Abstract
BLT mice, constructed by surgical implantation of human fetal thymus–liver tissues and intravenous delivery of autologous CD34+ haematopoietic stem cells into adult non-obese diabetic/severe combined immunodeficiency mice, were evaluated for vaccine-induced humoral immune responses. Following engraftment, these mice developed a human lymphoid system; however, the majority of the peripheral human B lymphocytes displayed an immature phenotype as evidenced by surface CD10 expression. Over 50% of the human B cells in the periphery but not in the bone marrow also expressed the CD5 antigen, which is found only infrequently on mature follicular B cells in humans. A single intramuscular immunization with recombinant viral envelope antigens, e.g. HIVgp140 and West Nile Virus envelope proteins, together with the immune stimulatory IC31® adjuvant resulted in seroconversion characterized by antigen-specific human antibodies predominantly of the IgM isotype. However, repeated booster immunizations did not induce secondary immune responses as evidenced by the lack of class switching and specific IgM levels remaining relatively unchanged. Interestingly, the peripheral CD19+ CD5+ but not the CD19+ CD5− human B lymphocytes displayed a late developing CD27+ IgM+ memory phenotype, suggesting that the CD5+ B-cell subset, previously implicated in ‘natural antibody’ production, may play a role in the vaccine-induced antibody response. Furthermore, human T lymphocytes from these mice demonstrated suboptimal proliferative responses and loss of co-stimulatory surface proteins ex vivo that could be partially reversed with human interleukin-2 and interleukin-7. Therefore, vaccine-induced immune responses in BLT mice resemble a T-cell-independent pathway that can potentially be modulated in vivo by the exogenous delivery of human cytokines/growth factors.
Keywords: BLT, HIVgp140, humanized mice, IC31, West Nile virus envelope
Introduction
A recent surge in research involving various mouse models of the human immune system (also termed ‘humanized’ mice) underscores the need for a reliable and validated in vivo system to study complex biological processes, such as haematopoiesis, autoimmunity, regenerative medicine, infectious diseases and vaccine development.1 The different human (Hu)-mouse chimeric models reported thus far can be broadly categorized into (i) Hu-PBL-SCID, where various immunodeficient mouse strains [non-obese diabetic/severe combined immunodeficiency (NOD/SCID), NOD/SCID/γc−/−, BALB/c-Rag2−/−-γc−/−)] are repopulated with human peripheral blood lymphocytes (PBL); (ii) Hu-SRC-SCID, where similar mouse strains are injected with SCID repopulating cells (SRC), e.g. CD34+ haematopoietic stem cells (HSC) isolated from fetal or adult human tissues; and (iii) second-generation SCID-Hu models, in which sub-renal implantation of human fetal tissues like thymus and liver fragments is performed with the co-delivery of autologous SRC.2 Research over the past decade has revealed specific differences among these mouse models in terms of overall chimerism and usefulness for specific translational studies. Transient levels of engraftment with human cells along with a reduced lifespan of the Hu-PBL-SCID mouse models led to the development of the Hu-SRC-SCID models, which offered improvement in both these aspects. One of the most studied mouse models of the latter category, the humanized NOD/SCID/γc−/− mice (also described as NSG or NOG) have been demonstrated to achieve high levels of chimerism when transplanted with human HSCs that differentiate into multi-lineage haemato-lymphoid cells;3 however, development of human T lymphocytes is delayed. Notably, this deficiency has been apparently corrected by HSC engraftment of neonatal instead of adult immunodeficient mice where high levels of human T-cell development are observed.4,5 In spite of this, the functionality of these ‘human’ T cells is unclear as they develop and are educated in the mouse thymic stroma. Although the NSG, and other strains of mice similarly reconstituted, have been used to establish virus infection models including important human pathogens like HIV6,7 and Dengue virus,8 the adaptive immune response generated in the context of infection or immunization appears to be restricted in terms of overall strength and breadth. Cellular and humoral immune response to HIV9 and Epstein–Barr virus infection5 in HSC reconstituted NSG and Rag2−/−-γc−/− mice has been noted to be weak and there has been only one report where the presence of low titre virus neutralizing antibodies to Dengue virus infection was demonstrated.10 Antigen-specific antibody response has been demonstrated to be predominantly IgM in nature with low levels of specific IgG even when immunized with highly antigenic keyhole limpet haemocyanin, ovalbumin or tetanus toxoid in both adult and neonatal humanized mouse models.4,11,12 In-depth phenotypic and functional analyses of the reconstituted human immune system in the Hu-SRC-SCID mice have uncovered several abnormalities, the salient ones being (i) large numbers of peripheral human B lymphocytes that express the CD5 antigen, a signature marker associated with murine B-1 B cells;13,14 (ii) anergic human T lymphocytes in the periphery;12 and (iii) poor responsiveness of the human cells to the mouse stroma-derived growth factors and cytokines, e.g. BLyS (B lymphocyte stimulator)15 and interleukin-7 (IL-7).16
The second-generation SCID-Hu mouse models, e.g. NOD/SCID/BLT (bone marrow/liver/thymus) or NOD/SCID/γc−/−/BLT (or simply BLT) mice were developed to provide a more appropriate microenvironment for human T-cell development. These mice were simultaneously engrafted with human fetal thymic and liver tissues along with autologous CD34+ HSC that resulted in a robust repopulation with human T lymphocytes.17,18 The development and selection of human T lymphocytes in the BLT mice were suggested to occur in a relevant human thymic microenvironment as the regenerated T cells were shown to mount human MHC class I and II restricted immune responses.18,19 The BLT mice have also been used to (i) establish a small animal model for HIV-1 infection,20,21 (ii) evaluate anti-retrovirals and other investigative therapeutic agents,22–25 and (iii) study immune correlates of HIV-1 infection.26 Although T-cell-mediated immune responses have been demonstrated in this mouse model, humoral immunity against infectious agents remains poorly studied. Additionally, there have been no reports so far on the use of this humanized mouse model for pre-clinical testing of human vaccine candidates. In a recent study investigating the immunocompetence of the BLT mouse model, a robust cellular immunity in the form of delayed type hypersensitivity response against a challenge with tetanus toxoid was demonstrated; however, the tetanus toxoid-specific antibody response was significantly delayed and predominantly IgM in nature.27 Given the inability of the BLT mice to demonstrate robust antibody class-switching despite booster immunizations, it becomes important to investigate whether the deficiencies reported for the Hu-SRC-SCID models still persist in the second-generation SCID-Hu models.
In the current study, a thorough assessment of the developmental and functional status of the B-cell compartment in response to vaccination in the BLT mice was undertaken. Specifically, a comprehensive analysis of the regenerated human B lymphocytes and, to a limited extent, human T lymphocytes in the BLT mouse was performed and compared with their counterpart in normal human subjects. These studies revealed profound differences, as evidenced by the abundance of a population of ‘B-1-like’ B cells (CD19+CD5+) in the BLT mouse. Analysis of HIVgp140 and West Nile virus envelope (WNV-E) protein immunized BLT mice implicated this CD5+ B-cell subset in a vaccine-induced T-cell-independent antibody response characterized by the predominant generation of antigen-specific IgM and relative unresponsiveness of the BLT mouse-derived human T cells to specific antigenic stimulation. Finally, this report provides evidence for the first time that ex vivo treatment of human T cells with species-specific cytokines leads to partial restoration of co-stimulatory molecules required for an optimal humoral immune response. This suggests that in vivo delivery of cytokines and growth factors may improve the current second-generation SCID-Hu models for human vaccine development studies.
Materials and methods
Construction of the humanized BLT mouse model
Methods for surgical implantation of human fetal tissues and injection of haematopoietic stem cells into immunodeficient NOD/SCID mice to construct the BLT mouse model were described previously.17,18,26 Briefly, adult female NOD/SCID mice 3–5 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME), acclimated for at least 7 days and myelo-ablated by sublethal whole body irradiation (325 rads) with a Gammacell 40 Exactor (Best Theratronics, Ottawa, ON, Canada) before surgery. The animals under isoflurane anaesthesia were surgically implanted with fragments of human thymus and liver tissues (∼ 1 mm3) from the same fetal donor of gestational age 17–20 weeks (Advanced Bioscience Resources, Alameda, CA) under the left kidney capsule. Subsequently, each mouse was injected via the tail vein with 0·25 × 106 CD34+ HSC in 200 μl PBS. The CD34+ HSC were isolated from the remaining portion of the same fetal liver using anti-human CD34 microbeads (Miltenyi, Auburn, CA) with > 98% purity determined by flow cytometry after staining with anti-CD34-PE (Miltenyi). All engrafted mice were housed under Biosafety Level-2 conditions and provided with autoclaved food and water supplemented with Baytril (Bayer, Shawnee Mission, KS). All animal experiments were approved by the Institutional Animal Care and Research Committee and the Office of Human Subjects Research at the Dana Farber Cancer Institute (DFCI), Boston, MA.
Determination of human immune reconstitution by flow cytometry
To measure the levels of human immune reconstitution in different tissues of engrafted animals, representative mice were killed 16 weeks post-engraftment. Peripheral blood, bone marrow, spleen and the human thymus–liver tissue implant were harvested. Blood was collected following cardiac puncture and mononuclear cells were purified on a Ficoll density gradient. Blood was also obtained from live animals via the mandibular route. Bone marrow cells were obtained by flushing the femoral cavity with an insulin syringe (Becton Dickinson, Franklin Lakes, NJ) containing cold RPMI-1640 medium (Invitrogen, Carlsbad, CA), and the cell suspension was passed through a 70-μm cell strainer. Splenocytes were prepared after homogenizing the spleen using a plunger and straining the cell suspension as described above. The fetal thymus–liver tissue implant was excised from the kidney and digested in RPMI-1640 medium supplemented with 1 mg/ml collagenase (Roche, Nutley, NJ), homogenized and the mononuclear cell suspension was separated after straining as described earlier. Human peripheral blood mononuclear cells (PBMC) were purified from leukapheresis blood collars obtained at the Kraft Family Blood Donor Center, DFCI, following standard Ficoll density gradient centrifugation techniques. Immunophenotyping was performed by staining the mononuclear cells with fluorochrome-conjugated antibodies to different cell surface markers, followed by multi-colour flow cytometry using a FACSCantoII or LSRII (BD Biosciences, San Jose, CA). The following fluorochrome-conjugated antibodies were used: anti-human CD45-allophycocyanin (APC) (clone H130), CD19-phycoerythrin (PE) (MB19-1), CD3-PECy5 (UCHT1), CD10-APC (CB-CALLA), CD27-PECy7 (O323), CD4-fluorescein isothiocyanate (FITC) (OKT-4), CD8-PE (OKT-8), anti-mouse CD45 (30-F11) (all from eBioscience, San Diego, CA), anti-human IgM-PECy5 (G20-127), IgG-FITC (G18-145), IgD-PE (IA6-2) (all from BD Biosciences) and CD5-FITC (UCHT2) (Biolegend, San Diego, CA). Gating was performed on viable lymphoid cells based on the forward and side scatter profiles of the total cells, and stained cells were analysed within the lymphoid gate. A comparison between the percentages of human CD45+ and endogenous mouse CD45+ was performed to measure the level of immune reconstitution in BLT mice, and other markers were used to analyse the different human B-lymphocyte subsets as described later. Background staining was determined using the corresponding isotype controls or staining cells isolated from non-engrafted animals. Data were analysed using flowjo version 8·6·3 (Tree Star, Ashland, OR).
Immunization of BLT mice with recombinant viral envelope antigens
Recombinant HIV-1 envelope gp140 antigen was expressed from the plasmid gp140Δ683(–/FT), a kind gift from Xingzhen Yang (DFCI), and the protein was purified as described by Doria-Rose et al.28 This plasmid, encoding amino acids 1 to 683 of HIVgp140 fused with a T4 phage fibritin trimerization domain and a C-terminal histidine tag, was transiently transfected in 293F suspension cells using 293Fectin transfection reagent (Invitrogen). The culture supernatant was harvested 4 days after transfection, and the Env trimer protein was purified using Nickel Sepharose 6 Fast Flow (GE Healthcare, Piscataway, NJ). Following elution, concentration and dialysis against PBS, the conformation of the protein was analysed on SDS–PAGE and functional activity was confirmed by ELISA using monoclonal antibodies newb12 and 4E10 to the HIV-1 envelope glycoprotein (data not shown). Recombinant WNV-E protein was a kind gift from Dr Michel Ledizet (L2 Diagnostics, New Haven, CT). As described earlier,29 the WNV-E protein (virus strain 2741) corresponding to amino acids 1 to 406 was expressed and purified from stably transfected Drosophila S2 cells.
BLT mice, approximately 5 months post-engraftment, and those that had at least 20% human CD45+ cells in the periphery were immunized with the recombinant viral envelope antigens mixed with a synthetic adjuvant IC31®, which has been reported to augment immune responses in neonatal and aged mice.30,31 IC31® was prepared by combining a cationic antimicrobial immunostimulatory peptide NH2-KLKLLLLLKLK-COOH (Chi Scientific, Maynard, CA) and negatively charged CpG containing oligodexoynucleotide ODN1a, 5′-ICI CIC ICI CIC ICI CIC ICI CIC IC-3′ (Integrated DNA Technologies, Coralville, IA). For every immunization, 10 μg of each antigen was mixed with 100 nmol KLKL5KLK and 5 nmol ODN1a in a final volume of 200 μl PBS and administered intramuscularly in both quadriceps muscles (100 μl per site). Mice were immunized on days 0, 21 and 45 and bled on days 0 (pre-immune), 15, 30, 45 and 90 to monitor seroconversion and antigen-induced B-cell differentiation.
Human IgM and IgG ELISA
Following bleeding via the mandibular route, whole blood was diluted 1 : 1 with PBS buffer containing 2 mm EDTA. The preparation was centrifuged at 500 g for 10 min and the cell-free plasma fraction was collected for ELISA. Total human IgM and IgG levels in the plasma of BLT mice were analysed using the Human IgM and IgG ELISA kits, respectively (Bethyl Laboratories, Montgomery, TX). Seroconversion following immunizations was analysed by standard ELISA techniques to determine titres of antigen-specific human IgM or IgG in individual or pooled BLT plasma samples. Briefly, 96-well plates were coated overnight at 4° with 1 μg/ml recombinant HIVgp140 or WNV-E protein in coating buffer (eBioscience). Plates were washed with PBS with 0·05% Tween-20 (PBST) and then blocked with 1% BSA in PBS for 1 hr at 37°. After removal of blocking buffer, different dilutions of BLT plasma were added and incubated for 2 hr at 37°. After at least six washes with PBST, the plates were incubated for another hour at 37° with anti-human IgG or anti-human IgM, both conjugated with horseradish peroxidase (Thermo Scientific, Rockford, IL). After extensive washing as before, the reaction was developed with SureBlue TMB Microwell substrate and stopped with TMB Stop Solution (both from KPL, Gaithersburg, MD). The absorbance was read at 450 nm using a Benchmark Plus microwell spectrophotometer (BioRad, Hercules, CA).
In vitro lymphocyte proliferation assay
To analyse antigen/mitogen-induced stimulation, splenocytes and PBMC were isolated as described earlier from BLT mice and normal human donors, respectively, and labelled with carboxyfluorescein succinimidyl ester (CFSE; Invitrogen). 1 × 106 cells/ml were incubated with 5 μm CFSE at 37° for 7–8 min and the reaction was stopped with cold complete RPMI-1640 medium. After washing three times, the labelled cells were cultured in round-bottom 96-well plates (Corning, Lowell, MA) for 5 days at 1 × 105 cells/well with 0·1–10 μg/ml phytohaemagglutinin (Sigma, St Louis, MO), recombinant HIVgp140 or WNV-E proteins. Cells were stained with anti-CD3 PECy5, and proliferation was measured as a function of CFSE dilution in CD3+ gated cells analysed by flow cytometry. To rule out the issue of assay sensitivity, thymidine incorporation assay to detect cellular proliferation was also performed as described3. Cells were plated in triplicate in the presence of specific antigen/mitogen as described and cultured for 72 hr following which they were pulsed with 1 μCi [3H]thymidine/well overnight. Cells were then washed, lifted on a filter paper (Filtermate Harvester, Perkin Elmer, Waltham, MA) and [3H]thymidine incorporation was measured using a scintillation counter (1450 Microbeta Trilux; Perkin Elmer). In another experiment, T cells were first enriched from human PBMC or BLT splenocytes using the EasySep Human T cell purification kit (Stem Cell Technologies, Vancouver, BC, Canada). Cells were cultured in medium alone or with human T-cell cytokines (R&D Systems, Minneapolis, MN), e.g. IL-2 at 20 U/ml and IL-7 (50 ng/ml). After 7 days in culture, cells were stained with anti-human CD3-PECy5 and other T-cell-specific surface markers and their expression pattern was analysed by flow cytometry.
Statistical analysis
All statistical calculations were performed using graphpad prism version 5 (Graphpad software, La Jolla, CA). Mann–Whitney U-tests (two-tailed) were performed to determine statistically significant differences between median values of each data set. P values < 0·005 were considered significant.
Results
Construction of BLT mice
Previous reports demonstrated reconstitution of adult NOD/SCID mice with human lymphocytes following surgical implantation of human fetal liver and thymus tissues under one or both kidney capsules along with intravenous delivery of autologous CD34+ HSC. In contrast to Melkus et al.18 where irradiation and HSC delivery was performed 3 weeks following surgical implantation of the mice, all the procedures in our study (initial conditioning via sublethal irradiation followed by implantation of the fetal liver and thymus tissue sandwich under the left kidney capsule and HSC delivery) were performed within a 12-hr period. The implantation scheme was also slightly different from that reported by Brainard et al.,26 where implantations of fetal tissues were performed bilaterally under both kidney capsules. By 16 wk post-engraftment, a significant repopulation of human CD45+ lymphocytes in the peripheral blood (PB) and other tissues, including bone marrow (BM), spleen and the thymus–liver implant (Fig. 1a–d) was observed. This observation is consistent with one of the first reports of humanized BLT mouse construction17 where maximal reconstitution with human cells was observed between 12 and 18 weeks after engraftment of adult NOD/SCID mice. The objective was to study the humoral immune response in this model, so the phenotypic and functional analysis of the human B and T lymphocytes generated in the BLT mice were characterized in detail. There was considerable variation between animals in the level of reconstitution with human CD45+ cells in spite of the fact that the fetal tissues and HSC for engraftment were derived from a single donor. When measured at 16 wk post-engraftment, total percentages of human CD45+ lymphocytes in the PB ranged between 24 and 87% (median 50%, n = 14). Similar variability was also observed in the percentages of CD45+ CD19+ human B cells (range < 1 to 27%; median 17%) and CD45+ CD3+ human T cells (range 5–78%; median 27%) in the periphery of the BLT mice. The distributions of B and T lymphocytes in the primary (bone marrow) and secondary (spleen) lymphoid tissues were as expected. In the representative animals (n = 4) that were sacrificed, the percentages of T lymphocytes (range 81–87%; median 85%) were considerably higher in the spleen compared with in B lymphocytes (range 11–16%; median 13%). However, the reverse was observed in the bone marrow where the median reconstitution level for T lymphocytes was 13% (range 1–33%) versus 72% (range 28–75%) for B lymphocytes. The fetal thymic tissue engrafted under the kidney capsule was also harvested and the lymphoid fraction constituted > 98% human T cells. Long-term stability of the human haematopoietic graft in the BLT model was also analysed. At ∼ 8 months post-engraftment that marked the end of the present study, the median levels of peripheral human CD45+, CD3+ and CD19+ cells in the BLT mice were 62% (range 36–84%, n = 10), 44% (range 7–65%) and 14% (range 2–49%), respectively. These observations suggested that at a gross phenotypic level and in terms of tissue localization, the human B and T lymphocytes generated in the humanized BLT mice closely approximate that found in normal humans and that the engraftment was relatively stable over a prolonged time period.
Figure 1.
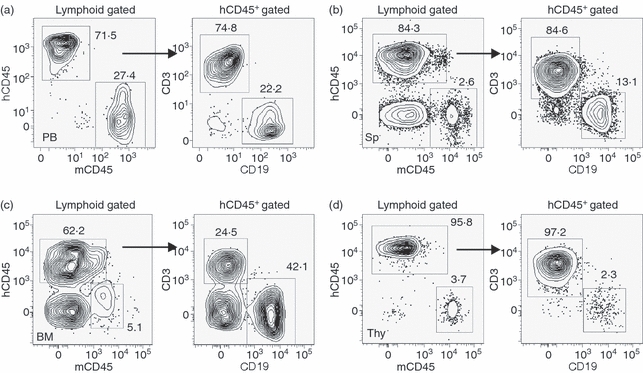
Development of human lymphoid cells in NOD/SCID mice engrafted with human fetal thymus–liver tissues and autologous CD34+ haematopoietic stem cells (BLT mice). (a–d) Flow cytometry analysis of the total human haematopoietic cells (hCD45+) along with human B (CD19+) and T (CD3+) lymphocytes in the peripheral blood (PB), spleen (Sp), bone marrow (BM) and the thymus–liver implant (Thy) of a representative BLT mouse 16 weeks after engraftment. Numbers indicate percentages of the total gated (indicated above each plot) cell populations. Percentages of endogenous mouse haematopoietic cells (mCD45+) are also shown in the different tissues of the BLT mice. Except for anti-mCD45, all antibodies were specific for the indicated human cell surface markers.
Elevated levels of CD5+ B cells in BLT mice
Naive B lymphocytes in both mice and humans are predominantly follicular B cells, also called B-2 cells. In mice, a B-cell subset, distinct from the follicular B cells in terms of lineage and anatomical localization, has also been described. This latter class of B cells, also known as B-1 cells, are characterized by the surface expression of the CD5 antigen that is not found on B-2 cells and are known producers of natural antibodies, predominantly of the IgM subclass.32 The murine B-1 cells have been found to be mostly localized in the peritoneal and pleural cavities and are scarce in the circulating blood or other lymphoid tissues.33 It was previously reported that NOD/SCID mice engrafted with human cord-blood-derived HSC generate high levels of CD19+ CD5+ human B lymphocytes in the lymphoid tissues.14 It is possible that the fetal progenitor cells (cord blood or fetal-liver-derived HSC) in comparison with their adult counterpart (mobilized peripheral blood or bone-marrow-derived HSC) has a higher potential to differentiate into CD5+ B cells; however, it has also been demonstrated that the lack of optimal T-cell–B-cell signalling may bias the B-cell developmental pathway towards the production of this B-cell subset.34 As implantation of human fetal thymus in the BLT model resulted in apparently normal T-cell development (determined by surface marker expression, see supplementary material, Fig. S1), it was investigated whether normal human B-cell ontogeny is recapitulated in these animals. A comparative flow cytometry analysis of the BLT-derived human B cells generated 16 weeks post-engraftment with those recovered from peripheral blood of healthy human adults is shown in Fig. 2. The majority (∼ 95%) of the B cells from a representative normal adult human PBMC donor displayed a CD19+ CD5− phenotype, typical of follicular B cells (Fig. 2a, left panel). A small percentage (5·8%) of the CD19+ CD5+ B -ell population was also observed. In striking contrast to the cellular distribution in human PBMC, markedly elevated levels of circulating CD19+ CD5+ human B cells were observed in the BLT mice (Fig. 2b, left panel). The median percentage of CD5+ human B-cell levels in the BLT PB was ∼ 10-fold higher (median 59%; range 50–65%, n = 5) than seen in a typical adult human donor.
Figure 2.
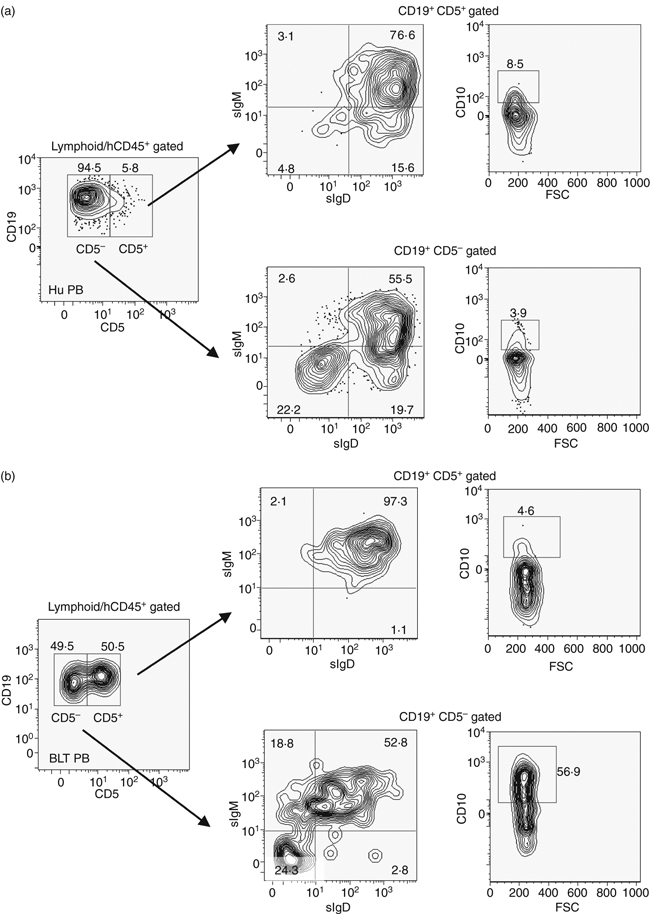
Comparative distribution of CD5+ and CD5− human B lymphocytes in a normal human donor and BLT mice. (a) Human peripheral blood mononuclear cells (Hu PB) and (b) peripheral blood mononuclear cells from a representative BLT mouse (n = 5) 16 weeks after engraftment (BLT PB) were analysed by flow cytometry following gating as indicated above each plot to enumerate the CD19+ CD5+ and CD19+ CD5− B lymphocytes. Each of these two populations was further analysed (indicated by arrows) for the expression of surface IgM (sIgM), surface IgD (sIgD) (middle panels) and CD10 (right panels).
The maturity levels of these human B-cell populations were investigated by analysing the surface expression of IgM, IgD and CD10. Mature follicular B cells are generally CD19+ IgM+ IgD+, whereas CD10 expression defines an immature phenotype and is found at high levels in the bone marrow, the site of B-cell development in the adult. Normal human peripheral B cells, either CD5+ or CD5−, are predominantly IgM+ IgD+, with < 10% of total B cells expressing the CD10 marker. Analysis of the human PBMC showed that a majority of the peripheral human B cells were mature as expected, with only 8·5% and 4% of CD5+ and CD5− cells expressing the CD10 marker, respectively (Fig. 2a, right panels). In the BLT mice, a marked difference was observed between the levels of IgM+ IgD+ human B cells in the peripheral CD5+ (median 91%; range 85–98%) versus the CD5− (median 51%; range 49–56%) B-cell subsets (Fig. 2b, middle panels). Interestingly, a significantly larger number of CD19+ CD5− cells stained strongly for surface CD10 expression (median 54%; range 44–57%) compared with that of CD19+ CD5+ cells (median 7%; range 4.6–12%), indicating a possible developmental deviation of human B-cell ontogeny in the BLT mice (Fig. 2b, right panel).
We also investigated whether the CD5+ CD10+ B-cell subset represented a transitional/pre-naive B-cell population emigrating from the bone marrow because of aberrations in bone marrow checkpoint control mechanisms.35 In such a scenario, equivalent levels of CD10+ CD5+ human B cells in the bone marrow and in the periphery may be expected. However, as seen in Fig. 3a, left panel, the number of CD5+ B cells in the bone marrow was significantly lower than that found in the spleen of BLT mice. The median percentage of CD5+ human B cells in the bone marrow was < 10% (range 3·4–20%; n = 4), whereas > 99% of the total B cells expressed CD10, as expected (Fig. 3a, right panel). In contrast, > 50% of total CD19+ B cells in the spleen stained strongly for CD10 (Fig. 3b). The relative expression of CD10 in the splenic CD19+ CD5+/CD19+ CD5− subsets was similar to that found in the PB (Fig. 2b, right panel) with CD10 expression higher in the CD5− (median 69·5%; range = 25–73%) compared with the CD5+ B-cell population (median 5%; range 4–7·5%) (data not shown). Intriguingly, this CD19+ CD5+ B-cell subset and not the ‘normal’ CD5− B cells appeared to have attained a comparatively more mature state, at least phenotypically, as evidenced by higher numbers of IgM+ IgD+ cells as well as lower levels of CD10 expression. Therefore, our observations suggest that a majority of the peripheral CD5+ B cells represent a distinct subset from the BM CD5+ B cells. This pool of peripheral B cells may have arisen as a result of acquisition of the CD5 marker during later stages of B cell development occurring outside the bone marrow. This is also supported by previous reports of higher numbers of CD5+ human B cells found in the spleen of reconstituted NOD/SCID mice compared with that in the bone marrow.14
Figure 3.
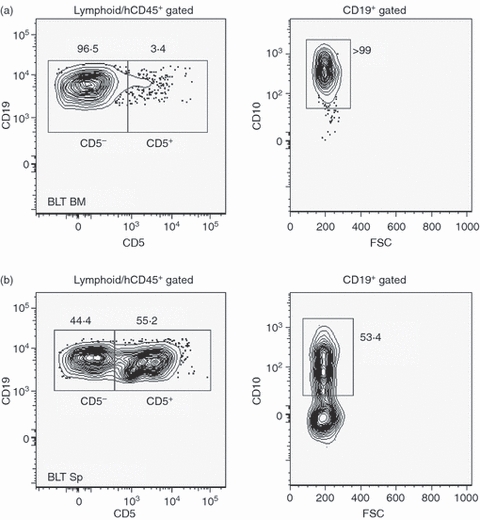
Comparative distribution of CD19+ CD10+ immature human B cells in BLT mice. Single cell suspensions prepared from (a) the bone marrow and (b) the spleen of a representative BLT mouse (BLT BM and BLT SP, respectively, n = 4) were analysed by flow cytometry following gating as indicated above the plots to enumerate the CD19+ CD5+ and CD19+ CD5− B lymphocytes. Expression of CD10 was also evaluated in the total CD19+ B-cell population in the BM and Sp (right panels). Numbers indicate percentages of the total gated cell populations. All antibodies were specific for the indicated human cell surface markers. FSC, forward scatter.
Generation of predominantly human IgM in humoral immune responses
To interrogate the antibody response elicited by the CD19+ CD5+ B-lymphocyte population, BLT mice were immunized with recombinant HIVgp140 and WNV-E protein complexed with a two component adjuvant IC31® delivered intramuscularly 5 months after engraftment and then analysed for the generation of antigen-specific human IgM and IgG. Previous reports demonstrated that immunization with either of these two immunogens elicited high-affinity antigen-specific as well as virus neutralizing IgG antibodies in BALB/c and C57BL/6 mice.29,36 The activation of Toll-like receptors on B cells is suggested to play an important role in the generation of antigen-specific antibody responses,37 and IC31® was reported to act via the Toll-like receptor 9 pathway.31 Additionally, IC31® was chosen because conventional adjuvants like alum or complete Freund's adjuvant have failed previously to elicit a strong antigen-specific IgG response in the BLT mice.27 As shown in Fig. 4, a single immunization induced a significant level of seroconversion measured 2 weeks following antigen delivery. Both HIVgp140 and WNV-E antigen binding human IgM and IgG could be detected in the plasma of immunized BLT mice at levels significantly higher than the pre-immune plasma from the same animals (P<0·0001). The mean absorbance (450 nm) readings for human IgM binding to HIVgp140 and WNV-E were both ∼ 3·5-fold, and the corresponding IgG readings were greater than twofold higher than that obtained from the pre-immune samples. However, it was evident that the antibody response was largely mediated via the IgM subclass with levels of IgG being only slightly detectable.
Figure 4.
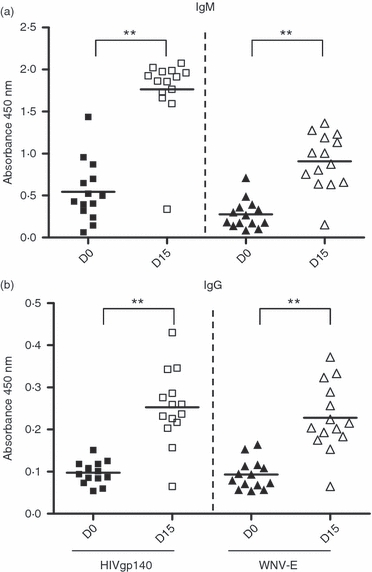
Antigen-specific antibody responses in immunized BLT mice. (a) HIVgp140 (left) and West Nile virus envelope (WNV-E) (right) specific human IgM response as detected by ELISA in BLT plasma samples obtained on day 0 before immunizations (D0) and on day 15 following the first immunization with recombinant viral antigens (D15). (b) HIVgp140 (left) and WNV-E (right) specific human IgG responses in D0 and D15 BLT plasma samples as detected by ELISA. Each symbol is representative of a single BLT mouse. **P<0·0001 determined by two-tailed Mann–Whitney U-test to analyse significant differences between median values of the datasets (D0 versus D15). Individual plasma samples were tested at a 1 : 100 dilution.
Flow-cytometry-based analysis was performed to investigate whether the B cells in immunized BLT mice were able to undergo an antigen-induced differentiation process that is normally manifested by expression of a CD27+ memory phenotype.38 Surface expression of IgG, an indicator of antibody class switching, was also analysed by multi-colour flow cytometry. As antigen presentation and subsequent antigen-induced B-lymphocyte differentiation occurs in secondary lymphoid organs, splenocytes from immunized BLT mice (n = 4) at 30 days after the primary immunization were analysed for the presence of the relevant surface markers. As shown in Fig. 5a, BLT splenocytes were categorized into CD19+ CD5+/CD19+ CD5− human B-cell subsets and the expressions of CD27 and surface IgM and IgG were analysed. As a positive control, human PBMC from a representative normal donor were similarly stained for the expressions of CD27 and surface IgM and IgG (Fig. 5b). Human peripheral blood B cells expressing a memory phenotype were observed at the expected levels. However, the BLT spleen-derived human B cells demonstrated extremely low levels of CD27 expression. Surface IgG was undetectable in both the B-cell subsets; however, the CD5+ B-cell subset had at least greater than threefold higher levels of surface IgM (median 30%; range 23–45%; n = 4) compared with the CD5− subset (median 14%; range 9–16%) (Fig. 5a). In comparison, higher numbers of CD19+ CD27+ B cells were found in the CD5− compared with the CD5+ subset in the representative human donor peripheral blood (∼ 35% versus ∼ 9%), and antibody class switching was also observed at higher levels in the CD5− subset (∼ 20% versus ∼ 4% in the CD5+ subset) as expected (Fig. 5b).
Figure 5.
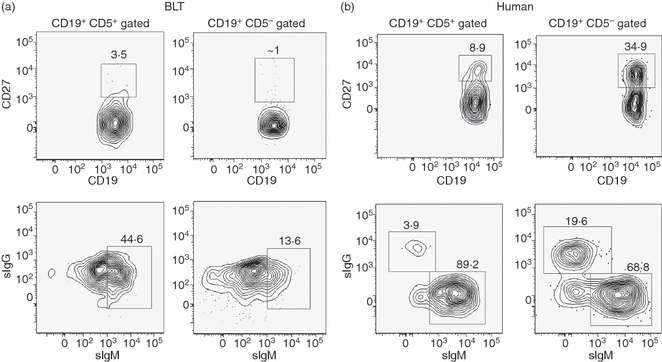
Analysis of a memory phenotype and surface immunoglobulin expression in the CD19+ CD5+ and CD19+ CD5− human B-lymphocyte subsets in splenocytes from immunized BLT mice (30 days following primary immunization) and normal human peripheral blood mononuclear cells (PBMC). (a) Representative BLT (n = 4) splenocytes and (b) PBMC from a normal human donor were isolated as described in the Materials and methods, and live cells were gated on CD19+ CD5+ or CD19+ CD5− expression as in Fig. 2. Each subset was analysed by flow cytometry for the expression of surface CD27 (upper panel) or surface IgM (sIgM) and surface IgG (sIgG) (bottom panel). Numbers indicate percentages of the total gated (indicated above each plot) cell populations. All antibodies were specific for the indicated human cell surface markers. FSC, forward scatter.
Booster immunizations were performed to investigate whether any further enhancement in the antibody response could be obtained. Mice were boosted on days 21 and 45 following primary immunization with the same antigens and dose as the initial inoculation. To investigate whether secondary immune responses were generated, e.g. increased antibody titres, antibody class switching, etc., titration of pooled plasma samples was performed following every immunization and up to a period of 3 months. As shown in Fig. 6 (upper panel), a modest increase in antigen-specific IgM titres was observed following the second immunization, which remained relatively stable for the entire period of the study. By contrast, a gradual decrease in specific IgG titres was observed after day 30 after the primary immunization (lower panel).
Figure 6.
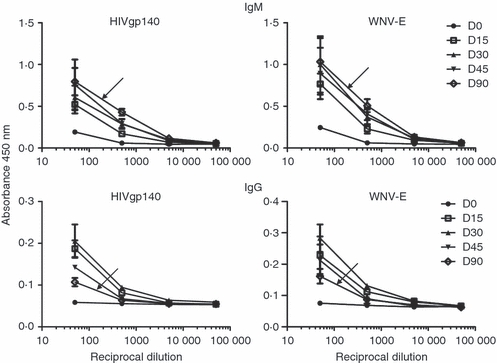
Antigen-specific antibody titres in immunized BLT mice. Mice immunized with recombinant HIVgp140 and West Nile virus envelope protein (WNV-E) on days 1, 21 and 45 were bled on days 0, 15, 30, 45 and 90, and pooled plasma samples (n ≥ 5) were titrated against respective antigens (indicated on top of each graph) and analysed for antigen-specific human IgM (top panel) and IgG titres (bottom panel). Samples were assayed in duplicate, and the means ± standard deviations are shown. The arrowhead indicates the titration curve for the pooled plasma sample obtained on day 90 after primary immunization.
Plasma levels of total human IgM and IgG were measured in the immunized BLT mice at similar time-points, as indicated in Fig. 6, to determine a correlation, if any, to the observed kinetics of vaccine-induced antibody response. Total IgM levels in individual mouse plasma increased from 15 ± 4 μg/ml in the pre-immune (n = 4) to ∼ 180 ± 37 μg/ml at day 15 post-immunization, which remained relatively stable until day 45 with a decline (∼ 50 ± 10 μg/ml) noted at day 90. Total IgG levels remained significantly lower throughout the duration of the experiment. Although it was barely detectable in the pre-immune plasma and at day 90, the levels increased to 5–10 μg/ml between days 15 and 45 after vaccination. Therefore, overall low levels of total IgG corresponded to the weak antigen-specific IgG response. Similar low levels of human IgG in the plasma of humanized mice have been reported earlier.11
Normally, B cells receiving antigenic stimulation become activated via cognate T-cell ‘help’, followed by differentiation into memory and plasma cells and specific antibody production. Results in Fig. 6 indicated no significant improvement in antibody titres over time, suggesting an impediment in the process of affinity maturation of antigen-stimulated B cells. A robust class switching mechanism to an IgG subclass was apparently absent, indicating no or reduced participation of follicular B cells in generating the humoral immune response. To determine whether the human B-cell differentiation as a result of antigenic stimulation required a longer time and repeated antigenic encounter, PBMC or splenocytes of the immunized mice were examined for surface CD27 antigen expression on day 90 after primary immunization (Fig. 7b). High levels (> 80%) of late developing CD19+ CD27+ cells were observed in the PBMC of the immunized BLT mice. This differentiation process apparently occurred only with multiple immunizations, because a single immunization did not induce significant memory B-cell generation (Fig. 7a). Furthermore, the CD27 expression co-localized with surface IgM-expressing B cells but not with surface IgG-expressing B cells. This was expected because antibody class switching was absent in the BLT mice. Interestingly, this CD19+ CD27+ IgM+ non-switch memory B-cell population was entirely of the CD5+ expressing B-cell subset.
Figure 7.
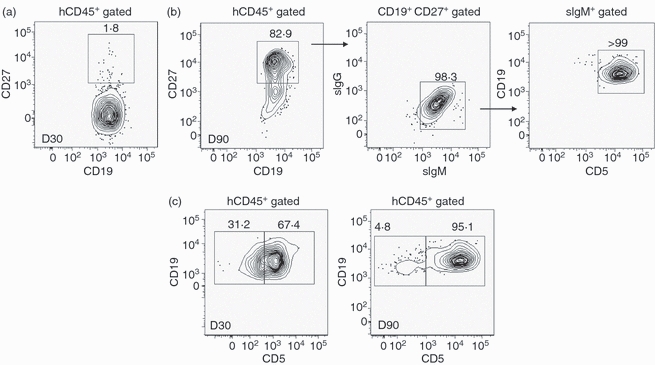
Characterization of surface expressed CD27-associated memory phenotype in human B cells in immunized BLT mice. (a) Peripheral blood lymphocytes from a representative BLT mouse (n = 3) were analysed for the presence of CD27+ CD19+ double-positive human memory B cells by flow cytometry 30 days (D30) after immunization. (b) Peripheral blood lymphocytes from a representative BLT mouse were analysed for the presence of CD27+ CD19+ double-positive human memory B cells by flow cytometry on D90 after immunization. The doublet cell population was further analysed for the surface expression of human IgG/IgM/CD5. The representative response shown here was observed in two out of three BLT mice tested. (c) Peripheral blood lymphocytes from a representative BLT mouse (n = 3) on D30 and D90 samples after immunization were analysed for the presence of total numbers of CD19+ CD5+ and CD19+ CD5− B lymphocytes. Numbers indicate percentages of the total gated (indicated above) cell populations. All antibodies were specific for the indicated human cell surface markers.
Surprisingly, no CD19+ CD27+ IgM+ cells were detected in the splenocytes at the same time-point in the same animals (data not shown). Therefore, the observed differentiation to a memory phenotype by the peripheral CD5+ B cells may not be a result of a typical germinal centre reaction, which is an outcome of T-cell-dependent B-cell responses occurring in secondary lymphoid organs. It cannot be ruled out that this differentiation to an IgM+ memory phenotype occurred in other lymphoid tissues, e.g. draining lymph nodes (not examined); however, these tissues appeared to be poorly developed. The overall CD19+ CD5+ B-cell subpopulation also increased over time (Fig. 7c). Taken together, our observations suggest a prominent role of the CD5+ B-cell subset in generating the observed humoral immune response in the BLT mice that appeared to occur in a T-cell-independent way.
Lack of T-cell ‘help’ in BLT mice
It was reported previously that in a humanized NOG/NSG mouse model, human T lymphocytes developed normal phenotypic markers and were responsive to mitogenic stimulation but were non-responsive to specific immunogens.12 This led us to hypothesize that the predominantly IgM restricted antibody response in the BLT mice occurred as a result of deficiencies in T-cell homeostasis and signalling. The overall functionality in terms of the proliferative potential of the human T lymphocytes in the immunized BLT mice in response to specific antigenic stimulation was measured. Splenocytes were harvested from BLT mice 2 weeks after booster immunization, labelled with CFSE and cultured in the presence of different concentrations of recombinant HIVgp140 or WNV-E proteins for 5 days. No significant proliferative responses in the CD3+ gated cell population were observed between cells cultured in medium alone or with 1–10 μg the recombinant viral envelope proteins. Similar results were obtained in a [3H]thymidine incorporation assay with cells cultured as described above (data not shown). Proliferation of the T lymphocytes was observed when BLT splenocytes were cultured in the presence of phytohaemagglutinin, a T-cell mitogen for the same duration (Fig. 8a, lower panel). However, a 10-fold higher concentration of phytohaemagglutinin (10 μg/ml) was required to obtain the same level of proliferation with BLT splenocytes as with human PBMC cultured similarly (Fig. 8a, upper panel).
Figure 8.
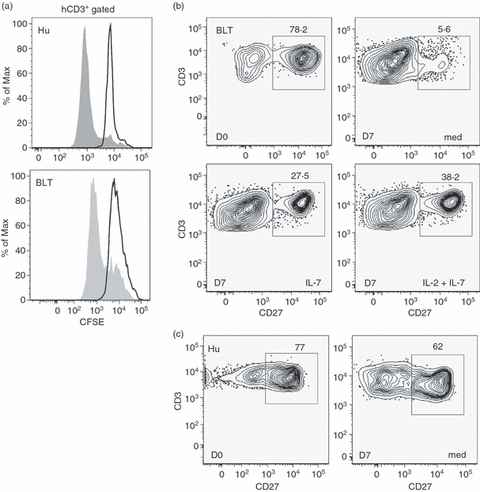
In vitro analysis of human T cells from BLT mice. (a) Human (Hu, top) and a representative BLT mouse (BLT, bottom) peripheral blood lymphocytes and splenocytes, respectively, were labelled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with phytohaemagglutinin in culture for 5 days as described in the Materials and methods. Cell proliferation in the CD3+ gated population was analysed by flow cytometry as a function of CFSE dilution (shaded histogram). (b) Representative BLT mouse splenocytes and (c) normal human donor peripheral blood lymphocytes were enriched for human T lymphocytes and cultured in vitro for 7 days in medium alone (med) or in the presence of human cytokines, interleukin-7 (IL-7) and IL-2, individually or in combination as indicated. Levels of surface-expressed B-cell co-stimulatory factor CD70 ligand (CD27) on CD3+ T lymphocytes were analysed by flow cytometry pre-culture (D0) and after 7 days in culture (D7) under various conditions (med, IL-7 or IL-2 + IL-7). Numbers indicate percentages of the total cell populations represented in the graphs. All antibodies were specific for the indicated human cell surface markers. All experiments were repeated three times.
Additionally, the profile of human T cells purified from different tissues of the BLT mice (spleen, peripheral blood and bone marrow) and cultured in vitro under different conditions for 7 days was examined. The results from a representative experiment are shown in Fig. 8(b). Approximately 13-fold lower surface expression of the CD27 molecule was observed in the T cells isolated from BLT spleen and cultured in medium for 7 days (day 7) compared with pre-culture conditions (day 0). The interaction between CD27 on T cells and CD70 on B cells has been reported to be a major co-stimulatory signal leading to activation of B lymphocytes and antibody production.39 Interestingly, when the BLT-derived splenic T cells were cultured in the presence of human T-cell cytokines such as IL-7 or a combination of IL-2 and IL-7, the loss of CD27 expression could be partially rescued. Similar restorative effects of the T-cell cytokines were noted with T cells purified from peripheral blood and bone marrow of the BLT mice (data not shown). In comparison, T cells purified from normal human PBMC did not show significant loss of the CD27 co-stimulatory molecule when cultured in vitro (Fig. 8c). Hence, our results suggest that there may be an inherent defect in the human T cells developed and matured in the xenogeneic environment and that T-cell function may be potentially restored by treatment with human cytokines.
Discussion
The humanized BLT mouse model offers a tremendous potential in translational human biomedical studies; however, as our current study demonstrates, further improvements in the model are needed for it to become a robust platform for infectious disease vaccine research where the generation of neutralizing antibodies largely determines vaccine efficacy. While human lymphocytes are detectable in these mice following engraftment with human CD34+ stem cells and tissues and gross examination shows localization of these regenerated human cells in the expected niches, the overall composition and functionality of this engrafted immune system deviates from that of normal humans in several ways. This study indicates that one of the major differences in the human B-lymphocyte composition is the high incidence of a distinct CD19+ IgM+ CD5+ B-cell subset in the periphery and spleen of the BLT mice. High levels of circulating and splenic human B lymphocytes expressing surface CD5 antigen were previously reported in NOD/SCID and NOD/SCID/γc−/− mice transplanted with CD34+ HSC, regardless of the stem cell source (cord blood, bone marrow or mobilized PBMC).11,13,14 Hence, it is important to address whether the CD5+ B cell noted in all the different humanized mouse models represents a distinct B-cell subset (e.g., B-1 B cells) or a pool of immature B cells emigrating from the bone marrow as a result of a defective developmental pathway in the mouse environment. CD5-expressing human peripheral B lymphocytes have been non-uniformly categorized as either being derived from functionally ‘immature transitional’ B lymphocytes with reduced responsiveness to antigenic stimulation and impaired in immunoglobulin secretion40 or as ‘pre-naive’ B cells with functionalities comparable to naive mature B cells.41 Notably, both of these types of B cells have been found to be expanded in immune disorders such as X-linked lymphoproliferative disease40 and systemic lupus erythematosus,41 as well as in subjects recovering from stem cell transplantation.40 On the other hand, a prevailing theory of B-1 cell ontogeny describes a phenomenon of ‘induced differentiation’ where B-2 precursors or mature B cells may be induced to express the CD5 antigen.42 The present study provides evidence that the peripheral CD19+ CD5+ B-cell subset in the BLT mice was not generated in the bone marrow and that the CD5 marker was acquired peripherally. The possibility that the acquisition of the CD5 marker occurs outside the bone marrow environment is supported by a previous report showing that the co-culture of CD19+ IgM− CD5− bone marrow cells with CD19+ IgM+ CD5+ splenic cells led to expression of CD5 on the bone marrow cells.13
In human patients following allogeneic blood stem cell transplantation, an increase in CD5-expressing B cells is observed in the circulation (20–30% of total B lymphocytes), which gradually decreases over time.43 However, the situation is different in the humanized mice where engraftment of human tissues and cells and differentiation of the transplanted cells occur in a xenogeneic environment. Therefore, there may be other complex physiological processes, e.g. lack of species-specific growth factors, which lead to the generation of the CD5+ B cells. A recent study described the generation of human monoclonal antibodies from humanized BALB/c Rag2−/−γc−/− mice vaccinated with commercially available hepatitis B virus vaccine and tetanus vaccine.44 In that study, the authors demonstrated levels of CD27+ B-cell differentiation as well as evidence of class switching higher than what we have observed in the present study. One possible explanation for this difference is that the authors used newborn mice for their reconstructions, and therefore the human immune cells were introduced into a less differentiated background than in the adult mice used in our studies. It is noteworthy that in spite of the higher level of B-cell differentiation observed in the neonatal model, human B-cell clones expressing antigen-specific IgM only but not IgG were recovered from the immunized mice. A possible prevalence of a CD5-expressing B-cell subpopulation and their role in the IgM antibody response was not addressed. Presence of CD27-expressing B cells in neonatal humanized mouse models have also been described elsewhere.45
Clearly, additional studies are required to reveal the true nature of the CD19+ CD5+ B cells in the BLT mice as described in the present study. Given that these cells do not resemble bone-marrow-derived immature B cells, the question is whether they represent a human equivalent of murine B-1 cells. The presence of such a B-cell subset in humans had been under debate until a recent report46 where human B-1 cells were detected and functionally characterized in adult human peripheral blood. The CD5 marker, although not exclusively, was found to be associated with this B-cell subset. Interestingly, these cells that also secreted antibodies of the IgM subclass in a T-cell-independent manner formed a minor subset within the CD27+ memory B-cell population.46
The present study also demonstrates the BLT mice elicit a predominantly antigen-specific IgM antibody response, apparently with minimal T-cell involvement. The peripheral CD5+ human B cells play a major role in the vaccine-induced immune response as evidenced by predominant IgM production, no increase in antibody titre in response to booster immunizations and absence of antibody class switching, all hallmarks of B-1 cell function. Interestingly, we observed a striking level of differentiation of the PB CD5+ but not splenic B cells into a CD27+ memory phenotype in the immunized mice. Localization of this CD5+ B-cell subset in lymphoid organs by immunohistology and other means should provide further characterization of the microenvironment of such differentiation. These observations suggest that a fraction of the peripheral human CD5+ B lymphocytes in the BLT mouse model may be similar to human B-1 cells.
The generation of high levels of CD5+ B cells in the xenogeneic environment was previously attributed to suboptimal signalling by T cells.14 Our in vitro analysis of the human T lymphocytes isolated from the BLT mice also indicate a major functional deficiency when compared with T cells from normal humans. A loss in the expression of the CD27 co-stimulatory molecule on T cells has been associated with dampened primary and secondary immune responses.47 The fact that this loss could be partially restored with T-cell cytokines indicates that the microenvironment in the BLT mice may be unfavourable for complete development and differentiation of human cells. Indeed, previous studies have shown partial restoration of immune system function by exogenous delivery of species-specific factors. A modest IgG response was generated in the humanized NOD rag1−/−Prf1−/− mice upon immunization with Pneumovax (T-independent) and tetanus toxoid (T-dependent) antigens only when BLyS, a factor required for B-cell survival and proliferation, was used as an adjuvant.15 More recently, expression of human cytokines delivered by intravenous hydrodynamic injection of plasmid DNA vectors was shown to modulate overall reconstitution levels in the hNSG model and improve immune function.48 In further relevance to our observation, enhancement of in vivo human T-cell development was observed in BALB/c Rag2−/−γc−/− mice when human IL-7 was exogenously delivered as recombinant protein3,16 or via a lentivirus-based delivery system.49 Importantly, in the latter study the authors did not observe vaccine-induced human IgG generation in both control and IL-7-treated mice, suggesting the need for additional factors to impact the overall engraftment, human cell reconstitution process and B-cell development in the xenogeneic environment.
The current study suggests that BLT may be an ideal mouse model to study human B-1-cell-driven T-cell-independent humoral immune responses. This is clearly an important topic that merits further investigation as it has been reported that B-1 and B-2 cells act in concert to provide full immune protection against microbial challenge.50 Additionally, disease susceptibility with aging has been associated with the gradual decline of the number of B-1 cells and loss of natural antibodies, which are a major component of the innate immune system.46 Therefore, our studies highlight the potential of using the BLT model for experimental modulation of this class of antibodies to expand the breadth of the innate immune response. Finally, we illustrate that there are species-specific deficiencies impacting the homeostasis of the repopulating human cells in the BLT humanized mouse model. Various approaches, including delivery of human cytokines and other growth factors in vivo are currently being undertaken in our laboratory to develop a more complete and robust adaptive humoral immune system in these humanized mice.
Acknowledgments
The authors are grateful to Michel Ledizet (L2 Diagnostics, LLC, New Haven, CT) and Erol Fikrig (Yale University School of Medicine) for generously providing the recombinant WNV-E antigen and to Xingzhen Yang (DFCI) for providing the plasmid DNA construct expressing the HIVgp140 trimer. The monoclonal antibodies to HIV-1 gp41 (4E10 from Hermann Katinger) and gp120 (b12 from Dennis Burton) were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergic and Infectious Diseases (NIAID; National Institutes of Health, Germantown, MD). The authors also thank the staff of the Animal Research Facility at DFCI for excellent animal care and housing. This work was supported by NIAID grants UO1 AI0703431 to E.F., 5U01 AI061318 and 1R21 AI091557 to W.A.M. E.F. is an Investigator of the Howard Hughes Medical Institute. Financial assistance from the National Foundation of Cancer Research is also acknowledged.
Glossary
Abbreviations
- BLT
bone marrow/liver/thymus
- BM
bone marrow
- E
envelope
- HSC
haematopoietic stem cells
- Hu
human
- NOD/SCID
non-obese diabetic/severe combined immunodeficiency
- PB
peripheral blood
- Sp
spleen
- WNV
West Nile virus
Disclosures
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Flow cytometry analysis was performed on the lymphoid fraction prepared from thy-liv implant (Thy) (left panel), peripheral blood (PB) (middle) and spleen (Sp) (right) to enumerate the relative proportion of the CD4+/CD8+ human T cell subsets in the CD45+CD3+ gated population. Numbers indicate percentages of the total gated cell population. All antibodies were specific for the indicated human cell surface markers. DP, double positive immature thymocytes expected at sites of active T cell lymphopoiesis.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat Immunol. 2009;10:1039–42. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- 2.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 3.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 6.Gorantla S, Sneller H, Walters L, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2−/−γc−/− mice. J Virol. 2007;81:2700–12. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe S, Ohta S, Yajima M, et al. Humanized NOD/SCID/IL2Rγ(null) mice transplanted with hematopoietic stem cells under nonmyeloablative conditions show prolonged life spans and allow detailed analysis of human immunodeficiency virus type 1 pathogenesis. J Virol. 2007;81:13259–64. doi: 10.1128/JVI.01353-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rγnull mice. PLoS ONE. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baenziger S, Tussiwand R, Schlaepfer E, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. Proc Natl Acad Sci U S A. 2006;103:15951–6. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2−/−γc−/− (RAG-hu) mice. Virology. 2007;369:143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Lepus CM, Gibson TF, Gerber SA, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe Y, Takahashi T, Okajima A, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/γc(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–58. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 13.Matsumura T, Kametani Y, Ando K, et al. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/γ(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31:789–97. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 14.Novelli EM, Ramirez M, Leung W, Civin CI. Human hematopoietic stem/progenitor cells generate CD5+ B lymphoid cells in NOD/SCID mice. Stem Cells. 1999;17:242–52. doi: 10.1002/stem.170242. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS ONE. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Lent AU, Dontje W, Nagasawa M, et al. IL-7 enhances thymic human T cell development in ‘human immune system’ Rag2−/−IL-2Rγc−/− mice without affecting peripheral T cell homeostasis. J Immunol. 2009;183:7645–55. doi: 10.4049/jimmunol.0902019. [DOI] [PubMed] [Google Scholar]
- 17.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–92. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 18.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 19.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–6. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoddart CA, Maidji E, Galkina SA, et al. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ−/− (NSG) BLT mice. Virology. 2011;417:154–60. doi: 10.1016/j.virol.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Denton PW, Estes JD, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–14. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denton PW, Krisko JF, Powell DA, et al. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS ONE. 2010;5:e8829. doi: 10.1371/journal.pone.0008829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denton PW, Othieno F, Martinez-Torres F, et al. One percent tenofovir applied topically to humanized BLT mice and used according to the CAPRISA 004 experimental design demonstrates partial protection from vaginal HIV infection, validating the BLT model for evaluation of new microbicide candidates. J Virol. 2011;85:7582–93. doi: 10.1128/JVI.00537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar P, Ban HS, Kim SS, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–86. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol. 2009;83:7305–21. doi: 10.1128/JVI.02207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajesh D, Zhou Y, Jankowska-Gan E, Roenneburg DA, Dart ML, Torrealba J, Burlingham WJ. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rγnull mice. Hum Immunol. 2010;71:551–9. doi: 10.1016/j.humimm.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doria-Rose NA, Klein RM, Manion MM, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–99. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledizet M, Kar K, Foellmer HG, Wang T, Bushmich SL, Anderson JF, Fikrig E, Koski RA. A recombinant envelope protein vaccine against West Nile virus. Vaccine. 2005;23:3915–24. doi: 10.1016/j.vaccine.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. IC31, a two-component novel adjuvant mixed with a conjugate vaccine enhances protective immunity against pneumococcal disease in neonatal mice. Scand J Immunol. 2009;69:194–202. doi: 10.1111/j.1365-3083.2008.02225.x. [DOI] [PubMed] [Google Scholar]
- 31.Riedl K, Riedl R, von Gabain A, Nagy E, Lingnau K. The novel adjuvant IC31 strongly improves influenza vaccine-specific cellular and humoral immune responses in young adult and aged mice. Vaccine. 2008;26:3461–8. doi: 10.1016/j.vaccine.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–62. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–68. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small TN, Keever CA, Weiner-Fedus S, Heller G, O'Reilly RJ, Flomenberg N. B-cell differentiation following autologous, conventional, or T-cell depleted bone marrow transplantation: a recapitulation of normal B-cell ontogeny. Blood. 1990;76:1647–56. [PubMed] [Google Scholar]
- 35.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 36.Bower JF, Li Y, Wyatt R, Ross TM. HIV-1 Envgp140 trimers elicit neutralizing antibodies without efficient induction of conformational antibodies. Vaccine. 2006;24:5442–51. doi: 10.1016/j.vaccine.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 37.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 38.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–6. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 39.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27–CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci U S A. 1995;92:11249–53. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, Tangye SG. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176:1506–16. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–26. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 42.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–55. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Veneri D, Franchini M, de Sabata D, Ledro S, Vella A, Ortolani R, Pizzolo G, Benedetti F. Peripheral blood CD5-positive B lymphocytes (B-1a cells) after allogeneic stem cell transplantation for acute myeloid leukaemia in humans. Blood Transfus. 2008;6:220–4. doi: 10.2450/2008.0010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker PD, Legrand N, van Geelen CM, et al. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS ONE. 2010;5:e13137. doi: 10.1371/journal.pone.0013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–42. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 48.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106:21783–8. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connell RM, Balazs AB, Rao DS, Kivork C, Yang L, Baltimore D. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS ONE. 2010;5:e12009. doi: 10.1371/journal.pone.0012009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192:271–80. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


