Abstract
The intestinal epithelium is rich in γδ T cells and the gut is a site of residence for a wide variety of pathogens, including nematodes. Although CD4+ T-cell receptor (TCR) -αβ+ T helper type 2 T cells are essential for the expulsion of intestinal nematodes, little information is available on the function of γδ T cells in this type of infection. Here, we demonstrate two major functions of γδ T cells as a potently protective T-cell population against Nippostrongylus brasiliensis infection using γδ T-cell-deficient (TCR-δ−/−) mice. First, γδ T cells are required to initiate rapid expulsion of adult worms from the intestine and to limit egg production. Second, γδ T cells prevent the pathological intestinal damage associated with nematode infection, evident by increased clinical disease and more severe microscopic lesions in infected TCR-δ−/− mice. γδ T-cell deficiency led to delayed goblet cell hyperplasia in association with reduced expression of phosphorylated STAT6, MUC2, Trefoil factor-3 (TFF3) and T helper type 2 cytokines including interleukin-13 (IL-13). TCR-δ−/− mice also produced more interferon-γ than wild-type mice. Within the intraepithelial lymphocyte compartment, γδ T cells produced IL-13. Adoptive transfer of γδ T cells or administration of recombinant IL-13 to TCR-δ−/− mice successfully reduced the egg production by N. brasiliensis. Collectively, these data provide strong evidence that γδ T cells play an important role in controlling infection with intestinal nematodes and limiting infection-induced pathology.
Keywords: goblet cells, interleukin-13, intestinal intraepithelial T lymphocytes, Nippostrongylus brasiliensis, T-cell receptor-γδ
Introduction
The impact of gastrointestinal nematode parasites is evident at two levels; first, there is a direct impact on human health with more than one billion people afflicted by these parasites and second, livestock are affected, leading to reduced productivity and increased costs.1,2 Immune protection against nematode infection is associated with T helper type 2 (Th2) responses, which are characterized by accumulation of CD4+ T cells and production of Th2 cytokines, such as interleukin-4 (IL-4) and IL-13 with elevated serum IgE, eosinophilia and increased numbers of mucosal mast cells.3 Infection with Nippostrongylus brasiliensis induces strong Th2 cytokine responses, goblet cell hyperplasia and increased mucus production co-incidentally with the time of nematode expulsion.4–6 Goblet cells are specialized epithelial cells that produce mucus to protect epithelial tissues7 and Th2 cytokines promote the differentiation of goblet cells.8,9 Interleukin-4 and IL-13 are Th2 cytokines that induce the phosphorylation of signal transducer and activator of transcription 6 (STAT6).10 The IL-13/IL-4-mediated STAT6 signalling is required to produce effective hyperplasia of goblet cells.11 Moreover, STAT6−/− mice are highly susceptible to infection with N. brasiliensis, being unable to expel the adult worms.12
The intestinal epithelium has evolved immunologically and structurally to form a tissue integrity with a barrier function in the antigen-rich environment of the lumen. Maintaining epithelial integrity is critical to avoid the incursion of pathogenic microbes into the body and the intestine is heavily populated by immune cells in discrete compartments. Intestinal intraepithelial lymphocytes (IEL) are dominated by T cells located in tight association with the epithelial cells of the intestine. Many studies have reported interactions between intestinal epithelial cells (EC) and T-cell subsets that alter the physiology and activation status of both populations.13–15 An important characteristic of the IEL population is the high frequency of γδ T cells although the function of these cells is less well understood than with the classical αβ T cells. Intestinal γδ T cells have been shown to regulate epithelial barrier function, EC turnover, and EC expression of MHC class II,14,16–18 and to protect against enteric lesions after exposure to protozoan pathogens.14,19
T-cell receptor-γδ (TCR-γδ) T cells can mediate immunity to infection and have been demonstrated to play a role in limiting the numbers of intracellular protozoa in the gut via an interferon-γ (IFN-γ) -dependent mechanism.20 Nonetheless, γδ T cells can also be a source of Th2 cytokines, including IL-4,21 which raises the possibility that these cells may influence infection with enteric nematodes. Hence, it is important to determine whether γδ T cells are involved in immunity or immunopathogenesis during infection with intestinal nematodes. In this study, we employed TCR-δ−/− mice to define a protective role for intestinal γδ T cells during infection with N. brasiliensis. This protective capacity was associated with γδ T-cell production of Th2 cytokines, maintenance of epithelial barrier function, modulation of goblet cell numbers and mucin production.
Materials and methods
Mice
Wild-type (WT) male C57BL/6 mice were purchased at the age of 7 weeks from Japan SLC (Hamamatsu, Japan). The TCR-δ−/− mice were a kind gift from Professor Itohara (Kyoto University, Japan). All mice were used between 8 and 12 weeks of age, and the protocols were approved by the institutional review board for animal experiments of the University of Miyazaki.
Infection with N. brasiliensis and enumeration of eggs
Mice were inoculated subcutaneously with 500 viable third-stage larvae of N. brasiliensis. Faeces were collected to determine the number of eggs using a McMaster chamber. Adult worm numbers were counted by examining the gut contents of killed mice using a dissecting microscope. Groups of mice were killed for preparation of IEL from day 6 to day 15 after inoculation of N. brasiliensis and for histological analysis of the intestine on day 9.
Antibodies
Anti-Mucin (MUC)2, anti-trefoil factor 3 (TFF3), anti-STAT6 and anti-IL-13 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti-tyrosine phosphorylated STAT6 antibody from Cell Signaling (Danvers, MA), and anti-Ki67 antibody (Abcam, Cambridge, UK). These antibodies were used in immunohistochemical and Western blotting analyses.
Histological analysis
Paraffin-embedded sections of 10% formalin-fixed tissues were stained with haematoxylin & eosin. Immunohistochemical analysis was carried out by using paraffin-embedded sections. For antigen retrieval, deparaffinized and rehydrated specimens were microwaved in a Retrieval kit (BD, San Jose, CA). The slides were then incubated with the primary antibody at 4° overnight. Subsequently, these were incubated at room temperature with a biotinylated secondary antibody, peroxidase-conjugated streptavidin and localized using and 3, 3′-diaminobenzidine, followed by counterstaining with haematoxylin. For immunofluorescent staining, the slides that were reacted with the primary antibodies were stained with Alexa 556-conjugated rabbit IgG antibody and mounted in ProLong® Gold anti-fade reagent with DAPI (Invitrogen, Carlsbad, CA) for detection by fluorescence microscope (Olympus, Tokyo, Japan).
Cell preparation
Mucosal lymphocytes were isolated and prepared according to a modification of previously published methods.15,18 Briefly, dissected small segments of the intestines were incubated at 37° for 30 min in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) containing 10% fetal calf serum and 1 mm dithiothreitol with vigorous shaking. The tissue suspension was passed through a nylon mesh to remove debris and centrifuged through a 25/40/75% discontinuous Percoll (Sigma-Aldrich) gradient at 600 g at 20° for 20 min. The fraction of cell collected from the interface of 40/75% was IEL. To isolate lamina propria lymphocytes (LPL), after removal of EC and IEL, tissues were incubated for 30 min at 37° in RPMI-1640 containing collagenase type VIII (Sigma-Aldrich). The cell suspension was centrifuged through a 40/75% discontinuous Percoll gradient, and the cells at the interface were used as LPL. To isolate αβ or γδ IEL for culture, IEL were incubated with biotin-conjugated anti-TCR-β antibody or anti-TCR-γδ antibody (BD), following streptavidin microbeads and negatively sorted by using an MS+ column and magnetic antibody cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany). The αβ or γδ T cells in lamina propria were positively sorted by MACS. To extract total RNA for quantitative real-time PCR, the αβ IEL and γδ IEL were purified by a two-step procedure with MACS. In brief, for sorting γδ IEL, first, αβ IEL were depleted using anti-FITC MicroBeads after incubation with FITC-conjugated anti-TCR-β monoclonal antibody (mAb; H57-597, BD), then γδ IEL were positively sorted by using anti-phycoerythrin MicroBeads after treatment with phycoerythrin-conjugated anti-TCR-γδ mAb (GL3, BD). Similarly, for sorting αβ IEL, γδ IEL were depleted, and then αβ IEL were positively sorted.
Cell culture and cytokine analysis
Whole unsorted and magnetically sorted αβ or γδ IEL (1 × 106/ml) were added to a 96-well plate precoated with 2·5 μg anti-CD3 mAb (145-2C11; BD) and were cultured for 48 hr in RPMI-1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 U/ml streptomycin at 37°, in 5% CO2 for 48 hr. The supernatants were collected to determine the cytokine content by ELISA to detect mouse IFN-γ, IL-4 and tumour necrosis factor-α (e-bioscience, San Diego, CA) with protocol according to the manufacturer's instructions. For detection of IL-13 production by Western blot analysis, lysates were prepared from IEL after 48 hr of stimulation with plate-bound anti-CD3 mAb supplemented during the final 5 hr with PMA (50 ng/ml) and ionomycin (1 μg/ml) and Brefeldin A (40 μg/ml). For analysis of mRNA expression with real-time RT-PCR, the cells were cultured with plate-bound anti-CD3 mAb for 6 hr before extraction of total RNA.
Real-time PCR
Total RNA was extracted from the small intestine of WT and TCR-δ−/− mice using the RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was synthesized using the Reverse Transcription System (Applied Biosystems, Foster, CA). The primer sets used in this study were as follows; IL-13 forward: 5′-CTC TTG CTT GCC TTG GTG GTC TC-3′, IL-13 reverse: 5′-AGG GAA TCC AGG GCT ACA CAG AA-3′, 18s forward: 5′-GTA ACC CGT TGA ACC CCA TT-3′, 18s reverse: 5′-CCA TCC AAT CGG TAG TAG CG-3′. Expression of mRNA was assessed by quantitative PCR using an SYBR Green PCR Master Mix (Applied Biosystems) and ABI PRISM 7700 Sequence Detector (Applied Biosystems). Threshold cycle numbers (Ct) were determined with Sequence Detector software (version 1·7; Applied Biosystems) and transformed using the ΔCt/ΔΔCt method, with ribosomal 18s used for the calibration.
Western blot analysis
Lysates of ileal mucosa or stimulated IEL were prepared and analysed by Western blotting according to a previously published method.14
Adoptive transfer of γδ IEL or administration of recombinant IL-13 to TCR-δ−/− mice
Adoptive transfer of IEL was carried out according to a previously published method.18 Donor γδ IEL from the small intestine of WT mice were prepared by MACS using a negative sorting strategy to remove αβ IEL. These IEL (3 × 106) were injected intraperitoneally into the recipient TCR-δ−/− mice, which were irradiated with 5 Gy from a caesium source γ irradiator. In some studies, TCR-δ−/− mice received daily intraperitoneally injections of 10 μg of recombinant IL-13 (rIL-13) (BioLegend, San Diego, CA) from 4 days to 10 days post-infection (p.i.). The magnitude of infection was assessed by counting faecal egg production at 6 and 10 days p.i.
Statistics
Student's t-test was used to determine significant differences. A P-value < 0·05 was considered significant.
Results
TCR-δ−/− mice are highly susceptible to infection with N. brasiliensis
To determine the role of intestinal γδ T cells against N. brasiliensis infection, we compared the course of infection in WT and TCR-δ−/− mice challenged with 500 third-stage N. brasiliensis larvae. Seventy per cent of the TCR-δ−/− mice succumbed between 6 and 16 days p.i., whereas 100% of WT mice survived (Fig. 1a). All TCR-δ−/− mice exhibited exacerbated clinical symptoms, such as diarrhoea, in contrast to WT mice (data not shown). Nematode eggs were first detected in WT and TCR-δ−/− mice at 6 days p.i. with peak egg production at 7 days p.i. in WT mice and 9–10 days p.i. in TCR-δ−/− mice. Egg production was significantly higher in TCR-δ−/− mice than WT mice at between 6 and 10 days p.i. (Fig. 1b). Faecal egg production ceased in WT mice on day 11 p.i., whereas eggs were detected in the faeces of TCR-δ−/− mice until 27 days p.i. Next, we counted the number of adult worms in the intestine of WT and TCR-δ−/− mice. Similar numbers of adult worms were detected in the intestinal contents at post-mortem of WT and TCR-δ−/− mice at 6 days p.i. (Fig. 1c). At 10 days p.i., the WT mice had cleared all adult worms from the intestine, whereas the TCR-δ−/− mice retained a high worm burden. These results indicated that TCR-δ−/− mice are highly susceptible to N. brasiliensis infection and exhibit delayed expulsion of adult worms compared with WT mice.
Figure 1.
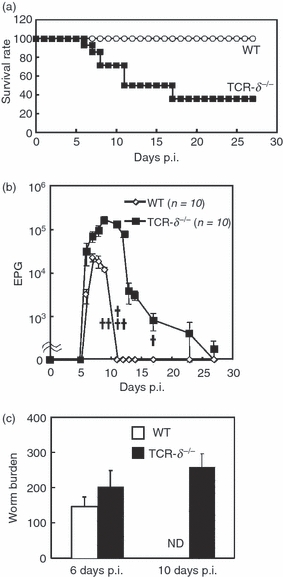
T-cell receptor-δ-deficient (TCR-δ−/−) mice exhibit increased susceptibility to infection with Nippostrongylus brasiliensis. (a) Survival of N. brasiliensis-infected wild-type (WT) and TCR-δ−/− mice (n = 20). Values represent the mean + SEM. (b) Faecal egg production in WT and TCR-δ−/− mice were counted at the times indicated. EPG, eggs per gram of faeces. Each cross indicates a number of TCR-δ−/− mouse that succumbed to infection. (c) Numbers of N. brasiliensis adults in the intestine of WT and TCR-δ−/− mice at 6 and 10 days post-infection (p.i.; three mice per group). Values represent the mean + SEM.
Considering the differences in the clinical outcome of infection, we performed a histological analysis of the small intestines of the WT and TCR-δ−/− mice before and after N. brasiliensis infection. At 9 days p.i., the duodenum was swollen and dilated in both WT and TCR-δ−/− mice but this observation was more evident in the TCR-δ−/− mice (Fig. 2a i–iv). Compared with WT mice, microscopic intestinal damage after the infection was more severe in the TCR-δ−/− mice, being associated with active invasion of adult worms between the villi and into the lamina propria (Fig. 2a v,vi). Adult N. brasiliensis infection was largely restricted to the duodenum of the WT mice, whereas worms were evident throughout the small intestine of TCR-δ−/− mice. The worms and eggs were readily detected in sections of the jejunum and ileum of TCR-δ−/− mice and were associated with intestinal lesions (Fig. 2b iii,iv). Significant crypt hyperplasia and villus erosion were evident in the infected TCR-δ−/− mice (Fig. 2b iii,iv,2c). No lesions were detected in the jejunum or ileum of the WT mice (Fig. 2b i,ii). The proliferation of intestinal epithelial cells has been reported to be lower in uninfected TCR-δ−/− mice compared with WT mice.17,22 To determine the effect of γδ T-cell deficiency on the proliferation of epithelial cells during infection of N. brasiliensis, we examined Ki67 staining of tissue sections at 9 days p.i. As shown in Fig. 2d, the numbers of Ki67-positive cells were considerably lower in TCR-δ−/− than WT mice, indicating that the deficiency in epithelial cell proliferation is induced in the absence of TCRγδ T cells during N. brasiliensis infection.
Figure 2.
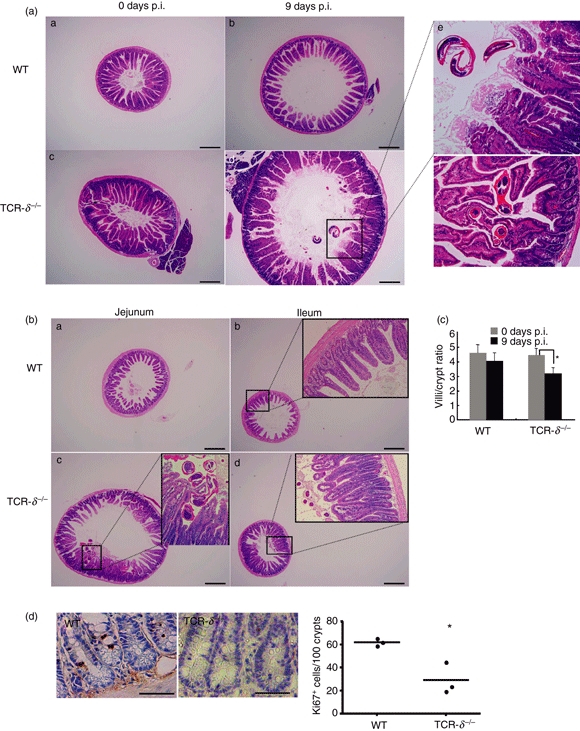
Exacerbated intestinal lesions in Nippostrongylus brasiliensis-infected T-cell receptor-δ-deficient (TCR-δ−/−) mice. (a) Haematoxylin & eosin-stained sections of duodenal tissue from uninfected and infected [9 days post-infection (p.i.)] wild-type (WT) and TCR-δ−/− mice. Panels i, ii, iii and iv; magnification × 40, scale bar, 500 μm. Panels v and vi are duodenums in TCR-δ−/− mice at 9 days p.i.; magnification × 400. (b) Haematoxylin & eosin staining of jejunum and ileum of WT and TCR-δ−/− mice at 9 days p.i. Panels i, ii, iii and iv; magnification × 40, scale bar, 500 μm. Enlarged panels within ii, iii and iv; magnification × 200. (c) Ratio of villus/crypt length of WT and TCR-δ−/− mice before and after N. brasiliensis infection at 9 days p.i. (d) Ki67-stained sections of duodenal tissue from infected (9 days p.i.) WT and TCR-δ−/− mice (magnification × 400, scale bar, 50 μm) (left). Quantification of ki67-positive cells per 100 crypts (right). Values represent the mean + SEM of three mice per group. *P< 0·05.
Reduced goblet cell hyperplasia in TCR-δ−/− mice
The observation that TCR-δ−/− mice were susceptible to N. brasiliensis infection and the link between this nematode infection and goblet cell hyperplasia23,24 led us to examine the role for γδ T cells in this response. The duodenal and ileal sections of the uninfected TCR-δ−/− mice showed a constitutively lower number of periodic acid Schiff/Alcian blue-stained goblet cells than those of WT mice (Fig. 3a,b). Goblet cells release mucins in the intestine, which provide a protective barrier for the underlying epithelium.5,24 MUC2 is the dominant gel forming mucin in the small intestine25 and MUC2 expression was substantially lower in the small intestinal epithelium of the uninfected TCR-δ−/− mice compared with that of the WT mice (Fig. 3c). Trefoil factor family 3 peptide is co-expressed with MUC2 in goblet cells.25 Coincident with MUC2 expression, TFF3 was also expressed at lower levels in the TCR-δ−/− mice than in the WT mice (Fig. 3c). These differences in goblet cell hyperplasia and expression of MUC2 and TFF3 may be important in determining the increased susceptibility of TCR-δ−/− mice.
Figure 3.
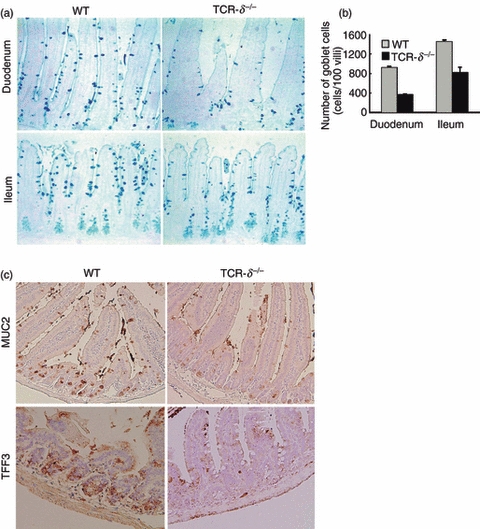
Reduced goblet cell numbers in T-cell receptor-δ-deficient (TCR-δ−/−) mice. (a) Alcian blue staining of duodenum and ileum in the wild-type (WT) and TCR-δ−/− mice at 12 weeks of age (magnification × 100). (b) Number of Alcian blue-positive cells was counted in duodenum and ileum of the WT and TCR-δ−/− mice. Values represent the mean + SEM of 100 villus units. (c) MUC2 and TFF3-staining of the small intestine in the WT and TCR-δ−/− mice (magnification × 100).
Decreased production of Th2-type cytokines by intestinal T cells of TCR-δ−/− mice
It is well documented that Th2-type cytokines are necessary for the development and hyperplasia of goblet cells, and for the induction of effector function against nematode infection.8,9,12 As TCR-δ−/− mice were highly susceptible to N. brasiliensis infection and experienced reduced goblet cell hyperplasia, we examined cytokine production in IEL from infected and uninfected WT and TCR-δ−/− mice. The IEL derived from uninfected WT mice produced very low levels of IL-4 and IFN-γ, whereas those from TCR-δ−/− mice produced higher levels of IFN-γ (Fig. 4). The IEL derived from infected WT mice produced increased levels of IL-4 from day 6 p.i. with the peak production between 9 and 12 days p.i. In contrast, TCR-δ−/− mice produced lower levels of IL-4 than WT mice, although these levels remained elevated at day 15 p.i. (Fig. 4). Production of IFN-γ in IEL derived from WT mice increased after infection, with a similar profile to that seen with IL-4. The IEL from uninfected TCR-δ−/− mice produced a higher level of IFN-γ than that seen with IEL from uninfected WT mice and the levels increased further during infection. These results suggested that γδ T cells in uninfected mice regulate IFN-γ production and the increased levels seen in uninfected TCR-δ−/− mice may interfere with the induction of rapid IL-4 responses after infection with N. brasiliensis.
Figure 4.
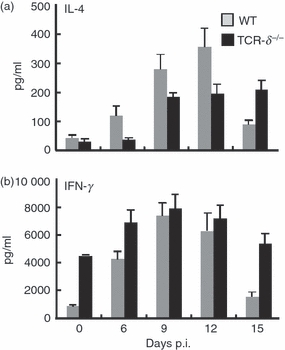
The temporal pattern of interferon-γ (IFN-γ) and interleukin-4 (IL-4) production by intestinal intraepithelial T lymphocytes (IEL) isolated from Nippostrongylus brasiliensis-infected wild-type (WT) and T-cell receptor-δ-deficient (TCR-δ−/−) mice. Freshly isolated IEL were cultured with plate-bound anti-CD3 monoclonal antibody for 48 hr, and the supernatants were collected to determine the concentration of IFN-γ and IL-4 by ELISA. Three mice were used in each point. Values represent the mean + SEM of three individual experiments in a triplicate assay. *P< 0·05.
To determine the cellular source of cytokines produced by IEL subpopulations, αβ and γδ IEL from WT mice and αβ IEL from TCR-δ−/− mice were sorted and assessed for cytokine production. In uninfected WT mice, αβ IEL produced higher levels of IFN-γ than γδ IEL whereas the αβ IEL from TCR-δ−/− mice produced greater amounts of IFN-γ than the αβ IEL from WT mice (Fig. 5a). γδ IEL from WT mice produced higher levels of IL-5 and IL-13 than αβ IEL from WT or TCR-δ−/− mice although IL-4 production was similar in both αβ and γδ IEL populations. We also examined mRNA and protein expression of IL-13 in αβ IEL and γδ IEL of WT mice and αβ IEL of TCR-δ−/− mice. The γδ IEL expressed higher levels of IL-13 mRNA and protein than αβ IEL from WT mice or αβ IEL from TCR-δ−/− mice (Figs. 5b,c). The LPL were also isolated, separated into TCR-αβ or TCR-γδ IEL fractions and tested for capacity to produce IL-13 and IFN-γ as a pool of cells from 10 mice. The TCR-γδ LPL from WT mice produced IL-13 (16 pg/ml), whereas this was undetectable in the TCR-αβ LPL from WT and TCR-δ−/− mice. In contrast, IFN-γ was detected in supernatants from both TCR-αβ (2010 pg/ml) and TCR-γδ (1600 pg/ml) LPL from WT mice as well as in LPL from TCR-δ−/− mice (2520 pg/ml). These results demonstrated that γδ IEL are capable of producing significant amounts of Th2-type cytokines, in particular, IL-13.
Figure 5.
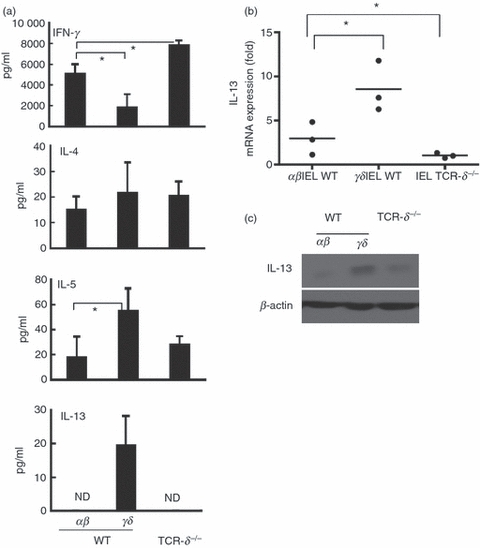
Cytokine production by γδ and αβ T cells derived from the intestinal intraepithelial T lymphocytes (IEL) of uninfected wild-type (WT) and T-cell receptor-δ-deficient (TCR-δ−/−) mice. (a) IEL bearing T-cell receptor-αβ (TCR-αβ) and TCR-γδ of naive WT mice were purified by magnetic antibody cell sorting and IEL of TCR-δ−/− mice, which consisted of TCR-αβ alone, was used unseparated. The IEL were treated with anti-CD3 monoclonal antibody (mAb) for 3 days and cytokine production was analysed by ELISA for interferon-γ (IFN-γ), interleukin-4 (IL-4), IL-5 and IL-13. Values represent the mean + SEM of three independent experiments. *P< 0·05. (b) IL-13 mRNA expression in the IEL of uninfected WT and TCR-δ−/− mice. The IEL were treated with anti-CD3 mAb for 6 hr and mRNA expression level of IL-13 was detected by quantitative RT-PCR. The data are normalized to 18s mRNA content and plotted as mean fold over IEL in TCR-δ−/− mice in three independent experiments. (c) IL-13 production in the IEL of uninfected WT and TCR-δ−/− mice by Western blot analysis.
Delayed goblet cell hyperplasia in TCR-δ−/− mice
Although TCR-δ−/− mice were susceptible to N. brasiliensis infection and exhibited exacerbated clinical signs, the worms were expelled from the surviving mice, albeit much later than was observed in WT mice (Fig. 1a,b). Following the infection with N. brasiliensis, we noticed that mucus levels increased in the small intestinal lumen of the TCR-δ−/− mice. Histological analysis of the small intestines in TCR-δ−/− and WT mice at the latter stages of infection (9 days p.i.) revealed an increase in goblet cell numbers in TCR-δ−/− mice that exceeded the numbers present in WT mice. We also observed larger numbers of adult worms in the intestine of TCR-δ−/− mice, including some in tight association with the intestinal villi (Fig. 6, arrow). These results indicated that the defect in worm expulsion seen in γδ T-cell-deficient mice is related to a delay in goblet cell hyperplasia in response to N. brasiliensis infection rather than to a failure in this response.
Figure 6.
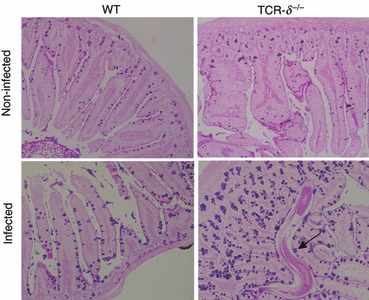
T-cell receptor-δ-deficient (TCR-δ−/−) mice showed delayed induction of mucins following Nippostrongylus brasiliensis infection. Periodic acid Schiff/Alcian blue staining of small intestine in uninfected and infected (9 days p.i.) wild-type (WT) and TCR-δ−/− mice. Arrow indicates an adult N. brasiliensis.
Delayed up-regulation of STAT6 activation in the intestinal mucosa of TCR-δ−/− mice
Using Western blot, we examined the expression of MUC2, TFF3 and Relmβ, which is restricted in bowel goblet cells and functions as an inflammatory regulator.26 These data confirm the alteration in the MUC2 and TFF3 expression levels observed through the immunohistochemical data (Fig. 3c) with greater levels of MUC2 and TFF3 at 5 days p.i. in WT mice than TCR-δ−/− mice (Fig. 7a). Resistin-like molecule (Relm)β expression was induced to comparable levels in WT and TCR-δ−/− mice after N. brasiliensis infection.
Figure 7.
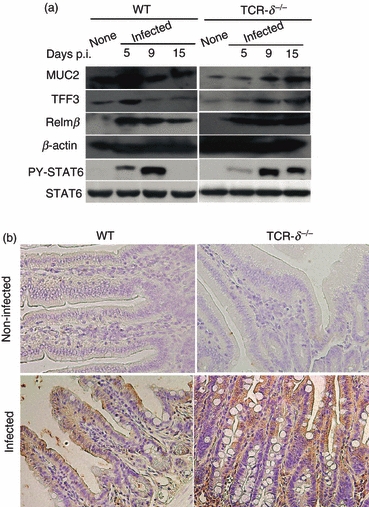
Modulation of signal transducer and activator of transcription 6 (STAT6) activation, goblet cell associated factors in the T-cell receptor-δ-deficient (TCR-δ−/−) mice. (a) Western blotting analysis of intestinal lysates for phosphorylated STAT6, mucin-related factors (MUC2, TFF3 and Relmβ) before and after Nippostrongylus brasiliensis infection at 5, 9 and 15 days post-infection (p.i.) in the wild-type (WT) and TCR-δ−/− mice. (b) Immunohistochemical analysis of phosphorylated STAT6 in uninfected and infected WT and TCR-δ−/− mice (9 days p.i.).
The Th2 cytokines have a pivotal role in regulating goblet cell hyperplasia with both IL-4 and IL-13 receptors triggering signal transduction via phosphorylation of STAT6.27,28 Changes in the level of phosphorylated STAT6 were detected in the intestinal mucosa of WT mice at 5 days p.i., which had returned to pre-infection levels at 15 days p.i. In contrast, the phosphorylation of STAT6 occurred later in TCR-δ−/− than WT mice and remained at high levels throughout the sampling period (Fig. 7a). The distribution of phosphorylated STAT6 was more widespread in EC and the lamina propria of the TCR-δ−/− mice at 9 days p.i., whereas the staining pattern was less intense and largely restricted to the EC of the infected WT mice (Fig. 7b). These data indicated that TCR-δ−/− mice experience a delayed but more sustained level of STAT6 activation after infection with N. brasiliensis than WT mice.
Adoptive transfer of γδ IEL or administration of recombinant IL-13 confers resistance to N. brasiliensis infection in TCR-δ−/− mice
To confirm the protective role of γδ T cells against N. brasiliensis infection, we isolated γδ IEL from WT mice and adoptively transferred these cells into TCR-δ−/− recipient mice before infection with N. brasiliensis. The number of eggs produced in TCR-δ−/− mice that received γδ IEL was significantly lower than that in TCR-δ−/− mice that received no cells (Fig. 8a). At 6 days p.i., the γδ IEL-reconstituted TCR-δ−/− mice produced almost 10-fold fewer eggs than unmanipulated TCR-δ−/− mice. This difference in egg production increased to 200-fold at 10 days p.i. As the γδ IEL were a major source of IL-13, we administered rIL-13 to TCR-δ−/− mice during the infection. The rIL-13 treatment dramatically reduced the number of eggs produced by TCR-δ−/− mice (Fig. 8a). Histological analysis revealed that rIL-13 administration or adoptive transfer of γδ IEL led to recovery of villi/crypt structure and MUC2 expression in TCR-δ−/− mice (Fig. 8b). Collectively, these data indicated that the exacerbated infection associated with γδ T-cell deficiency could be overcome by adoptive transfer of γδ T cells or by administration of rIL-13.
Figure 8.

Adoptive transfer of γδ intestinal intraepithelial T lymphocytes (IEL) or injection of recombinant interleukin-13 (rIL-13) reduces the reproductive success of Nippostrongylus brasiliensis in T-cell receptor-δ-deficient (TCR-δ−/−) mice. TCR-δ−/− mice received daily intraperitoneal (i.p.) injection of 10 μg of rIL-13 from 4 days post-infection (p.i.) to 10 days p.i. or single i.p. transfer of donor γδ IEL (3 × 106) isolated from the small intestine of wild-type (WT) mice. (a) The number of N. brasiliensis eggs produced in the faeces of treated or untreated TCR-δ−/− mice was at 6 and 10 days p.i. Values represent the mean + SEM of five mice per each group. *P< 0·01, **P< 0·001. (b) Haematoxylin & eosin, periodic acid Schiff/Alcian blue staining and immunohistochemistry for MUC2 of small intestine of TCR-δ−/− mice that received rIL-13 or adoptive transfer of γδ IEL, samples taken at 10 days p.i.
Discussion
This work provides the first evidence that γδ T cells play a protective role against infection with the intestinal nematode, N. brasiliensis. The TCR-γδ T-cell deficiency was also associated with reduced expression of goblet cells and IL-13 production as well as enhanced pathology during infection. These results indicate that γδ T cells are capable of producing Th2-type cytokines and play an important role to maintain epithelial integrity in the intestine.
Natural infections with N. brasiliensis involve larval penetration of the skin followed by migration to the lung before entering the intestine. It might be possible that extra-intestinal γδ T cells in the lung may impact on the biology of N. brasiliensis. However, considering the infection dynamics seen with TCR-δ−/− mice, it is unlikely that γδ T cells exert significant effects on the extra-intestinal tissue migratory stage of N. brasiliensis because similar numbers of worms were detected in WT and TCR-δ−/− mice at 6 days p.i. (Fig. 1c). The major γδ T-cell-mediated impact on N. brasiliensis was evident by the survival of worms in the intestine and a reduction in the numbers of eggs produced in the faeces. For example, infection with WT mice was resolved and egg production was terminated before 12 days p.i., whereas TCR-δ−/− mice delayed clearance until 27 days p.i. (Fig. 1b). Adoptive transfer of γδ IEL from WT mice into TCR-δ−/− mouse recipients substantially reduced faecal egg production, confirming a protective role for these cells in limiting the reproductive success of N. brasiliensis. The mechanisms of nematode clearance from the intestine have been studied extensively, and a predominant role for TCR-αβ Th2 CD4+ T cells is well established.3 The γδ T cells may directly participate in protective immunity or act by modulation of the developing αβ T-cell response. A simple explanation may be that the increased level of IFN-γ production by IEL in uninfected TCR-δ−/− mice alters the enteric environment and inhibits the development of strong Th2 responses. Indeed, our data revealed that TCR-δ−/− mice were capable of responding to infection with an increase in Th2 cytokines, but this response was delayed compared with WT mice because of the Th1-biased microenvironment. TCR-δ−/− mice have been reported to exhibit a lower turnover of enteric epithelial cells than WT mice.17,22 Changes in epithelial turnover are considered an important mechanism of nematode expulsion, so our finding that both crypt/villus ratio and number of Ki67-positive cells were lower in the intestine of infected TCR-δ−/− mice at 9 days p.i. (Fig. 2c,d) consistent with a hypothesis that the effect of γδ T-cell deficiency may, at least in part, also be the result of an altered epithelial cell response.
As γδ IEL are a source of IL-13, a cytokine with a prominent role in clearance of N. brasiliensis,12,29γδ T cells may be directly responsible for some protective effect against the nematode. Both IL-13 and IL-4 exhibit a functional overlap in many of their biological activities with shared components of the IL-4R and IL-13R and a common signalling pathway involving STAT6 phosphorylation.10 Disruption of STAT6 signalling pathways dramatically delays worm expulsion.12 STAT6 phosphorylation was detected earlier in the intestine of WT mice than in that of TCR-δ−/− mice (Fig. 7), an effect consistent with the defect of IL-4-mediated or IL-13-mediated clearance event in TCR-δ−/− mice. Indeed, administration of rIL-13 successfully cleared egg production in TCR-δ−/− mice, confirming the importance of this cytokine in limiting the reproduction of N. brasiliensis (Fig. 8) and supporting the hypothesis that the γδ T-cell deficiency relates to altered IL-13 availability. Although CD4+ TCR-αβ T cells are widely regarded as a major source of IL-13, these cells are rare in the IEL and other cell populations including NK cells are reported to produce IL-13 after infection with Trichinella spiralis.29 Hence it is important to consider the potential role for non-classical IL-13-producing effector cells such as NK cells or γδ IEL in immunity to intestinal infections. Intestinal nematode infections induce goblet cell hyperplasia and these changes are considered important in the expulsion of many nematodes, including N. brasiliensis.23,24 Lack of γδ T cells was associated with a marked decrease in duodenal and ileal goblet cells in uninfected mice that may also be related to the deficiency in IL-13 production by IEL. Goblet cells are specialized mucus-secreting epithelial cells induced by Th2 cytokines.8,9 Mucus secretion and mucin gene expression were not affected by the absence of IL-4, IFN-γ or tumour necrosis factor-α30 but were reduced in IL-4Rα-deficient mice.31 Interleukin-4Rα represents a common component of both IL-4 and IL-13 receptors,32 indicating a dominant role for IL-13 in N. brasiliensis-induced goblet cell hyperplasia.
Goblet cells produced a variety of mucins and other proteins, such as MUC2, TFF3 and Relmβ. MUC2 is a major gel-forming mucin produced in the intestine.25 TFF proteins are important factors for epithelial repair and are produced by goblet cells in the intestine.33,34 Relmβ is a member of the resistin-like molecules family, produced by goblet cells and is known to play a role in nematode expulsion by binding to chemosensory organs of intestinal worms.35 During infection with N. brasiliensis, TCR-δ−/− mice expressed a lower level of MUC2 and TFF3 than WT mice. Increased levels of Relmβ were detected in the intestines of WT and TCR-δ−/− mice (Fig. 7). This finding was surprising, because Relmβ is known to be affected by the IL-13 level and TCR-δ−/− mice exhibited a deficiency in IL-13 production and reduced STAT6 phosphorylation. Moreover, Relmβ is produced by goblet cells and normally its level is coincidentally varied by goblet cell.35 The TCR-δ−/− mice experienced less robust goblet cell hyperplasia and less MUC2 and TFF3 expression than WT mice but retained Relmβ expression in the intestinal tissue, suggesting that worm expulsion requires multiple factors to act in a coordinated manner.
In addition to the defects in controlling infection, TCR-δ−/− mice also experienced worse clinical symptoms and more exacerbated intestinal pathology during N. brasiliensis infection. Clinical symptoms were associated with the intestinal phase of infection and included diarrhoea with some TCR-δ−/− mice succumbing to infection between 6 and 17 days p.i. Histologically, the intestine of the infected TCR-δ−/− mice exhibited greater thickening, oedema, villus erosion and crypt hyperplasia than that of infected WT mice. The lesions were more widespread in TCR-δ−/− mice, encompassing the whole of the small intestine, and were associated with a wider distribution of adult worms. Enhanced intestinal pathology associated with γδ T-cell deficiency has been reported with other infectious36 and non-infectious disease models.18
In summary, we documented a clear functional role for γδ T cells during infection with the intestinal nematode, N. brasiliensis. These cells are involved in restricting the reproductive success and promoting clearance of the adult worms as well as moderating the intestinal pathology associated with infection. Mechanistically, the absence of γδ T cells leads to delayed induction of a protective Th2 immune response, delayed goblet cell hyperplasia as well as delayed production of MUC2 and TFF3. All of these features are known to contribute to protective immunity with enteric nematodes and we propose that γδ T cells are important in coordinating these responses.
Acknowledgments
We are grateful to Professor Goro Matsuzaki (University of the Ryukyus) for longstanding support, critical reading of the manuscript and constructive discussion. This work is supported by grants from Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17590373, 19590432) (K.I-O). A.L.S. is a Jenner Fellow and receives support from the Jenner Institute and the John Fell Fund at the University of Oxford.
Glossary
Abbreviations
- EC
intestinal epithelial cells
- IEL
intestinal intraepithelial T lymphocytes
- IFN
interferon
- IL
interleukin
- LPL
lamina propria lymphocytes
- mAb
monoclonal antibody
- N. brasiliensis
Nippostrongylus brasiliensis
- p.i.
post-infection
- STAT
signal transducer and activator of transcription
- TCR
T-cell receptor
- Th
T helper cells
- Trefoil factor
TFF
- WT
wild-type
Disclosures
The authors have no potential conflict of interest.
References
- 1.Claerebout E, Vercruysse J. The immune response and the evaluation of acquired immunity against gastrointestinal nematodes in cattle: a review. Parasitology. 2000;120(Suppl.):S25–42. doi: 10.1017/s0031182099005776. [DOI] [PubMed] [Google Scholar]
- 2.Newton SE, Meeusen EN. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. 2003;25:283–96. doi: 10.1046/j.1365-3024.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 4.Miller HR, Huntley JF. Protection against nematodes by intestinal mucus. Adv Exp Med Biol. 1982;144:243–5. doi: 10.1007/978-1-4615-9254-9_37. [DOI] [PubMed] [Google Scholar]
- 5.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–93. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 6.Theodoropoulos G, Hicks SJ, Corfield AP, Miller BG, Carrington SD. The role of mucins in host–parasite interactions: part II – helminth parasites. Trends Parasitol. 2001;17:130–5. doi: 10.1016/s1471-4922(00)01775-x. [DOI] [PubMed] [Google Scholar]
- 7.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 8.Dohi T, Fujihashi K, Koga T, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124:672–82. doi: 10.1053/gast.2003.50092. [DOI] [PubMed] [Google Scholar]
- 9.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–83. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–9. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 11.Khan WI, Motomura Y, Blennerhassett PA, Kanbayashi H, Varghese AK, El-Sharkawy RT, Gauldie J, Collins SM. Disruption of CD40–CD40 ligand pathway inhibits the development of intestinal muscle hypercontractility and protective immunity in nematode infection. Am J Physiol Gastrointest Liver Physiol. 2005;288:G15–22. doi: 10.1152/ajpgi.00159.2004. [DOI] [PubMed] [Google Scholar]
- 12.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–64. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Fujihashi K, Kawabata K, McGhee JR, Kiyono H. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J Immunol. 1998;160:2188–96. [PubMed] [Google Scholar]
- 14.Inagaki-Ohara K, Dewi FN, Hisaeda H, et al. Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun. 2006;74:5292–301. doi: 10.1128/IAI.02024-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki-Ohara K, Sasaki A, Matsuzaki G, et al. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut. 2006;55:212–9. doi: 10.1136/gut.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, Nawa Y. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun. 2005;331:977–83. doi: 10.1016/j.bbrc.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Komano H, Fujiura Y, Kawaguchi M, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc Natl Acad Sci U S A. 1995;92:6147–51. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inagaki-Ohara K, Chinen T, Matsuzaki G, et al. Mucosal T cells bearing TCRγδ play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–8. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- 19.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AL, Hayday AC. Genetic dissection of primary and secondary responses to a widespread natural pathogen of the gut, Eimeria vermiformis. Infect Immun. 2000;68:6273–80. doi: 10.1128/iai.68.11.6273-6280.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 22.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial γδ T cells. Science. 1994;266:1253–5. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 23.Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol. 1995;17:485–91. doi: 10.1111/j.1365-3024.1995.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller HR. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology. 1987;94(Suppl.):S77–100. doi: 10.1017/s0031182000085838. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M, Ikeda H, Nakanuma Y. Expression profiles of MUC mucins and trefoil factor family (TFF) peptides in the intrahepatic biliary system: physiological distribution and pathological significance. Prog Histochem Cytochem. 2007;42:61–110. doi: 10.1016/j.proghi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Steppan CM, Brown EJ, Wright CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502–6. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izuhara K, Shirakawa T. Signal transduction via the interleukin-4 receptor and its correlation with atopy. Int J Mol Med. 1999;3:3–10. doi: 10.3892/ijmm.3.1.3. [DOI] [PubMed] [Google Scholar]
- 28.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 29.McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol. 2005;175:3207–13. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 30.Shekels LL, Anway RE, Lin J, Kennedy MW, Garside P, Lawrence CE, Ho SB. Coordinated Muc2 and Muc3 mucin gene expression in Trichinella spiralis infection in wild-type and cytokine-deficient mice. Dig Dis Sci. 2001;46:1757–64. doi: 10.1023/a:1010622125040. [DOI] [PubMed] [Google Scholar]
- 31.Horsnell WG, Cutler AJ, Hoving JC, et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Rα-deficient mice. PLoS Pathog. 2007;3:e1. doi: 10.1371/journal.ppat.0030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor α chain. J Biol Chem. 1996;271:29265–70. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 33.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–9. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 34.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–5. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 35.Artis D, Wang ML, Keilbaugh SA, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2004;101:13596–600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egan CE, Dalton JE, Andrew EM, Smith JE, Gubbels MJ, Striepen B, Carding SR. A requirement for the Vγ1+ subset of peripheral γδ T cells in the control of the systemic growth of Toxoplasma gondii and infection-induced pathology. J Immunol. 2005;175:8191–9. doi: 10.4049/jimmunol.175.12.8191. [DOI] [PubMed] [Google Scholar]


