Abstract
BACKGROUND AND PURPOSE
Although 3α-hydroxy, 5α-reduced pregnane steroids, such as allopregnanolone (AlloP) and tetrahydrodeoxycorticosterone, are endogenous positive modulators of postsynaptic GABAA receptors, the functional roles of endogenous neurosteroids in synaptic transmission are still largely unknown.
EXPERIMENTAL APPROACH
In this study, the effect of AlloP on spontaneous glutamate release was examined in mechanically isolated dentate gyrus hilar neurons by use of the conventional whole-cell patch-clamp technique.
KEY RESULTS
AlloP increased the frequency of glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) in a dose-dependent manner. The AlloP-induced increase in sEPSC frequency was completely blocked by a non-competitive GABAA receptor blocker, tetrodotoxin or Cd2+, suggesting that AlloP acts on presynaptic GABAA receptors to depolarize presynaptic nerve terminals to increase the probability of spontaneous glutamate release. On the other hand, γ-cyclodextrin (γ-CD) significantly decreased the basal frequency of sEPSCs. However, γ-CD failed to decrease the basal frequency of sEPSCs in the presence of a non-competitive GABAA receptor antagonist or tetrodotoxin. In addition, γ-CD failed to decrease the basal frequency of sEPSCs after blocking the synthesis of endogenous 5α-reduced pregnane steroids. Furthermore, γ-CD decreased the extent of muscimol-induced increase in sEPSC frequency, suggesting that endogenous neurosteroids can directly activate and/or potentiate presynaptic GABAA receptors to affect spontaneous glutamate release onto hilar neurons.
CONCLUSIONS AND IMPLICATIONS
The modulation of presynaptic GABAA receptors by endogenous neurosteroids might affect the excitability of the dentate gyrus-hilus-CA3 network, and thus contribute, at least in part, to some pathological conditions, such as catamenial epilepsy and premenstrual dysphoric disorder.
Keywords: allopregnanolone, neurosteroids, GABAA receptors, presynaptic modulation, hippocampus
Introduction
3α-Hydroxy, 5α-reduced pregnane steroids, such as allopregnanolone (AlloP) and tetrahydrodeoxycorticosterone (THDOC), are de novo synthesized by 5α-reductase and 3α-hydroxysteroid dehydrogenase (3α-HSD) from progesterone and deoxycorticosterone, respectively (Mellon and Griffin, 2002), and are widely distributed in the brain including the hippocampus and cortex (Saalmann et al., 2007). These neurosteroids have inhibitory actions against neuronal excitability, thus exhibiting anxiolytic, sedative and anticonvulsant effects (Wieland et al., 1991; Kokate et al., 1994). These inhibitory actions would mainly be mediated by the positive modulation of GABAA receptors, as AlloP, at nanomolar concentrations, potentiated the GABAA receptor-mediated currents (Zhu and Vicini, 1997; Stell et al., 2003; Akk et al., 2005). At somewhat higher concentrations (≥30 nM), however, these neurosteroids directly open GABAA receptors in the absence of ligand (e.g. GABA) binding (Majewska et al., 1986; Hosie et al., 2006). The levels of 3α-hydroxy, 5α-reduced pregnane steroids might be locally regulated by de novo synthesis and/or generated from circulating progesterone within the brain Moreover, the neurosteroids can reach concentrations approaching 100 nM during the oestrous cycle as well as during acute stress (Purdy et al., 1991; Corpéchot et al., 1993). Therefore, changes in the levels of endogenous 3α-hydroxy, 5α-reduced pregnane steroids might be closely related to premenstrual and post-partum dysphoric disorders (Follesa et al., 2004), depression (Uzunova et al., 1998), ethanol withdrawal (Gililland-Kaufman et al., 2008) and catamenial epilepsy (Reddy and Rogawski, 2000).
Growing evidence has shown that GABAA receptors are also expressed on presynaptic terminals in a variety of brain regions, and that their activation induces presynaptic depolarization in order to modulate neurotransmitter release from nerve terminals (Eccles et al., 1963; Segev, 1990; Cattaert and El Manira, 1999; Jang et al., 2001; 2002; Turecek and Trussell, 2002), including hippocampal mossy fibres (Ruiz et al., 2003; Jang et al., 2006; Nakamura et al., 2007). Presynaptic GABAA receptors can be targets for some pharmacological agents (Han et al., 2009), as GABAA receptors have multiple allosteric binding sites for benzodiazepines, general anaesthetics, synthetic and endogenous neurosteroids, alcohols and divalent metal ions (Barnard et al., 1998; Belelli et al., 1999; Mohler et al., 2002; Whiting, 2003). For example, a recent study has shown that exogenously applied 3α-hydroxy, 5α-reduced pregnane steroids enhances action potential-dependent Ca2+ transients and facilitates glutamatergic transmission to CA3 pyramidal neurons (Ruiz et al., 2010). In addition, our previous studies have shown that presynaptic GABAA receptors expressed on hippocampal glutamatergic nerve terminals are highly sensitive to muscimol, a GABAA receptor agonist (Jang et al., 2006; Han et al., 2009). Given that mRNAs of both 5α-reductase and 3α-HSD are exclusively expressed on hippocampal glutamatergic neurons (Agís-Balboa et al., 2006), and that 3α-hydroxy, 5α-reduced pregnane steroids, even at low nanomolar concentrations, potentiate GABAA receptors, the higher sensitivity of presynaptic GABAA receptors to agonists might be due to the intrinsic modulation of GABAA receptors by endogenous 3α-hydroxy, 5α-reduced pregnane steroids. In the present study, therefore, we have investigated the functional roles of AlloP and endogenous neurosteroids in glutamatergic transmission in acutely isolated dentate gyrus (DG) hilar neurons.
Methods
Preparation of neurons
All animal care and experimental procedures complied with the guidelines for the care and use of animals approved by the Council of the Physiological Society of Korea and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize both the number of animals used and their suffering.
Sprague Dawley rats (12- to 16-day-old), except where indicated, were decapitated under pentobarbital anaesthesia (50 mg·kg−1, i.p.). The brain was dissected and transversely sliced at a thickness of 400 µm using a microslicer (VT1000S; Leica, Nussloch, Germany). Slices containing the hippocampus were kept in an incubation medium (or bath solution, see Solutions) saturated with 95% O2 and 5% CO2 at room temperature (22–24°C) for at least 1 h before the mechanical dissociation. For dissociation, slices were transferred into a 35 mm culture dish (Primaria 3801; Becton Dickinson, Rutherford, NJ, USA) containing a standard external solution (see below), and the hilar region of the hippocampus was identified under a binocular microscope (SMZ-1; Nikon, Tokyo, Japan). Details of the mechanical dissociation have been described previously (Rhee et al., 1999). Briefly, mechanical dissociation was accomplished using a custom-built vibration device and a fire-polished glass pipette oscillating at about 50–60 Hz (0.3–0.5 mm) on the surface of the hilar region, except where indicated. The slices were removed and the mechanically dissociated neurons were allowed to settle and adhere to the bottom of the dish for 15 min. These dissociated neurons lost the most distal processes but retained a short portion (∼50 µm in a length) of their proximal dendrites (Lee et al., 2009).
In a subset of experiments, the effect of AlloP on glutamate release was tested using the slice preparation. For the slice preparation, the hippocampus was transversely sliced at a thickness of 300 µm by use of a microslicer. Slices containing the hippocampus were kept in the bath solution (see below) saturated with 95% O2 and 5% CO2 at room temperature (22–24°C) for at least 1 h. Thereafter a slice containing the hippocampus was transferred into a recording chamber, and the hilar region was identified under an upright microscope (E600FN, Nikon, Tokyo, Japan) with a water-immersion objective (X 40). The bath solution was perfused at 2 mL·min−1 using a peristaltic pump (MP-1000, EYELA, Tokyo, Japan).
Electrophysiological recordings
All electrophysiological recordings were performed using conventional whole-cell patch recordings and a standard patch-clamp amplifier (Axopatch 200B; Molecular Devices, Union City, CA, USA). Neurons were voltage-clamped at a holding potential (VH) of −60 mV. Patch pipettes were made from borosilicate capillary glass (1.5 mm outer diameter, 0.9 mm inner diameter; G–1.5; Narishige, Tokyo, Japan) by use of a pipette puller (P-97; Sutter Instrument Co., Novato, CA, USA). The resistance of the recording pipettes filled with an internal (pipette) solution was 4–6 MΩ. The liquid junction potential (∼−11 mV, measured by exchanging bath solution from internal solution to standard external solution) and pipette capacitance were compensated for. Neurons were viewed under phase contrast on an inverted microscope (TE-2000; Nikon, Tokyo, Japan). Membrane currents were filtered at 2 kHz (Axopatch 200B), digitized at 10 kHz, and stored on a computer equipped with pCLAMP 10 (Molecular Devices). When recording, 10 mV hyperpolarizing step pulses (30 ms in duration) were periodically delivered to monitor the access resistance. All experiments were performed at room temperature (22–24°C).
In the slice preparation, electrical measurements were performed using conventional whole-cell patch recordings with a patch-clamp amplifier (MultiClamp 700B; Molecular Devices). To record action potential-dependent EPSCs (eEPSCs), a glass stimulation pipette (∼10 µm diameter), filled with a bath solution, was positioned around the granule cell layer. Brief paired pulses (500 µs, 100–200 µA, 10 Hz) were applied by the pipette at a stimulation frequency of 0.05 Hz using a stimulator (SEN-7203, Nihon Kohden, Tokyo, Japan) equipped with an isolator unit (SS-701J, Nihon Kohden). Data were filtered at 2 kHz and digitized at 10 kHz.
Data analysis
The amplitude of eEPSCs was calculated by subtracting the baseline from the respective peak amplitude. Spontaneous EPSCs (sEPSCs) were counted and analysed using the MiniAnalysis program (Synaptosoft, Inc., Decatur, GA, USA), as described previously (Jang et al., 2002; 2006;). Briefly, sEPSCs were screened automatically using an amplitude threshold of 10 pA and then visually accepted or rejected based upon the rise and decay times. The average values of both the frequency and amplitude of sEPSCs during the control period (5–10 min) were calculated for each recording, and the frequency and amplitude of all the events during the AlloP or muscimol application (1–2 min) were normalized to these values. The effects of AlloP or muscimol were quantified as a percentage increase in sEPSC frequency compared with the control values. The inter-event intervals and amplitudes of a large number of synaptic events obtained from the same neuron were examined by constructing cumulative probability distributions and compared using the Kolmogorov–Smirnov (K-S) test with Stat View software (SAS Institute, Inc., Cary, NC, USA). Numerical values are provided as the mean ± SEM using values normalized to the control. Significant differences in the mean amplitude and frequency were tested using a Student's paired two-tailed t-test with absolute values rather than normalized ones. Values of P < 0.05 were considered significant.
Solutions
The ionic composition of the incubation medium consisted of (in mM) 124 NaCl, 3 KCl, 1.5 KH2PO4, 24 NaHCO3, 2 CaCl2, 1.3 MgSO4 and 10 glucose saturated with 95% O2 and 5% CO2. The pH was about 7.45. The standard external solution was (in mM) 150 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES, and was adjusted to a pH of 7.4 with Tris-base. For recording sEPSCs, these standard external or bath solutions routinely contained 50 µM DL-2-amino-5-phosphonovaleric acid (APV) to block NMDA receptors. The ionic composition of the internal solution consisted of (in mM) 135 CsF, 5 TEA-Cl, 5 CsCl, 2 EGTA, 5 QX-314, 2 ATP-Mg and 10 HEPES with a pH adjusted to 7.2 with Tris-base.
Materials
The compounds used in the present study were AlloP, muscimol, tetrodotoxin (TTX), 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid HBr (SR95531), strychnine, kainic acid (KA), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), QX-314, APV, EGTA, bumetanide, picrotoxin, bicuculline, γ-cyclodextrin (γ-CD), finasteride, ZnCl2 and ATP-Mg (from Sigma, St. Louis, MO, USA). All solutions containing drugs were applied using the ‘Y–tube system’ for rapid solution exchange (Murase et al., 1989).
Results
AlloP acts presynaptically to facilitate spontaneous glutamate release onto hilar neurons
After brief mechanical dissociation of the hippocampal hilar region, large neurons (>20 µm in somatic diameter) that had variously shaped somata, including fusiform, triangular and multipolar forms were observed (see also Lee et al., 2009), which were similar to previously described neurons in the hilar region (Acsády et al., 1998; Johnston and Amaral, 2004). When these neurons were held at a VH of −60 mV using the whole-cell patch-clamp technique, the spontaneous synaptic currents, including unusually large ones (>100 pA, up to 600 pA), were recorded in the presence of 50 µM APV, a selective NMDA receptor antagonist. These spontaneous currents were completely and reversibly blocked by 20 µM CNQX (n = 6), an AMPA/KA receptor blocker, indicating that these spontaneous inward synaptic currents are AMPA/KA receptor-mediated sEPSCs (Figure 1A). The generation of GABAergic spontaneous inhibitory postsynaptic currents was minimized by the use of CsF-based and low Cl- pipette solution (Lee et al., 2009).
Figure 1.
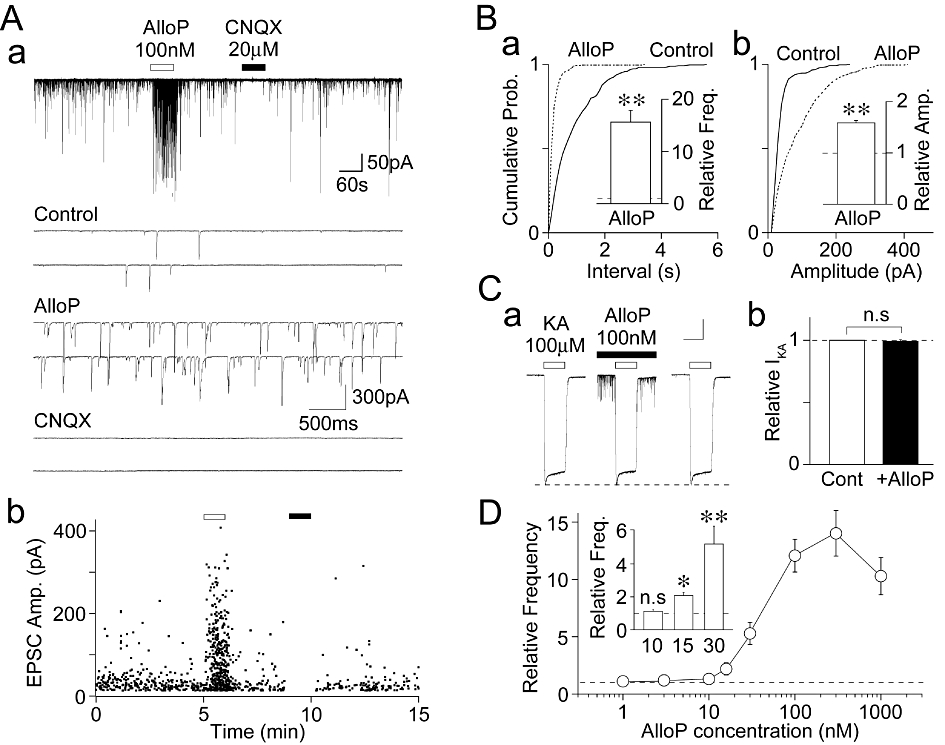
Effect of AlloP on glutamatergic sEPSCs in hilar neurons. (Aa) A typical time course of glutamatergic sEPSC frequency before, during and after the application of 100 nM AlloP or 20 µM CNQX. Insets; typical traces of sEPSCs in the absence (control) and the presence of AlloP or CNQX with an expanded time scale. (Ab) An all points scatter plot of sEPSCs shown in (Aa). 921 events were plotted. (B) Cumulative probability distributions for the inter-event interval (a; P < 0.01, K-S test) and current amplitude (b; P < 0.01, K-S test) of glutamatergic sEPSCs shown in (A). 401 for the control and 338 events for AlloP were plotted. Insets, 100 nM AlloP-induced changes in sEPSC frequency (left) and amplitude (right). Each column is expressed as the mean ± SEM from 18 experiments. **P < 0.01. (Ca) Typical traces of KA (100 µM)-induced currents (IKA) before, during and after the application of 100 nM AlloP (left). (b) Each column and error bar represents the mean ± SEM from five experiments (right). Scale bars; 15 s, 100 pA. n.s, not significant. (D) Concentration–response relationship of sEPSC frequency facilitation against AlloP concentration. Each point and error bar represents the mean ± SEM from 6 to 12 experiments. Inset; AlloP (10, 15, 30 nM)-induced changes in sEPSC frequency. Each column is expressed as the mean ± SEM from 8–16 experiments. n.s, not significant, *P < 0.05, *P < 0.01.
Under these conditions, we first observed the effect of exogenously applied AlloP on glutamatergic sEPSCs in order to elucidate whether the neurosteroid AlloP does affect spontaneous glutamatergic transmission onto hilar neurons. AlloP (100 nM) greatly increased sEPSC frequency, and seemed to increase the frequency of the large sEPSCs in particular (Figure 1A and B). As shown in Figure 1B, AlloP (100 nM) significantly shifted the cumulative distributions of inter-event interval and amplitude of sEPSCs to the left and right, respectively (P < 0.01, K-S-test), consistent with increases in the frequency and amplitude of glutamatergic sEPSCs. In 18 neurons in which the AlloP effects on sEPSCs were fully analysed, 100 nM AlloP increased both the frequency (1563 ± 226% of the control, n = 18, P < 0.01) and amplitude (159 ± 5% of the control, n = 18, P < 0.01) of glutamatergic sEPSCs (Figure 1B). This increase in sEPSC amplitude was not due to a change in the sensitivity of AMPA/KA receptors by AlloP, as 100 nM AlloP had no effect on the KA (100 µM)-induced currents (IKA) (Figure 1C). We also examined the concentration–response relationship of AlloP for the sEPSC frequency facilitation (Figure 1D). AlloP at a concentration as low as 15 nM increased sEPSC frequency (204 ± 3% of the control, n = 16, P < 0.05), but at 10 nM it hardly affected sEPSC frequency (110 ± 6% of the control, n = 8, P = 0.16, Figure 1D inset). Together, these results suggest that AlloP acts presynaptically to increase spontaneous glutamate release onto mechanically isolated hilar neurons.
AlloP modulation of presynaptic GABAA receptors
Although AlloP can affect a number of ligand-gated ion channels (Mellon and Griffin, 2002), at nanomolar concentrations, it predominantly affects GABAA receptors (Majewska et al., 1986; Zhu and Vicini, 1997; Stell et al., 2003; Akk et al., 2005; Hosie et al., 2006). Therefore, we observed the effects of GABAA receptor antagonists on the AlloP-induced increase in spontaneous glutamate release. SR95531 (10 µM), a competitive GABAA receptor antagonists, did not change the cumulative distributions of inter-event interval (P = 0.72, K-S test) and amplitude (P = 0.79, K-S test) of basal sEPSCs (Figure 2A and B). In addition, SR95531 (10 µM) had no effect on the mean frequency (102 ± 5% of the control, n = 8, P = 0.62) and amplitude (104 ± 7% of the control, n = 7, P = 0.59) of basal sEPSCs (data not shown). Neither 100 µM SR95531 nor 1 µM strychnine, a glycine receptor blocker, changed the basal frequency of sEPSCs (data not shown). Although the AlloP-induced facilitation of sEPSC frequency was slightly reduced in the presence of 10 µM SR95531 (977 ± 189% of the control and 705 ± 133% of the SR95531 condition, n = 7, respectively, Figure 2E and Fa), this change was not significant statistically (P = 0.08). In contrast, picrotoxin (30 µM), a non-competitive GABAA receptor blocker, shifted the cumulative distribution of inter-event interval of basal sEPSCs to the right (P < 0.01, K-S test), but it did not change the cumulative distribution of the amplitude of basal sEPSCs (P = 0.22, K-S test) (Figure 2C and D). In addition, picrotoxin (30 µM) significantly decreased the mean frequency (76 ± 5% of the control, n = 10, P < 0.01), without affecting the mean amplitude (94 ± 8% of the control, n = 10, P = 0.32) of basal sEPSCs (data not shown). In the presence of 30 µM picrotoxin, however, the AlloP-induced facilitation of sEPSC frequency (898 ± 203% of the control) was completely prevented (n = 10, P < 0.01, Figure 2E and Fb). We also investigated whether Zn2+ modulated the AlloP-induced increase in spontaneous glutamate release. The application of Zn2+ (30 µM) hardly affected the basal sEPSC frequency (97 ± 13% of the control, n = 6, P = 0.56), as shown in our previous study (Han et al., 2009). In the presence of 30 µM Zn2+, the AlloP-induced facilitation of sEPSC frequency (1361 ± 320% of the control) was not affected (1109 ± 228% of the values with Zn2+ alone, n = 6, P = 0.77, data not shown).
Figure 2.
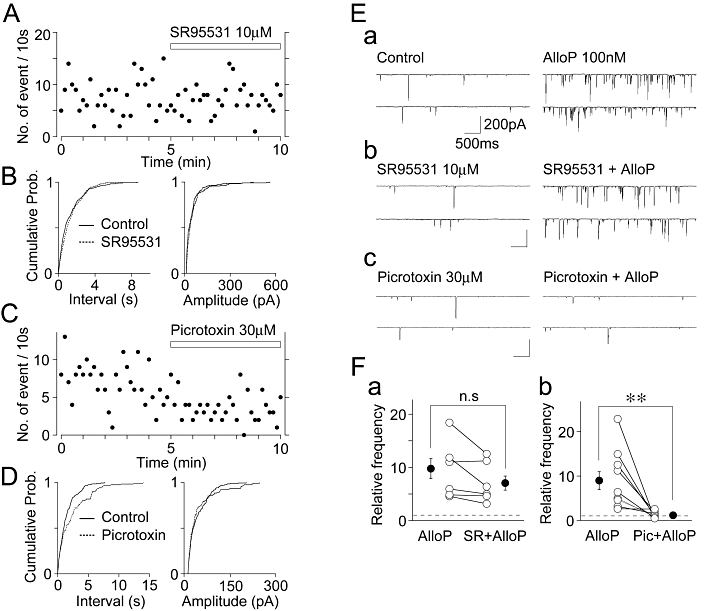
Effects of GABAA receptor antagonists on the basal frequency of sEPSCs and the AlloP-induced increase in sEPSC frequency. (A) A typical time course of sEPSC frequency before and during the application of 10 µM SR95531. The number of events in every 10 s period was summed and plotted. (B) Cumulative probability distributions for the inter-event interval (left) and amplitude (right) of basal glutamatergic sEPSCs shown in (A). 232 for the control and 223 events for SR95531 were plotted. (C) A typical time course of sEPSC frequency before and during the application of 30 µM picrotoxin. The number of events in every 10 s period was summed and plotted. (D) Cumulative probability distributions for the inter-event interval (left) and amplitude (right) of basal glutamatergic sEPSCs shown in (C). 208 for the control and 122 events for picrotoxin were plotted. (E) Typical traces of sEPSCs recorded before (left) and during (right) the application of 100 nM AlloP in the control external solution (a), in the presence of 10 µM SR95531 (b), and in the presence of 30 µM picrotoxin (c). (F) Changes of the AlloP-induced facilitation of sEPSC frequency in the absence and presence of 10 µM SR95531 (a, n = 7) and 30 µM picrotoxin (b, n = 10). Open circles and connected lines represent the individual results, whereas closed circles and error bars indicate the mean ± SEM.
Because AlloP at low nanomolar concentrations potentiates the GABAA receptor-mediated currents in postsynaptic neurons (Zhu and Vicini, 1997; Stell et al., 2003; Akk et al., 2005), we investigated whether AlloP potentiates presynaptic GABAA receptors activated by a GABAA receptor agonist. In order to test this, we used 10 nM AlloP, in which concentration AlloP had no facilitatory effect on sEPSC frequency as shown in Figure 1D. Muscimol, a GABAA receptor agonist, increased sEPSC frequency in a concentration-dependent manner as shown in the concentration–response relationship (Figure 3B), where at 100 nM it increased sEPSC frequency (382 ± 49% of the control, n = 6, P < 0.01, Figure 3A and B). In the presence of 10 nM AlloP, the muscimol (100 nM)-induced increase in sEPSC frequency was significantly increased to 737 ± 126% of the AlloP condition (n = 6, P < 0.05, Figure 3A and B). In contrast, AlloP reduced the extent of muscimol (≥1 µM)-induced increase in sEPSC frequency (Figure 3B). The data shown in, Figure 3B demonstrates that AlloP shifted the concentration–response relationship of muscimol to the left (Figure 3B), suggesting that AlloP potentiates the action of muscimol on presynaptic GABAA receptors.
Figure 3.
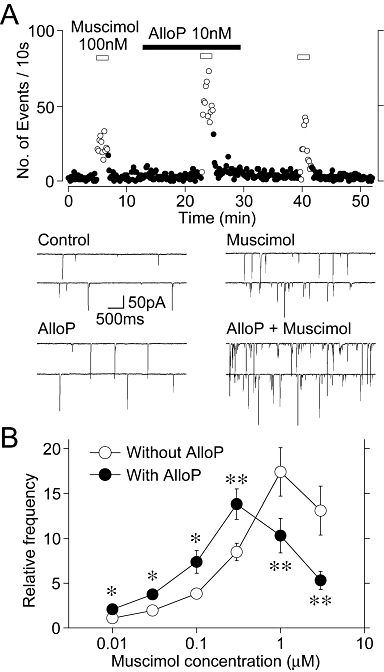
Effect of AlloP on the muscimol-induced increase in sEPSC frequency. (A) A typical time course of sEPSC frequency before, during and after the application of 100 nM muscimol in the absence and presence of 10 nM AlloP. The number of events in every 10 s period was summed and plotted. Inset traces represent typical sEPSPs in numbered regions with an expanded time scale. (B) Changes of the muscimol-induced increase in sEPSC frequency in the absence and presence of 10 nM AlloP. Each point and error bar represents the mean ± SEM from 5–8 experiments. *P < 0.05, **P < 0.01. Note that AlloP shifted the concentration–response relationship of muscimol to the left.
Mechanisms underlying the AlloP-induced facilitation of spontaneous glutamate release
We next examined the mechanisms underlying the AlloP-induced facilitation of sEPSC frequency. The activation of presynaptic GABAA receptors modulates neurotransmitter release by eliciting a presynaptic depolarization, which activates either voltage-dependent Na+ channels (VDNCs) or voltage-dependent Ca2+ channels (VDCCs) (Jang et al., 2001; 2002; 2006;). Therefore, we investigated the effects of TTX, a specific VDNC blocker, and Cd2+, a general VDCC blocker, on the AlloP-induced facilitation of sEPSCs frequency in the presence of 10 µM SR95531. The application of 300 nM TTX significantly decreased the sEPSC frequency (52 ± 11% of the control, n = 8, P < 0.01). In the presence of TTX, however, the action of AlloP on sEPSC frequency was greatly reduced to 189 ± 56% of the TTX condition (n = 8, P < 0.01, Figure 4A and B). The application of 100 µM Cd2+ also significantly decreased the sEPSC frequency (43 ± 12% of the control, n = 7, P < 0.01). In the presence of Cd2+, the AlloP action on sEPSC frequency was greatly reduced to 94 ± 14% of the Cd2+ condition, n = 7, P < 0.01, Figure 4A and B). These results suggest that the AlloP-induced activation of presynaptic GABAA receptors induces a presynaptic depolarization, and subsequently increases Ca2+ influx into the presynaptic nerve terminals via VDNCs and VDCCs, thus resulting in the increase of spontaneous glutamate release.
Figure 4.
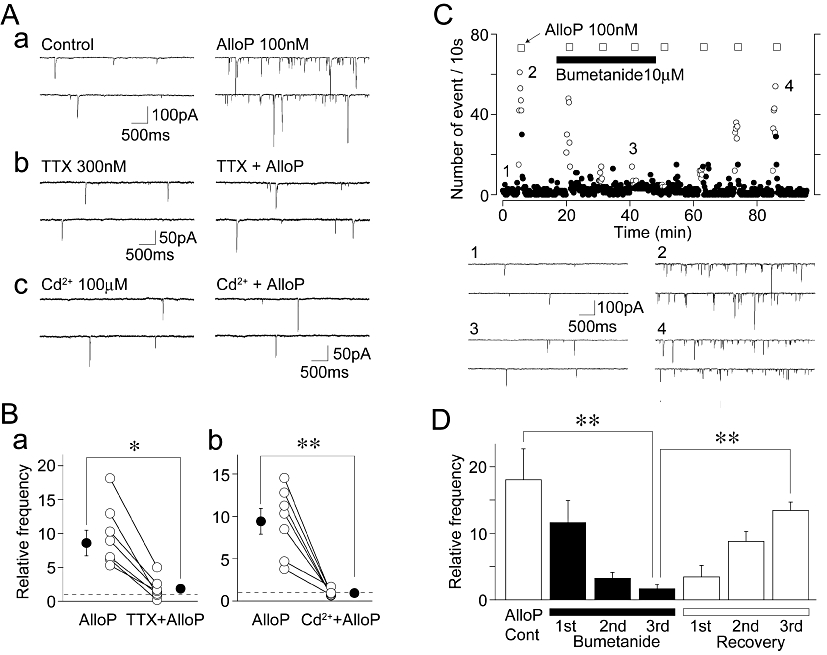
AlloP depolarizes glutamatergic nerve terminals. (A) Typical traces of sEPSCs recorded before (left) and during (right) the application of 100 nM AlloP in the control external solution (a), in the presence of 300 nM TTX (b) and in the presence of 100 µM Cd2+ (c). (B) The changes in AlloP-induced increase in sEPSC frequency in the presence of TTX (a) or Cd2+ (b). Each column and error represents was the mean and SEM from 8 for TTX and 7 for Cd2+ experiments. Open circles and connected lines represent the individual results, whereas closed circles and error bars indicate the mean ± SEM. *P < 0.05, **P < 0.01. (C) A typical time course of sEPSC frequency before, during and after the application of 100 nM AlloP in the absence and presence of 10 µM bumetanide. The number of events in every 10 s period was summed and plotted. Inset traces represent typical sEPSPs in the numbered regions with an expanded time scale. (D) Changes of the AlloP-induced increase in sEPSC frequency in the absence and presence of bumetanide. Each column and error bar represents the mean ± SEM from five experiments. **P < 0.01.
Given that presynaptic GABAA receptors on glutamatergic nerve terminals induce a presynaptic depolarization that activates VDNCs, the intracellular Cl- concentration ([Cl-]i) within glutamatergic nerve terminals should be maintained at a higher level than that predicted for passive Cl- distribution. This would be mediated by inwardly directed Cl- co-transporters such as Na-K-Cl co-transporter type 1 (NKCC1), as reported previously (Jang et al., 2001; 2002; Lee et al., 2009). To confirm that AlloP indeed induces a presynaptic depolarization via a similar mechanism, we tested the effect of bumetanide, a selective NKCC1 blocker (Haas, 1989), on the AlloP-induced facilitation of sEPSC frequency (Figure 4C and D). The extent of AlloP-induced facilitation of sEPSC frequency (1805 ± 462% of the control, n = 5, P < 0.05) was gradually attenuated in the prolonged presence of 10 µM bumetanide (3rd application: 168 ± 62%, n = 5, P = 0.43). However, the effect of AlloP slowly recovered after the washout of bumetanide (30 min later: 1343 ± 126% of the control, n = 5, P < 0.01, Figure 4C and D).
Presynaptic GABAA receptors are tonically activated by endogenous neurosteroids
We next examined whether presynaptic GABAA receptors are tonically activated by endogenous 3α-hydroxy, 5α-reduced pregnane steroids, because both 5α-reductase and 3α-HSD are exclusively expressed on hippocampal glutamatergic neurons (Agís-Balboa et al., 2006). In order to evaluate this hypothesis, we investigated the effects of γ-CD (a membrane-impermeable and efficient sequestering agent (which binds several types of naturally occurring neurosteroids including AlloP and THDOC; Shu et al., 2007), on the basal frequency of sEPSCs in the presence of 10 µM SR95531. If endogenous 3α-hydroxy, 5α-reduced pregnane steroids act on presynaptic GABAA receptors to facilitate the frequency of basal sEPSCs, exogenously applied γ-CD would be expected to decrease the frequency of basal sEPSCs. As expected, the application of 500 µM γ-CD significantly shifted the cumulative distribution of inter-event interval sEPSCs to the right (P < 0.01, K-S test) and decreased the mean frequency of basal sEPSCs to 64 ± 14% of the control (n = 7, P < 0.01) (Figure 5Ba). However, γ-CD did not change the cumulative distribution (P = 0.21, K-S test) and the mean amplitude (96 ± 7% of the control, n = 7, P = 0.72) of sEPSCs (Figure 5Bb). In the presence of 500 µM γ-CD the AlloP (100 nM)-induced facilitation of sEPSC frequency (735 ± 144% of the control, n = 7) was completely attenuated to 140 ± 23% of the γ-CD condition (n = 7, P < 0.01, Figure 5C), ascertaining that γ-CD efficiently sequesters AlloP.
Figure 5.
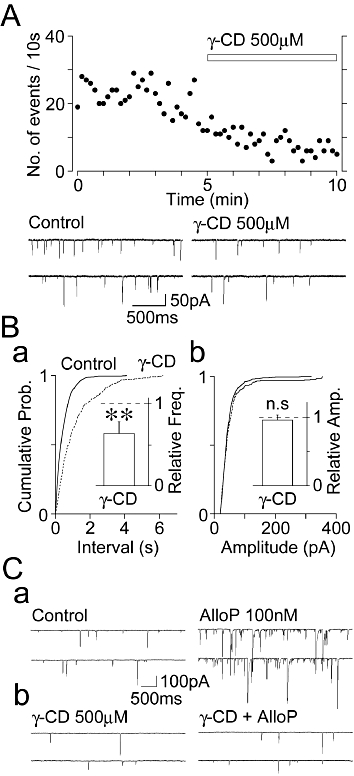
Effects of γ-CD on the basal frequency of sEPSCs. (A) A typical time course of sEPSC frequency before and during the application of 500 µM γ-CD in the control condition. The number of events in every 10 s period was summed and plotted. Insets; typical traces of sEPSCs in the absence (control) and the presence of γ-CD with an expanded time scale. (B) Cumulative probability distribution for the inter-event interval (a) and amplitude (b) of glutamatergic sEPSCs shown in (A). 664 for the control and 261 events for γ-CD were plotted. Insets, the γ-CD-induced changes in the basal frequency (a) and amplitude (b) of sEPSCs. Columns are the mean ± SEM from seven experiments. **P < 0.01. (C) Typical traces of sEPSCs recorded before (left) and during (right) the application of 100 nM AlloP in the control external solution (a) and in the presence of 500 µM γ-CD (b).
To confirm whether presynaptic GABAA receptors are involved in the decrease in sEPSC frequency induced by γ-CD, we examined the effect of picrotoxin on γ-CD-induced decrease in basal sEPSC frequency in the presence of 10 µM SR95531, because picrotoxin itself decreased the basal frequency of sEPSCs as shown in Figure 2. In presence of 30 µM picrotoxin, γ-CD (500 µM) failed to decrease basal sEPSC frequency (94 ± 10% of the picrotoxin treatment, n = 10, P = 0.23, Figure 6A and D). We also examined the effect of TTX on γ-CD-induced decrease in basal sEPSC frequency, because TTX completely blocked the AlloP-induced increase in sEPSC frequency as described above. In the presence of 300 nM TTX, γ-CD (500 µM) again failed to decrease basal sEPSC frequency (100 ± 5% of the TTX condition, n = 10, P = 0.46, Figure 6B and D). In order to determine whether γ-CD decreased the basal frequency of sEPSCs after the synthesis of endogenous 3α-hydroxy, 5α-reduced pregnane steroids was blocked, we examined the effect of finasteride, a specific 5α-reductase inhibitor, on the basal frequency of sEPSCs. However, the application of 10 µM finasteride for 10 min did not change the basal sEPSC frequency (97 ± 12% of the control, n = 5, P = 0.56, data not shown). This observation might be due to a slow (>1 h) neosynthesis of endogenous 5α-reduced pregnane steroids, as shown in a previous study (Keller et al., 2004). Therefore, we incubated hippocampal slices with 10 µM finasteride immediately after making the slices, and examined the effect of γ-CD on the basal frequency of sEPSCs. In the continued presence of 10 µM finasteride, γ-CD (500 µM) failed to decrease the basal frequency of sEPSCs (100 ± 5% of the finasteride condition, n = 7, P = 0.61, Figure 6C and D). Together, these results suggest that endogenous neurosteroids such as AlloP and THDOC, which can be trapped by extracellularly applied γ-CD, might contribute to the tonic activation of presynaptic GABAA receptors expressed on glutamatergic nerve terminals.
Figure 6.
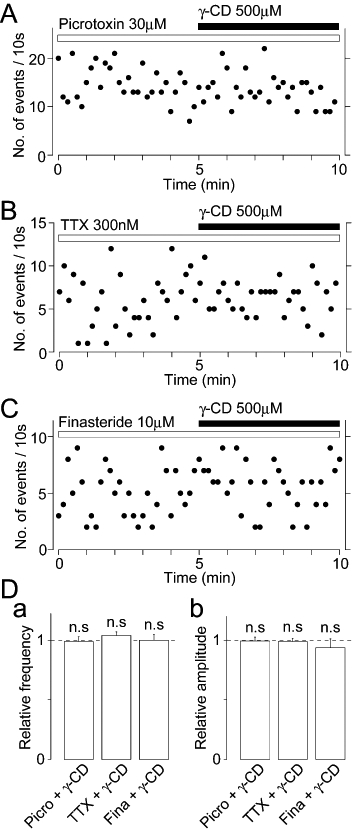
Effects of picrotoxin, TTX and finasteride on the γ-CD-induced decrease in basal sEPSC frequency. (A) A typical time course of sEPSC frequency before and during the application of 500 µM γ-CD in the presence of 30 µM picrotoxin. The number of events in every 10 s period was summed and plotted. (B) A typical time course of sEPSC frequency before and during the application of 500 µM γ-CD in the presence of 300 nM TTX. The number of events in every 10 s period was summed and plotted. (C) A typical time course of sEPSC frequency before and during the application of 500 µM γ-CD in the presence of 10 µM finasteride. The number of events in every 10 s period was summed and plotted. Note that finasteride (10 µM) was added in the incubation solution immediately after making hippocampal slices. (D) Changes of the basal frequency (a) and amplitude (b) of sEPSCs by γ-CD in the absence and presence of 30 µM picrotoxin (Picro; n = 10), 300 nM TTX (n = 10) and 10 µM finasteride (Fina; n = 7).
Our previous studies have shown that presynaptic GABAA receptors expressed on hippocampal glutamatergic nerve terminals are highly sensitive to muscimol (Jang et al., 2006; Han et al., 2009). Based on the present results showing that AlloP potentiates the muscimol-induced increase in spontaneous glutamate release (Figure 3) and that endogenous 3α-hydroxy, 5α-reduced pregnane steroids might modulate presynaptic GABAA receptors (Figures 5 and 6), it is possible that the higher sensitivity of presynaptic GABAA receptors to muscimol might be due to the intrinsic modulation of GABAA receptors by endogenous neurosteroids. To test this hypothesis, we examined the effect of γ-CD on the muscimol-induced increase in spontaneous glutamate release. The muscimol (100 nM)-induced increase in sEPSC frequency (353 ± 34% of the control, n = 8) was significantly reduced in the presence of 500 µM γ-CD (230 ± 29% of the γ-CD condition, n = 8, P < 0.05, Figure 7A and B). However, γ-CD had no inhibitory effect on the muscimol (≥1 µM)-induced increase in sEPSC frequency (Figure 7B). These data indicate that γ-CD shifted the concentration–response relationship of muscimol to the right (Figure 7B). On the other hand, it is possible that γ-CD directly inhibits GABAA receptors and thus it attenuates the facilitatory action of muscimol on sEPSC frequency. Therefore, we examined the direct effect of γ-CD on GABAA receptors in acutely isolated DG granule cells, which are parent neurons of mossy fibres. However, γ-CD (500 µM) had no inhibitory effect on the IMus induced by 30 nM and 300 nM muscimol (Figure 7C), suggesting that γ-CD had no inhibitory effect on GABAA receptors themselves.
Figure 7.
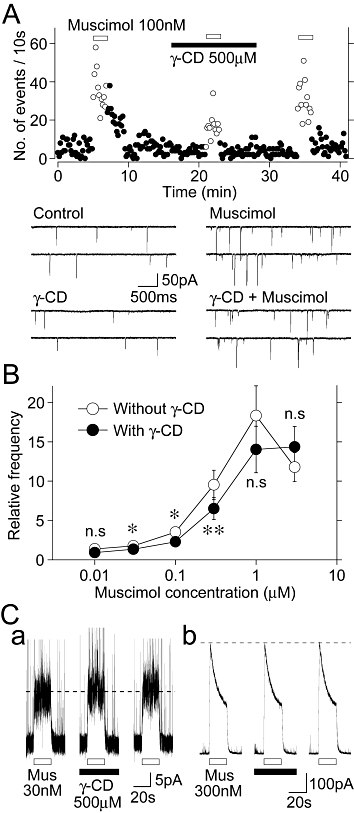
Effects γ-CD on the muscimol-induced increase in sEPSC frequency. (A) A typical time course of sEPSC frequency before, during and after the application of 100 nM muscimol in the absence and presence of 500 µM γ-CD. The number of events in every 10 s period was summed and plotted. Inset traces represent typical sEPSPs in numbered regions with an expanded time scale. (B) Changes of the muscimol-induced increase in sEPSC frequency in the absence and presence of 500 µM γ-CD. Each point and error bar represents the mean ± SEM from 5–8 experiments. *P < 0.05, **P < 0.01. Note that γ-CD shifted the concentration–response relationship of muscimol to the right. (C) Representative traces of IMus induced by 30 nM (a) or 300 nM muscimol (Mus; b) in the absence and presence of 500 µM γ-CD. Muscimol was applied at 4 min intervals and γ-CD was preincubated 1 min before the muscimol application. Note that γ-CD had no effect on the IMus.
Effects of AlloP and γ-CD on action potential-dependent glutamate release
To elucidate the physiological significance of the present findings, we examined the effect of AlloP on action potential-dependent glutamate release in the slice preparation. Glutamatergic eEPSCs were recorded in the presence of 10 µM SR95531 and 50 µM APV. In six of nine neurons tested, bath applied AlloP (100 nM) slightly but significantly increased the amplitude of eEPSCs (120 ± 4% of the control, n = 6, P < 0.05) and decreased the paired-pulse ratio (PPR) from 1.65 ± 0.08 to 1.52 ± 0.07 (92 ± 1% of the control, n = 6, P < 0.01, Figure 8A and C), suggesting that AlloP acts presynaptically to increase the probability of glutamate release from mossy fibre terminals. In the remaining three neurons, AlloP had no facilitatory effect on glutamatergic eEPSCs. In a subset of experiments, we also observed the effect of γ-CD on action potential-dependent glutamate release. In 7 of 11 neurons tested, bath applied γ-CD (500 µM) significantly decreased the amplitude of eEPSCs (82 ± 4% of the control, n = 7, P < 0.05) and increased the PPR from 1.60 ± 0.04 to 1.91 ± 0.07 (117 ± 5% of the control, n = 7, P < 0.05, Figure 8B and C). In the remaining four neurons, γ-CD had no inhibitory effect on glutamatergic eEPSCs. The results suggest that endogenous neurosteroids, which can be sequestered by γ-CD, act on presynaptic GABAA receptors to decrease the probability of glutamate release from mossy fibre terminals.
Figure 8.
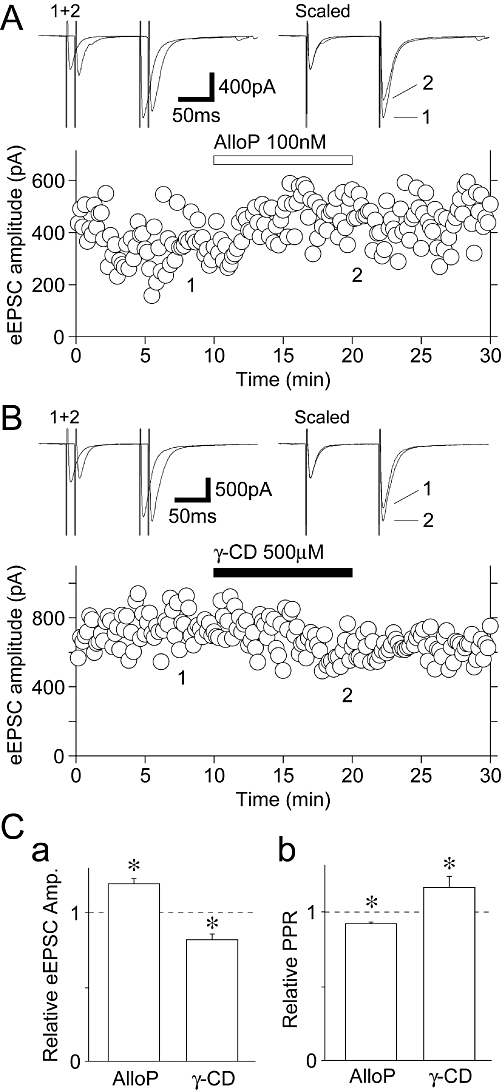
Effects of AlloP and γ-CD on eEPSCs in the slice preparation. (A) A typical time course of eEPSC amplitude before, during and after application of 100 nM AlloP. Inset traces represent typical eEPSCs in numbered regions with an expanded time scale. (B) A typical time course of eEPSC amplitude before, during and after application of 500 µM γ-CD. Inset traces represent typical eEPSCs in numbered regions with an expanded time scale. (C) AlloP- and γ-CD-induced changes in the amplitude of eEPSCs (a) and the PPR (b). Each column and error bar represents the mean ± SEM from 6–7 experiments. *P < 0.05.
Discussion
In the present study, we found that AlloP acts presynaptically to increase sEPSC frequency on acutely isolated hilar neurons. Although AlloP also increased the mean amplitude of sEPSCs, the AlloP-induced increase in sEPSC amplitude might be due to an increase in the frequency of large sEPSCs (see also Jang et al., 2006). The facilitatory action of AlloP should result from the direct activation of presynaptic GABAA receptors rather than the interaction of agonist binding sites, because the AlloP action was significantly blocked by non-competitive rather than competitive GABAA receptor antagonists. Although a few studies have shown that the neurosteroid-mediated Cl- currents are sensitive to SR95531, such inhibitory effects have been tested for the Cl- currents induced by higher (≥300 nM) concentrations of neurosteroids (Ueno et al., 1997; Shu et al., 2004). Similarly, we have recently shown that while SR95531 significantly reduced the Cl- currents induced by 1 µM AlloP, it had no inhibitory effect on the Cl- currents induced by ≤100 nM AlloP (Park et al., 2011). In addition, the AlloP-induced increase in sEPSC frequency was hardly affected by 30 µM Zn2+, as presynaptic GABAA receptors expressed on putative mossy fibre terminals are highly insensitive to Zn2+ (Han et al., 2009). These pharmacological data strongly suggest that AlloP at nanomolar concentrations directly activates presynaptic GABAA receptors to facilitate spontaneous glutamate release onto hilar neurons. Although the AlloP concentration for direct GABAA receptor activation is in a range of 300 nM to 10 µM, which is substantially higher than that for the modulatory action (Belelli et al., 2002; Shu et al., 2004; Hosie et al., 2006), our recent study has shown that AlloP at ≥30 nM concentrations can elicit the Cl- currents in hippocampal neurons (Park et al., 2011). On the other hand, AlloP also increased the muscimol-induced facilitation of sEPSC frequency and shifted the concentration–response relationship to the left. Taken together, it is likely that AlloP, even at low nanomolar concentrations, has dual effects (direct activation and potentiation) on presynaptic GABAA receptors.
Given that AlloP directly activates presynaptic GABAA receptors as described above, AlloP should depolarize the terminal membrane as the activation of presynaptic GABAA receptors, which elicits the Cl- conductance, is also known to depolarize mossy fibres (Ruiz et al., 2003; Jang et al., 2006; Nakamura et al., 2007). In the present study, the AlloP-induced increase in sEPSC frequency was completely blocked by either TTX or Cd2+, suggesting that AlloP directly depolarizes the terminal membrane and contributes to the activation of VDNCs and VDCCs. As a result, the Ca2+ influx through presynaptic VDCCs would be responsible for the AlloP-induced increase in spontaneous glutamate release. If AlloP depolarizes the terminal membrane via the direct activation of presynaptic GABAA receptors, the Cl- equilibrium potential must be positive with respect to the resting membrane potential of presynaptic terminals, that is, glutamatergic nerve terminals innervating hilar neurons should have a higher intraterminal [Cl-] than predicted for passive distribution. This effect could be accomplished by inwardly directed Cl- co-transporters, such as NKCC1 (Plotkin et al., 1997; Clayton et al., 1998; Jang et al., 2001; 2002;). In the present study, the AlloP-induced increase in sEPSC frequency was gradually reduced in the presence of bumetanide, a specific NKCC1 blocker (Haas, 1989), which has no inhibitory effect on GABAA receptors (Jang et al., 2001). These results suggest that bumetanide-sensitive NKCC1 contributes to the AlloP-induced presynaptic depolarization of glutamatergic nerve terminals projecting to hilar neurons.
One important aim of this study was to elucidate the possible roles of endogenous neurosteroids in presynaptic GABAA receptor function. In the present study, we found that γ-CD decreased the basal frequency of sEPSCs, and that non-competitive but not competitive GABAA receptor antagonists reduced the basal frequency of sEPSCs. In addition, as the γ-CD did not affect the basal frequency of sEPSCs in the presence of TTX or after blocking the synthesis of 5α-reduced neurosteroids, both endogenous neurosteroids and GABAA receptors seem to be closely related to basal spontaneous glutamate release. The depletion of membrane cholesterol rather than neurosteroids by γ-CD changes the probability of spontaneous glutamate release. However, γ-CD is less effective than β-CD in sequestering cholesterol from membranes (Ohtani et al., 1989). A previous study has also shown that the depletion of cholesterol by methyl-β-CD increases, rather than decreases, Ca2+-dependent asynchronous transmitter release (Zamir and Charlton, 2006). These results suggest that endogenous 3α-hydroxy, 5α-reduced pregnane steroids act on presynaptic GABAA receptors to change the excitability of glutamatergic nerve terminals and regulate the probability of basal spontaneous glutamate release. On the other hand, the origin of endogenous AlloP in mechanically isolated hippocampal neurons is unknown. Given that both 3α-hydroxy, 5α-reduced pregnane steroids and 5α-reductase are strongly expressed in glutamatergic principal neurons including DG granule cells but not hilar neurons (Agís-Balboa et al., 2006; Saalmann et al., 2007), endogenous neurosteroids might be synthesized in glutamatergic nerve terminals rather than postsynaptic hilar neurons. Although the actual concentration of endogenous AlloP around glutamatergic nerve terminals remains to be elucidated, other neuroactive steroids having a positive effect on GABAA receptors, such as THDOC or 3α-hydroxy, 5β-reduced pregnane steroids, might synergistically act on presynaptic GABAA receptors to affect basal glutamatergic transmission.
The higher sensitivity of presynaptic GABAA receptors to muscimol might be due to the subunit composition of GABAA receptors, as GABAA receptors containing α5 or δ subunits are known to be more sensitive to GABA (Mtchedlishvili and Kapur, 2006). Previous reports have shown that δ subunit-containing GABAA receptors, especially α4β2δ subunit combinations, have a high sensitivity to GABA (Sundstrom-Poromaa et al., 2002) and might contribute to the tonic inhibition of DG granule cells (Wei et al., 2003). In addition, δ subunit-containing GABAA receptors are more sensitive to 3α-hydroxy, 5α-reduced pregnane steroids (Stell et al., 2003). However, our previous study has suggested that presynaptic GABAA receptors expressed on hippocampal glutamatergic nerve terminals presumably contain γ subunits, according to the diazepam and Zn2+ sensitivities (Han et al., 2009). For example, γ subunit-containing GABAA receptors are much less sensitive to Zn2+ (α1β1; IC50: 1.2 µM, α1β1γ2; IC50: >600 µM, α1β1δ; IC50: 16.3 µM) (Draguhn et al., 1990; Krishek et al., 1998; Brown et al., 2002; Mtchedlishvili and Kapur, 2006). In addition, DG granule cells express multiple types of GABAA receptors, such as α1βγ2, α4βγ2 and α4βδ GABAA receptors (Wei et al., 2003; Lindquist and Birnir, 2006). In contrast, α5 subunit-containing GABAA receptors would contribute to the higher sensitivity of presynaptic GABAA receptors as DG granule cells are likely to express α5 subunits (Glykys et al., 2008; but see also Sperk et al., 1997). In the present study, we also found that γ-CD decreased the extent of muscimol-induced increase in sEPSC frequency and shifted the concentration–response relationship of muscimol to the right, suggesting that γ-CD reduces the sensitivity of presynaptic GABAA receptors to muscimol. The depletion of membrane cholesterol by γ-CD can also change the sensitivity of presynaptic GABAA receptors to muscimol, as cholesterol depletion lowers the EC50 value of GABA (Sooksawate and Simmonds, 2001a). However, the depletion of membrane cholesterol by methyl-β-CD increases the potentiation of GABA by pregnanolone (Sooksawate and Simmonds, 2001b). Furthermore, we found that γ-CD had no effect on GABAA receptor-mediated Cl- currents in DG granule cells, suggesting that γ-CD has no direct inhibitory effect on GABAA receptors themselves. Given that endogenous neurosteroids act on presynaptic GABAA receptors as described above, it is highly feasible that endogenous 3α-hydroxy, 5α-reduced pregnane steroids contribute, at least in part, to the higher sensitivity of presynaptic GABAA receptors to agonists.
The hippocampus is closely related to a variety of pathophysiological conditions including stress, epilepsy, and learning and memory (Bliss and Collingridge, 1993; Coulter, 2000). In the hippocampus, mossy fibres arising from DG granule cells innervate hilar neurons, excitatory mossy cells and inhibitory basket cells, before entering the CA3 region of the hippocampus (Acsády et al., 1998; Johnston and Amaral, 2004), suggesting that a significant portion of sEPSCs recorded from acutely isolated hilar neurons may originate from mossy fibres (see also Lee et al., 2009). Mossy fibre-derived spontaneous excitatory postsynaptic potentials (sEPSPs) are sufficiently large to trigger action potentials in postsynaptic neurons (Henze et al., 2002; Jang et al., 2006), and hilar neurons are known to receive large sEPSPs that can trigger bursts of action potentials (Scharfman and Schwartzkroin, 1988). In this regard, our present results suggest that AlloP through the direct activation and/or potentiation of presynaptic GABAA receptors might have an excitatory effect on postsynaptic hilar neurons because AlloP greatly increased sEPSC frequency, especially the frequency of large sEPSCs. However, considering that mossy fibres project to more inhibitory interneurons than do excitatory neurons in the DG as well as CA3 regions (Seress et al., 2001), and that neuronal death in the hilar region induces the hyperexcitability of hippocampal neural networks (McNamara, 1994), the functional roles of endogenous neurosteroids in the modulation of glutamatergic transmission is likely to be complex in the context of the intrinsic hippocampal circuitry.
In conclusion, our present results suggest that presynaptic GABAA receptors and their activation and/or potentiation by endogenous neurosteroids might affect the excitability of the DG-hilus-CA3 network, and thus contribute, at least in part, to some pathological conditions, such as catamenial epilepsy and premenstrual dysphoric disorder.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (Nos. 2010–0015468 and 2010–0029459).
Glossary
Abbreviations
- 3α-HSD
3α-hydroxysteroid dehydrogenase
- γ-CD
γ-cyclodextrin
- AlloP
allopregnanolone
- APV
DL-2-amino-5-phosphonovaleric acid
- [Cl-]I
intracellular Cl- concentration
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DG
dentate gyrus
- eEPSCs
action potential-dependent EPSCs
- KA
kainic acid
- K-S
Kolmogorov–Smirnov
- NKCC1
Na-K-Cl cotransporter type 1
- PPR
paired-pulse ratio
- sEPSCs
spontaneous EPSCs
- SR95531
6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid HBr
- THDOC
tetrahydrodeoxycorticosterone
- TTX
tetrodotoxin
- VDCCs
voltage-dependent Ca2+ channels
- VDNCs
voltage-dependent Na+ channels
Conflicts of interest
None.
References
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, et al. Neurosteroid access to the GABAA receptor. J Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Pistis I, Peters JA, Lambert JJ. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol Sci. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing α4β3δ human GABAA receptors. Brit J Phamacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaert D, El Manira A. Shunting versus inactivation: analysis of presynaptic inhibition mechanisms in primary afferents of the crayfish. J Neurosci. 1999;19:6079–6089. doi: 10.1523/JNEUROSCI.19-14-06079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl cotransporter expression in rat neocortex. Brain Res Dev Brain Res. 1998;109:281–292. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Corpéchot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, et al. Neurosteroids: 3α-hydroxy-5α-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Mossy fiber zinc and temporal lobe epilepsy: pathological association with altered ‘epileptic’ gamma-aminobutyric acid A receptors in dentate granule cells. Epilepsia. 2000;41(Suppl 6):S96–S99. doi: 10.1111/j.1528-1157.2000.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Pharmacological study of presynaptic inhibition. J Physiol (Lond) 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–425. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Gililland-Kaufman KR, Tanchuck MA, Ford MM, Crabbe JC, Beadles-Bohling AS, Snelling C, et al. The neurosteroid environment in the hippocampus exerts bi-directional effects on seizure susceptibility in mice. Brain Res. 2008;1243:113–123. doi: 10.1016/j.brainres.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of Na-K-Cl cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Han JW, Nakamura M, Choi IS, Cho JH, Park HM, Lee MG, et al. Differential pharmacological properties of GABAA receptors in axon terminals and soma of dentate gyrus granule cells. J Neurochem. 2009;109:995–1007. doi: 10.1111/j.1471-4159.2009.06018.x. [DOI] [PubMed] [Google Scholar]
- Henze DA, McMahon DBT, Harris KM, Barrionuevo G. Giant miniature EPSCs at the hippocampal mossy fiber to CA3 pyramidal cell synapse are monoquantal. J Neurophysiol. 2002;87:15–29. doi: 10.1152/jn.00394.2001. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Jang IS, Jeong HJ, Akaike N. Contribution of the Na-K-Cl co-transporter on GABAA receptor-mediated presynaptic depolarization in excitatory nerve terminals. J Neurosci. 2001;21:5962–5972. doi: 10.1523/JNEUROSCI.21-16-05962.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Jeong HJ, Katsurabayashi S, Akaike N. Functional roles of presynaptic GABAA receptors on glycinergic nerve terminals in the rat spinal cord. J Physiol (Lond) 2002;541:423–434. doi: 10.1113/jphysiol.2001.016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Nakamura M, Ito Y, Akaike N. Presynaptic GABAA receptors facilitate spontaneous glutamate release from presynaptic terminals on mechanically dissociated rat CA3 pyramidal neurons. Neuroscience. 2006;138:25–35. doi: 10.1016/j.neuroscience.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford: Oxford University Press; 2004. pp. 455–498. [Google Scholar]
- Keller AF, Breton JD, Schlichter R, Poisbeau P. Production of 5α-reduced neurosteroids is developmentally regulated and shapes GABAA miniature IPSCs in lamina II of the spinal cord. J Neurosci. 2004;24:907–915. doi: 10.1523/JNEUROSCI.4642-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Krishek BJ, Moss SJ, Smart TG. Interaction of H+ and Zn2+ on recombinant and native rat neuronal GABAA receptors. J Physiol (Lond) 1998;507:639–652. doi: 10.1111/j.1469-7793.1998.639bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EA, Cho JH, Choi IS, Nakamura M, Park HM, Lee JJ, et al. Presynaptic glycine receptors facilitate spontaneous glutamate release onto hilar neurons in the rat hippocampus. J Neurochem. 2009;109:275–286. doi: 10.1111/j.1471-4159.2009.05960.x. [DOI] [PubMed] [Google Scholar]
- Lindquist CE, Birnir B. Graded response to GABA by native extrasynaptic GABA receptors. J Neurochem. 2006;97:1349–1356. doi: 10.1111/j.1471-4159.2006.03811.x. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol. 2006;69:564–575. doi: 10.1124/mol.105.016683. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Sekino Y, Manabe T. GABAergic interneurons facilitate the mossy fiber excitability in the developing hippocampus. J Neurosci. 2007;27:1365–1373. doi: 10.1523/JNEUROSCI.4672-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186:17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Park HM, Choi IS, Nakamura M, Cho JH, Lee MG, Jang IS. Multiple effects of allopregnanolone on GABAergic responses in single hippocampal CA3 pyramidal neurons. Eur J Pharmacol. 2011;652:46–54. doi: 10.1016/j.ejphar.2010.10.097. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brain: a possible mechanism underlying GABA's excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–915. [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N. Calcium channels in the GABAergic presynaptic nerve terminals projecting to meynert neurons of the rat. J Neurochem. 1999;72:800–807. doi: 10.1046/j.1471-4159.1999.0720800.x. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampul mossy fiber. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Campanac E, Scott RS, Rusakov DA, Kullmann DM. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat Neurosci. 2010;13:431–438. doi: 10.1038/nn.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kirkcaldie MT, Waldron S, Calford MB. Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Electrophysiology of morphologically identified mossy cells of the dentate hilus recorded in guinea pig hippocampal slices. J Neurosci. 1988;8:3812–3821. doi: 10.1523/JNEUROSCI.08-10-03812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol. 1990;63:987–998. doi: 10.1152/jn.1990.63.5.987. [DOI] [PubMed] [Google Scholar]
- Seress L, Abrahám H, Paleszter M, Gallyas F. Granule cells are the main source of excitatory input to a subpopulation of GABAergic hippocampal neurons as revealed by electron microscopic double staining for zinc histochemistry and parvalbumin immunocytochemistry. Exp Brain Res. 2001;136:456–462. doi: 10.1007/s002210000601. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAAreceptors. J Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Zeng CM, Wang C, Covey DF, Zorumski CF, Mennerick S. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br J Pharmacol. 2007;150:164–175. doi: 10.1038/sj.bjp.0706973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABAA receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001a;40:178–184. doi: 10.1016/s0028-3908(00)00159-3. [DOI] [PubMed] [Google Scholar]
- Sooksawate T, Simmonds MA. Influence of membrane cholesterol on modulation of the GABAA receptor by neuroactive steroids and other potentiators. Br J Pharmacol. 2001b;134:1303–1311. doi: 10.1038/sj.bjp.0704360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Reciprocal developmental regulation of presynaptic ionotropic receptors. Proc Natl Acad Sci USA. 2002;99:13884–13889. doi: 10.1073/pnas.212419699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, House CR, Istvan M. Perisynaptic localization of subunit-δ containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ. GABAA receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Brain Res. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- Zamir O, Charlton MP. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol (Lond) 2006;571:83–99. doi: 10.1113/jphysiol.2005.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


