Abstract
BACKGROUND AND PURPOSE
The endocannabinoid system appears to play a pivotal role in mediating the rewarding and reinforcing effects of nicotine. Recent studies have shown that the inhibition of fatty acid amide hydrolase (FAAH) attenuates reinstatement of nicotine-seeking induced by nicotine priming and nicotine-associated cues. FAAH hydrolyses the endogenous endocannabinoid anandamide, as well as other non-cannabinoid ligands such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). As OEA and PEA can attenuate both nicotine-taking and nicotine-seeking behaviour, the specific role of anandamide remains unclear. In this study, we have tested the selective anadamide uptake inhibitor, VDM11, which elevates anandamide levels without affecting levels of OEA/PEA, on nicotine-taking and nicotine-seeking behaviour.
EXPERIMENTAL APPROACH
We used a nicotine intravenous self-administration model in rats to assess the effect of VDM11, given i.p., on nicotine taking using fixed and progressive ratio schedules of reinforcement as well as on reinstatement of nicotine-seeking induced by nicotine priming and nicotine-associated cues.
KEY RESULTS
VDM11 did not affect levels of responding for nicotine under fixed-ratio and progressive-ratio schedules of reinforcement. In contrast, VDM11 dose-dependently attenuated reinstatement of nicotine-seeking behaviour induced by nicotine-associated cues and nicotine priming.
CONCLUSIONS AND IMPLICATIONS
These results indicate that ligands elevating anandamide levels could have therapeutic value for preventing relapse into nicotine-seeking behaviour and should be tested in humans trying to quit smoking.
Keywords: nicotine, anandamide, cannabinoid, self-administration, VDM11
Introduction
The endocannabinoid system is involved in drug-taking behaviour and relapse for various drugs of abuse, including nicotine (De Vries and Schoffelmeer, 2005; Le Foll and Goldberg, 2005; Le Foll et al., 2008). The endocannabinoid system consists of the endocannabinoids [mostly anandamide and 2-arachidonoylglycerol (2-AG)], the target receptors for those endocannabinoids (cannabinoid CB1 and CB2 receptors, but also non-cannabinoid targets for anandamide; receptor nomenclature follows Alexander et al., 2009), enzymatic degradation systems (through fatty acid amide hydrolase (FAAH) for anandamide and monoacylglycerol lipase for 2-AG) and a putative transport uptake system (Di Marzo et al., 1998; 2001; Mechoulam et al., 1998; Piomelli et al., 2000; Sugiura and Waku, 2002; Piomelli, 2003; 2005; Di Marzo, 2006). Preclinical and human clinical studies (Le Foll et al., 2008) have indicated that blocking endocannabinoid transmission by CB1 receptor antagonists/inverse agonists could be a useful strategy for the treatment of human smokers. Rimonabant, a CB1 receptor inverse agonist used in Europe for the treatment of obesity (Scheen et al., 2006; Burch et al., 2009; Leite et al., 2009), increased the smoking cessation rates in controlled trials (Le Foll et al., 2008). Unfortunately, the use of rimonabant has been associated with increased risk of anxiety and depression (Moreira et al., 2009; Nathan et al., 2010) and hence, rimonabant was withdrawn from the European market at the end of 2008 (Le Foll et al., 2009)
Interestingly, we have recently discovered that elevating endogenous anandamide levels could be an alternative therapeutic strategy. We first evaluated the effects of URB 597 (a FAAH inhibitor) (Fegley et al., 2005) as pharmacological blockade of FAAH activity prolonged many behavioural and neurobiological effects of anandamide. We found that URB 597 administration reversed abuse-related behavioural and neurochemical effects of nicotine in rats (Scherma et al., 2008; Forget et al., 2009). URB 597 elevates not only brain levels of anandamide, but also those of the non-cannabinoid acylethanolamides, oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) which act on α-type peroxisome proliferator-activated receptors (PPARα) (Bond et al., 1995; Fegley et al., 2005; Astarita et al., 2006; Mascia et al., 2011). As the effects of URB 597 could be mediated by either elevated levels of anandamide, by elevated levels of OEA and PEA, or by combinations of these three endogenous ligands, there is a need to explore further the effects of anandamide alone on nicotine-taking and nicotine-seeking behaviour.
In this study, we evaluated the effect of the selective anadamide uptake inhibitor, VDM11 on nicotine-taking and nicotine-seeking behaviour. VDM11 elevates anandamide levels with minimal effects on levels of OEA, PEA or 2-AG (the other endogenous cannabinoid ligand) both in vitro (De Petrocellis et al., 2000) and in vivo (Van der Stelt et al., 2006). Our aim was to study the effect of selectively elevating levels of anandamide in the brain on i.v. nicotine self-administration under fixed-ratio (FR) and progressive-ratio (PR) schedules of reinforcement, as well as nicotine-seeking behaviour induced by nicotine-associated cues and nicotine priming. The anandamide uptake inhibitors were of potential therapeutic benefit in the treatment of pain, motor impairments and anxiety (Fernandez-Espejo et al., 2004; Bortolato, et al., 2006; La Rana et al., 2006). If proven to modulate reinstatement of nicotine-seeking behaviour, this class of ligands could have a potential therapeutic benefit as smoking-cessation therapy.
Methods
Animals
All animal care and experimental procedures described in this report complied with the guidelines of the Canadian Council on Animal Care (compatible with NIH guidelines), and were reviewed and approved by the institutional Animal Care Committee. Male Long Evans rats (Charles River, Lachine, PQ, Canada), experimentally naive at the start of the study and initially weighing 250–275 g, were used. All rats were individually housed in a temperature-controlled environment on a 12 h reverse light/dark cycle (lights off from 07:00 h to 19:00 h). Before any experimental manipulation, animals were given a minimum of 7 days to habituate to the colony room, during which they were weighed, handled and received unlimited access to both food and water. After habituation, all rats were diet restricted to five pellets or 20 g daily and had free access to water.
Apparatus
Nicotine i.v. self-administration studies were carried out in commercially available experimental chambers (Med Associates, St Albans, VT, USA) located in sound-attenuating boxes and equipped with two levers, a house light and two cue lights, one located above each lever. For half the animals, the left lever was the active lever and for the other half, the right lever was the active lever.
Experimental procedures
Food-maintained behaviour
Techniques for initial acquisition of food-maintained behaviour and surgery were similar to those already reported (Corrigall and Coen, 1989; Forget et al., 2009; Gamaleddin et al. 2011; Khaled et al., 2010) Animals learned to lever press for food reinforcement on a continuous reinforcement schedule, in which each press on the active lever resulted in the delivery of a 45 mg food pellet. During these acquisition sessions, the house light was on, with no illumination of the cue lights above the levers. Daily 1 h acquisition sessions were conducted for 5 days. Once food-maintained behaviour was acquired, i.v. catheters were surgically implanted.
Implantation of i.v. catheters
Surgical procedures for implantation of chronic i.v. catheters were similar to those already reported (Corrigall et al., 1989; Forget et al., 2009; Khaled et al., 2010). Briefly, catheters were implanted into the jugular vein, exiting between the scapulae. Surgery was performed under anaesthesia induced by xylazine (10 mg·kg−1, i.p.) and ketamine hydrochloride (90 mg·kg−1, i.p.). Incision sites were infiltrated s.c. with a local anaesthetic, marcaine (0.125%). Buprenorphine was given for post-operative analgesia (0.03 mg·kg−1, s.c.), and a single dose of penicillin (30 000 units, i.m.) was administered at the completion of surgical procedures. Animals were allowed to recover for 1 week before drug self-administration sessions were begun.
Self-administration procedures
Acquisition of nicotine self-administration was performed under an FR schedule of reinforcement at a unit dose of 30 µg·kg−1 per infusion of nicotine base. Session duration was 60 min. The start of each 60 min session was signalled by illumination of the house light. In the presence of the illuminated house light, 1–5 active lever presses resulted in the delivery of a nicotine infusion. Each infusion was followed by a time out period of 60 s, during which the house light was dimmed, the cue light above the active lever illuminated and lever press responses had no programmed consequences. During the first week of acquisition, response requirements were FR1 (i.e. each active lever press during the time-in period resulted in the delivery of a nicotine infusion). Response requirements were then gradually increased to reach a final value of FR5, by which time self-administration behaviour was stable and the animals had a 15–20 day history of nicotine self-administration. Self-administration sessions occurred mostly 5 days a week.
Testing under the FR5 schedule of reinforcement
Animals were considered to have acquired stable nicotine self-administration when they pressed the active lever more than twice the number of times they pressed the inactive lever, received a minimum of 10 infusions per 60 min session and had less than 20% variation in the number of infusions earned per session during two consecutive sessions.
Once stability was reached, the animals were given i.p. injections of vehicle (tocrisolve) to habituate them to the injection procedure for an additional 3 days. Rats (n = 18) were then tested using i.p. injections of vehicle or VDM11 at doses of 1, 3 and 10 mg kg−1 given 30 min before the start of the session, in a counterbalanced, within-subject design.
Testing under the PR schedule of reinforcement
The same group of animals (n = 14) (four animals were excluded due to blocked catheters) was used to self-administer nicotine (30 µg·kg−1 per infusion) under the FR schedule and then was directly switched to a PR schedule where the response requirement increased with each successive injection. The response requirement progression was based on the formula 5e(0.25 ×[inj.number + 3]), with the first two values replaced by 5 and 10 (modified from Roberts and Bennett, 1993). Thus, the response requirements for successive injections were 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, etc. The break point was defined as the highest ratio completed prior to the first 30 min period without a response on the active lever. PR sessions lasted a maximum of 4 h. The animals were allowed 10 days of nicotine self-administration under the PR schedule before testing with the pharmacological compounds began. Testing of VDM11 (1–10 mg·kg−1, 30 min before the session) was performed using a counterbalanced within-subject design.
Extinction
Reinstatement testing was performed on another group of animals (this experiment started with 18 rats, but only 14 rats completed testing on all doses of VDM11 on cue-induced reinstatement due to attrition). After acquisition of nicotine self-administration as described previously, an extinction phase was conducted by withholding nicotine and its associated cues (house light stayed on and cue lights stayed off throughout the session). Responses on the active and inactive lever were recorded, but had no programmed consequences. An extinction criterion was established for each animal individually and was defined as total active lever responses being less than 20 presses. The extinction criteria had to be maintained for two consecutive days in order to conduct testing. All animals reached extinction criteria within an average of eight extinction sessions. Both extinction and reinstatement sessions lasted for 60 min
Effects of VDM11 on cue-induced reinstatement of nicotine-seeking behaviour
All tests were carried out in a counterbalanced, within-subject design. After each test, extinction was re-established until extinction criteria were obtained for at least two consecutive days. Rats were pretreated 30 min before the session with vehicle or 1, 3 and 10 mg·kg−1 VDM11 in a counterbalanced order to measure the effects of VDM11 on cue-induced reinstatement of nicotine-seeking behaviour. Cue-induced reinstatement tests were conducted under conditions identical to that of self-administration, except that responses on the active lever (on an FR5 schedule) resulted in contingent presentation of the cues (light above the active lever on and house light off for 60 s) without nicotine availability (no infusions). Responses on the inactive lever were recorded but had no programmed consequences. The testing sessions lasted 1 h.
Effects of VDM11 on nicotine-induced reinstatement of nicotine-seeking-behaviour
A new group of animals (n = 7) were given same training and extinction and was subsequently used to determine the effects of VDM11 (1, 3, and 10 mg·kg−1 i.p. 30 min before the session) on nicotine-induced reinstatement. Nicotine priming was performed as described by Forget et al., (2010a,b;) by administering 0.15 mg·kg−1 nicotine s.c., 10 min before the test session. During the extinction and nicotine-induced reinstatement testing sessions, the cue light above the active lever was always off.
Data analysis
The number of active and inactive lever presses or nicotine infusions was recorded and analysed. To analyse difference in responses between the active and inactive levers along time in the acquisition of nicotine self-administration under FR and PR schedules and during extinction, we used two-way anova. To analyse the effects of VDM11 on the number of nicotine infusions earned under the FR and the PR schedule of reinforcement, one-way anova was performed. For reinstatement studies, one-way repeated measures anova was used to assess the reinstatement effect and the effects of VDM11 on reinstatement induced by nicotine priming and nicotine-associated cues.
Materials
(-)Nicotine hydrogen tartrate (Sigma-Aldrich, St Louis, MO, USA) was dissolved in saline, the pH was adjusted to 7.0 (±0.2) and the solution was filtered through a 0.22 mm syringe filter (Fisher Scientific, Pittsburgh, PA, USA) for sterilization purposes. All nicotine doses are reported as free base concentrations. Nicotine was administered i.v. in a volume of 100 µL·kg−1 per injection for self-administration studies or was administered s.c. at the dose of 0.15 mg·kg−1 for reinstatement studies.
VDM11[(5Z,8Z,11Z,14Z)-N-(4-hydroxy-2-methylphenyl)-5,8,11,14-eicosatetraenamide] (Tocris Bioscience, Ellisville, MO, USA) was dissolved in Tocrisolve and injected i.p. at a volume of 1 mL·kg−1 30 min before the start of the session.
Results
Acquisition of nicotine self-administration behaviour under FR schedule of reinforcement
During the first week of acquisition, responding on the active lever, which had previously been reinforced by food and was now reinforced by nicotine infusion, decreased to relatively low levels. When response requirement was then increased to reach a final value of FR5 over the next 2 weeks, responding on the active lever that was reinforced by nicotine infusion increased to the high levels previously maintained by food, while responding on the inactive lever remained low (Figure 1A). anova revealed a significant effect of time (F14,644 = 13.35; P < 0001), a significant effect of lever (i.e. active vs. inactive; F1,46 = 44.16; P < 0001) and a significant interaction between time and lever (F14,644 = 7.77; P < 0001).
Figure 1.
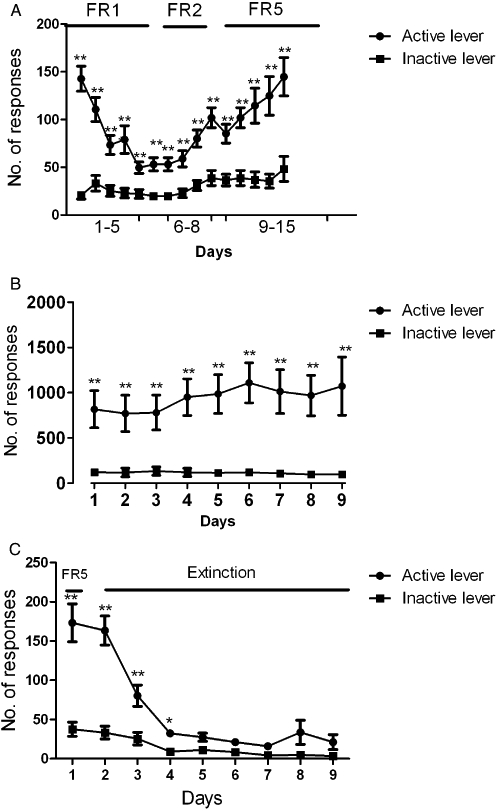
(A) Acquisition of nicotine (30 µg·kg−1 per infusion) self-administration under an FR schedule of reinforcement. A: the number of active and inactive lever presses (mean ± SEM) received in each session under the different schedules of reinforcement (FR-1, FR-2, FR-5) anova showed a significant effect of time (P < 0.0001) and active versus inactive lever (P < 0.0001), and a significant interaction between lever presses and time (P < 0.0001). (B) Acquisition of nicotine (30 µg·kg−1/infusion) self-administration under PR schedule of reinforcement. B: the number of active and inactive lever presses (mean ± SEM) received in each session under PR schedule of reinforcement. anova showed no significant effect of time (P = 0.37), a significant difference between active and inactive levers (P < 0.0001), and no significant interaction between lever presses and time (P = 0.31). (C) Extinction of nicotine self-administration behaviour before reinstatement testing. C: the number of active and inactive lever presses (mean ± SEM) received in each extinction session. anova performed on the 8 days of extinction showed a significant effect of time (P < 0.0001) and lever (P < 0.0001), and interaction between lever presses and time (P < 0.0001). LSD post hoc analysis showed no significant difference between responses on the active and inactive levers after the 3rd day of extinction (P = 0.65); *P < 0.05; **P < 0.01.
Acquisition of nicotine self-administration behaviour under PR schedule of reinforcement
Animals achieved stable self-administration under the PR schedule of reinforcement within 9 days, after which subsequent testing with VDM11 started. anova performed on acquisition of nicotine self-administration under PR schedule of reinforcement showed a non-significant effect of time (F8,208 = 1.08; P = 0.37), a significant effect of lever (i.e. active vs. inactive levers, F1,26 = 27.36; P < 0.0001) and no significant interaction between time and lever (F8,208 = 1.17; P = 0.31) (Figure 1B).
Extinction
The data presented in Fig 1C reflect the extinction pattern for the group of animals (n = 16) used in the cue-induced reinstatement testing (only 14 animals completed testing on cue-induced reinstatement). Animals were subjected to extinction sessions that lasted for 8 days, during which most of the animals reached extinction criteria and testing with VDM11 on reinstatement was started (extinction training was pursued for the remaining rats until they reach the extinction criteria). anova performed on the 8 days of extinction showed a significant effect of time (F8,240 = 48.19; P < 0.0001), a significant effect of lever (F1,30 = 27.10; P < 0.0001) and a significant interaction between time and lever (F8,240 = 27.10; P < 0.0001). LSD post hoc analysis showed no significant difference between responses on the active and inactive levers after the third day of extinction (P = 0.65)
Effects of VDM11 on nicotine self-administration under the FR5 schedule
anova showed no effect of VDM11 pretreatment on the number of nicotine infusions (F3,51 = 0.07087, P = 0.97), and pairwise comparisons with baseline level indicated that pre-treatment with VDM11 (1, 3 and 10 mg·kg−1) did not affect the number of nicotine infusions received during the session (Figure 2A).
Figure 2.
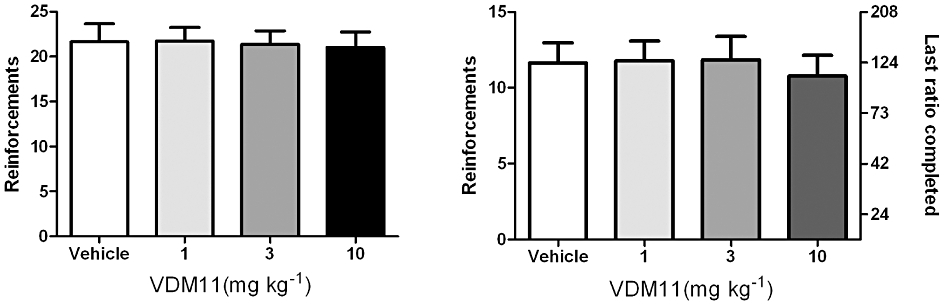
Effect of VDM11 on nicotine self-administration under FR5 and PR schedules of reinforcement. (A) Effects of pre-treatment with VDM11 (1, 3 and 10 mg·kg−1, i.p.) on nicotine (30 µg·kg−1 per infusion) self-administration under the FR5 schedule. Data are expressed as mean ± SEM of the number of infusions obtained during the 60 min session. VDM11 did not affect responding versus vehicle pre-treatment (n = 18); P = 0.97. (B) Effects of pre-treatment with VDM11 (1, 3 and 10 mg kg–1, i.p.) on nicotine (30 µg·kg−1 per infusion) self-administration under PR schedule. Data are expressed as mean ± SEM of the number of infusions obtained during the 4 h sessions. VDM11 did not affect BP P > 0.05 versus vehicle pre-treatment (n = 14); P = 0.15.
Effects of VDM11 on nicotine self-administration under the PR schedule
anova showed no effect of VDM11 pretreatment on the number of nicotine infusions (F3,39 = 1.843, P > 0.05). VDM11 (1, 3 and 10 mg·kg−1) at the various doses tested failed to produce any change in the break point values, compared with vehicle (Figure 2B).
Effects of VDM11 on reinstatement of nicotine seeking induced by nicotine-associated cues
anova performed on active lever presses indicated a main effect of cues per se on reinstatement of nicotine seeking compared with baseline conditions (P < 0.001). anova performed on the active lever presses indicated a main effect of VDM11 (F4,52 = 14.22; P < 0.05) and Newman–Keuls post hoc analysis showed that pre-treatment with 3 and 10 mg·kg−1 VDM11, 30 min before the start of the session, attenuated the reinstatement induced by nicotine-associated cues (P < 0.05), as compared with reinstatement under vehicle pre-treatment. Neither presentation of nicotine-associated cues nor VDM11 administration had a significant effect on responding on the inactive lever (F4,52 = 1.019; P = 0.40) (Figure 3A).
Figure 3.
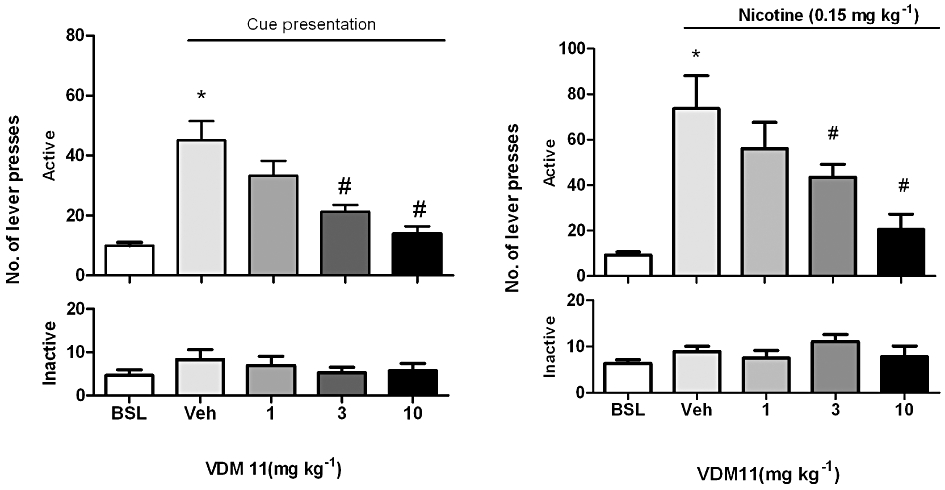
(A) Effects of VDM11 on reinstatement of nicotine-seeking behaviour induced by presentation of nicotine associated cues and by (B) priming doses of nicotine. (A) Effects of pretreatment with VDM11 (1, 3 and 10 mg·kg−1, i.p.) on cue-induced reinstatement of nicotine-seeking behaviour. A significant reinstatement of nicotine-seeking behaviour was produced by presentation of nicotine-associated cues alone (*P < 0.001). Newman–Keuls post hoc analysis after significant anova showed that pre-treatment with VDM11 (3 and 10 mg·kg−1, i.p.) significantly reduced cue-induced reinstatement of nicotine-seeking behaviour (#P < 0.05), n = 14. (B) A Significant reinstatement of nicotine-seeking was also produced by pre-treatment with nicotine (0.15 mg·kg−1) (*P < 0.001). Newman–Keuls post hoc analysis after significant anova showed that VDM11 (3 and 10 mg·kg−1, i.p.) significantly reduced the reinstatement of nicotine-seeking behaviour induced by a priming injection of 0.15 mg·kg−1 nicotine administered 10 min before the session (#P < 0.05). Data are expressed as mean ± SEM of the number of lever presses: BSL, during extinction; Veh, vehicle (Veh) pre-treatment (visual cues); n = 7.
Effects of VDM11 on reinstatement of nicotine seeking induced by nicotine priming
anova performed on active lever presses indicated a main effect of priming with nicotine (0.15 mg·kg−1) on nicotine-seeking behaviour, as compared with baseline conditions (P < 0.001: Figure 3B). anova analysis of the active lever presses indicated a main effect of VDM11 (F4,24 =,9.963; P < 0.05) and Newman–Keuls post hoc analysis showed that pre-treatment with 3 and 10 mg·kg−1 VDM11, 30 min before the start of the session, attenuated the effect of nicotine priming (P < 0.05), compared with reinstatement under vehicle pretreatment. Neither priming injections of nicotine nor VDM11 (1,3 and 10 mg·kg−1) treatment had any significant effect on responding on the inactive lever (F4,24 = 0.947; P = 0.43) (Figure 3B).
Discussion
Anandamide transport inhibitors represent a novel class of ligands that modulate endocannabinoid neurotransmission. Our results indicate that elevation of anandamide levels by the selective uptake inhibitor VDM11 reduced reinstatement of nicotine-seeking behaviour induced by either presentation of nicotine-associated cues or by nicotine priming. In contrast, VDM11 did not have an effect on responding for nicotine under FR or PR schedules of reinforcement.
Our findings with VDM11 are in agreement with our previous results with URB597. We reported that URB597 attenuated nicotine-induced reinstatement of nicotine seeking using both i.v. nicotine self-administration and conditioned place preference paradigms (Scherma et al., 2008; Forget et al., 2009). URB597 also attenuated acquisition of nicotine self-administration under an FR schedule (Scherma et al., 2008). In contrast, URB597 did not alter nicotine self-administration behaviour under a PR schedule (Forget et al., 2009). The present findings obtained with VDM11 indicates that this ligand has a similar profile; that is, it is able to disrupt nicotine seeking induced by both cues and priming, but does not affect established nicotine-taking behaviour. Further studies will be required to evaluate whether VDM11 can affect acquisition of nicotine self-administration behaviour, as it has been reported that URB597 significantly decreases acquisition of nicotine self-administration (Scherma et al., 2008).
As mentioned earlier, inhibition of FAAH increases levels of several endogenous substances in the brain, including the endocannabinoid anandamide and the non-cannabinoid fatty acid acylethanolamides OEA and PEA, which are ligands for PPARα (Scherma et al., 2008). We recently reported that ligands acting selectively as PPARα agonists can reduce reward-related effects of nicotine (Mascia et al., 2011). In those studies, PPARα agonists dose-dependently decreased nicotine self-administration and nicotine-induced reinstatement, but did not alter food- or cocaine-reinforced operant behaviour (Mascia et al., 2011). Therefore, the effects observed with URB597 could have been mediated by only PPARα activation by OEA and PEA. It is interesting to note that VDM11 exhibited a different profile from that of the PPARα agonists, being unable in the present experiments to affect established nicotine self-administration behaviour.
One possible limitation of our findings is the fact that the effects could be modulated by non-specific effects on motor response. Beltramo et al., (2000) have previously reported that intracerebroventricular injection of AM404 (a VDM11 analogue) induced mild hypokinesia as shown by the increase in immobility time and decreased motor behaviour stimulated by dopamine D2 receptor agonists. However, such effects are unlikely to mediate the effects observed here with VDM11 as we found no significant effect on the ability of the animals to lever press for nicotine under both FR and PR schedules (Figure 2A,B).
Another limitation of our study is that we tested only one anandamide uptake inhibitor. Further studies evaluating the ability of other anandamide uptake inhibitors, such as AM404, which also elevate anandamide levels selectively, are needed to validate our findings. Interestingly, it has been reported that AM404 administration reduces alcohol self-administration under an FR schedule of reinforcement but it did not affect reinstatement of alcohol-seeking, suggesting that either there may be differences between AM404 and VDM11, or that anandamide may cause differential effects on drug-taking and drug-seeking depending on the substance under study (Cippitelli et al., 2007).
It is also possible that VDM11 could have an inhibitory effect on FAAH enzymatic activity in vitro (De Petrocellis et al., 2000), however, such an effect has not been reported consistently. Any potential inhibitory effects of VDM11 on FAAH activity could be assay-related and also dependent on the source of the compound or the pH used in the assay, as well as the concentration of fatty acid-free BSA used in the experiment (Fowler et al., 2004)
Anandamide and 2-AG are the two main endocannabinoids in the brain (Bisogno et al., 2000; Hanus et al., 2001; Huang et al., 2002; Porter et al., 2002; Di Marzo, 2006). Anandamide not only binds to cannabinoid CB1 receptors with high affinity (Ki = 52 nM) (Devane et al., 1992; Felder et al., 1993; Childers et al., 1994; Terranova et al., 1995), but also acts on CB2 receptors and may also have non-cannabinoid-mediated effects, notably through the transient receptor potential vanilloid (TRPV) ion channels (Di Marzo et al., 2000). The present experiments do not provide information on the downstream mechanisms by which VDM11 acts to produce its effects on nicotine reinstatement. Activation at CB1, CB2 and TRPV receptors are possible mechanisms. Interestingly, like anandamide, 2-AG also binds to cannabinoid CB1 receptors, but with lower affinity (Ki = 15 µM). 2-AG acts as a full agonist at CB1 receptors (Devane et al., 1992; Felder et al., 1993; Childers et al., 1994; Terranova et al., 1995), whereas anandamide acts as a partial agonist (Sugiura et al., 2002). Because 2-AG is a full agonist and anandamide is a partial agonist, it is possible that anandamide may oppose some effects of 2-AG (Maccarrone et al., 2008). It may appear surprising that an experimental approach which increases endocannabinoid tone produces opposing effects. However, the administration of a full cannabinoid agonist, such as WIN 55,212–2, has been shown to increase motivation for nicotine and produce reinstatement of nicotine-seeking behaviour (Gamaleddin et al., in press). Moreover, such opposite effects have also been described in experiments utilising nicotine full agonists and partial agonists, with nicotine (full agonist) being able to induce nicotine-seeking (see Figure 3B and Scherma et al., 2008), while varenicline (a nicotinic partial agonist) reducing the motivation to self-administer nicotine and decreasing cue-induced reinstatement of nicotine-seeking (Le Foll et al., abstract SRNT 2011). These hypotheses will be evaluated through further ongoing studies that will investigate the separate roles of anandamide and 2-AG on nicotine-seeking behaviour.
Our results indicate that elevation of anandamide levels reduced reinstatement to nicotine seeking, induced by nicotine-associated cues as well as by exposure to a priming dose of nicotine. As the ligands elevating anandamide levels are likely to be devoid of the psychotropic side effects of the CB1 inverse agonist rimonabant (Le Foll et al., 2009), this class of ligands may have some therapeutic potential to prevent relapse in abstinent smokers.
Acknowledgments
This research was supported by a grant from the Heart & Stroke Foundation, grant # NA 6901 and Egyptian Ministry of Higher Education – Ain Shams University and by the Intramural Research Program, National Institute on Drug Abuse, NIH, DHHS, Baltimore, MD, USA.
Glossary
Abbreviations
- 2AG
2-arachidonoylglycerol
- AM404
N-(4-hydroxyphenyl)-arachidonamide
- anova
analysis of variance, CRF, continous reinforcement
- FAAH
fatty acid amide hydrolase
- FR
fixed ratio
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PPAR
peroxisome proliferator-activated receptor
- PR
progressive ratio
- TO
time out
- TRPV
transient receptor potential vanilloid
- URB597
cyclohexyl carbamic acid 3′-carbamoyl-3-yl ester
- VDM11
[(5Z,8Z,11Z,14Z)-N-(4-hydroxy-2-methylphenyl)-5,8,11,14-eicosatetraenamide]
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita G, Di Giacomo B, Gaetani S, Oveisi F, Compton TR, Rivara S, et al. Pharmacological characterization of hydrolysis-resistant analogs of oleoylethanolamide with potent anorexiant properties. J Pharmacol Exp Ther. 2006;318:563–570. doi: 10.1124/jpet.106.105221. [DOI] [PubMed] [Google Scholar]
- Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, et al. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, et al. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351(Pt 3):817–824. [PMC free article] [PubMed] [Google Scholar]
- Bond AR, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, et al. Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the?α2-adrenoreceptor. Nature. 1995;374:272–276. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al. Rimonabant for the treatment of overweight and obese people. Health Technol Assess. 2009;13(Suppl. 3):13–22. doi: 10.3310/hta13suppl3/03. [DOI] [PubMed] [Google Scholar]
- Childers SR, Sexton T, Roy MB. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol. 1994;47:711–715. doi: 10.1016/0006-2952(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Gorriti MA, Navarro M, Massi M, Piomelli D, et al. The anandamide transport inhibitor AM404 reduces ethanol self-administration. Eur J Neurosci. 2007;26:476–486. doi: 10.1111/j.1460-9568.2007.05665.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 2006;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, et al. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. Endocannabinoids Part I: molecular basis of endocannabinoid formation, action and inactivation and development of selective inhibitors. Expert Opin Ther Targets. 2001;5:241–265. doi: 10.1517/14728222.5.2.241. [DOI] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Espejo E, Caraballo I, Rodriguez de Fonseca F, Ferrer B, El Banoua F, Flores JA, et al. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology. 2004;29:1134–1142. doi: 10.1038/sj.npp.1300407. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration – comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry. 2010a;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010b;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, Lopez-Rodriguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis – a difficult issue to handle. Eur J Pharmacol. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, et al. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00314.x. doi: 10.1111/j.1369-1600.2011.00314.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, et al. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- La Rana G, Russo R, Campolongo P, Bortolato M, Mangieri RA, Cuomo V, et al. Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 [N-(4-hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide] J Pharmacol Exp Ther. 2006;317:1365–1371. doi: 10.1124/jpet.105.100792. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Forget B, Aubin HJ, Goldberg SR. Blocking cannabinoid CB1 receptors for the treatment of nicotine dependence: insights from pre-clinical and clinical studies. Addict Biol. 2008;13:239–252. doi: 10.1111/j.1369-1600.2008.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009;205:171–174. doi: 10.1007/s00213-009-1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Chatterjee M, Lev-Ran S, Barnes C, Gamaleddin I, Pushparaj A, et al. Ontario, Canada: SRNT Poster session; 2011. The Nicotinic Partial Agonist Varenicline Blocks Motivation for Nicotine and Significantly Reduces Cue-Induced Reinstatement of Nicotine-Seeking in Rats. [Google Scholar]
- Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV. Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep. 2009;61:217–224. doi: 10.1016/s1734-1140(09)70025-8. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, et al. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23:133–144. doi: 10.1016/j.beem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, O'Neill BV, Napolitano A, Bullmore ET. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2010.00172.x. doi: 10.1111/j.1755-5949.2010.00172.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA. Heroin self-administration in rats under a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1993;111:215–218. doi: 10.1007/BF02245526. [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Van Gaal LG, Despres JP, Pi-Sunyer X, Golay A, Hanotin C. [Rimonabant improves cardiometabolic risk profile in obese or overweight subjects: overview of RIO studies] Rev Med Suisse. 2006;2:1916–1923. [PubMed] [Google Scholar]
- Scherma M, Panlilio LV, Fadda P, Fattore L, Gamaleddin I, Le Foll B, et al. Inhibition of anandamide hydrolysis by URB597 reverses abuse-related behavioral and neurochemical effects of nicotine in rats. J Pharmacol Exp Ther. 2008;327:482–490. doi: 10.1124/jpet.108.142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M, Mazzola C, Esposito G, Matias I, Petrosino S, De Filippis D, et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem. 2002;132:7–12. doi: 10.1093/oxfordjournals.jbchem.a003200. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]


