Abstract
BACKGROUND AND PURPOSE
Epigenetic modifications are thought to play an important role in the neurobiology of depression. Antidepressant treatment induces histone acetylation in the hippocampus, which is associated with transcriptional activation, whereas stress increases DNA methylation, which is associated with transcriptional repression. Because the specific involvement of DNA methylation in the regulation of depressive-like behaviours is not yet known, we have investigated the effects induced by systemic or intra-hippocampal administration of inhibitors of DNA methyltransferase (DNMT) in rats submitted to a range of behavioural tests.
EXPERIMENTAL APPROACH
Rats received i.p. injections of 5-aza-2-deoxycytidine (5-azaD, 0.1–0.8 mg·kg−1), 5-azacytidine (5-azaC, 0.4–3.2 mg·kg−1), imipramine (15 mg·kg−1) or vehicle and were submitted to the forced swimming test (FST) or open field test (OFT). Other groups of rats received intra-hippocampal injection of DNMT inhibitors.
KEY RESULTS
Systemic administration of DNMT inhibitors induced a dose-dependent antidepressant-like effect, which was followed by decreased DNA methylation and increased brain-derived neurotrophic factor (BDNF) levels in the hippocampus. Hippocampal inhibition of DNA methylation induced similar behavioural effects. No treatment induced any locomotor effects in the OFT. Antidepressant-like effects of 5-azaD were confirmed in mice submitted to the FST or the tail suspension test.
CONCLUSIONS AND IMPLICATIONS
Systemic, as well as hippocampal, inhibition of DNA methylation induced antidepressant-like effects. These effects could be associated with increased hippocampal expression of BDNF. Our data give further support to the hypothesis that DNA methylation is an important epigenetic mechanism involved in the development of depressive-like behaviours.
Keywords: epigenetic, 5-azacytidine, 5-aza-2-deoxy-cytidine, RG-108, DNA methylation, hippocampus, forced swimming test, tail suspension test
Introduction
Depression is a common and serious illness with the potential of becoming the leading cause of disability worldwide (Kessler et al., 2003; 2008;). Its lifetime prevalence rate is in the range of 8–20% throughout different populations, and it is expected to increase in the next years (Kessler et al., 2003; 2008;). These facts contribute to the high economic burden of depression, including direct treatment costs, lost earnings due to depression-related suicide and indirect workplace costs (Greenberg et al., 2003).
The earliest treatments for depression were based upon the serendipitous discovery of monoamine oxidase inhibitors and tricyclic antidepressants, which inhibit the metabolism and the reuptake of monoamines, respectively (Castren, 2005; Krishnan and Nestler, 2008). Since then, the pathophysiology of depression has been dominated by the monoamine hypothesis and newer antidepressants have been developed based on this mechanism of action (Hindmarch, 2002; Castren, 2005; Racagni and Popoli, 2008). However, despite the advances in drug discovery and therapeutic options to treat depression, there are still some shortcomings that need to be improved. For example, a significant symptom improvement is only observed after 2–4 weeks of treatment and only 60–65% of patients respond to the initial regimen, with less than half of these patients reaching remission or becoming symptom-free (Rosenzweig-Lipson et al., 2007). Therefore, the study of the neurobiology of depression could lead to the discovery of new targets, other than those based on the biogenic amines, for the development of more effective therapies for depression.
The findings that chronic antidepressant treatment induces neuronal plastic changes, most likely as an attempt to counteract stress-induced effects in brain circuitry, have supported a new hypothesis about the neurobiology of this disorder, the so called ‘neurotrophic (or molecular) hypothesis of depression’ (Castren, 2005; Duman and Monteggia, 2006; Krishnan and Nestler, 2008). It is mainly based on observations that exposure to stress decreases the expression of brain-derived neurotrophic factor (BDNF), induces dendritic atrophy and reduces neurogenesis, particularly in the hippocampus. These neuroplastic changes are attenuated by chronic antidepressant treatment (Castren, 2005; Duman and Monteggia, 2006; Krishnan and Nestler, 2008). Moreover, antidepressant-induced hippocampal neurogenesis and BDNF expression are thought to be necessary for their behavioural effects (Saarelainen et al., 2003; Adachi et al., 2008).
New insights into the neurobiology of depression have derived from recent evidence that gene expression changes related to neural plasticity processes, such as adult hippocampal neurogenesis (Covic et al., 2010) and activity-driven long-lasting plasticity (Levenson et al., 2006), are modulated by complex ‘epigenetic’ mechanisms (Tsankova et al., 2006; Krishnan and Nestler, 2008; 2010;). Epigenetic changes refers to changes in DNA packing and chromatin structure that control gene expression without changing the original DNA sequence, thus leading to different cellular phenotypes without a change in genotype (Jones and Takai, 2001). Therefore, epigenetic modifications are important mechanisms by which environmental experience can modify gene function in the absence of DNA sequence changes and produce long-lasting changes in protein availability and brain function (Tsankova et al., 2007).
The epigenetic modifications include covalent changes to DNA (methylation) and post-translational modifications of histone N-terminal tails (acetylation, methylation, phosphorylation and ubiquitinylation), as well as non-transcriptional gene silencing mechanisms (micro-RNAs, for example) (Kim et al., 2009).
Among the processes that modulate chromatin structure and gene expression, modifications in histone tails have been the most studied in relation to the neurobiology of depression. For example, histone acetylation of lysines, which is most often associated with transcription activation through its ability to relax condensed areas of chromatin, is induced by chronic antidepressant treatment. This mechanism is thought to account to its behavioural effects (Tsankova et al., 2006). In addition, inhibition of histone deacetylase induces antidepressant-like effects in animals (Schroeder et al., 2007; Covington et al., 2009). On the other hand, histone methylation, depending on the number of methyl groups and position of lysine residues, could be involved with reduced gene expression and stress-induced behavioural consequences (Hunter et al., 2009).
Methylation of DNA cytosines (at the 5-position) is another important epigenetic mechanism for gene transcription regulation in the nervous system. DNA methylation is a process accomplished by DNA methyltransferases (DNMTs), which catalyse a covalent addition of a methyl group to the 5-position of cytosines, almost exclusively at cytosines within cytosine-guanine dinucleotides (CpGs) (Weber and Schubeler, 2007). Methylated sites can be targeted by methylated DNA-binding proteins, triggering additional phenomena that result in condensed chromatin states and transcriptional repression (Weber and Schubeler, 2007).
Besides its recognized role in development and differentiation (Kim et al., 2009), recent evidence has suggested that DNA methylation is also an important epigenetic mechanism that modulates synaptic plasticity in the adult brain (Levenson et al., 2006; Miller and Sweatt, 2007; Covic et al., 2010). This assumption is further supported by evidence that exposure to different stressors, such as social defeat (LaPlant et al., 2010), footshocks (Miller and Sweatt, 2007) and low maternal care (Zhang et al., 2010), increases the expression of DNMTs in different brain regions and this is accompanied by increased DNA methylation and decreased expression of genes that regulate synaptic plasticity and neurotransmission. On the other hand, there is evidence that stress decreases DNA methylation of specific genes involved in stress responses, indicating that DNA methylation could be a dynamic process involved in stress-induced behavioural and physiological outcomes (Murgatroyd et al., 2009; Elliott et al., 2010).
Despite these pieces of evidence, little is known about the role played by DNA methylation in the aetiology of psychiatric disorders such as depression. However, considering that stress increases DNA methylation in the hippocampus and other brain regions (Miller and Sweatt, 2007; Zhang et al., 2010) and that higher levels of DNA methylation have also been described in specific genomic loci at the hippocampus of suicide victims (McGowan et al., 2008; 2009; Poulter et al., 2008), it is plausible to suggest that stress-induced DNA methylation could contribute to the pathophysiology of depression. In this regard, apart from the work recently reported by LaPlant et al., (2010), describing inhibition of DNA methylation in the nucleus accumbens inducing antidepressant-like effects in rodents, there is no other work studying the involvement of DNA methylation in the modulation of depressive-like behaviours.
Therefore, we aimed at investigating the effects induced by single or repeated systemic administration of 5-aza-2′-deoxycytidine (5-azaD) and 5-azacytidine (5-azaC), two potent inhibitors of DNMTs (Yoo et al., 2004; Stresemann and Lyko, 2008), in rats and mice submitted to the forced swimming or tail suspension tests (TSTs), two widely used animal model predictive of antidepressant activity (Cryan et al., 2002). Besides, we also investigated a possible site of action for these drugs by injecting them into the dorsal hippocampus of rats submitted to the same procedures, as neural plasticity in this brain region has been related to the behavioural effects induced by antidepressant drugs (Saarelainen et al., 2003; Santarelli et al., 2003; Adachi et al., 2008). In addition, considering that BDNF is proposed to mediate the behavioural effects of antidepressant drugs (Saarelainen et al., 2003) and its gene expression is regulated by methylation (Roth et al., 2011), the effects of DNMT inhibition on the hippocampal levels of this neurotrophin were also assessed.
Methods
Animals
All animal care and experimental procedures complied with the Brazilian Society of Neuroscience and Behaviour guidelines for the care and use of laboratory animals and were approved by the local Ethical Committee (protocol number 10.1.136.53.2). All efforts were made to minimize animal suffering. Male Wistar rats weighing 200–220 g at the beginning of each experiment were housed in pairs in a temperature-controlled room (24 ± 1°C) under standard laboratory conditions with free access to food and water and a 12 h light/12 h dark cycle (lights on at 06:30 h am). Male Swiss mice weighing 25–30 g at the beginning of each experiment were housed in 6–10 per cage (570 cm2) under the same conditions.
Drug treatments
Imipramine hydrochloride (Sigma-Aldrich®, USA) was dissolved in sterile isotonic saline and administered i.p. (15 mg·kg−1; Joca and Guimarães, 2006). 5-azaD (decitabine; Sigma-Aldrich, USA) (Christman, 2002; Oki et al., 2007), was dissolved in sterile isotonic saline and administered i.p. in a range of doses (0.1, 0.2, 0.3, 0.4, 0.6 or 0.8 mg·kg−1)(Pereira et al., 2004). 5-azaC (Sigma-Aldrich, USA) (Christman, 2002), was administered i.p. (0.4, 1.6 or 3.2 mg·kg−1) according to its potency in inhibiting DNA methylation compared to 5-azaD (Stresemann et al., 2006). For intra-hippocampal injections, 5-azaD (50, 100 or 200 nmol·per 0.5 µL) or the non-nucleoside DNMT inhibitor RG-108 (Tocris Biosciences,USA, 100 or 200 nmol·per 0.5 µL) were dissolved in sterile isotonic saline or DMSO, respectively, and administered bilaterally, according to (Miller and Sweatt, 2007). All drugs were freshly prepared and protected from light during the experimental session.
Open field test (OFT)
Independent groups of animals were submitted to the OFT in order to investigate if the treatments used could induce any significant exploratory/motor effect, which would interfere in the forced swimming test (FST) results. The protocol was done as previously described (Crestani et al., 2010; Scopinho et al., 2010). Briefly, the animals were placed individually in the centre of an open circular arena (diameter: 72 or 40 cm in diameter, for rats or mice, respectively, with a 50 cm high Plexiglas wall) located in a sound-attenuated, temperature-controlled room, illuminated with three 40 W fluorescent bulbs. The animals were left in the arena for 5 (rats) or 6 (mice) min. Their exploratory activity was videotaped and the behavioural analysis was carried out, without knowledge of the treatments given, using the Any-Maze software (Stoelting, USA). This software detects the position of the animal in the open arena and calculates the distance moved.
Stereotaxic surgery and intracerebral administration
Stereotaxic surgery was performed as described before (Joca et al., 2003). Briefly, rats were anaesthetized with 2,2,2-tribromoethanol (10 mg·kg−1, i.p., Aldrich Chemical, USA) and fixed in a stereotaxic frame. Stainless steel guide cannulae (0.7 mm OD) were implanted bilaterally aimed at the dorsal hippocampus (coordinates: AP = −4.0 mm from lambda, L = 2.8 mm, D = 2.1 mm), according to the Paxinos and Watson (1997) atlas. The cannulae tips were located 1.5 mm above the site of injection and the cannulae were attached to the skull bone with stainless steel screws and acrylic cement. An obturator inside the guide cannulae prevented obstruction.
Five to seven days after the surgery, intracerebral injections were performed with a thin dental needle (0.3 mm OD) that was introduced bilaterally through the guide cannula until its tip was 1.5 mm below the cannulae end. A volume of 0.5 µL per side was injected in 1 min, using a microsyringe (Hamilton) controlled by an infusion pump (Insight Equipamentos Científicos, Brazil). A polyethylene catheter (PE10) was interposed between the upper end of the dental needle and the microsyringe. The movement of an air bubble inside the polyethylene catheter confirmed drug flow.
FST
The FST procedure for rats was similar to that first described by Porsolt et al. (1978), with minor modifications according to Joca et al. (2007b). Animals were initially placed individually to swim in plastic cylinders (30 cm of diameter by 40 cm in height containing 25 cm of water at 24 ± 1°C) for 15 min (pretest). They were then removed and allowed to dry in a separate cage before returning to their home cages. Twenty-four hours later the animals were submitted to a 5 min session of forced swim. The procedure for mice consisted in placing the animals into glass cylinders (height 25 cm, diameter 17 cm) containing 10 cm of water maintained at 23–25°C. The animals were left in the cylinder for 6 min and the total duration of immobility was measured during the last 4 min period. During the test session the total amount of time in which animals remained immobile (except for small limb movements necessary for floating) was recorded. The water was changed after each trial to avoid the influence of alarm substances.
TST
The animals were suspended by the tail and fixed using a tape in a wood platform elevated 50 cm above the floor, according to the protocol described by Viana et al. (2010). Their behaviour was videotaped and the immobility time was recorded during 6 min by a trained observer. The mice were considered immobile when they remained suspended passively.
Histology
After the behavioural tests the rats were killed with deep urethane anaesthesia and perfused through the left ventricle of the heart with isotonic saline followed by 10% formalin solution. After that, a dental needle was inserted through the guide cannula and 0.5 µL of fast green was injected. The brains were removed and, after a minimum period of 3 days immersed in a 10% formalin solution, 40 µm sections was obtained in a Cryostat (Cryocut 1800). The injection sites were identified on diagrams from the Paxinos and Watson's atlas (Paxinos and Watson 1997). Rats that received injections outside the aimed area were excluded from analysis.
DNA methylation analysis
The animals were deeply anaesthetized with chloral hydrate (500 mg·kg−1) and decapitated. The hippocampus was dissected and the tissue stored at −80°C until the analysis. DNA was extracted using the AxyPrep Blood Genomic DNA Miniprep Kit (Axygen Biosciences, USA) according to the manufacturer's instructions. The purified DNA was digested with Nuclease P1 (#P2640, Sigma) (2 U·µg−1 of DNA, 4 h at 65°C in acetate buffer 20 mM pH 5.3) and with alkaline phosphatase (#N8630, Sigma) (1 U·µg−1 of DNA, 2 h at 65°C in Tris-HCl 20 mM pH 7.5). The digested DNA was precipitated in pure cold ethanol and NaCl 5 M at −20°C for 18 h and centrifuged at 20000×g for 15 min. The pellet was resuspended in TE buffer (5 mM Tris-HCl, 0.1 mM EDTA, pH 8.5) and the methylated DNA was quantified using the DNA Methylation EIA kit (#589324, Cayman Chemicals), according to the manufacturer's instructions. The absorbance produced in the assay was measured by VictorX3 plate reader and software (Perkin Elmer). Several concentrations of purified 5-methyl-2’-deoxycytidine (provided by the kit) were used to construct the standard curve. The concentration of the sample (ng·mL−1) was calculated based on the 4-parameter logistic equation of the standard curve.
Quantification of BDNF levels
The animals were deeply anaesthetized with chloral hydrate 500 mg·kg−1 and decapitated. The hippocampus was dissected and homogenized in lysis buffer (20 mM Tris-HCl pH 8.0, 137 mM NaCl, 10% glycerol supplemented with protease inhibitor cocktail, P2714, Sigma) and centrifuged at 10000×g 4°C for 15 min. The supernatant was collected and BDNF measured with the BDNF Emax® ImmunoAssay System (#G7610, Promega, USA), according with the manufacturer's instructions. The concentration of the samples was calculated based on the standard curve, constructed with purified recombinant human BDNF. The levels of total protein content were determined by the reaction with Bradford reagent (#B6916, Sigma), following the manufacturer's instructions, and used to normalize the BDNF results. The final result was expressed as pg BDNF per µg total proteins.
Experimental design
Experiment one: effects of systemic administration of 5-azaD, 5-azaC or imipramine in rats submitted to the FST
Rats were submitted to the pretest and afterwards received two injections 5-azaD, 5-azaC, imipramine or respective vehicles (0 and 5 h later). The third injection was given 1 h before the test session.
Experiment two: effects of systemic administration of 5-azaD or 5-azaC in rats submitted to the OFT
Rats received three injections 5-azaD, 5-azaC, or respective vehicles, 1, 19 and 24 h before the test session.
Experiment three: effects of single systemic administration of 5-azaD in rats submitted to the FST
In order to investigate whether a single injection of the drug would be able to induce antidepressant-like effects, independent groups of rats were submitted to the pretest and received a single injection of 5-azaD immediately or 5 h after the pretest, or 1 h before the test.
Experiment four: effects of 5-azaD in the levels of methylated DNA and BDNF in the hippocampus of rats submitted to the FST
Independent groups of animals were submitted to the experimental protocol as described in Experiment 1 and were killed immediately after or immediately before the test, in order to have their hippocampus dissected and the levels of methylated DNA and of BDNF, respectively, analysed. The levels of BDNF were measured in animals that underwent pretest 24 h before but were not submitted to the test in order to avoid that the protein levels could be influenced by the exposure to the test session. Because we wanted to know how BDNF levels would be at the moment that the animal performed the test, we believe that this experimental design would avoid false-negative results and allow us to identify even modest differences of BDNF protein levels.
Experiment five: effects of intra-hippocampal administration of 5-azaD or RG-108 in rats submitted to the FST
Rats were submitted to the pretest and, immediately afterwards, received a bilateral injection of 5-azaD (50, 100 or 200 nmol·per 0.5 µL), or vehicle and were tested 24 h later. An independent group of animals was submitted to the same experimental protocol but received intra-hippocampal injections of RG-108 (100 or 200 nmol·per 0.5 µL), a non-nucleoside inhibitor of DNMTs, which, unlike 5-azaD, does not need to be incorporated into DNA to inhibit DNMTs (Szyf, 2009).
Experiment six: effects of systemic administration of 5-azaD in mice submitted to the FST, TST or OFT
Independent groups of mice received a single i.p. injection of 5-azaD or vehicle and were submitted to the behavioural tests 1 h later.
Statistical analysis
The treatment effects were compared using one-way anova followed by Dunnett's test for post hoc comparisons. Data from Experiment 2 were analysed by a two-way anova with the factors being treatment and time. Probability less than 0.05 was accepted as significant.
Results
Experiment one: effects of systemic administration of 5-azaD, 5-azaC or imipramine to rats submitted to the FST
Systemic treatment with 5-azaD, at the dose of 0.4 mg·kg−1, or with imipramine significantly reduced immobility time (F7,40 = 8.97, P < 0.0001; Dunnett's test, P < 0.05; Figure 1). Systemic injection of 5-azaC induced similar effects, with the dose of 3.2 mg·kg−1 being the most effective in reducing immobility time (F4,33 = 10.86, P < 0.0001; Dunnett's test, P < 0.05; Figure 1).
Figure 1.
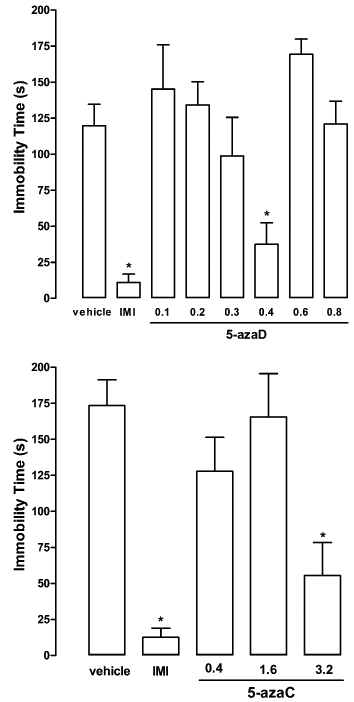
Systemic injection of 5-azaD, 5-azaC or imipramine (IMI) in rats reduced the immobility time in the FST. Animals were submitted to the pretest session and received three i.p. injections (0, 5 and 23 h later) of vehicle, 5-azaD (0.1–0.8 mg·kg−1) or 5-azaC (0.4–3.2 mg·kg−1). The immobility time was recorded 1 h after the last injection in a 5 min test session. Data are expressed as mean ± SEM (n = 5–8 per group). *P < 0.05, significant difference from vehicle-treated group (anova followed by Dunnett's test).
Experiment two: effects of systemic administration of 5-azaD or 5-azaC in rats submitted to the OFT
Two-way anova indicated that the distance moved in the OFT decreased with time but it did not differ among groups treated with 5-azaD or vehicle (treatment factor: F4,100 = 1.63, P > 0.05; time factor: F4,100 = 65.14, P < 0.001; interaction: F16,100 = 2.31, P > 0.05). Similar effects were observed for the groups treated with 5-azaC or vehicle (treatment factor: F3,76 = 1.63, P > 0.05; time: factor F4,76 = 48.33, P < 0.001). Although there was an interaction between factors (F12,76 = 8.92, P < 0.05), post hoc analysis indicated that there were no significant difference between treatments at any time of the test (5-azaC vs. vehicle, Bonferroni, P > 0.05). None of the treatments induced any significant difference in the total distance moved in the OFT (5-azaD: F4,25 = 0.54, P > 0.05; 5-azaC: F3,20 = 0.57, P > 0.05; Figure 2).
Figure 2.
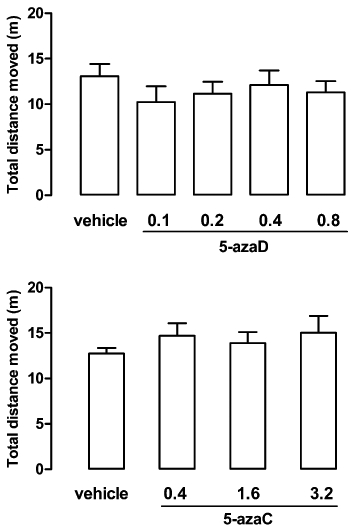
Systemic injection with 5-azaD or 5-azaC in rats did not modify locomotor activity in the OFT. Animals received 3 i.p. injections of 5-azaD (0.1–0.8 mg·kg−1), 5-azaC (0.4–3.2 mg·kg−1) or vehicle (24, 19 and 1 h before the test) and had their locomotor activity evaluated during 5 min. Data are expressed as mean ± SEM (n = 6–10 per group). No significant differences between groups were detected (P > 0.05, two-way anova).
Experiment three: effects of single systemic administration of 5-azaD in rats submitted to the FST
5-azaD reduced immobility time when injected immediately or 5 h after pretest (F3,24 = 17.25, P < 0.01, Dunnett's test, P < 0.05, Figure 3), but not when administered 1 h before the test (Dunnett's test, P > 0.05, Figure 5).
Figure 3.
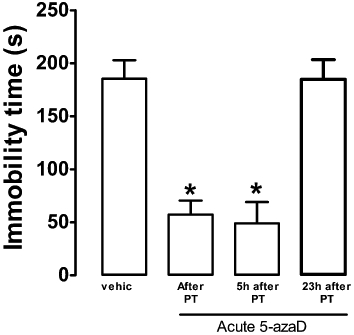
Single systemic injection of 5-azaD in rats, immediately or 5 h after the pretest (PT), reduced the immobility time in the FST. Animals were submitted to the pretest session and received an i.p. injection (0, 5 or 23 h after) of vehicle or drug. The immobility time was recorded in a 5 min test session, 24 h after pretest. Data are expressed as mean ± SEM (n = 5–7 per group). *P < 0.05, significant difference from vehicle-treated group (Student's t-test).
Figure 5.
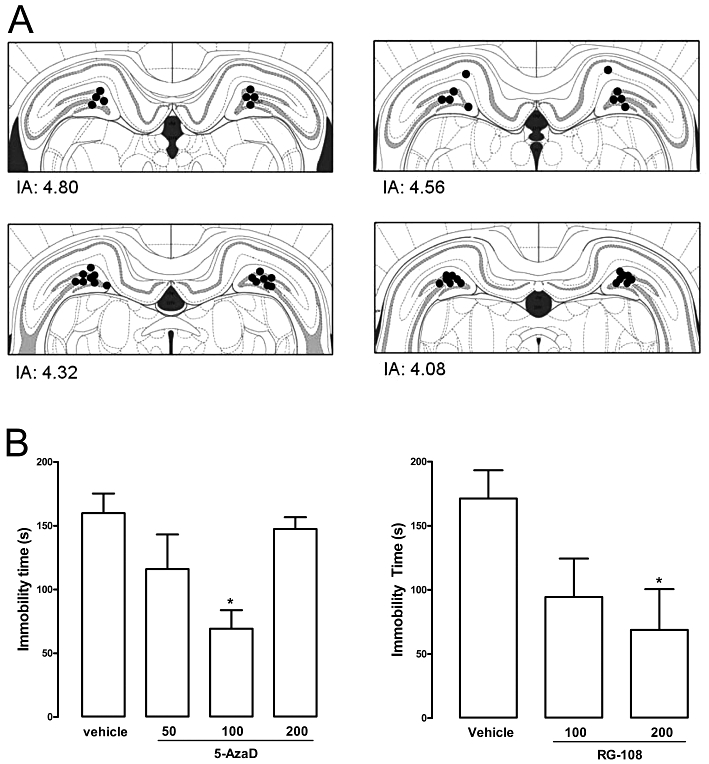
Post-stress intra-hippocampal administration in rats of 5-azaD or RG-108 reduced the immobility time in the FST. Animals received a bilateral intra-hippocampal injection (0.5 µL) of drug or vehicle immediately after the pretest session and were tested 24 h later. (A) Diagram representing injection sites aimed at the dorsal hippocampus (dots indicate the localization of the injection site visualized by the dye injection). IA, interaural distance. (B) Effects of 5-azaD (50–100 nmol·per 0.5 µL) or RG-108 (100, 200 nmol·per 0.5 µL) on the immobility time in the FST. Data are expressed as mean ± SEM (n = 6–9 per group). *P < 0.05, significant difference from vehicle-treated group (anova followed by Dunnett's test).
Experiment four: effects of 5-azaD in the levels of methylated DNA and BDNF in the hippocampus of rats submitted to the FST
As in experiment one, systemic treatment with 5-azaD, at the dose of 0.4 mg·kg−1 significantly reduced immobility time (mean ± epm of saline and 5-azaD respectively: 135 ± 20 and 47.8 ± 31, t10 = 2.434, P < 0.05). This same treatment reduced hippocampal levels of methylated DNA (t10 = 3.10, P < 0.05, Figure 4) and BDNF protein (t11 = 2.35, P < 0.05, Figure 4).
Figure 4.
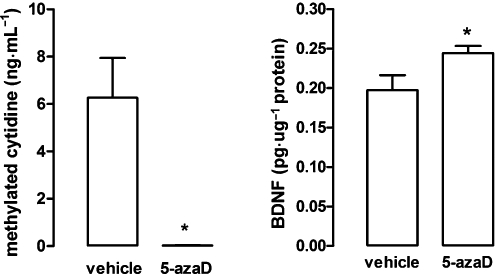
Systemic injection of 5-azaD in rats reduced global DNA methylation and increased BDNF levels, in the hippocampus. Animals were submitted to the pretest session and received three i.p. injections (0, 5 and 23 h after) of vehicle or drug and were killed 1 h after the last injection, immediately after (DNA methylation) or before (BDNF levels) the test session. Data are expressed as mean ± SEM (n = 5–7 per group). *P < 0.05, significant difference from vehicle-treated group (Student's t-test).
Experiment five: effects of intra-hippocampal administration of 5-azaD or RG-108 in rats submitted to the FST
Microinjection sites in the dorsal hippocampus can be seen in Figure 5. Intra-hippocampal injection of 5-azaD significantly reduced immobility time at a dose of 100 nmol·per 0.5 µL (F3,28 = 3.59, P < 0.05; Dunnett's test, P < 0.05; Figure 5). RG-108 produced similar effects at adose of 200 nmol·per 0.5 µL (F2,18 = 3.42, P < 0.05; Dunnett's test, P < 0.05; Figure 5).
Experiment six: effects of 5-azaD systemic administration in mice submitted to the FST, TST or OFT
5-azaD, at the doses of 0.2 and 0.8 mg·kg−1 for the FST and TST, respectively, decreased immobility time. Similar effects were found for imipramine (FST: F4,25 = 2.85; TST: F4,31 = 6.96, P < 0.05; Dunnett's test P < 0.05; Figure 6). The drug also decreased the total distance moved in the OFT (F3,20 = 8.10, P < 0.05; Dunnett's test, P < 0.05; Figure 6).
Figure 6.

Single systemic injection of 5-azaD (0.2–0.8 mg·kg−1) in mice decreased immobility time in the FST (upper panel) and TST (middle panel). The drug also decreased the total distance travelled in the OFT (lower panel). Experiments were conducted with independent groups of animals that received i.p. injections of 5-azaD, imipramine or vehicle and were submitted to the behavioural tests 1 h later. Data are expressed as mean ± SEM (n = 5–9 per group). *P < 0.05, significant difference from vehicle-treated group (anova followed by Dunnett's test).
Discussion
The results of the present study showed that systemic administration of inhibitors of DNA methylation decreased immobility time in the FST, an antidepressant-like effect. These effects were not likely to be related to non-specific motor changes, as the same treatments did not modify locomotor activity of rats in the OFT. Although 5-azaD did induce a decrease in distance moved by mice in this model, this effect would, by favouring increased immobility, actually prevent rather than induce antidepressant-like activity in the FST. 5-AzaD also decreased immobility time in mice submitted to the tail suspension test (TST), another widely employed animal model of depression (Cryan et al., 2002), thus confirming the antidepressant-like effects observed in the FST. The behavioural effects induced by 5-azaD was accompanied by decreased DNA methylation and increased BDNF levels in the dorsal hippocampus. Moreover, intra-hippocampal injections of 5-azaD induced antidepressant-like effects in the FST. Together the results show that prevention of DNA methylation induces antidepressant-like effects and suggest that the dorsal hippocampus could be an important site of action for such effects.
Several DNMTs have been identified, and grouped into three major classes (DNMT1, DNMT3A and DNMT3B) depending on their substrate preference and resulting function (Klose and Bird, 2006). DNMT1 has a preference for hemi-methylated substrates and has been designated as a maintenance methyltransferase that copies the pattern of methylation from the paternal strand to the nascent strand during cell division, whereas DNMT3A and DNMT3B show equal preference for unmethylated and hemi-methylated DNA in vitro, and were proposed as de novo methyltransferases (Klose and Bird, 2006; Kim et al., 2009).
DNA methylation can be prevented by 5-azaD (decitabine), a unique cytosine analogue that inhibits DNMT1, reverses methylation and reactivates silenced genes (Oki et al., 2007). It does so by being incorporated into DNA in place of cytosine, which covalently trap DNA methyltransferases and eventually leads to degradation of DNMTs (Szyf, 2009). 5-azaD has shown therapeutic activity in patients with myelodysplastic syndrome or with acute and chronic leukaemias, effects that are likely to be related to its hypomethylating activity of tumour-suppressor genes (Oki et al., 2007).
In the present study, the involvement of DNA methylation in the modulation of stress-induced depressive-like behaviour was investigated by systemic injection of 5-azaD to animals submitted to the FST. The results showed that this drug treatment induced an antidepressant-like effect that was significant at a dose comparable to that used in a previous report (0.4 mg·kg−1) and able to induce a significant decrease in DNA methylation in rats (Pereira et al., 2004). However, higher doses produced no significant effect in the FST. This U-shape profile could be attributed to the fact that while low doses of 5-azaD inhibit DNMTs, higher doses can induce toxic effects due to inhibition of DNA synthesis and cell cycle arrest (Oki et al., 2007).
5-azaC is another cytidine analogue, which, although less potent then 5-azaD (Stresemann et al., 2006; Stresemann and Lyko, 2008), can also induce DNMT inhibition (Christman, 2002). Therefore, we used this drug as an attempt to further strengthen the possibility that the effects induced by 5-azaD would be due to DNMT inhibition, as these two drugs share this common mechanism of action. The results showed that systemic administration of 5-azaC to rats also induced antidepressant-like effects, in a dose-dependent manner. It is noteworthy that the effective dose of 5-azaC was four times smaller than the effective dose of 5-azaD, in agreement with their relative potency to inhibit DNA methylation in vitro (Stresemann et al., 2006; Stresemann and Lyko, 2008).
In the FST performed in rats the immobility time is responsive to systemic antidepressant treatment only after repeated drug administration over a period of at least 24 h (Porsolt et al., 1978; Cryan et al., 2002). However, the results presented here suggest that DNMT inhibitors might have a different pharmacological profile, with a faster onset of action, as a single administration of the drug within a short time window after the pretest session significantly reduced the immobility time in the FST. This suggests that in rats the increased gene and protein expression that follow the pretest swimming session is necessary to counteract stress-induced behavioural consequences or mediate behavioural adaptation during the test session. The involvement of DNA methylation in the modulation of depressive-like behaviours was also supported by our findings that a single injection of 5-azaD induced antidepressant-like effects in mice submitted to the FST or the TST. In this case, however, the drug was effective when administered before the test. However, different from rats, the FST and TST in mice are known to be sensitive to pretest acute treatment with antidepressants (Cryan et al., 2002). The reasons for this species difference is unknown, but the results suggest that at least in mice DNA methyltransferases are involved in the acute stress response.
Because the drugs used in this study can readily cross the blood–brain barrier (Chabot et al. 1983), it is probable that these effects depend on DNA methylation inhibition in brain regions related to stress coping responses. Considering that DNMTs are constitutively expressed in the adult hippocampus (Brown et al., 2008), an important brain region involved in behavioural adaptation to stress and in depression physiopathology (Graeff et al., 1996; Castren, 2005; Duman and Monteggia, 2006; Joca et al., 2007a; Krishnan and Nestler, 2010), we hypothesized that the hippocampus could be an important site of action for the antidepressant-like effects induced by systemic administration of DNMT inhibitors. Corroborating this proposal, intra-hippocampal administration of 5-azaD, immediately after stress, induced antidepressant-like effect in the rat FST. In addition, the administration of the non-nucleoside DNMT inhibitor RG1-108 that, unlike 5-azaD, does not need to be incorporated into DNA to inhibit DNMTs (Szyf, 2009), induced similar results. This further confirms that DNA methylation in the hippocampus modulates depressive-like behaviour and suggests that this effect is not restricted to cells undergoing mitosis in this brain structure.
Altogether, these data suggest that increased DNA methylation in specific genomic loci within the hippocampus could reduce the expression of genes that would be important to regulate its functioning under aversive situations and, therefore, predispose to the development of stress-induced behavioural/emotional outcomes. This hypothesis is supported by evidence that exposure to social adversity early in life (Brown et al., 2008; Zhang et al., 2010) or to stressful situations in adulthood (Miller and Sweatt, 2007) increases DNA methylation in the rat hippocampus. Moreover, hypermethylation of ribosomal DNA promoter sequences have been found in the hippocampus of suicide victims with a history of childhood abuse or neglect (McGowan et al., 2008). Because ribosomal RNAs encode the building blocks for the protein synthesis machinery of the cell, its reduced expression is likely to impair cellular functions in the affected hippocampi (Akbarian, 2008).
Impairments in hippocampal structure, such as atrophy of apical dendrites, increased cell death and decreased neurogenesis, have been consistently found in stressed animals and humans (Pittenger and Duman, 2008; Lucassen et al., 2010; McEwen, 2010), and are suggested to contribute to the hippocampal dysfunction described in depressed humans (Sheline et al., 1996; 1999;). Because DNA methylation of genes involved in structural and neurotrophic functions seems to affect adult neurogenesis (Covic et al., 2010), as well as activity-driven neuroplasticity in the hippocampus (Levenson and Sweatt, 2006; Levenson et al., 2006), it is possible that increased hippocampal DNA methylation of such genes could contribute to the stress-induced neuroplasticity within the region and its functional consequences. Consistent with this, stress exposure increased DNA methylation and decreased the expression of BDNF in the hippocampus (Roth et al., 2009), an important neurotrophin for cell survival and proliferation (Arancio and Chao, 2007) and for antidepressant-induced behavioural effects in the FST (Saarelainen et al., 2003; Duman and Monteggia, 2006; Adachi et al., 2008). Corroborating this idea, in the present work the antidepressant-like effect induced by 5-azaD was accompanied by decreased global methylation in cytidine residues of DNA and increased BDNF levels in the hippocampus. It is possible that this latter effect is contributing to the antidepressant-like effects induced by 5-azaD. However, in addition to BDNF, methylation is known to regulate the expression of other genes involved in the neurobiology of depression, such as those that encode the glucocorticoid (McGowan et al., 2009) and the GABAA receptors (Poulter et al., 2008). The involvement of these genes in the present results remains to be investigated. Moreover, increased DNA methylation in other brain structures could also be involved in the development of stress-induced depressive-like behaviours. It has been recently shown, for example, that DNMT3a inhibition in the nucleus accumbens induces antidepressant-like effects in the chronic social defeat stress model (LaPlant et al., 2010), suggesting that inhibition of DNA methylation in this nucleus could also have contribute to the antidepressant-like effects induced by systemic administration of 5-azaD and 5-azaC.
In conclusion, the present results suggest that systemic or hippocampal administration of DNA methylation inhibitors induced antidepressant-like effects and gives further support for the involvement of DNA methylation in the neurobiology of depression.
Acknowledgments
The authors acknowledge Flávia Salata, Juliana Vercesi and José Carlos de Aguiar for their helpful technical assistance. We are also thankful to Dr L. Resstel for his helpful discussions of this manuscript. This research was supported by grants from FAPESP (2009/18372–6; 2007/03685–3) and CNPq.
Glossary
Abbreviations
- 5-azaC
5-azacytidine
- 5-azaD
5-aza-2′-deoxy-cytidine
- BDNF
brain-derived neurotrophic factor
- DNMT
DNA methyltransferase
- FST
forced swimming test
- OFT
open field test
- TST
tail suspension test
Conflicts of interest
The authors declare no conflicts of interest.
References
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S. Approaching the molecular pathology of suicide. Biol Psychiatry. 2008;64:643–644. doi: 10.1016/j.biopsych.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Brown SE, Weaver IC, Meaney MJ, Szyf M. Regional-specific global cytosine methylation and DNA methyltransferase expression in the adult rat hippocampus. Neurosci Lett. 2008;440:49–53. doi: 10.1016/j.neulet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- Chabot GG, Rivard GE, Momparler RL. Plasma and cerebrospinal fluid pharmacokinetics of 5-Aza-2’-deoxycytidine in rabbits and dogs. Cancer Res. 1983;43:592–597. [PubMed] [Google Scholar]
- Covic M, Karaca E, Lie DC. Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity. 2010;105:122–134. doi: 10.1038/hdy.2010.27. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct. 2010;6:30. doi: 10.1186/1744-9081-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A. Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci. 2010;13:1351–1353. doi: 10.1038/nn.2642. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, McEwen BS. Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA. 2009;106:20912–20917. doi: 10.1073/pnas.0911143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca SR, Guimarães FS. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 2006;185:298–305. doi: 10.1007/s00213-006-0326-2. [DOI] [PubMed] [Google Scholar]
- Joca SR, Padovan CM, Guimaraes FS. Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res. 2003;978:177–184. doi: 10.1016/s0006-8993(03)02943-3. [DOI] [PubMed] [Google Scholar]
- Joca SR, Ferreira FR, Guimaraes FS. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress. 2007a;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Joca SR, Guimaraes FS, Del-Bel E. Inhibition of nitric oxide synthase increases synaptophysin mRNA expression in the hippocampal formation of rats. Neurosci Lett. 2007b;421:72–76. doi: 10.1016/j.neulet.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38:365–374. doi: 10.1017/S0033291707002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Samaranayake M, Pradhan S. Epigenetic mechanisms in mammals. Cell Mol Life Sci. 2009;66:596–612. doi: 10.1007/s00018-008-8432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking Molecules to Mood: New Insight Into the Biology of Depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms: a common theme in vertebrate and invertebrate memory formation. Cell Mol Life Sci. 2006;63:1009–1016. doi: 10.1007/s00018-006-6026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006a;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, Huang TC, Unterberger A, Suderman M, Ernst C, et al. Promoter-wide hypermethylation of the ribosomal RNA gene promoter in the suicide brain. PLoS ONE. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Oki Y, Aoki E, Issa JP. Decitabine–bedside to bench. Crit Rev Oncol Hematol. 2007;61:140–152. doi: 10.1016/j.critrevonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sidney, Australia: Academic Press; 1997. [Google Scholar]
- Pereira MA, Wang W, Kramer PM, Tao L. DNA hypomethylation induced by non-genotoxic carcinogens in mouse and rat colon. Cancer Lett. 2004;212:145–151. doi: 10.1016/j.canlet.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Du L, Weaver IC, Palkovits M, Faludi G, Merali Z, et al. GABAA receptor promoter hypermethylation in suicide brain: implications for the involvement of epigenetic processes. Biol Psychiatry. 2008;64:645–652. doi: 10.1016/j.biopsych.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci. 2008;10:385–400. doi: 10.31887/DCNS.2008.10.4/gracagni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Beyer CE, Hughes ZA, Khawaja X, Rajarao SJ, Malberg JE, et al. Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther. 2007;113:134–153. doi: 10.1016/j.pharmthera.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, Scopinho M, Lisboa SF, Correa FM, Guimaraes FS, Joca SR. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behav Brain Res. 2010;214:437–442. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]
- Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F. Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 2006;66:2794–2800. doi: 10.1158/0008-5472.CAN-05-2821. [DOI] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Viana AF, Maciel IS, Dornelles FN, Figueiredo CP, Siqueira JM, Campos MM, et al. Kinin B1 receptors mediate depression-like behavior response in stressed mice treated with systemic E. coli lipopolysaccharide. J Neuroinflammation. 2010;7:98. doi: 10.1186/1742-2094-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Schubeler D. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr Opin Cell Biol. 2007;19:273–280. doi: 10.1016/j.ceb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Yoo CB, Cheng JC, Jones PA. Zebularine: a new drug for epigenetic therapy. Biochem Soc Trans. 2004;32:910–912. doi: 10.1042/BST0320910. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


