Abstract
α1,3-Galactosyltransferase gene-knockout pigs transgenic for porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig) have been produced to reduce T-cell-mediated rejection following xenotransplantation. The level of soluble pCTLA4-Ig in their blood was greatly in excess of the therapeutic level in patients, rendering the pigs immune-incompetent. Soluble pCTLA4-Ig produced by these transgenic pigs was evaluated for binding to porcine and human (h) B7 molecules, and for its inhibitory effect on allogeneic and xenogeneic human T-cell responses. Porcine CTLA4-Ig-expressing peripheral blood mononuclear cells (PBMCs) and aortic endothelial cells (AECs) were evaluated for their direct inhibitory effect on hCD4+ T-cell responses. Soluble pCTLA4-Ig and purified hCTLA4-Ig showed similar binding to pB7 molecules, but pCTLA4-Ig showed significantly less binding to hB7 molecules. The pCTLA4-Ig and hCTLA4-Ig inhibited the response of hCD4+ T cells to pAECs equally, but pCTLA4-Ig was less successful in inhibiting the human allogeneic response. The hCD4+ T-cell response to PBMCs from pCTLA4-Ig pigs was significantly lower than that of non-pCTLA4-Ig pigs. Although pCTLA4-Ig was detected in the cytoplasm of pCTLA4-Ig-expressing pAECs, only a minimal level of soluble pCTLA4-Ig was detected in the supernatant during culture, and pCTLA4-Ig-expressing pAECs did not inhibit the xenogeneic direct human T-cell response. High-level tissue-specific production of pCTLA4-Ig may be required for sufficient immunosuppression for organ or cell (e.g. islets) transplantation.
Keywords: co-stimulatory molecules, human CD4+ T cells, porcine CTLA4-Ig, xenotransplantation
Introduction
Since the production of α1,3-galactosyltransferase gene-knockout (GTKO) pigs,1 hyperacute rejection related to the presence of anti-Galα1,3Gal antibodies in the host has been largely prevented.2 However, there are several immunological barriers that must be overcome before long-term survival of such grafts can be achieved in pig-to-primate xenotransplantation (xenoTx).3 Cell-mediated rejection (previously obscured by antibody-mediated rejection) is incompletely understood, and its control will be necessary.4
In pig-to-primate xenoTx, both direct and indirect T-cell immune responses are involved in xenograft rejection.5,6 It is notable that whereas a short course of cytotoxic T-lymphocyte antigen 4-immunoglobulin (CTLA4-Ig) frequently induced indefinite survival of human (h) islet xenografts in mice, anti-hB7 antibody was also effective, indicating that much of the immune response was initiated against donor-type antigen-presenting cells (APCs).7 Dendritic cells are potent APCs. Donor-derived dendritic cells migrate into host lymphoid organs after xenograft placement and may be responsible for initiating graft rejection through the direct pathway (as in allograft rejection).8
For instance, the immunogenicity of xenogeneic pancreatic islets is enhanced by passenger leucocytes and endothelial cells (ECs) that are capable of providing co-stimulation.9,10 In addition, direct xeno-specific T-cell responses were inhibited by donor-specific co-stimulation blocking antibody.11,12 Porcine aortic ECs (pAECs), unlike hAECs, constitutively express CD80/86, and are fully capable of stimulating a human T-cell response through the direct pathway,13–17 providing the potential for full human T-cell activation at the donor EC surface. Hence, intensive suppression of the direct human T-cell response to pig organs/cells will be required. The current standard clinical immunosuppressive protocols used to prevent allograft rejection do not uniformly prevent xenograft rejection, and high-dose and/or additional immunosuppressive agents are required because the human T-cell xenoresponse to pig cells is greater than to allo human cells.18,19
Purified pCTLA4-Ig from pCTLA4-Ig-transfected Chinese hamster ovary K1 cells exhibits a clearly preferential binding to pB7 molecules in comparison to hB7 molecules in vitro.20 This is associated with the substitution of methionine for leucine in the B7 binding motif of hCTLA4-Ig.20–23 Accordingly, in mixed lymphocyte reaction (MLR) assay, purified pCTLA4-Ig and hCTLA4-Ig equivalently inhibit the direct xenogeneic response of human T cells, but pCTLA4-Ig does not inhibit the indirect xenogeneic response.20 These results indicated a low binding of purified pCTLA4-Ig for hB7 molecules. Nevertheless, intraperitoneal injection of purified pCTLA4-Ig delays rejection of pig islet xenografts in a diabetic murine model of xenoTx.12
In xenoTx, there is the possibility of modulating the anti-graft immune responses by genetic modification of the pig organ and tissues/cells. GTKO pigs transgenic for pCTLA4-Ig (GTKO/pCTLA4-Ig pigs) have been produced as potential sources of organs for xenoTx.24 Over-expression of the porcine form of CTLA4-Ig (versus hCTLA4-Ig) in these transgenic pigs provides the potential to inhibit only the direct hCD4+ T-cell-mediated xenogeneic response, hence providing local immune protection in the donor tissue without systemic immunosuppression of the recipient. These pigs exhibited marked expression of pCTLA4-Ig in each organ studied, with high levels of soluble pCTLA4-Ig in the serum. However, they proved to have impaired immunity, which was associated with a susceptibility to infection, necessitating euthanasia. The detailed immunological profile of these pigs has not been reported. For example, it is not fully understood whether soluble pCTLA4-Ig from these pigs is effective in suppressing the human T-cell responses to APCs (allogeneic/xenogeneic) or whether organ-specific or tissue/cell-specific expression of pCTLA4-Ig protects donor tissues from recipient T-cell-mediated rejection.
To address these issues, we first evaluated whether soluble pCTLA4-Ig from pCTLA4-Ig transgenic pigs could bind to B7 molecules (CD80/CD86) on pAECs, and inhibit the xenogeneic direct response of hCD4+ T cells to pAECs. The results were compared with the effects of hCTLA4-Ig. We also explored the potential of pPBMCs and pAECs expressing pCTLA4-Ig to protect themselves from the hCD4+ T-cell-mediated xenogeneic response.
Materials and methods
Reagents
Abatacept (Orencia®/Bristol Myers Squibb, Princeton, NJ) and hCTLA4-Ig (R&D Systems, Minneapolis, MN) were obtained commercially. Each product (500 μg/ml) was prepared with sterile saline for abatacept or sterile saline containing 0·1% bovine serum albumin for hCTLA4-Ig, and stored at −80° until use.
Sources of soluble pCTLA4-Ig
Sera from GTKO/CTLA4-Ig pigs as a source of soluble pCTLA4-Ig were collected as previously described,24 and were stored at −80° until use. Sera from GTKO pigs were also stored as a negative control.
Cell culture
Thoracic aortas were excised from GTKO and GTKO/pCTLA4-Ig pigs, all provided by Revivicor, Inc. (Blacksburg, VA). Human AECs were purchased from Cambrex (Walkersville, MD). Porcine AECs and hAECs were cultured as previously described.25 Activation of sub-confluent pAECs and hAECs was carried out by culture for 48 hr in recombinant porcine interferon-γ (IFN-γ; 40 ng/ml, Serotec, Raleigh, NC) and hIFN-γ (50 ng/ml, Serotec), respectively.
Isolation of hPBMCs and hCD4+ T cells
The PBMCs were isolated from buffy coats from multiple human donors (Institute for Transfusion Medicine, Pittsburgh, PA) as previously described.19,26 Human CD4+ T cells were isolated from PBMCs by negative selection with the CD4+ T-cell isolation kit II (Miltenyi Biotec, Auburn, CA) as previously described.19 CD4+ T-cell purity was > 98% by flow cytometric analysis. To activate hPBMCs, hPBMCs were incubated in culture medium supplemented with lipopolysaccharide (LPS; 10 μg/ml; Sigma-Aldrich, St Louis, MO) for 24 hr.
RT-PCR
RNA was extracted from unstimulated and pIFN-γ-stimulated GTKO pAECs using Trizol (Invitrogen, Carlsbad, CA). Messenger RNA was then reverse transcribed into cDNA, and followed by a PCR. The pCD80, pCD86 and pGAPDH cDNA were amplified by 35 cycles of PCR, using the primer pairs (Table 1). The PCR products were then run on 2% UltraPure™ agarose (Invitrogen) gels and visualized under ultraviolet light by ethidium bromide (Invitrogen) staining.
Table 1.
Target-specific nucleotide sequences of oligonucleotide primers for reverse transcription-PCR
| Target gene | Primer sequence | |
|---|---|---|
| pCD80 | Forward | 5′-TCTGTTCAGGCATCGTTCAG-3′ |
| Reverse | 5′-CTCATACTTGGGCCACACCT-3′ | |
| pCD86 | Forward | 5′-TTTGGCAGGACCAGGATAAC-3′ |
| Reverse | 5′-GCCCTTGTCCTTGATTTGAA-3′ | |
| pGAPDH | Forward | 5′-GGGCATGAACCATGAGAAGT-3′ |
| Reverse | 5′-TGTGGTCATGAGTCCTTCCA -3′ |
Quantification of soluble pCTLA4-Ig by ELISA
Soluble pCTLA4-Ig in either serum or supernatant of cultured AECs from GTKO/pCTLA4-Ig pigs was quantified by ELISA as previously described.24 Briefly, multiple dilutions of serum or supernatant from cultured AECs from GTKO/pCTLA4-Ig or GTKO (as negative control) pigs were loaded in triplicate on plates coated with goat anti-hIgG1 antibodies (The Binding Site, San Diego, CA) at 5 μg/ml and incubated for 1 hr at room temperature. In addition, PBS (Invitrogen) was loaded as negative background. A standard curve was obtained using hCTLA4-Ig (R&D Systems). A goat anti-hIgG1 conjugated with horseradish peroxidase (The Binding Site) was added and incubated for 1 hr at room temperature. Peroxidase substrate 3,3,5,5-tetramethylbenzidine (Sigma) was added for colour development, which was stopped by adding sulphuric acid. The optical density for each sample was read with a Victor 3 Multilabel Plate Reader (Perkin Elmer, Waltham, MA) at 450 nm.
Flow cytometry
Surface expressions of swine leucocyte antigen (SLA) class I and II antigens was detected by BD™ LSR II flow cytometer (Beckton Dickinson, Franklin Lakes, NJ), as previously described.25,27 The expression of B7 molecules on the pAECs, hAECs and hPBMCs was determined using a hamster anti-mouse CD80 monoclonal antibody (clone 16-10A1, BD), which has been reported to cross-react with pCD80 16 and hCTLA4-Ig (R&D Systems), followed by FITC-labelled goat anti-hIgG-Fc polyclonal antibody (Bethyl Laboratories, Montgomery, TX). The expressions of CD80 and CD86 on hAECs and hPBMCs were also tested using mouse anti-hCD80 (clone L307.4, BD) or CD86 (clone FUN-1, BD) monoclonal antibody. The expressions of CD80/86 on hPBMCs were gated with anti-hCD14 monoclonal antibodies (clone TÜK4; Miltenyi Biotec, Auburn, CA and clone RMO52; Beckman Coulter, Miami, FL).
Binding of (i) hCTLA4-Ig, (ii) abatacept, (iii) serum from a GTKO/pCTLA4-Ig pig, or (iv) GTKO pig serum to pAECs and hPBMCs was detected by indirect staining. These cells were incubated with either no antibody, secondary antibody alone, or multiple concentrations of CTLA4-Ig (each at doses ranging from 0·02 to 1250 ng/1 × 105 cells for pAECs, 1 × 106 cells for human monocytes), followed by the addition of FITC-conjugated secondary antibody.
Mixed lymphocyte reaction
The MLRs were carried out as previously described.19,27,28 Briefly, isolated hCD4+ T cells as responders (2 × 105 cells/well) were co-cultured with irradiated (2500 cGy) pAECs (2 × 104 cells/well) with/without activation by pIFN-γ or hPBMCs 5 × 105 cells/well with or without activation by LPS. All assays were performed in 96-well round-bottom plates (Corning, Lowell, MA) with AIM V Medium (Invitrogen). The cells were cultured at 37° in 5% CO2 for 6 days (pAECs stimulator) and 5 days (PBMCs stimulator), and [3H]thymidine (1 μCi/well) (NEN Life Science, Boston, MA) was added to each well during the last 15 hr of incubation. Cells were harvested on glass-fibre filters (Perkin Elmer) with a cell harvester, and were analysed by beta-scintillation counting on a liquid scintillation counter (TopCount NXT; Perkin Elmer). The mean of triplicate results was expressed as [3H]thymidine uptake.
For suppression MLR, pAECs or hPBMCs were resuspended with staining buffer containing PBS, 0·5% BSA (Sigma) and 2 mm EDTA (Sigma). Stimulator cells were incubated with several concentrations (from 500 to 0·008 μg/ml) of hCTLA4-Ig, abatacept, GTKO/pCTLA4-Ig serum, or GTKO pig serum for 1 hr. Cells were washed twice with staining buffer and were resuspended with serum-free medium, AIM V medium (Invitrogen). These stimulator cells were irradiated and co-cultured with responder hCD4+ T cells as addressed above.
Western blot analysis
Cells were homogenized in the presence of protease inhibitors (Thermo Scientific, Rockford, IL), followed by the addition of SDS at 1% as a final concentration. Thereafter, cell lysates were centrifuged to remove any residual debris. Protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Heat-denatured and β-mercaptoethanol-reduced samples (20 μg protein/lane) were electrophoresed on 4–20% BisTris–SDS gradient gels (Invitrogen). A recombinant hCTLA4-Ig fusion protein (R&D Systems) was employed as a positive control protein. After electrophoresis, proteins were then transferred into a polyvinylidene fluoride membrane and blocked with casein-blocking buffer (Sigma). The blocked membrane was incubated in goat anti-hIgG–horseradish peroxidase (Invitrogen). Positive signals were detected with Pierce ECL Western Blotting Substrate (Thermo Scientific) and photographic imaging.
Statistical methods
All results are expressed as mean ± SEM. The statistical significance of differences was determined by Student's t-test or non-parametric tests, as appropriate, using GraphPad Prism version 4 (Graphpad Software, San Diego, CA). A P-value of < 0·05 was considered to be statistically significant.
Results
Expression of B7 molecules on pAECs and hAECs
Differences in expression of co-stimulatory molecules CD80/86 on both inactivated and activated AECs between pigs and humans were assessed by flow cytometry. The pAECs constitutively expressed CD80 (Fig. 1a). Although there was no difference in CD80 expression on quiescent or activated pAECs (Fig. 1a-i), binding of hCTLA4-Ig to pCD80/86 on pAECs was up-regulated when cells were activated (Fig. 1a-ii), indicating that CD86 expression on pAECs might be up-regulated after activation. [In a preliminary study, we could not find a significant further increase of pCD80 on pAECs even at concentrations up to 400 ng/ml of pIFN-γ (data not shown)]. In contrast to pAECs, there was no expression of CD80 and CD86 on either quiescent or activated hAECs (Fig. 1b).
Figure 1.
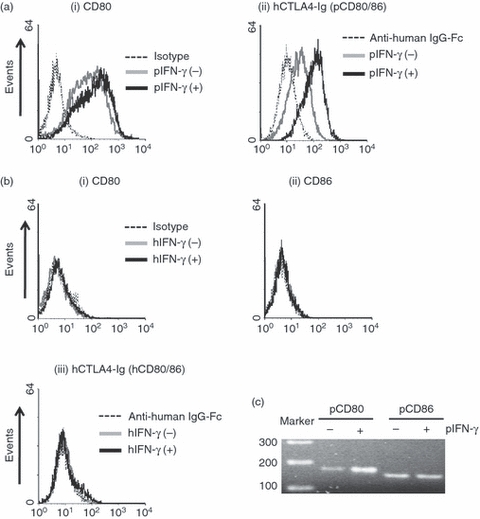
Constitutive expression of B7 molecules (CD80/86) on porcine aortic endothelial cells (pAECs), but not on human (h) AECs. Detection of B7 molecules on (a) pAECs and (b) hAECs by flow cytometry. The pAECs and hAECs [both inactivated and activated with interferon-γ (IFN-γ)] were stained with (i) FITC-conjugated anti-hamster CD80 monoclonal antibodies (mAbs) to detect CD80 expression, or with (ii) human cytotoxic T-lymphocyte antigen 4 immunoglobulin (hCTLA4-Ig) followed by adding FITC-conjugated goat anti-hIgG antibody to detect B7 molecules (CD80/86). (iii) The expression of hCD86 was also measured using anti-hCD86 mAbs. Isotype control or secondary antibody only (dotted line), inactivated (grey line) and activated (solid line) cells. Results are representative of at least three independent experiments. (c) Detection of mRNA levels for pCD80/86 in quiescent and activated pAECs were investigated by reverse transcription-PCR using specific primers for pCD80 and pCD86.
As there are no commercially available specific antibodies against pCD86, we alternatively evaluated pCD80/86 mRNA levels using RT-PCR. Both CD80 and CD86 mRNA in quiescent pAECs were detected (Fig. 1c). The expression of CD80 mRNA was up-regulated in activated pAECs although surface expression of pCD80 did not change after activation. The expression of pCD86 mRNA in activated pAECs was similar to that of quiescent pAECs (Fig. 1c).
These results indicated that, unlike hAECs, which lack expression of B7 molecules, pAECs constitutively express both CD80 and CD86.
Comparison of binding of soluble pCTLA4-Ig and purified hCTLA4-Ig to pAECs
To compare the binding of soluble pCTLA4-Ig for pB7 molecules with that of commercially available hCTLA4-Ig and abatacept, several concentrations (ranging from 500 to 0·008 μg/ml) of these CTLA4-Ig products were incubated with inactivated or pIFN-γ-activated GTKO pAECs. In ELISA, the original concentration of pCTLA4-Ig in frozen-stored serum of a GTKO/pCTLA4-Ig pig was 400 μg/ml (data not shown). With diluting concentrations of these CTLA4-Ig products, binding of soluble pCTLA4-Ig to both inactivated (Fig. 2a) and activated (Fig. 2b) GTKO pAECs was similar to those of hCTLA4-Ig and abatacept. Binding of soluble pCTLA-Ig, hCTLA4-Ig and abatacept to activated pAECs was higher than to inactivated pAECs, suggesting that the expression of pCD80/86 on pAECs was up-regulated after activation. The maximal positive percentage binding to pAECs was identical between each CTLA4-Ig. These results indicated that soluble pCTLA4-Ig and hCTLA4-Ig have similar binding for pCD80/86.
Figure 2.
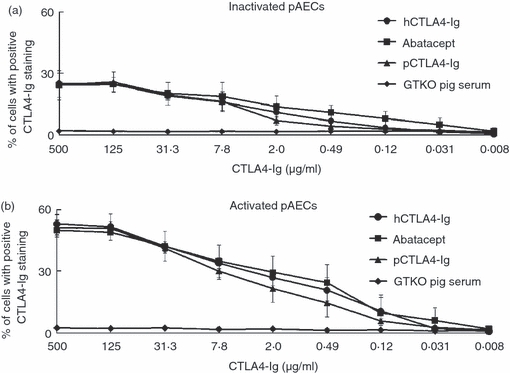
Similar binding of porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig), human (h) CTLA4-Ig, and abatacept for pB7 molecules. Binding of serially diluted soluble pCTLA4-Ig derived from a α1,3-galactosyltransferase gene-knockout (GTKO)/pCTLA4-Ig pig, purified hCTLA4-Ig, and abatacept to (a) inactivated and (b) activated GTKO porcine aortic endothelial cells (pAECs) was compared by flow cytometry. Sera from GTKO pigs (with the same volume as for GTKO/pCTLA4-Ig serum) were used as a negative control. Dilution of these CTLA4-Ig preparations ranged from 500 to 0·008 μg/ml. Data are expressed as mean + SEM obtained from three independent experiments.
Comparison of inhibitory effect of soluble pCTLA4-Ig and purified hCTLA4-Ig on the hCD4+ T-cell response to pAECs
To evaluate the effect of soluble pCTLA4-Ig on the response of hCD4+ T cells to activated pAECs, MLRs were carried out in the presence of soluble pCTLA4-Ig or hCTLA4-Ig (Fig. 3). The response of hCD4+ T cells to GTKO pAECs treated with GTKO pig serum was equivalent to that in an untreated control group (data not shown); serum from a GTKO pig had no inhibitory effect on xenogeneic CD4+ T-cell responses. Soluble pCTLA4-Ig in the serum from pCTLA4-Ig/GTKO pigs significantly inhibited the response of hCD4+ T cells to activated GTKO pAECs at a concentration of 20 μg/ml (Fig. 3). Both hCTLA4-Ig and abatacept also significantly inhibited the xenogeneic hCD4+ T-cell response at a concentration of 20 μg/ml (Fig. 3). There was no statistically significant difference in inhibition between each type of CTLA4-Ig at concentrations of 20 and 125 μg/ml. These results indicated that pCTLA4-Ig was capable of inhibiting the hCD4+ T-cell direct response to pAECs as efficiently as hCTLA4-Ig.
Figure 3.
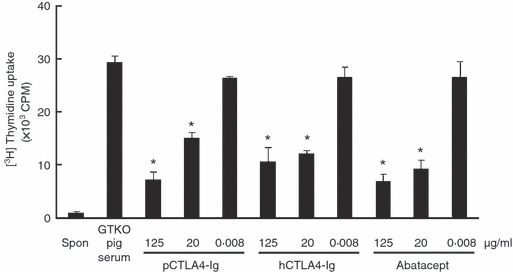
Porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig) is capable of inhibiting the hCD4+ T-cell response to α1,3-galactosyltransferase gene-knockout (GTKO) porcine aortic endothelial cells (pAECs) co-stimulated by pB7 molecules. Human (h) CD4+ T cells were co-cultured with activated GTKO pAECs treated with serial concentrations (125, 20 and 0·008 μg/ml) of (i) soluble pCTLA4-Ig, (ii) purified hCTLA4-Ig, or (iii) abatacept (n = 6). As a negative control, hCD4+ T cells were cultured alone (spon). As a control for soluble pCTLA4-Ig derived from a GTKO/pCTLA4-Ig pig, GTKO pig serum was used at a high concentration (125 μg/ml). (*P< 0·05 versus GTKO pig serum).
Comparison of binding of soluble pCTLA4-Ig and purified hCTLA4-Ig to hB7 molecules
As hAECs do not express CD80/86 molecules even after activation (Fig. 1b), hPBMCs, including APCs (e.g. monocytes), were used to evaluate the differences in binding between pCTLA4-Ig and hCTLA4-Ig for hB7 molecules. Human PBMCs were activated with LPS to up-regulate HLA class II and co-stimulatory molecules. Human CD14+ monocytes constitutively expressed HLA class II (Fig. 4a-i) and CD86 (Fig. 4a-iii), but weakly expressed CD80 (Fig. 4a-ii). Although there was increased expression of HLA-class II on CD14+ monocytes after activation with LPS, there was minimal difference in CD80 and CD86 expression on these cells; confirmed by specific markers for CD80 or CD86 (Fig. 4a-ii and a-iii) and hCTLA4-Ig (Fig. 4b).
Figure 4.
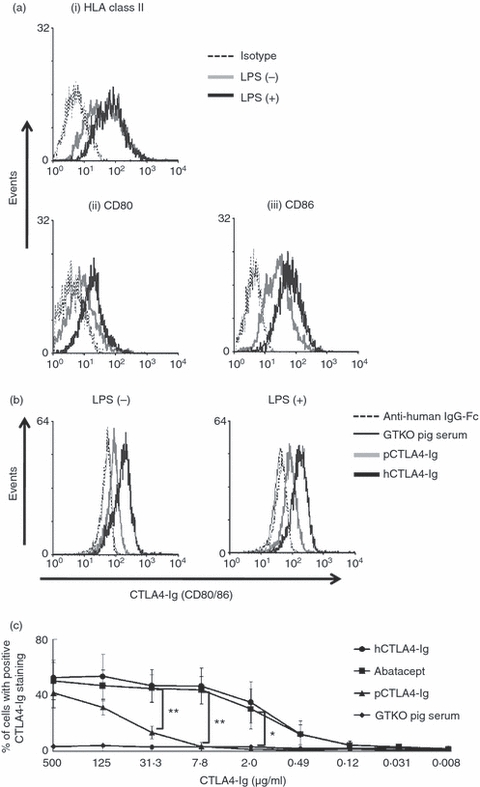
Lower binding of porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig) for human (h) B7 molecules compared with hCTLA4-Ig and abatacept. (a) Expression of (i) HLA class II, (ii) hCD80, and (iii) hCD86 on CD14+ human monocytes when inactivated or after activation with lipopolysaccharide (LPS; 10 μg/ml) for 24 hr was investigated by flow cytometry. Results are representative of three independent experiments. Isotype control (dotted line), inactivated (grey line), and activated (solid line) cells. (b and c) Binding of pCTLA4-Ig to hB7 molecules on CD14+ human monocytes with/without activation by LPS was compared with that of hCTLA4-Ig at several concentrations. (b) Inactivated and activated human peripheral blood mononuclear cells (PBMCs) were incubated with soluble pCTLA4-Ig (125 μg/ml) (grey line) or hCTLA4-Ig (125 μg/ml) (solid line) followed by FITC-conjugated anti-human IgG-Fc. The PBMCs were further stained with anti-human CD14-PE antibody. Binding of CTLA4-Ig to monocytes was investigated by gating on CD14+ cells. α1,3-Galactosyltransferase gene-knockout (GTKO) pig serum (dotted line), instead of pCTLA4-Ig and secondary antibodies only (black thin line), was tested as a negative control. Results are representative of three independent experiments. (c) Binding of serially diluted soluble pCTLA4-Ig, purified hCTLA4-Ig, and abatacept to activated hPBMCs was compared by flow cytometry. Binding of CTLA4-Ig to monocytes was investigated by gating on CD14+ cells. Sera from GTKO pigs (with the same volume as for GTKO/pCTLA4-Ig serum) were used as a negative control. Dilution of these CTLA4-Ig preparations ranged from 500 to 0·008 μg/ml. Data are expressed as mean + SEM obtained from three independent experiments. (*P< 0·05, **P< 0·01 versus soluble pCTLA4-Ig serum).
Binding assays on monocytes in hPBMCs were carried out using multiple dilutions of pCTLA4-Ig, and compared with the binding of hCTLA4-Ig and abatacept (Fig. 4c). Although soluble pCTLA4-Ig significantly bound to human monocytes at the highest concentration (500 μg/ml), soluble pCTLA4-Ig displayed significantly less binding to hB7 molecules (mainly hCD86) than either hCTLA4-Ig or abatacept at or below a concentration of 31·3 μg/ml (Fig. 4c). Whereas both hCTLA4-Ig and abatacept showed approximately 40–50% binding at concentrations ranging from 500 to 7·8 μg/ml, soluble pCTLA4-Ig showed only 13·4% and 3·4% binding at 31·3 and 7·8 μg/ml, respectively. As anticipated, at pCTLA4-Ig concentrations of 500 to 31·3 μg/ml, there were significant differences (P< 0·05) in binding in comparison to binding of serum from GTKO pigs (Fig. 4c). These results indicated that species differences (known DNA sequence changes) between porcine and human CTLA4 influence binding to hB7 molecules.
Comparison of the inhibitory effect of soluble pCTLA4-Ig and purified hCTLA4-Ig on the response of hCD4+ T cells to hAPCs
To determine whether the differences in binding between pCTLA4-Ig and hCTLA4-Ig for hB7 molecules resulted in functional differences in the T-cell response, we compared hCD4+ T-cell direct allo-immune responses to hAPCs (LPS-activated hPBMCs) treated with pCTLA4-Ig or hCTLA4-Ig. As expected, GTKO pig serum failed to inhibit hCD4+ T-cell allo responses compared with untreated controls (data not shown). The pCTLA4-Ig significantly inhibited the hCD4+ T-cell allo response compared with that of GTKO serum (Fig. 5). However, compared with hCTLA4-Ig, an inhibitory effect by pCTLA4-Ig was seen only at the highest concentration used (125 μg/ml), and no suppression of the response to hAPCs was seen at lower concentrations (e.g. 20 μg/ml). In contrast, both hCTLA4-Ig and abatacept significantly inhibited hCD4+ T cells responses to hAPCs even at 20 μg/ml (Fig. 5) (P< 0·05 versus pCTLA4-Ig at 20 μg/ml). These and previous results indicated that, although pCTLA4-Ig is capable of inhibiting hCD4+ T-cell responses co-stimulated by pB7 molecules, pCTLA4-Ig only weakly inhibits similar responses co-stimulated by hB7 molecules (associated with lower binding of pCTLA4-Ig for the hB7 molecules). Furthermore, these experiments provide indirect evidence that soluble pCTLA4-Ig will be efficiently capable of inhibiting the hCD4+ T-cell direct immune response to pAPCs, but not the hCD4+ T-cell indirect immune response to pig antigens presented by hAPCs, which also express hB7 molecules.
Figure 5.
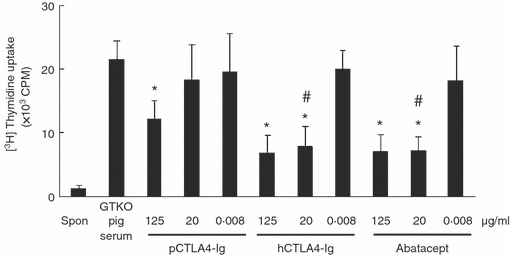
Porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig) is less able to inhibit the human (h) CD4+ T-cell response to human peripheral blood mononuclear cells (PBMCs) co-stimulated by hB7 molecules. The hCD4+ T cells were incubated with lipopolysaccharide (LPS) -activated hPBMCs treated with serial dilutions of soluble pCTLA4-Ig, purified hCTLA4-Ig, or abatacept for 5 days (n = 5). α1,3-Galactosyltransferase gene-knockout (GTKO) pig serum was evaluated as a control. (*P< 0·05, **P< 0·01 versus. GTKO pig serum. #P< 0·05 versus soluble pCTLA4-Ig).
Direct suppression of hCD4+ T cells by pCTLA4-Ig transgenic pig cells
To investigate the direct effect of pCTLA4-Ig-expression on the cells, PBMCs from pCTLA4-Ig transgenic pigs (on both wild-type and GTKO backgrounds), previously shown to produce and secrete significant levels of soluble pCTLA4-Ig into the serum, were co-cultured with hCD4+ T cells in the absence of previous treatment with pCTLA4-Ig. Human CD4+ T-cell responses to pCTLA4-Ig PBMCs were significantly lower than to PBMCs from control non-transgenic pigs (Fig. 6).
Figure 6.
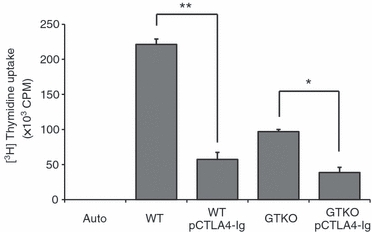
Direct suppression of the human (h) CD4+ T-cell response to pig peripheral blood mononuclear cells (PBMCs) expressing porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig). Human CD4+ T cells (2 × 105 cells/well) were co-cultured with irradiated (2500 cGy) PBMCs (5 × 105 cells/well) either from pCTLA4-Ig-transgenic pigs or wild-type (WT) or α1,3-galactosyltransferase gene-knockout (GTKO) non-transgenic pigs for 5 days (n = 3). (*P< 0·05, **P< 0·01).
To confirm the results obtained by pCTLA4-Ig transgenic PBMC stimulators, we also evaluated the potential of GTKO pAECs expressing pCTLA4-Ig to inhibit the response of hCD4+ T cells to these cells. Western blot analysis showed a specific positive band in lysates of GTKO/pCTLA4-Ig pAECs, but not in lysates of control GTKO pAECs (Fig. 7a). In contrast, only a minimal level of soluble pCTLA4-Ig protein (< 3μg/ml) was detected by ELISA in the supernatant obtained from GTKO/pCTLA4-Ig pAECs during culture (Fig. 7b).
Figure 7.
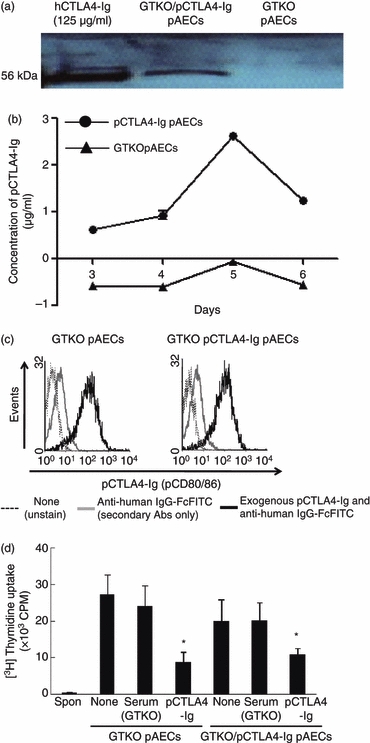
Absence of a direct suppressive effect on the human (h) CD4+ T-cell response to porcine aortic endothelial cells (AECs) expressing porcine cytotoxic T-lymphocyte antigen 4 immunoglobulin (pCTLA4-Ig) was associated with weak of production of soluble pCTLA4-Ig during culture. (a) Detection of pCTLA4-Ig protein in pAECs from α1,3-galactosyltransferase gene-knockout (GTKO)/pCTLA4-Ig pigs by Western blot analysis. A specifically positive band (56 000 molecular weight) was observed in lysates of GTKO/pCTLA4-Ig pAECs, but not in lysates of GTKO pAECs. Purified hCTLA4-Ig (125 μg/ml) was loaded as a positive control. (b) Detection by ELISA of pCTLA4-Ig in the supernatant from cultured pAECs expressing pCTLA4-Ig. GTKO/pCTLA4-Ig and GTKO pAECs (1 × 106 cells) were cultured in a 75T flask from 3 days to 6 days without changing the culture medium. Approximately 500 μl supernatant was collected from the cultured cells at 3, 4, 5 and 6 days after culture. Levels of pCTLA4-Ig were measured by ELISA with serial dilution. (c) Detection by flow cytometry of pCTLA4-Ig bound to pAECs. GTKO/pCTLA4-Ig and GTKO pAECs were cultured in 75T flasks until confluence. The pAECs were harvested and tested to determine whether soluble pCTLA4-Ig produced by GTKO/pCTLA4-Ig pAECs bound to these pAECs in an autocrine fashion during culture. Porcine AECs were stained with/without FITC-conjugated anti-human IgG-Fc antibodies, and fluorescence intensity in pAECs was compared between unstained (solid line) and stained (with FITC-conjugated anti-human IgG-Fc antibodies) (grey line), and between GTKO pAECs and GTKO/pCTLA4-Ig pAECs. The expression of pB7 molecules on GTKO and GTKO/pCTLA4-Ig pAECs was also compared by staining with pCTLA4-Ig (500 μg/ml) followed by FITC-conjugated anti-human IgG-Fc antibodies (solid thick line). Results are representative of three independent experiments. (d) The direct effect of pAECs expressing pCTLA4-Ig on the hCD4+ T-cell xenogeneic response was investigated by mixed lymphocyte reaction. The hCD4+ T cells were co-cultured with GTKO and GTKO/pCTLA4-Ig pAECs untreated or treated with either GTKO serum or soluble pCTLA4-Ig (500 μg/ml) (n = 5). Results were compared with hCD4+ T-cell responses to GTKO pAECs and GTKO serum-treated stimulators. (*P< 0·05 versus GTKO serum-treated).
To eliminate the possibility that soluble pCTLA4-Ig was being produced by cultured GTKO/pCTLA4-Ig pAECs, but immediately binding to pB7 molecules on the same cells during culture, the intensity of pCTLA4-Ig expression on GTKO/pCTLA4-Ig cells was investigated by flow cytometry by adding anti-human IgG-Fc secondary antibodies, and compared with the absence of secondary antibodies. There was no difference in fluorescence intensity between GTKO/pCTLA4-Ig and GTKO pAECs, suggesting that cultured GTKO/pCTLA4-Ig pAECs did not bind soluble pCTLA4-Ig during culture (Fig. 7c). To further confirm these results, the expression of pB7 molecules was also compared between GTKO/pCTLA4-Ig and GTKO pAECs by adding exogenous soluble pCTLA4-Ig. There was similar expression of pB7 molecules between GTKO/pCTLA4-Ig and GTKO pAECs (Fig. 7c). These results indicated that GTKO/pCTLA4-Ig pAECs, unlike GTKO/pCTLA4-Ig PBMCs, did not produce higher concentrations of soluble pCTLA4-Ig during culture, although intracellular expression of pCTLA4-Ig was confirmed.
Therefore, we hypothesized that pAECs from GTKO/pCTLA4-Ig pigs would not inhibit the xenogeneic response of hCD4+ T cells directly. To address this, hCD4+ T cells were co-cultured with GTKO/pCTLA4-Ig and GTKO pAECs with or without exogenous pCTLA4-Ig at a concentration of 125 μg/ml. There was no direct inhibitory effect on the response of hCD4+ T cells to GTKO/pCTLA4-Ig pAECs compared with that of GTKO pAECs (Fig. 7d). The addition of soluble pCTLA4-Ig at 125 μg/ml inhibited this xenoresponse by approximately 60%, which was a similar result to that obtained from the MLR assay (consisting of hCD4+ T responder cells to GTKO pAEC stimulator cells in the presence of soluble pCTLA4-Ig) (Fig. 7d). These results indicated that cultured pAECs from GTKO/pCTLA4-Ig pigs did not have a direct suppressive effect on the hCD4+ T-cell response (because of only a minimal level of production of exogenous pCTLA4-Ig).
Discussion
Over the past decade, many advances have been made in the development of selective immunomodulatory agents. In particular, co-stimulatory blockade has provided a novel therapeutic approach for autoimmune disease and Tx.29–31 CTLA4-Ig has been shown to successfully inhibit CD4+ T-cell proliferation by blocking co-stimulatory CD28/B7 activation of allo and xeno CD4+ T cells both in vitro and in vivo in rodent7,12,32,33 and non-human primate34–36 alloTx models. In addition, CTLA4-Ig can suppress the T-cell-dependent humoral response.34,37,38
Specific blockade of pig-derived B7 molecules is required to maintain effective inhibition of the xenoresponse as pB7 molecules expressed on pAECs are crucial for mediating the response of recipient T cells to xenogeneic donor cells. It would be predicted that treatment with hCTLA4-Ig or the Tx of an organ from an hCTLA4-Ig transgenic pig would prevent both direct and indirect T-cell responses following pig organ xenoTx into a primate. However, in such situations, long-term treatment with hCTLA4-Ig would not be desirable, particularly as it would increase susceptibility to infectious pathogens. In comparison, treatment with soluble pCTLA4-Ig or the presence of an organ from a pCTLA4-Ig-transgenic pig would be safer as it would preferentially prevent the direct response without inhibiting the host cellular response to pathogens.
In the present study, two types of commercially available hCTLA4-Ig were used to compare with pCTLA4-Ig. One (obtained from R&D Systems) does not have mutations in the immunoglobulin tail, and can be used only for in vitro studies. The other, abatacept (a CTLA4-Ig fusion protein comprising the extracellular domain of hCTLA4 fused to a mutated IgG1 Fc tail domain) represents a new therapeutic approach in rheumatoid arthritis.39,40 The therapeutic blood level of abatacept in patients with rheumatoid arthritis has been reported to be approximately 20 μg/ml.41,42 In an allogeneic pancreatic islet Tx model in non-human primates, a serum level of hCTLA4-Ig of approximately 50–100 μg/ml was associated with prolonged graft survival.34 Furthermore, Larsen et al.36 carried out renal alloTx in rhesus monkeys using belatacept (a modified version of abatacept that binds with two- to four-fold higher avidity to CD80/86) at a serum level of > 20 μg/ml. This group also used belatacept in a phase III clinical study of kidney Tx,43,44 in which they administered a dose of belatacept that had been successful in a non-human primate study; blood levels were not reported. Belatacept binds to pCD80/86 and suppresses T-cell responses in vitro.45
For the first time, our results demonstrate that soluble pCTLA4-Ig derived from pCTLA4-Ig-transgenic pigs is effective in terms of both binding and functionality against pB7 molecules on pAECs, and is equivalent to hCTLA4-Ig (Figs. 2 and 3). As expected, soluble pCTLA4-Ig showed significantly weaker binding to hB7 molecules on APCs (e.g., monocytes) compared with hCTLA4-Ig (Fig. 4c). Soluble pCTLA4-Ig preferentially inhibited the xenogeneic direct response of hCD4+ T cells to pAECs, whereas hCTLA4-Ig broadly inhibited both xenogeneic (pB7 molecules) and allogeneic (hB7 molecules) T-cell responses (Figs. 3 and 5). Although similar findings have previously been reported by Vaughan et al.,20 who demonstrated that pCTLA4-Ig has low binding for hB7 molecules, these authors employed purified pCTLA4-Ig from a transfected cell line, but not soluble pCTLA4-Ig produced by pCTLA4-Ig-transgenic pigs. Our results suggest that, if only the T-cell direct immune response to pig organ/cells needs to be suppressed after xenoTx, the serum trough level of pCTLA4-Ig in the recipient should be maintained at approximately 20 μg/ml. A higher trough level of pCTLA4-Ig (> 125 μg/ml) would suppress both direct (Fig. 3) and indirect (Fig. 5) pathways, resulting in a greater risk of infection.
The result demonstrating that hCTLA4-Ig had a similar binding/inhibitory capacity to pB7 molecules as pCTLA4-Ig suggests that leucine at position 97 is not crucial for CTLA4 species-specific interaction between hB7 and pB7 molecules. A full investigation of the possible role of the amino acid substitution in abrogating hCTLA4-Ig binding to pB7 molecules, and of the regions important for interaction with pB7 molecules, will require mutational analysis of the main residues in the human amino acid sequences and the production of mutant hCTLA4-Ig and hB7 molecules for binding studies.
For the GTKO/pCTLA4-Ig pigs used in this study, a strong constitutive expression system, consisting of a cytomegalovirus enhancer, a chicken β-actin promoter, and rabbit globin splice site (CAG), resulted in high-level systemic expression of pCTLA4-Ig. A high incidence of infection was observed in this line of transgenic pigs, possibly related to impaired development of B cells, resulting in a marked decrease in serum levels of both IgG and IgM.24 The serum concentration of pCTLA4-Ig observed in these pigs ranged from 380 to 1600 μg/ml, which greatly exceeds the level of hCTLA4-Ig considered clinically therapeutic (approximately 20–100 μg/ml). However, whether a single transplanted pCTLA4-Ig-transgenic pig organ will produce a level of pCTLA4-Ig in the blood after Tx high enough to be significantly immunosuppressive remains unknown. For instance, for an appropriate pCTLA4-Ig (20 μg/ml) level to be detected in the blood of the recipient after organ Tx, the source pig is likely to have a very high level of pCTLA4-Ig in the blood (e.g. > 200 μg/ml), and so may not remain healthy until Tx. GTKO/pCTLA4-Ig transgenic pigs which use an endothelial cell-specific expression system, where pCTLA4-Ig expression is localized to blood vessel endothelium in the organ, rather than constitutive expression, may overcome this issue.
We used one clinically applicable agent, abatacept, in this study. Abatacept has a similar binding for pB7 molecules on pAECs as does pCTLA4-Ig. This suggests that abatacept could be used for co-stimulatory blockade in a pig alloTx model. In fact, abatacept suppresses the pig-to-pig T-cell response in vitro in MLR (H. Hara et al., unpublished data). Pre-treatment with hCTLA4-Ig (e.g., abatacept, belatacept) in donor pigs before the organ is transplanted could be an alternative approach to reduce the early direct primate CD4+ T-cell response to a pig organ in the pig-to-primate xenoTx model (as the pig vascular endothelium constitutively expresses both CD80 and CD86).
To investigate the direct effect of pCTLA4-Ig-transgenic pig cells, we tested the hCD4+ T-cell response to cells from these pigs. The hCD4+ T-cell response to PBMCs from pCTLA4-Ig pigs was significantly reduced compared with the response to control pig PBMC stimulators. However, several factors must be considered with regard to this result.
First, the population of APCs in the stimulator cells may influence the MLR result. Unfortunately, we did not measure the populations of APCs (e.g. B cells, monocytes) in the PBMCs from pCTLA4-Ig pigs before having to kill these pigs. Serum levels of immunoglobulin, especially IgG, were significantly reduced in pCTLA4-Ig pigs compared with non-transgenic pigs,24 suggesting that the population of B cells (APCs) in the pCTLA4-Ig pigs might have been reduced, possibly resulting in a weaker hCD4+ T-cell response in MLR.
Second, B7 molecules on freshly isolated PBMCs from pCTLA4-Ig pigs might have been coated with pCTLA4-Ig in vivo (because of the high level of soluble pCTLA4-Ig in the blood), resulting in weaker hCD4+ T-cell responses. The PBMCs from pCTLA4-Ig pigs might therefore not be appropriate as stimulators for MLR. Therefore, pAECs were also tested as stimulators. Although high levels of soluble pCTLA4-Ig were detected in the sera of pCTLA4-Ig pigs,24 ELISA could only detect a minimal level of pCTLA4-Ig (< 3 μg/ml) in the culture supernatant of GTKO/pCTLA4-Ig pAECs (Fig. 7b). A comparison of the level of pCTLA4-Ig in the culture supernatant between AECs and PBMCs from GTKO/pCTLA4-Ig pigs was not possible because these pigs were no longer available. In contrast, Western blot analysis showed that the pCTLA4-Ig protein was positively detected in the cell lysates from GTKO/CTLA4-Ig pAECs (Fig. 7a). These data suggest that the cultured GTKO/pCTLA4-Ig pAECs produced only a minimal level of soluble pCTLA4-Ig, which would not be able to suppress the hCD4+ T-cell response. Indeed, there was no significant difference observed between the hCD4+ T-cell response to GTKO/pCTLA4-Ig-pAECs and to GTKO/pAECs (Fig. 6b). When soluble pCTLA4-Ig was administered exogenously, the direct T-cell xenoresponse was significantly inhibited, again suggesting that little or no pCTLA4-Ig was being produced by the pAECs constitutively expressing pCTLA4-Ig.
Potential explanations for this discrepancy between the clearly high production of pCTLA4-Ig in the pig and the low/minimum production of pCTLA4-Ig in the cultured pAECs can be considered. (i) The production of pCTLA4-Ig from cultured pig cells was very limited because of the small number of cells compared with those in an organ or tissues, and may have been diluted in the supernatant. (ii) Culturing of isolated pAEC over time could inhibit the secretory function of these cells. (iii) The particular integration site of the CAG-pCTLA4-Ig vector in the genome of this line of pigs, while resulting in high pCTLA4-Ig expression/secretion in most cells and tissues, did not support high-level expression specifically in the aortic endothelium. Nevertheless, the discrepancy between the high levels in the blood of living pigs and the minimum production of pCTLA4-Ig after EC culture remains an unresolved question, because vascular ECs would be anticipated to be one of the sources of the soluble CTLA4-Ig measured in the serum of the transgenic pigs.
Several groups have shown that site-specific delivery of immunosuppressive molecules can provide an alternative immunosuppressive strategy.46–49 Agents such as hCTLA4-Ig inhibit T-cell reactivity in the periphery, but may not be active in immune-privileged sites (e.g. brain, cornea) because of low permeability across the blood–brain and/or blood–aqueous barriers. Therefore, cells/tissue from CTLA4-Ig pigs could prove a good approach to local immune protection after neuronal cell50,51 or corneal52,53 xenoTx.
In the present study, a constitutive CAG vector was used for the systemic expression of pCTLA4-Ig. However, pCTLA4-Ig pigs were at high risk from infection. To reduce the risk of infection, transgenic pigs in which islet-specific expression of pCTLA4-Ig by an insulin promoter have been produced.54 These insulin-specific-expressing pCTLA4-Ig pigs are healthy, with no increased incidence of infection (C. Phelps, personal communication), and could provide islets for xenoTx into primates. Likewise, as discussed above, the production of GTKO/pCTLA4-Ig pigs with a promoter/vector expression system designed for endothelium-specific expression of pCTLA4-Ig could alleviate not only the infection risks to the donor animals, but also ensure that expression of the pCTLA4-Ig molecule was indeed present locally and specifically in the donor organ's vascular ECs where inhibition of the direct CD4+ T-cell immune response is desired.
Taken together, our study indicates the following points. (i) Soluble pCTLA4-Ig derived from GTKO/pCTLA4-Ig pigs is effective in terms of binding and functionality. Porcine CTLA4-Ig (20 μg/ml) only inhibits the human direct T-cell xenogeneic response, and this is probably associated with low binding for hB7 molecules. Porcine CTLA4-Ig might be a useful therapeutic agent in clinical xenotransplantation. (ii) As AECs from the current pCTLA4-Ig pigs show no direct effect on suppression of the human T-cell response because of minimal production of pCTLA4-Ig during culture, cells/tissue/organs from future GTKO/CTLA4-Ig pigs will need to produce a sufficient concentration of pCTLA4-Ig (at least 20 μg/ml) to suppress the direct T-cell response after Tx. (iii) Human CTLA4-Ig or other like-acting immunosuppressants will need to be administered simultaneously to the recipient to suppress the indirect immune response, though this can be discontinued if the risk of infection is considered high.
Acknowledgments
We thank Miss Cassandra Long for her excellent technical assistance. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by National Institutes of Health grants U01 (AI068642, DKCC), R21 (A1074844, DKCC), and U19 (AI090959, DKCC), by Olympus America Inc. (Center Valley, PA) (#2115, HH), and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA, USA.
Glossary
Abbreviations
- AEC
aortic endothelial cell
- APC
antigen-presenting cell
- ECs
endothelial cells
- GTKO
α1,3-galactosyltransferase gene-knockout
- h
human
- p
porcine
- SLA
swine leucocyte antigen
- Tx
transplantation
Disclosures
CP and DA are employees of Revivicor Inc, and own stock options in the company.
Authorship contributions
TK participated in the performance of the research, and in the writing of the paper. CP and DA provided genetically engineered pigs, data from these pigs, and contributed to the writing of the paper. JF, SE and MF participated in the performance of the research, and in the review of the paper. DKCC participated in research design and in the writing of the paper. HH participated in research design, in the performance of the research, and in the writing of the paper.
References
- 1.Phelps CJ, Koike C, Vaught TD, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DK, Dorling A, Pierson RN, III rd, et al. α1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7. doi: 10.1097/01.tp.0000260427.75804.f2. [DOI] [PubMed] [Google Scholar]
- 3.Pierson RN, III rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, Hering BJ, Cooper DK. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–80. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Bas-Bernardet S, Blancho G. Current cellular immunological hurdles in pig-to-primate xenotransplantation. Transpl Immunol. 2009;21:60–4. doi: 10.1016/j.trim.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Brouard S, Gagne K, Blancho G, Soulillou JP. T cell response in xenorecognition and xenografts: a review. Hum Immunol. 1999;60:455–68. doi: 10.1016/s0198-8859(99)00020-8. [DOI] [PubMed] [Google Scholar]
- 6.Davila E, Byrne GW, LaBreche PT, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13:31–40. doi: 10.1111/j.1399-3089.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4Ig. Science. 1992;257:789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 8.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci USA. 1984;81:3864–8. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirenda V, Le Mauff B, Boeffard F, Cassard A, Jugeau N, Soulillou JP, Anegon I. Intact pancreatic islet function despite humoral xenorecognition in the pig-to-monkey combination. Transplantation. 1998;66:1485–95. doi: 10.1097/00007890-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 11.Rogers NJ, Mirenda V, Jackson I, Dorling A, Lechler RI. Costimulatory blockade by the induction of an endogenous xenospecific antibody response. Nat Immunol. 2000;1:163–8. doi: 10.1038/77853. [DOI] [PubMed] [Google Scholar]
- 12.Mirenda V, Golshayan D, Read J, Berton I, Warrens AN, Dorling A, Lechler RI. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes. 2005;54:1048–55. doi: 10.2337/diabetes.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 13.Murray AG, Khodadoust MM, Pober JS, Bothwell AL. Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity. 1994;1:57–63. doi: 10.1016/1074-7613(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 14.Maher SE, Karmann K, Min W, Hughes CC, Pober JS, Bothwell AL. Porcine endothelial CD86 is a major costimulator of xenogeneic human T cells: cloning, sequencing, and functional expression in human endothelial cells. J Immunol. 1996;157:3838–44. [PubMed] [Google Scholar]
- 15.Rogers NJ, Jackson IM, Jordan WJ, Hawadle MA, Dorling A, Lechler RI. Cross-species costimulation: relative contributions of CD80, CD86, and CD40. Transplantation. 2003;75:2068–76. doi: 10.1097/01.TP.0000069100.67646.08. [DOI] [PubMed] [Google Scholar]
- 16.Wada M, Amae S, Sasaki H, Ishii T, Sano N, Nio M, Hayashi Y, Ohi R. The functional roles of porcine CD80 molecule and its ability to stimulate and regulate human anti-pig cellular response. Transplantation. 2003;75:1887–94. doi: 10.1097/01.TP.0000065298.81277.D9. [DOI] [PubMed] [Google Scholar]
- 17.Choi I, Kim SD, Cho B, et al. Xenogeneic interaction between human CD40L and porcine CD40 activates porcine endothelial cells through NF-kappaB signaling. Mol Immunol. 2008;45:575–80. doi: 10.1016/j.molimm.2007.06.161. [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249–56. [PubMed] [Google Scholar]
- 19.Lin YJ, Hara H, Tai HC, et al. Suppressive efficacy and proliferative capacity of human regulatory T cells in allogeneic and xenogeneic responses. Transplantation. 2008;86:1452–62. doi: 10.1097/TP.0b013e318188acb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan AN, Malde P, Rogers NJ, Jackson IM, Lechler RI, Dorling A. Porcine CTLA4-Ig lacks a MYPPPY motif, binds inefficiently to human B7 and specifically suppresses human CD4+ T cell responses costimulated by pig but not human B7. J Immunol. 2000;165:3175–81. doi: 10.4049/jimmunol.165.6.3175. [DOI] [PubMed] [Google Scholar]
- 21.Peach RJ, Bajorath J, Brady W, Leytze G, Greene J, Naemura J, Linsley PS. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J Exp Med. 1994;180:2049–58. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton PA, Fu XT, Stewart JA, et al. Differential effects of CTLA-4 substitutions on the binding of human CD80 (B7-1) and CD86 (B7-2) J Immunol. 1996;156:1047–54. [PubMed] [Google Scholar]
- 23.Metzler WJ, Bajorath J, Fenderson W, et al. Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat Struct Biol. 1997;4:527–31. doi: 10.1038/nsb0797-527. [DOI] [PubMed] [Google Scholar]
- 24.Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–85. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 25.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, Cooper DK. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–74. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 26.Long C, Hara H, Pawlikowski Z, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49:2418–29. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 27.Hara H, Koike N, Long C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically-engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–86. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzelarab M, Welchons D, Torres C, Hara H, Long C, Yeh P, Ayares D, Cooper DK. Atorvastatin down-regulates the primate cellular response to porcine aortic endothelial cells in vitro. Transplantation. 2008;86:733–7. doi: 10.1097/TP.0b013e3181821cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 30.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 31.Durrbach A, Francois H, Jacquet A, Beaudreuil S, Charpentier B. Co-signals in organ transplantation. Curr Opin Organ Transplant. 2010;15:474–80. doi: 10.1097/MOT.0b013e32833c1369. [DOI] [PubMed] [Google Scholar]
- 32.Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–5. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–6. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levisetti MG, Padrid PA, Szot GL, et al. Immunosuppressive effects of human CTLA4Ig in a non-human primate model of allogeneic pancreatic islet transplantation. J Immunol. 1997;159:5187–91. [PubMed] [Google Scholar]
- 35.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 37.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 38.Lane P, Burdet C, Hubele S, Scheidegger D, Muller U, McConnell F, Kosco-Vilbois M. B cell function in mice transgenic for mCTLA4-H gamma 1: lack of germinal centers correlated with poor affinity maturation and class switching despite normal priming of CD4+ T cells. J Exp Med. 1994;179:819–30. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–23. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- 40.Korhonen R, Moilanen E. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol. 2009;104:276–84. doi: 10.1111/j.1742-7843.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 41.Nogid A, Pham DQ. Role of abatacept in the management of rheumatoid arthritis. Clin Ther. 2006;28:1764–78. doi: 10.1016/j.clinthera.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Lin BR, Lin B, et al. Pharmacokinetics of CTLA4Ig fusion protein in healthy volunteers and patients with rheumatoid arthritis. Acta Pharmacol Sin. 2009;30:364–71. doi: 10.1038/aps.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10:547–57. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 44.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 45.Emamaullee JA, Merani S, Larsen CP, Shapiro AM. Belatacept and basiliximab diminish human antiporcine xenoreactivity and synergize to inhibit alloimmunity. Transplantation. 2008;85:118–24. doi: 10.1097/01.tp.0000296832.92128.94. [DOI] [PubMed] [Google Scholar]
- 46.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273:109–12. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 47.Gainer AL, Korbutt GS, Rajotte RV, Warnock GL, Elliott JF. Expression of CTLA4-Ig by biolistically transfected mouse islets promotes islet allograft survival. Transplantation. 1997;63:1017–121. doi: 10.1097/00007890-199704150-00019. [DOI] [PubMed] [Google Scholar]
- 48.Olthoff KM, Judge TA, Gelman AE, da Shen X, Hancock WW, Turka LA, Shaked A. Adenovirus-mediated gene transfer into cold-preserved liver allografts: survival pattern and unresponsiveness following transduction with CTLA4Ig. Nat Med. 1998;4:194–200. doi: 10.1038/nm0298-194. [DOI] [PubMed] [Google Scholar]
- 49.Takeda Y, Gotoh M, Dono K, et al. Protection of islet allografts transplanted together with Fas ligand expressing testicular allografts. Diabetologia. 1998;41:315–21. doi: 10.1007/s001250050909. [DOI] [PubMed] [Google Scholar]
- 50.Martin C, Plat M, Nerriere-Daguin V, et al. Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Res. 2005;14:373–84. doi: 10.1007/s11248-004-7268-4. [DOI] [PubMed] [Google Scholar]
- 51.Badin R, Padoan A, Vadori M, et al. Porcine embryonic xenografts transgenic for CTLA4-Ig enable longterm recovery in Parkinsonian macaques. Am J Transplant. 2010;10(S4):208. (Abstract LB01) [Google Scholar]
- 52.Hara H, Cooper DK. Xenotransplantation – the future of corneal transplantation? Cornea. 2010;30:371–8. doi: 10.1097/ICO.0b013e3181f237ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hara H, Cooper DK. The immunology of corneal xenotransplantation: a review of the literature. Xenotransplantation. 2010;17:338–49. doi: 10.1111/j.1399-3089.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 54.Phelps CJ, Vaught TD, Ball SF, et al. Multi-transgenic pigs designed for xenoislet transplants. Xenotransplantation. 2009;16:374. [Google Scholar]


