frequency of IgAD. We have therefore summarized the prevalence and onset of concomitant autoimmunity in a large cohort of MG patients. This patient material has also been tested for IgA serum level, and demonstrates that the incidence of IgAD is not elevated in the largest MG cohort examined to date. This finding shows that MG, albeit being a B8 associated disease, is clearly not similar to juvenile diabetes, SLE and CD, where the frequency of IgAD is markedly increased (1:40 (Smith et al. 1978), 1:20 (Rankin & Isenberg 1997) and 1:135 (McGowan et al. 2008), respectively). The strong linkage and association of the entire haplotype with the disorder suggest that the IgAD predisposing gene on the A1, B8, DR3, DQ2 haplotype may be located in the Class III/II, rather than the Class I, region (Cucca et al. 1998).
There is clearly an increase in HLA-B8 in EOMG patients, even in haplotypes not carrying HLA DR3, DQ2, which is in contrast to patients with IgAD. This supports the prevailing view that MG, and in particular EOMG, is primarily associated with genes close to HLA B8 and that the high frequency of HLA B8 in IgAD is due to the LD within this haplotype. In a previous publication, we determined that approximately 0.45% of all B8, DR3, DQ2 haplotypes in Sweden are associated with IgAD (Mohammadi et al.2010). Using this figure (1/222 haplotypes), we would expect 0–1 individuals in our MG cohort (110 B8, DR3, DQ2 haplotypes) to be IgAD based solely on presence of the haplotype. The incidence of IgAD in MG may thus simply be fully attributable to the presence of this particular haplotype in a deleterious form. The lack of appreciable differences in the SNP map of the MHC does not clarify if a mutated form of the haplotype exists in MG and IgAD patients. However, due to the low resolution (approximately 1 SNP/10,000 base pairs), additional fine mapping or even complete sequencing may be required to fully address this issue.
In heterozygous HLA-B8, DR3, DQ2 MG patients, DR1-DQ5 occurred in 5 of 15 individuals with concomitant autoimmune disorders. This suggests that this haplotype may act in concert with the B8, DR3, DQ2 haplotype to predispose individuals to autoimmunity. It would therefore be of interest to investigate in autoimmune disorders other than IgAD.
It has been suggested that treatment for MG, in particular thymectomy, may further perturb immunity, thereby increasing the chances for a patient to develop additional autoimmune disorders (Zonana et al. 2002). However, there is also evidence to the contrary (Pirskanen 1977). A borderline significance (p=0.049) may suggest that thymectomy increases susceptibility of MG patients to develop additional autoimmune disorders, since 35 of 57 (61.4%) thymectomized patients developed a disorder after thymectomy. This may, however, be partially explained due to recommendations of thymectomy in younger, early onset patients, with generalized symptoms. Although it has been suggested that thymectomy in MG patients may predispose to the development of SLE (Mevorach et al. 1995b);(Gerli et al. 1999), a similar percentage was observed post thymectomy, with 3 of the 5 cases with developing SLE after surgery.
Due to potential common mechanisms underlying autoimmunity, examination of overlapping disease predisposing genes may yield insights into the molecular basis of these diseases.
Supplementary Material
Acknowledgments
The study was supported by the Swedish Research Council, the Palle Ferb Foundation, and by a grant (U19AI067152) from the US National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammann AJ, Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine. 1971;50(3):223–236. [PubMed] [Google Scholar]
- Bachmann R. Studies on the serum gamma-A-globulin level. 3. The frequency of A-gamma-A-globulinemia. Scandinavian Journal of Clinical and Laboratory Investigation. 1965;17(4):316–320. doi: 10.3109/00365516509077057. [DOI] [PubMed] [Google Scholar]
- de Bakker PIW, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nature Genetics. 2006;38(10):1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan PO, Simpson JA, Behan WM. Letter: Decreased serum-IgA in myasthenia gravis. Lancet. 1976;1(7959):593–594. doi: 10.1016/s0140-6736(76)90398-6. [DOI] [PubMed] [Google Scholar]
- Bettencourt BF, et al. Evaluation of two methods for computational HLA haplotypes inference using a real dataset. BMC Bioinformatics. 2008;9:68. doi: 10.1186/1471-2105-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhinder S, Majithia V, Harisdangkul V. Myasthenia gravis and systemic lupus erythematosus: truly associated or coincidental-two case reports and review of the literature. Clinical Rheumatology. 2006;25(4):555–556. doi: 10.1007/s10067-005-0099-8. [DOI] [PubMed] [Google Scholar]
- Bramis J, et al. Serum-IgA in myasthenia gravis. The Lancet. 1976;307(7971):1243–1244. doi: 10.1016/s0140-6736(76)92193-0. [DOI] [PubMed] [Google Scholar]
- Castelli EC, et al. Evaluation of computational methods for the reconstruction of HLA haplotypes. Tissue Antigens. 2010;76(6):459–466. doi: 10.1111/j.1399-0039.2010.01539.x. [DOI] [PubMed] [Google Scholar]
- Christensen PB, et al. Associated autoimmune diseases in myasthenia gravis. A population-based study. Acta Neurologica Scandinavica. 1995;91(3):192–195. doi: 10.1111/j.1600-0404.1995.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Compston DA, Vincent A, et al. Clinical, pathological, HLA antigen and immunological evidence for disease heterogeneity in myasthenia gravis. Brain: A Journal of Neurology. 1980;103(3):579–601. doi: 10.1093/brain/103.3.579. [DOI] [PubMed] [Google Scholar]
- Congia M, et al. A gene dosage effect of the DQA1*0501/DQB1*0201 allelic combination influences the clinical heterogeneity of celiac disease. Human Immunology. 1994;40(2):138–142. doi: 10.1016/0198-8859(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Cucca F, et al. Evaluation of IgA deficiency in Sardinians indicates a susceptibility gene is encoded within the HLA class III region. Clinical and Experimental Immunology. 1998;111(1):76–80. doi: 10.1046/j.1365-2249.1998.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DB. Myasthenia gravis. The New England Journal of Medicine. 1994;330(25):1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- Engelmark M, et al. Affected sib-pair analysis of the contribution of HLA class I and class II loci to development of cervical cancer. Human Molecular Genetics. 2004;13(17):1951–1958. doi: 10.1093/hmg/ddh201. [DOI] [PubMed] [Google Scholar]
- Farid NR, Barnard JM, Marshall WH. The association of HLA with autoimmune thyroid disease in Newfoundland. The influence of HLA homozygosity in Graves’ disease. Tissue Antigens. 1976;8(3):181–189. doi: 10.1111/j.1399-0039.1976.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Ferreira RC, et al. High-density SNP mapping of the HLA region identifies multiple independent susceptibility loci associated with selective IgA deficiency. doi: 10.1371/journal.pgen.1002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RC, et al. Association of IFIH1 and other autoimmunity risk alleles with selective IgA deficiency . Nature Genetics. 42(9):777–780. doi: 10.1038/ng.644. [DOI] [PubMed] [Google Scholar]
- Gerli R, et al. Long-term immunologic effects of thymectomy in patients with myasthenia gravis. The Journal of Allergy and Clinical Immunology. 1999;103(5 Pt 1):865–872. doi: 10.1016/s0091-6749(99)70431-8. [DOI] [PubMed] [Google Scholar]
- Giraud M, Vandiedonck C, Garchon H-J. Genetic factors in autoimmune myasthenia gravis. Annals of the New York Academy of Sciences. 2008;1132:180–192. doi: 10.1196/annals.1405.027. [DOI] [PubMed] [Google Scholar]
- Hammarström L, Smith CI. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. Oxford: Oxford University Press; 2007. Genetic approach to common variable immunodeficiency and IgA deficiency; pp. 313–325. [Google Scholar]
- Hrycek A. Systemic lupus erythematosus and myasthenia gravis. Polskie Archiwum Medycyny Wewnȩ trznej. 2009;119(9):582–585. [PubMed] [Google Scholar]
- Jorgensen GH, et al. Familial aggregation of IgAD and autoimmunity. Clinical Immunology (Orlando, Fla.) 2009;131(2):233–239. doi: 10.1016/j.clim.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, et al. Clinical features of patients with myasthenia gravis associated with autoimmune diseases. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2007;14(12):1403–1404. doi: 10.1111/j.1468-1331.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- Leite MI, Jacob S, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain: A Journal of Neurology. 2008;131(Pt 7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S, Donnelly Peter, Gil McVean. A statistical method for predicting classical HLA alleles from SNP data. American Journal of Human Genetics. 2008;82(1):48–56. doi: 10.1016/j.ajhg.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau R, et al. The frequency of selective IgA deficiency in myasthenia gravis. Neurology. 1992;42(3 Pt 1):516–518. doi: 10.1212/wnl.42.3.516. [DOI] [PubMed] [Google Scholar]
- Lisak RP, Zweiman B. Serum immunogloblin levels in myasthenia gravis, polymyositis, and dermatomyositis. Journal of Neurology, Neurosurgery, and Psychiatry. 1976;39(1):34–37. doi: 10.1136/jnnp.39.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak, Robert P. Handbook of myasthenia gravis and myasthenic syndromes. Marcel Dekker; 1994. [Google Scholar]
- Marinó M, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. The Journal of Clinical Endocrinology and Metabolism. 1997;82(2):438–443. doi: 10.1210/jcem.82.2.3749. [DOI] [PubMed] [Google Scholar]
- McGowan KE, Lyon ME, Butzner JD. Celiac disease and IgA deficiency: complications of serological testing approaches encountered in the clinic. Clinical Chemistry. 2008;54(7):1203–1209. doi: 10.1373/clinchem.2008.103606. [DOI] [PubMed] [Google Scholar]
- Mevorach D, Perrot S, Buchanan NM, Khamashta M, Laoussadi S, Hughes GR, Menkes CJ. Appearance of systemic lupus erythematosus after thymectomy: four case reports and review of the literature. Lupus. 1995a;4(1):33–37. doi: 10.1177/096120339500400108. [DOI] [PubMed] [Google Scholar]
- Mevorach D, Perrot S, Buchanan NM, Khamashta M, Laoussadi S, Hughes GR, Menkes CJ. Appearance of systemic lupus erythematosus after thymectomy: four case reports and review of the literature. Lupus. 1995b;4(1):33–37. doi: 10.1177/096120339500400108. [DOI] [PubMed] [Google Scholar]
- Mohammadi J, et al. Human leukocyte antigens (HLA) associated with selective IgA deficiency in Iran and Sweden. Iranian Journal of Allergy, Asthma, and Immunology. 2008;7(4):209–214. [PubMed] [Google Scholar]
- Mohammadi J, et al. IgA Deficiency and the MHC: Assessment of Relative Risk and Microheterogeneity Within the HLA A1 B8, DR3 (8.1) Haplotype. Journal of Clinical Immunology. 2010 Jan;30(1):138–143. doi: 10.1007/s10875-009-9336-2. [DOI] [PMC free article] [PubMed]
- Monden Y, et al. Clinical characteristics and prognosis of myasthenia gravis with other autoimmune diseases. The Annals of Thoracic Surgery. 1986;41(2):189–192. doi: 10.1016/s0003-4975(10)62667-7. [DOI] [PubMed] [Google Scholar]
- Olerup O, Aldener A, Fogdell A. HLA-DQB1 and -DQA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Tissue Antigens. 1993;41(3):119–134. doi: 10.1111/j.1399-0039.1993.tb01991.x. [DOI] [PubMed] [Google Scholar]
- Omar HA, et al. Systemic lupus erythematosus after thymectomy for myasthenia gravis: a case report and review of the literature. [Accessed March 19, 2010];Clinical and Experimental Nephrology. 2010 doi: 10.1007/s10157-009-0256-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20054604. [DOI] [PubMed]
- Pirskanen R. Genetic aspects in myasthenia gravis. A family study of 264 Finnish patients. Acta Neurologica Scandinavica. 1977;56(5):365–388. [PubMed] [Google Scholar]
- Rankin EC, Isenberg DA. IgA deficiency and SLE: prevalence in a clinic population and a review of the literature. Lupus. 1997;6(4):390–394. doi: 10.1177/096120339700600408. [DOI] [PubMed] [Google Scholar]
- Rioux JD, et al. Mapping of multiple susceptibility variants within the MHC region for 7 immune-mediated diseases. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18680–18685. doi: 10.1073/pnas.0909307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser O, Morell A, Hitzig WH. Primary immunodeficiencies in Switzerland: first report of the national registry in adults and children. Journal of Clinical Immunology. 1988;8(6):479–485. doi: 10.1007/BF00916954. [DOI] [PubMed] [Google Scholar]
- Sahay BM, Blendis LM, Greene R. Relation between myasthenia gravis and thyroid disease. British Medical Journal. 1965;1(5437):762–765. doi: 10.1136/bmj.1.5437.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. Myasthenia gravis, a new hypothesis. Scottish Medical Journal. 1960;5:419–436. [Google Scholar]
- Skarsvåg S, et al. Distribution of HLA class II alleles among Scandinavian patients with systemic lupus erythematosus (SLE): an increased risk of SLE among non[DRB1*03,DQA1*0501,DQB1*0201] class II homozygotes? Tissue Antigens. 1992;40(3):128–133. doi: 10.1111/j.1399-0039.1992.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Smith WI, et al. Immunopathology of juvenile-onset diabetes mellitus. I. IgA deficiency and juvenile diabetes. Diabetes. 1978;27(11):1092–1097. doi: 10.2337/diab.27.11.1092. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68(4):978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, et al. Acetylcholine receptor antibodies in myasthenia gravis are associated with greater risk of diabetes and thyroid disease. Acta Neurologica Scandinavica. 2006;114(2):124–132. doi: 10.1111/j.1600-0404.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- Vaiopoulos G, et al. The association of systemic lupus erythematosus and myasthenia gravis. Postgraduate Medical Journal. 1994;70(828):741–745. doi: 10.1136/pgmj.70.828.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent Angela, et al. Myasthenia gravis seronegative for acetylcholine receptor antibodies. Annals of the New York Academy of Sciences. 2008;1132:84–92. doi: 10.1196/annals.1405.020. [DOI] [PubMed] [Google Scholar]
- Wetherall JD, Robinson J, Dawkins RL. Serum-Iga in myasthenia gravis. Lancet. 1976;2(7986):637. doi: 10.1016/s0140-6736(76)90709-1. [DOI] [PubMed] [Google Scholar]
- Zonana MF, Reyes E, Weisman AK. Coexistence of four autoimmune diseases in one patient: the kaleidoscope of autoimmunity. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases. 2002;8(6):322–325. doi: 10.1097/00124743-200212000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.