Abstract
Vitamin D3 is known to induce regulatory T (Treg) cells by rendering antigen-presenting cells tolerogenic, its direct effect on human naturally occurring Treg cells is unclear. Here, we investigated if and how 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] can directly affect the proliferation and function of human naturally occurring Treg cells in vitro. First, we demonstrated that these Treg cells express vitamin D receptors that were up-regulated following anti-CD3/CD28-bead stimulation. 1,25(OH)2D3 inhibited proliferation of Treg cells even when exogenous interleukin-2 was provided. Treg cells were more susceptible to the inhibitory effect of 1,25(OH)2D3 than conventional T cells. 1,25(OH)2D3 neither affected the anergic state nor the suppressive function of Treg cells but induced a subtle increase in interleukin-10-secreting cells. The cell-division-inhibiting effect of 1,25(OH)2D3 on Treg cells was also demonstrated in vivo by supplementing vitamin D-deficient HIV-1-infected patients with 2000 IU cholecalciferol (vitamin D3). Increased serum 1,25(OH)2D3 levels were associated with a drop in the number and percentage of Treg cells, which may be attributed to a decrease in the proliferating Foxp3+ Treg cell population. In conclusion, 1,25(OH)2D3 directly affects Treg cell growth and promotes interleukin-10 production without apparent effects on activation status and suppressive phenotype whereas in vivo, high serum 1,25(OH)2D3 levels are associated with reduced Treg cell proliferation and a reduced number of Treg cells.
Keywords: 1,25-dihydroxyvitamin D3; cholecalciferol; forkhead box protein 3; HIV; immunomodulation; regulatory T cells
Introduction
Vitamin D3 is mostly associated with its primary role in bone and calcium metabolism, but recent interest hinges on its potential as an immunomodulatory agent. Vitamin D3 is obtained by dietary intake or by cutaneous pre-vitamin D3 biosynthesis upon UVB exposure. Pre-vitamin D3 is subsequently hydroxylated into 25-hydroxyvitamin D3 [25(OH)D3] in the liver and further hydroxylated by 1α-hydroxylase in the kidney into the active metabolite, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3),1 which is the ligand for vitamin D receptor (VDR). Several mononuclear cells including T cells, express VDRs2,3 and can also produce 1,25(OH)2D3 by expressing 1α-hydroxylase.4,5 A role for vitamin D3 in immune regulation has been demonstrated both in vivo and in vitro; 1,25(OH)2D3 influences the growth, differentiation and cytokine production of various immune cells.6–8 As a result, 1,25(OH)2D3 has been proposed as a potential immunomodulatory agent in autoimmune diseases such as insulin-dependent diabetes mellitus, multiple sclerosis, inflammatory bowel disease and rheumatoid arthritis,9–11 as well as in cancer 12 and infectious diseases such as HIV infection.13
Regulatory T (Treg) cells are characterized by a CD4+ CD25high phenotype and signature transcription factor, forkhead box protein 3 (Foxp3). They are an integral part of the immune system and are crucial in the regulation of immune homeostasis.14 It has been demonstrated that the regulatory function of CD4+ CD25+ Treg cells is hampered in autoimmunity, allergy and infectious diseases, indicating that these cells play a crucial role in immune-mediated pathology. Over the past decade, evidence has mounted for the potential therapeutic application of Treg cells either in enhancing their regulatory activity in inflammatory diseases such as autoimmunity, allograft rejection, graft versus host disease and allergic diseases, or in blocking their suppressive activity in tumour immunity or vaccine development.15–18
Either Treg cells can be derived from the thymus, the so-called naturally occurring Treg (nTreg) cells, or they can be induced de novo in the periphery from CD4+ T cells. Vitamin D3 interferes with the maturation and differentiation of dendritic cells and may induce a so-called tolerogenic phenotype.19,20 This implies that 1,25(OH)2D3 can indirectly potentiate the differentiation of interelukin-10 (IL-10) -producing CD4+ CD25+ Treg cells by altering the function of antigen-presenting cells (APCs).21,22 1,25(OH)2D3 can also act directly on CD4+ CD25− T cells to generate Foxp3+ T cells expressing high levels of cytotoxic T-lymphocyte antigen-4 that are capable of immune suppression.23 In mouse models, 1,25(OH)2D3 enhanced the proliferative capacity of CD4+ CD25+ Treg cells24 and their ability to suppress T helper type 2 (Th2) activity.25 However, there is limited information on the direct effects of 1,25(OH)2D3 on human naturally occurring Treg cells. In patients with multiple sclerosis, there is controversy on how serum 25(OH)D3 levels correlate with the peripheral nTreg cell pool26,27 though it seems to be implicated in the enhancement of Treg cell suppressive function.27
In this study, we assessed the direct effect of 1,25(OH)2D3 on ex vivo stimulated human Treg cells. We show for the first time that naturally occurring human Treg cells express VDRs, and consequently that 1,25(OH)2D3 can exert its immunomodulatory effect directly on pre-existing Treg cells in the absence of APCs. The major effect of 1,25(OH)2D3 on pre-existing Treg cells is inhibition of proliferation. Other properties associated with suppressor capacity are left largely unaffected although IL-10 production by Treg cells was slightly enhanced. Our in vitro data on the reduced proliferative capacity of Treg cells are supported by a clinical study in which decreased numbers of peripheral blood Treg cells were found during treatment of vitamin-D-deficient HIV-infected patients with cholecalciferol.
Materials and methods
Cell isolation
Buffy coats were obtained from healthy donors (Sanquin Blood Bank, Region South East, the Netherlands) with written informed consent on scientific use, according to the Declaration of Helsinki. Peripheral blood mononuclear cells were isolated by density centrifugation with Lymphoprep (Axis-Shield AS, Oslo, Norway) and LeucoSep® (Greiner Bio-One, Frickenhausen, Germany). CD4+ T cells were purified from peripheral blood mononuclear cells by negative selection using monoclonal antibodies (mAbs) directed against CD8 (RPA-T8), CD14 (M5E2), CD16 (3G8), CD19 (4G7), CD33 (P67.6), CD56 (B159) and CD235a [GA-R2(HIR2)] (BD-Biosciences, Erembodegem, Belgium) combined with sheep anti-mouse immunoglobulin-coated magnetic beads (Dynal Biotech, Oslo, Norway). Bead–cell complexes were removed using a magnetic holder. The resultant CD4+ T-cell fraction, typically of > 90% purity, was incubated with phycoerythrin-conjugated anti CD25 (anti-CD25-PE; M-A251, BD Biosciences, New York, NY), anti-CD4-ECD (SFCI12T4D11) and PE-cyanin 5-conjugated anti-CD27 (anti-CD27-PC5; 1A4CD27) antibodies (both from Beckman Coulter Corporation, Miami, FL). CD4+ CD25high CD27+ Treg cells and CD4+ CD25neg CD27+ conventional T (Tconv) cells were isolated from purified CD4+ T cells by high-purity flow cytometric cell sorting (Altra Flow Cytometer; Beckman Coulter). The isolated CD4+ CD25high CD27+ Treg (routine yield of > 98% purity) and CD4+ CD25neg CD27+ Tconv cells were used immediately after isolation. A phenotypic analysis after isolation established that our target CD4+ CD25high CD27+ Treg population expressed high levels of Foxp3 whereas CD127 expression was lacking. In some experiments, CD4+ CD25+ and CD4+ CD25neg T cells were isolated from the negative isolated CD4+ population by magnetic antibody cell sorting, using 10 μl anti-CD25 magnetic microbeads for every 107 CD4+ T cells (Miltenyi Biotec, Bergisch Gladbach, Germany).
Cell proliferation assay
To study the effect of 1,25(OH)2D3 on cell proliferation, 2·5 × 104 Treg or Tconv cells were stimulated with 5 × 103 anti-CD3/anti-CD28 mAb-coated microbeads (Dynal Biotech, Invitrogen ASA) in 200 μl culture medium (RPMI-1640 supplemented with glutamax, 0·02 mm sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin; all from Gibco, Paisley, UK) and 10% human pooled serum. Exogenous recombinant human IL-2 (rhIL-2) 12·5 U/ml (Proleukine; Chiron, Amsterdam, the Netherlands) was added to the cell culture. The dose–response of 1,25(OH)2D3 (Fluka Biochemika, Sigma-Aldrich, MI), dissolved in absolute ethanol, was examined at 1, 10, 100 nm. No solvent effect was apparent in control experiments. Cell cultures were performed in 96-well round-bottom plates (Greiner, Frickenhausen, Germany) and incubated in a 37°, 95% humidity, 5% CO2 incubator. Cell proliferation was monitored by [3H]thymidine ([3H]TdR) incorporation using a gas scintillation counter (Matrix 96 Beta-counter; Canberra Packard, Meriden, CT) on days 3, 4 and 5. The cells were pulsed with 0·5 μCi of [3H]TdR (Amersham Biosciences, Piscataway, NJ) during the last 16–18 hr of culture. Incorporation of [3H]TdR is expressed as mean (± SD) counts per 5 min of triplicate measurements. Relative inhibition was calculated as: % inhibition = {1 – ([3H]TdR incorporation co-culture / [3H]TdR incorporation control mixed lymphocyte culture) × 100%}.
Suppression assay
The suppressive capacity of isolated or cultured Treg cells was studied in co-culture assays. In brief, graded doses of Treg cells were added in an increasing ratio to 2·5 × 104 responder cells (Tconv) and stimulated with anti-CD3/anti-CD28 mAb-coated microbeads. Cell proliferation was examined by [3H]TdR incorporation, as mentioned above, on day 4 of cultures. Relative suppression was calculated as: % suppression = {1 – ([3H]TdR incorporation co-culture / [3H]TdR incorporation control MLC) × 100%}. To study the suppressive capacity of Treg cells in the presence of 1,25(OH)2D3, co-cultures were conducted with or without the addition of 100 nm 1,25(OH)2D3.
Flow cytometry
Cells were phenotypically analysed by four-colour and five-colour flow cytometry (Coulter Epics XL and Coulter Cytomics FC 500; Beckman Coulter, Fullerton, FL) using Coulter Epics Expo 32 software. Cells were washed with PBS with 0·2% bovine serum albumin before being labelled with fluorochrome-conjugated mAbs. After incubation for 20 min at room temperature, in the dark, cells were washed twice to remove unbound antibodies and analysed. For cell surface staining, the following mAbs were used: CD25-PE (M-A251, BD Bioscience), CD4-ECD (SFCI12T4D11), CD27-PC5 (1A4CD27) (both from Beckman Coulter Corporation), CD25-PC5 (B1.49.9), CD69-PE (TP1.55.3) (both from Immunotech, Marseille, France) and CD27-PE (M-T271; Dako, Glostrup, Denmark). Appropriate isotype control mAbs were used for marker setting.
Intracellular cytokine staining was performed after 4 hr stimulation with PMA (12·5 ng/ml) and ionomycin (500 ng/ml) in the presence of brefeldin A (5 μg/ml; Sigma-Aldrich). Cells were fixed and permeabilized using Fix and Perm reagent (eBioscience, San Diego, CA) according to the manufacturer's recommendations. The following mAbs were used for staining: anti-interferon-γ-PC7 (4S.B3, eBioscience) and anti-IL-2-PE (MQ1-17H12), anti-IL-4-PE (8D4-8), anti-IL-10-PE (JES3-19F1); all from BD Bioscience, Intracellular Foxp3 expression was analysed after fixation and permeabilization using anti-human FoxP3 (FCH101) mAb, FITC-labelled or PE-labelled; all from eBioscience). Similarly, Ki-67 expression was examined by intracellular staining using anti-human Ki-67-FITC mAb (B56, BD Bioscience).
Expression of VDRs on T cells was analysed by an indirect staining method. After fixation and permeabilization of cell sample, cells were incubated with anti-human VDR mAb (NR1I1, R&D Systems, Minneapolis, MN) at 4° for 30 min. Cells were then washed twice with permeabilization buffer (eBioscience) followed by incubation with goat anti-mouse IgG-FITC (Dako) in the dark, at 4° for 30 min and analysed by flow cytometry. Appropriate isotype (IgG2A mAb) and conjugate control staining were included.
CFSE-based cell division analysis
Cell division was studied by carboxyfluorescein succinimidyl ester (CFSE) dilution analysis. To this end, high-purity sorted cells, Treg (0·5 × 106 to 2 × 106) and Tconv (10 × 106), were labelled with 0·25–0·5 μm and 4 μm CFSE (Molecular Probes, Leiden, the Netherlands), respectively. The cells were subsequently activated with anti-CD3/anti-CD28 mAb-coated microbeads with or without the addition of 100 nm 1,25(OH)2D3. Cell division accompanied by CFSE dilution was analysed by flow cytometry on days 1, 2 and 5.
Stimulation assay to study T-cell anergy
To study T-cell anergy, 2·5 × 104 sorted Treg and 2·5 × 104 Tconv cells were stimulated with anti-CD3/anti-CD28 mAb-coated microbeads, 25 U/ml rhIL-2 and 10 ng/ml IL-15 (Biosource International, Camarillo, CA); with or without 1,25(OH)2D3. After expansion, the cells were harvested on day 7, washed and rested for 2 days in culture medium containing 5% human pooled serum. Next, secondary cell cultures were performed whereby cells were stimulated with anti-CD3/anti-CD28 mAb-coated microbeads with and without the addition of 12·5 U/ml IL-2. Cell proliferation was examined by [3H]TdR incorporation, as described above, after 2 days.
In vivo study
An observational study was performed among vitamin-D-deficient HIV-1-seropositive patients visiting the Radboud University Nijmegen Medical Centre, the Netherlands. Vitamin D deficiency was defined as 25(OH)D3 levels below 25 or 35 nm, depending on the season. We prospectively studied the effect of cholecalciferol supplementation on circulating Treg cell numbers by flow cytometry. The subjects were treated with a daily dose of 2000 IU cholecalciferol (vitamin D3) during the first 12 weeks and thereafter for at least 48 weeks with a dose of 1000 IU daily. At baseline and after 24 and 48 weeks serum 25(OH)D3, 1,25-(OH)2D3, parathyroid hormone (PTH) levels and circulating Treg cell numbers were determined. This study was approved by the local ethics committee and written informed consent was given by all participating subjects.
Twenty subjects were included but two subjects discontinued cholecalciferol supplementation after 24 weeks for non-medical reasons. No adverse effects were reported or observed during the supplementation.
25(OH)D3, 1,25(OH)2D3 and PTH determination
Serum 25(OH)D3 was measured by HLPC with UV detection, after previous extraction on small SepPak columns. Tritiated 25-(OH)D3, collected from the HPLC system during passage of the UV peak, was used to correct for procedural losses. Serum 1,25(OH)2D3 was measured by radioreceptor assay with previous extraction and chromatographic purification with correction for recovery. For PTH assays the ELSA-PTH assay supplied by CIS BIO (Bedford, MA) was used first. This method was switched into the intact PTH assay performed by the Abbott Architect analyzer, when the CIS BIO assay was no longer available. The Abbott Architect assay was recalibrated on the CIS BIO assay to give identical measurement results.
Statistical analysis
Statistical analysis was performed using the Statistical Product and Services Solutions (SPSS) package version 16.0. The Wilcoxon rank-sum test was used to compare differences between groups (unless otherwise stated). The level of significance was set at P ≤ 0·05.
Results
Human regulatory T cells express VDRs
1,25(OH)2D3 can only exert its effect through binding to its receptor. Although the expression of VDRs by T lymphocytes has been reported,2,3 no information was available on the expression of VDRs by Treg cells. Using flow cytometric analysis, we here show that both freshly isolated CD4+ CD25high Treg cells and CD4+ CD25neg Tconv cells express VDRs (Fig. 1a). Expression of VDR is increased upon activation with anti-CD3/anti-CD28 mAb-coated microbeads in Treg (Fig. 1b) and Tconv (Fig. 1c) cells. The highest levels of VDR expression on Treg and Tconv cells were found on day 2 of culture.
Figure 1.
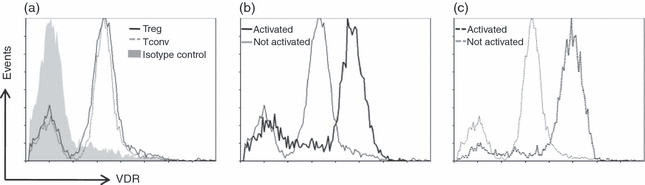
Flow cytometry of vitamin D receptor (VDR) expression by regulatory T (Treg) and conventional T (Tconv) cells. (a) Histogram overlay of VDR expression in Treg and Tconv cells with isotype staining. Histograms showing VDR expression (x-axis) in anti-CD3/anti-CD28 monoclonal antibody-coated microbeads stimulated versus unstimulated (b) Treg and (c) Tconv cells on day 2. Data are representative for three independent experiments performed with cells obtained from different donors.
1,25(OH)2D3 inhibits proliferation, but allows activation of Treg cells
Having established that Treg cells express VDRs, we assessed whether vitamin D3 can affect Treg cell proliferation in an APC-free system. The Treg cells were stimulated with anti-CD3/anti-CD28 mAb-coated microbeads, in the presence of exogenously added rhIL-2 and 1, 10 or 100 nm 1,25(OH)2D3. Treg cell proliferation as analysed by [3H]TdR incorporation was clearly inhibited in a 1,25(OH)2D3 dose-dependent fashion (Fig. 2a). Even at a low concentration of 1 nm 1,25(OH)2D3 there was a marked decrease in cell proliferation. Interestingly, we observed a significant difference in the inhibition of cell division between Treg and Tconv cells (Fig. 2b,e), indicating that Treg cell proliferation is more susceptible to the suppressive effect of vitamin D3. This difference between Treg and Tconv cells was consistently observed through all the concentrations of 1,25(OH)2D3 employed (Fig. 2b).
Figure 2.
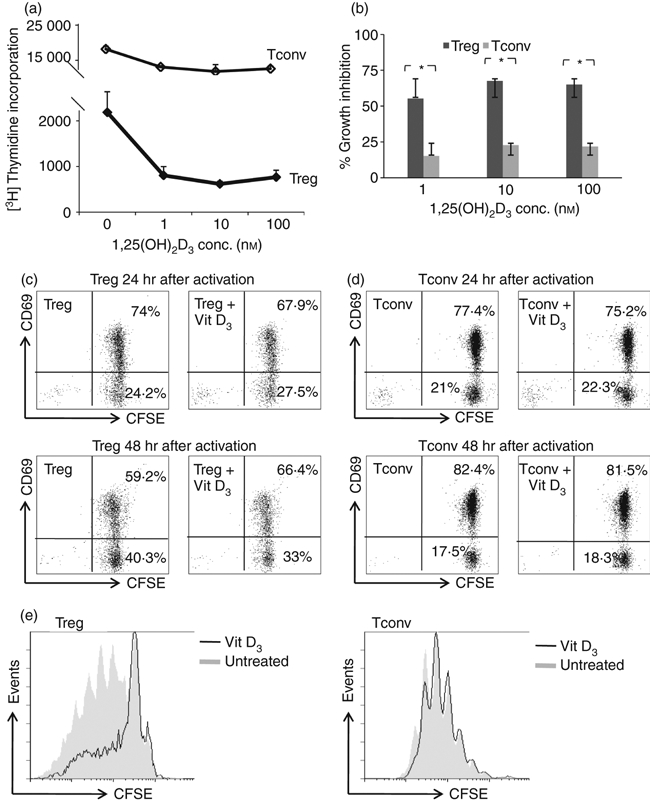
1,25-dihydroxyvitamin D3 [1,25(OH)2D3] inhibits regulatory T (Treg) and conventional T (Tconv) cell proliferation. (a) The effect of graded doses of 1,25(OH)2D3 on Treg or Tconv cell proliferation, stimulated with anti-CD3/anti-CD28 monoclonal antibody-coated microbeads and in the presence of interleukin-2 (IL-2). Cell proliferation as assayed by [3H]thymidine incorporation at day 4 of culture and expressed as mean ± SD. Data are representative for five independent experiments. (b) Percentage growth inhibition of Treg and Tconv cells upon treatment with 1,25(OH)2D3 at 1, 10 and 100 nm was calculated. Data are the cumulative results of five independent experiments using cells isolated by high purity flow cytometric cell sorting. *P< 0·05 when comparing the degree of growth inhibition between Treg and Tconv cells. Effect of 1,25(OH)2D3 (100 nm) on CD69 expression (y-axis), in (c) Treg and (d) Tconv cells labelled with carboxyfluorescein succinimidyl ester (CFSE; x-axis), 24 or 48 hr after activation. (e) Cell division of Treg and Tconv cells as analysed by CFSE dilution (x-axis) on day 5 with and without addition of 100 nm 1,25(OH)2D3. Data are representative for three independent experiments performed with cells isolated by high-purity flow cytometric cell sorting, obtained from different donors.
Addition of vitamin D3 significantly inhibited Treg cell proliferation, so we wondered whether this compound also interfered with early T-cell activation. To analyse this, we looked at cell activation status by measuring the expression of CD69, an inducible cell surface glycoprotein acquired very early during T-cell activation. We found that treatment with 1,25(OH)2D3 did not cause any difference in the expression of CD69 in Treg (Fig. 2c) or Tconv (Fig. 2d) cells within the first 24–48 hr after activation. Although 1,25(OH)2D3 allowed activation of Treg cells, cell cycle progression was inhibited (Fig. 2e).
Vitamin D3 treatment preserves Treg cell suppressor function, anergic phenotype and Foxp3 expression
Treg cells are characterized by suppressor function, anergic phenotype and constitutively high expression of the transcription factor Foxp3. Here, we assessed the influence of vitamin D3 on the aforementioned Treg cell characteristics. To examine whether 1,25(OH)2D3 treatment interferes with the suppressor activity of Treg cells, freshly isolated Treg cells were stimulated with anti-CD3/anti-CD28 mAb-coated microbeads in the presence of 1,25(OH)2D3 and cultured for 7 days. Thereafter, the cells were allowed to recuperate and tested for their suppressor function in a co-culture assay. After this ‘pre-treatment’ with 1,25(OH)2D3, Treg cells kept their suppressive capacity (Fig. 3a). We also demonstrated that the suppressive activity of Treg cells was not affected when a high concentration of 1,25(OH)2D3 was present during the co-culture assay (Fig. 3b).
Figure 3.
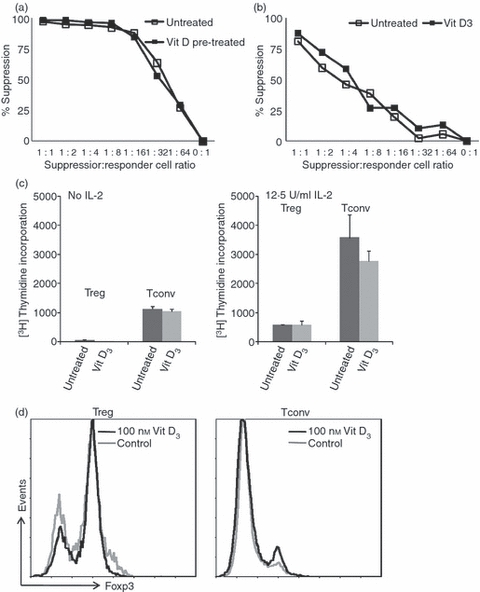
Effect of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] on regulatory T (Treg) cell suppressive function and phenotypic characteristics. (a) Treg cells were expanded with anti-CD3/anti-CD28 monoclonal antibody (mAb) -coated microbeads with or without 100 nm 1,25(OH)2D3 for 7 days. The cells were harvested and rested for 2 days. Co-culture suppression assays were conducted to analyse the suppressor potential of these 1,25(OH)2D3-pre-treated cells. Cell proliferation was determined by [3H]thymidine incorporation at day 4. Results are expressed as percentage suppression = {1 – ([3H]thymidine incorporation co-culture/[3H]thymidine incorporation control mixed lymphocyte culture) × 100%} (y-axis). (b) Suppression assay performed in the absence or presence of 100 nm 1,25(OH)2D3. Percentage suppression (y-axis) was measured and calculated as mentioned above. (c) Treg cells and conventional T (Tconv) cells were expanded with anti-CD3/anti-CD28 mAb-coated microbeads with or without 100 nm 1,25(OH)2D3 for 7 days. The cells were harvested and rested for 2 days. Proliferative capacity of expanded cells upon re-stimulation with anti-CD3/anti-CD28 mAb-coated microbeads was determined in the absence or presence of interleukin-2 by [3H]thymidine incorporation (y-axis). (d) Representative histogram overlay depicting the expression of Foxp3 (y-axis) in Treg and Tconv cells on day 8, as measured by flow cytometry. Data are representative for five or six independent experiments performed with cells isolated by high-purity flow cytometric cell sorting, obtained from different donors. *P < 0·05 as compared with respective cell culture without the addition of 1,25(OH)2D3.
Another classical characteristic of Treg cells is that they exhibit anergic behaviour in vitro. Classically, T-cell anergy is defined as a low proliferative capacity upon stimulation with antigen only, which can be (partially) reversed by addition of exogenous IL-2. This feature was examined in re-stimulation assays. We found that independent of the provision of 1,25(OH)2D3 to the primary cell culture, Treg cells retained their anergic state (Fig. 3c). In contrast to Tconv cells, the Treg cells did not proliferate in the secondary culture upon stimulation in the absence of IL-2, whereas the addition of IL-2 to the secondary culture restored their proliferative capacity.
Foxp3 expression was examined by flow cytometry 8 days after Treg cell stimulation with anti-CD3/anti-CD28 mAb-coated microbeads in the presence of 1,25(OH)2D3. 1,25(OH)2D3 did not affect Foxp3 expression in Treg cells. Neither the level of Foxp3 expression per cell as indicated by the mean fluorescence intensity nor the percentage of cells expressing Foxp3 was affected (Fig. 3d).
Vitamin D3 increases the percentage of Treg cells producing IL-10 in an APC-free system
Having established that 1,25(OH)2D3 diminished the proliferative capacity of Treg cells but spared their suppressive function, we evaluated the influence of 1,25(OH)2D3 on cytokine production of IL-2, interferon-γ, IL-4 and IL-10 in isolated Treg cells by intracellular flow cytometry. With the addition of 100 nm 1,25(OH)2D3, we found a statistically significant increase in the percentage of Treg cells producing IL-10 upon stimulation with anti-CD3/anti-CD28 mAb-coated microbeads, in an APC-free system (Fig. 4a). Production of IL-2 and interferon-γ in both Treg and Tconv cells was unaffected by 1,25(OH)2D3 (Fig. 4b).
Figure 4.
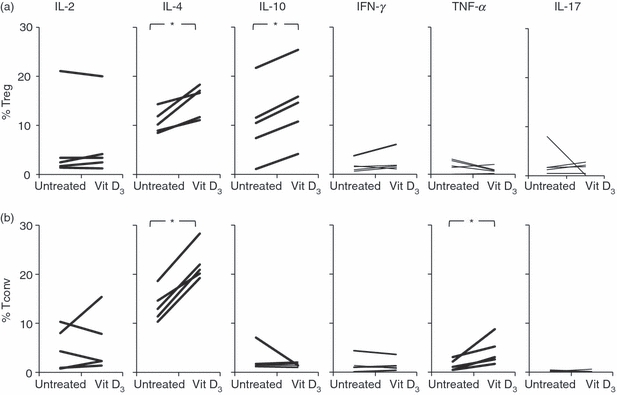
Effect of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] on cytokine production. Intracellular staining for cytokines in (a) regulatory T (Treg) cells and (b) conventional T (Tconv) cells. Both Treg and Tconv cells were stimulated with anti-CD3/anti-CD28 monoclonal antibody-coated microbeads with or without the addition of 100 nm 1,25(OH)2D3. At day 8 of cultures, the cells were stimulated with PMA plus ionomycin in the presence of brefeldin A and intracellularly stained with antibodies directed against the cytokines listed: IL, interleukin; IFN, interferon; TNF, tumour necrosis factor. Data are representative for five independent experiments performed with cells isolated by high-purity flow cytometric cell sorting, obtained from different donors. *P < 0·05 as compared with respective cell culture without the addition of 1,25(OH)2D3.
In patients supplemented with cholecalciferol, the proliferative capacity of Treg cells was reduced and lower numbers of Treg cells were found
Having established inhibitory effects of 1,25(OH)2D3 on Treg cell proliferation in vitro, we looked for evidence of this feature in vivo. To this end, we studied the effect of cholecalciferol (vitamin D3) supplementation on Treg cell division and Treg cell numbers in HIV-1-seropositive patients, who are known to have low serum levels of 25(OH)D3 and 1,25(OH)2D3.13 The subjects were supplemented with a daily dose of 2000 IU cholecalciferol during the first 12 weeks and thereafter for at least 48 weeks with a dose of 1000 IU daily. At baseline and after 24 and 48 weeks after supplementation, we determined the numbers and proliferative capacity of circulating CD4+ CD25high Treg cells and the serum levels of 25(OH)D3, 1,25(OH)2D3 and PTH.
Upon supplementation of cholecalciferol, the 25(OH)D3 serum concentration significantly increased after 24 and 48 weeks compared with baseline levels (Table 1). Also, 1,25(OH)2D3 levels significantly increased after 24 weeks of supplementation, but were not significantly different from baseline at 48 weeks (Table 1, Fig. 5a). As expected, PTH levels had an inverse relation to 1,25(OH)2D3 levels. Interestingly, the percentages and absolute numbers of CD4+ CD25high (CD127low Foxp3+) Treg cells were significantly lower after 24 weeks of supplementation, but recovered at 48 weeks when the dosage of cholecalciferol was lowered at week 12 (Fig. 5b). This suggests a direct and inverse relationship between the levels of 1,25(OH)2D3 and the number of circulating Treg cells. Next, we analysed the ex vivo proliferative capacity of Treg cells in cholecalciferol-supplemented HIV-infected patients by measuring the expression of the proliferation marker Ki-67 in CD4+ Foxp3+-expressing Treg cells using flow cytometry (Fig. 5c). At first, we compared the proliferation status of Treg cells in eight subjects after 24 and 48 weeks supplementation of cholecalciferol, which corresponded to reduced 1,25(OH)2D3 levels at week 48 compared with week 24. We found an increased expression of Ki-67 in the Treg cell population (Fig. 5d), but not in Tconv (i.e. CD4+ Foxp3−) cells (Fig. 5e) at week 48 compared with week 24. Together, these findings show that increased 1,25(OH)2D3 levels concur with reduced proliferative capacity of Treg cells in vivo.
Table 1.
Baseline data and week 24 and 48 data after cholecalciferol supplementation in 25-hydroxyvitamin D3-deficient HIV-1-positive subjects
| Median (IQR) | Median (IQR) | Median (IQR) | P-value | P-value | |
|---|---|---|---|---|---|
| Week 0 | Week 24 | Week 48 | Week 0–24 | Week 0–48 | |
| 25-Hydroxyvitamin D3 (nm) | 26·4 (19·0–29·1) | 98·5 (68·3–103·5) | 79·8 (62·9–98·4) | < .001 | < .001 |
| 1,25-Dihydroxyvitamin D3 (pm) | 142 (106–167) | 186 (108–215) | 132 (99–144) | 0·005 | 0·339 |
| Parathyroid hormone (pm) | 4·70 (4·0–6·1) | 4·2 (2·9–4·8) | 5·0 (3·4–6·4) | 0·021 | 0·398 |
| Treg cell number (cells/L) | 19 (12–28) | 9 (6–14) | 24 (11–38) | 0·005 | 0·129 |
| Treg cells in CD4 population | 4·3 (2·3–5·7) | 2·0 (1·5–3·1) | 5·6 (2·4–10·1) | 0·001 | 0·068 |
IQR, interquartile range; Treg cells, regulatory T cells
Figure 5.
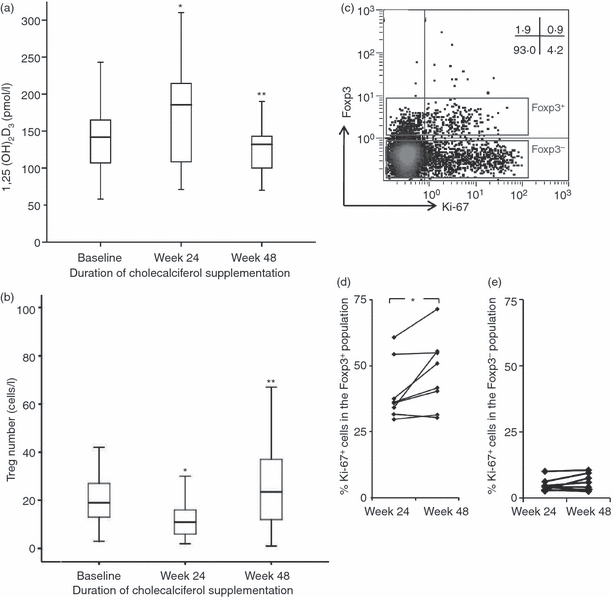
Effect of cholecalciferol supplementation in vitamin D3-deficient HIV-infected patients. (a) Changes in serum 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] concentrations and (b) regulatory T (Treg) cell numbers over time. *P < 0·05 compared with baseline, **P < 0·05 compared with week 24. The subjects were treated with a daily dose of 2000 IU cholecalciferol during the first 12 weeks and thereafter for at least 48 weeks with a dose of 1000 IU daily. (c) Representative flow cytometry dot plot showing intracellular Ki-67 (x-axis) and Foxp3 (y-axis) staining on CD4+ gated T cells. Cumulative data showing the percentages of Ki-67-expressing cells within the (d) CD4+ Foxp3+ and (e) CD4+ Foxp3− population at week 24 and week 48 in eight subjects, where serum 25(OH)D3 levels at week 48 were reduced compared with week 24. *P < 0·05 compared with week 24.
Discussion
In this study, we showed a direct effect of 1,25(OH)2D3 on Treg cells independent of APC. 1,25(OH)2D3 dose-dependently inhibited Treg cell proliferation in vitro and high levels of serum 25(OH)D3 and 1,25(OH)2D3 concur with low levels of Treg cells in vivo. Apart from its inhibitory effect on Treg cell proliferation, 1,25(OH)2D3 had no influence on either the expression of Foxp3 in Treg cells or on the anergic characteristic and suppressive function in vitro, and even induced a slight increase in IL-10 production by Treg cells in an APC-free system. Also, we showed an inverse relationship between in vivo 1,25(OH)2D3 levels and Treg cell numbers in HIV-infected patients supplemented with cholecalciferol. So, whereas vitamin D3 may de novo induce Treg cells via the induction of tolerogenic dendritic cells28,29 its direct effect on pre-existing Treg cells appears to be containment of the population size.
The active form of vitamin D, 1,25-(OH)2D3, exerts its biological effect through the VDR leading to modified gene expression. The VDRs are part of the steroid hormone nuclear receptor family that are expressed by various cell types including mononuclear cells. However, to our knowledge, no information is available regarding the expression of VDRs by Treg cells. In this study, we demonstrated that Treg cells, like Tconv, do express VDRs, and that the expression is up-regulated upon T-cell receptor stimulation. This implies that 1,25(OH)2D3 may exert its immunomodulatory effects directly on Treg cells as we show here for cytokine production and proliferation arrest. The interplay between VDR and TCR signalling has not been widely studied. Although not specific to Treg cells, one possible mechanism involves TCR signalling via protein kinase p38 pathway, which has been shown to induce up-regulation of VDR and phospholipase C-γ1 in CD4+ and CD8+ T cells.30 In addition, induction of phospholipase C-γ1 was found to be dependent on vitamin D and expression of the VDR and was vital for subsequent classical TCR signalling and T-cell activation.
Here, we show clearly that 1,25(OH)2D3 inhibits the proliferation of human Treg cells freshly isolated from peripheral blood. Interestingly, we observed that in comparison to Tconv cells, 1,25(OH)2D3 had a more pronounced repressive effect on Treg cells with regard to proliferation. Despite the fact that 1,25(OH)2D3 inhibited proliferation of Treg cells in vitro, it did not affect the number of Treg cells that became activated upon stimulation with anti-CD3/anti-CD28 mAb-coated microbeads, as indicated by the expression of CD69, nor did it induce cell death in Treg cells (data not shown).
Our finding adds to the already described immunomodulatory effects of 1,25(OH)2D3 on APCs and conventional T lymphocytes. In the presence of APCs, 1,25(OH)2D3 can induce a regulatory phenotype by influencing their maturation,22,31 hereby facilitating the induction of regulatory cells via modulation of the APC. In pre-existing Treg cells we show that 1,25(OH)2D3 inhibits proliferation, but spares the suppressive phenotype and to some extent increases the number of IL-10-producing cells, notably in an APC-free system employed by the present study. With regard to conventional T cells, it has been shown that 1,25(OH)2D3 and its analogues have the capacity to inhibit T-cell proliferation32–34 and to modulate cytokine production35,36 when activated with mitogens. Secretion of interferon-γ and IL-17 was inhibited,23 and IL-4 and IL-10 production was enhanced23,37–39 thereby favouring a bias towards Th2 differentiation.
In vivo we found suggestive evidence that cholecalciferol supplementation affects Treg cell numbers and proliferation. Increasing serum 25(OH)D3 and 1,25(OH)2D3 levels were paralleled by a drop in peripheral Treg cell numbers, whereas a subsequent reduction in 25(OH)D3 levels resulted in recovery of Treg cell numbers. This variation in 25(OH)D3 and 1,25(OH)2D3 levels concurred with changes in the number of dividing Foxp3+ cells, suggesting that regulation of Treg cell division was the underlying effect of cholecalciferol supplementation in vivo. Importantly, resting Treg cells have recently been identified as a reservoir for HIV replication. In vitro activation of these cells led to a detectable viral production.40 The authors suggested that resting Treg cells could release the virus when their anergy status is disturbed. We showed that vitamin D3 can inhibit Treg cell proliferation in vivo whereas in vitro it preserved anergy. Therefore, 1,25(OH)2D3 could be possibly beneficial in diminishing the latent viral pool. Having said that, this postulation requires further verification.
In conclusion, the present study demonstrated that nTreg cells express VDRs, which can be up-regulated upon activation. The immediate effect of 1,25(OH)2D3 on nTreg cells is growth inhibition without interference on its suppressive capacity, anergy status or the Foxp3 expression. Also, in vitro 1,25(OH)2D3 skewed cytokine secretion in nTreg cells towards IL-10-producing cells in the absence of APCs. As in vivo proof of principle that vitamin D3 inhibits Treg cell proliferation, we demonstrated that cholecalciferol supplementation in HIV-infected subjects restored 1,25(OH)2D3 and 25(OH)D3 levels resulting in decreased Treg cell numbers.
Acknowledgments
The authors thank Ronald van Beek and Esther van Rijssen for technical assistance and Jeroen van Velzen for his assistance in high-purity flow cytometric cell sorting. This work was supported by The Nutricia Research Foundation, the Netherlands. M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 3.Veldman CM, Cantorna MT, Deluca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374:334–8. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 4.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 5.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 6.van EE, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–85. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol Med. 2002;8:174–9. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 9.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–64. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92:49–59. doi: 10.1016/j.pbiomolbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64:226–33. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 14.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 16.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay S, Chakraborty NG, Mukherji B. Regulatory T cells and tumor immunity. Cancer Immunol Immunother. 2005;54:1153–61. doi: 10.1007/s00262-005-0699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 19.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 20.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorman S, Judge MA, Hart PH. Gene regulation by 1,25-dihydroxyvitamin D3 in CD4+CD25+ cells is enabled by IL-2. J Invest Dermatol. 2010;130:2368–76. doi: 10.1038/jid.2010.167. [DOI] [PubMed] [Google Scholar]
- 25.Gorman S, Judge MA, Burchell JT, Turner DJ, Hart PH. 1,25-dihydroxyvitamin D3 enhances the ability of transferred CD4+ CD25+ cells to modulate T helper type 2-driven asthmatic responses. Immunology. 2010;130:181–92. doi: 10.1111/j.1365-2567.2009.03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royal W, III, Mia Y, Li H, Naunton K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol. 2009;213:135–41. doi: 10.1016/j.jneuroim.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Smolders J, Thewissen M, Peelen E, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS ONE. 2009;4:e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 29.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 30.von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. 2010;11:344–9. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 31.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 32.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–5. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–5. [PubMed] [Google Scholar]
- 34.Muller K, Odum N, Bendtzen K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett. 1993;35:177–82. doi: 10.1016/0165-2478(93)90088-j. [DOI] [PubMed] [Google Scholar]
- 35.Muller K, Bendtzen K. Inhibition of human T lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3. Differential effects on CD45RA+ and CD45R0+ cells. Autoimmunity. 1992;14:37–43. doi: 10.3109/08916939309077355. [DOI] [PubMed] [Google Scholar]
- 36.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:1659–64. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1 alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 38.Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 2004;229:1136–42. doi: 10.1177/153537020422901108. [DOI] [PubMed] [Google Scholar]
- 39.Imazeki I, Matsuzaki J, Tsuji K, Nishimura T. Immunomodulating effect of vitamin D3 derivatives on type-1 cellular immunity. Biomed Res. 2006;27:1–9. doi: 10.2220/biomedres.27.1. [DOI] [PubMed] [Google Scholar]
- 40.Tran TA, de Goer de Herve MG, Hendel-Chavez H, et al. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS ONE. 2008;3:e3305. doi: 10.1371/journal.pone.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]


