There is increasing interest in exploring inappropriate activation of the renin-angiotensin-aldosterone system (RAAS) as a unifying mechanism between insulin resistance and other components of the metabolic syndrome (1,2). Recent data suggest that elevations in plasma aldosterone levels are associated with the insulin resistance independent of other components of angiotensin II (Ang II). This relationship has been observed in studies exploring the association between primary aldosteronism, in which there are low levels of renin activity and Ang II, and insulin resistance (1). In this issue Kumagai et al (3) explored increases in aldosterone in the normal range at baseline as a predictor of development of insulin resistance in a prospective cohort over 10 years. Insulin resistance was ascertained by a homeostatic model assessment (HOMA) of insulin resistance (HOMA-IR).
The authors utilized a Japanese cohort of 1,088 individuals without diabetes to explore the strength of the association between baseline aldosterone levels and development of insulin resistance. They then further excluded individuals with demonstrable insulin resistance at baseline and tracked the remaining 564 persons prospectively over 10 years. On univariate regression analysis at baseline, aldosterone levels correlated with age, measures of insulin resistance (fasting glucose and insulin), obesity [body mass index (BMI)/waist circumference (WC)], and dyslipidemia but not with the presence of hypertension or potassium level. Using a stepwise regression approach age, dyslipidemia, and HOMA-IR remained significantly correlated with aldosterone levels (table 2).
Unique to this report is the prospective analysis of the relationship between plasma aldosterone levels and the development of insulin resistance. In the prospective cohort, 151 individuals developed insulin resistance. Those who developed insulin resistance were older, had higher levels of aldosterone, BMI/WC, and triglycerides, compared to those who did not develop insulin resistance. The authors created tertiles for increasing levels of aldosterone using an ROC with c-statistic approach and determined relative risk for the development of insulin resistance within each aldosterone tertile (Table 5). The authors report an increased risk on unadjusted analysis in the 2nd and 3rd tertile that remained after adjustment for age, gender, and BMI (model 2) in the 2nd tertile and following adjustment for measured clinical variables (through model 6) in the 3rd tertile. Thus, these observations suggest that increasing levels of aldosterone in the normal range predict the development of insulin resistance.
Previous work in this area has evaluated the association between aldosterone and insulin resistance in cohorts mostly comprised of individuals with primary aldosteronism, hypertension, and other components of the metabolic syndrome (1,4,5). HOMA-IR has been employed to assess insulin resistance in those with primary aldosteronism and explore this correlative relationship (5, 6), and a few studies have explored improvements in insulin resistance post-resection of aldosterone-producing tumors (6,7). Results of the current investigation contrast with findings from a prospective analysis of Framingham data wherein increases in aldosterone in the normal range were similarly associated with insulin resistance at baseline; however, this relationship was lost in the longitudinal follow-up cohort. Disparate longitudinal results in these 2 studies may be due to differences in population characteristics (US versus Japanese) or analytic approach. In this Japanese cohort (3) increases in BMI and WC at 10 years, coupled with the strength of the association at baseline with determinants of dyslipidemia, suggest a strong relationship between aldosterone levels and metabolic risk. The exclusion of those insulin resistant at baseline and a non-significant relationship with hypertension suggest that dyslipidemia along with BMI/WC and plasma aldosterone levels at baseline (table 2) drove the risk for insulin resistance in this cohort. In this regard, in Japan obesity is defined as a BMI >25 (8) and not a BMI >30 used by the World Health Organization. The difference in definitions are derived from data suggesting Japanese adults have a higher percentage of body fat compared with caucasians at any given level of BMI, and that obesity-associated disorders begin in mild to moderate obesity adults in Japanese persons (9,10).
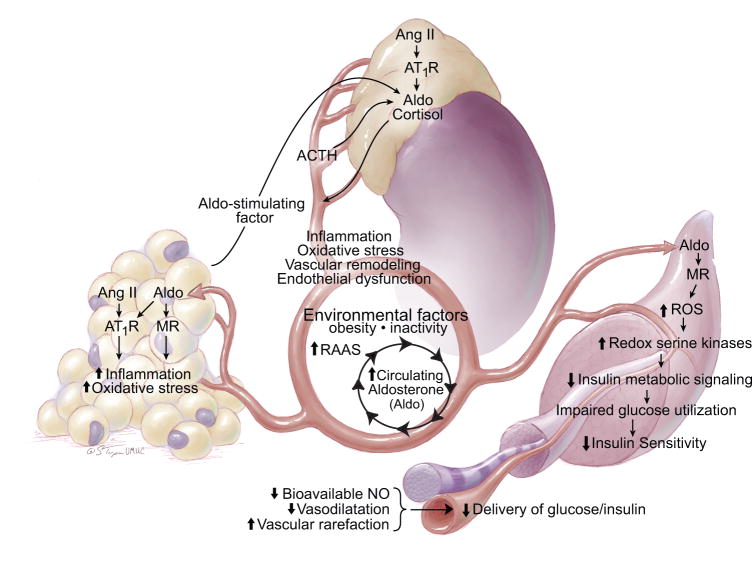
That plasma aldosterone levels predicts the development of insulin resistance in this Japanese cohort translates recent basic science data showing that increased non-genomic signaling through the mineralcorticoid receptor (MR) contributes to the development of insulin resistance (1,2). In this regard, activation of the MR in muscle, liver, and fat tissue promotes a pro-oxidative/pro-inflammatory milieu that contributes to impairments in glucose utilization and disposal that result in reductions in insulin sensitivity (Fig). In skeletal muscle tissue MR activation reduces insulin-dependent metabolic signaling as a result of activation of redox sensitive serine/threonine kinases, and associated serine/threonine phosphorylation of the insulin receptor substrate 1 (IRS-1). This, in turn, leads to IRS-1 degradation and/or reduced engagement of phosphoinositide-3–kinase (PI3-K)/downstream phosphorylation/activation of protein kinase B (Akt), which mediates a number of metabolic actions including GLUT-4 translocation to the plasma membrane as well as endothelial nitric oxide synthase (eNOS) phosphorylation/activation and increases in bioavailable NO (1). Thus, aldosterone can diminish insulin stimulated glucose up-take in skeletal muscle directly (GLUT 4 translocation) as well as indirectly by decreasing NO enhancement of insulin and glucose delivery to this tissue (Fig).
As recently summarized, there is also recent data suggesting that adipose tissue produces a lipid soluble factor that stimulates aldosterone secretion (1). There is also emerging evidence that MR activation further promotes adipogenesis and macrophage infiltration in visceral fat. Thus, the interaction of visceral fat, adrenal aldosterone production and reduced insulin sensitivity interact in a positive servo-regulatory manner to promote further adipogenesis, inflammation, increases in plasma aldosterone and insulin resistance (Fig).
In summary, there is an increasing basic science and clinical evidence that aldosterone adversely impacts insulin sensitivity. Indeed, the current longitudinal study (3) indicates in a young Japanese population followed over 10 years that high normal baseline levels of aldosterone predict risk for development of insulin resistance. However, the study included a relatively small Japanese cohort, and there is need for investigation in more diverse populations.
Figure.
The integrative relationship between aldosterone, visceral adipose tissue and skeletal muscle. RAAS – renin-angiotensin-aldosterone system; SNS – sympathetic nervous system; NO – nitric oxide; BP – blood pressure.
Acknowledgments
Sources of Funding: This research was supported by NIH (R01 HL73101-01A and R01 HL107910-01) to JRS and R-03 AG040638 and ASN-ASP Development Grant to AWC and the Veterans Affairs Merit System (0018) for JRS and CDA-2 for AWC.
The authors thank Brenda Hunter for her assistance in editing the manuscript.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement: The authors have no disclsoures.
References
- 1.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulakat L, Demarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell AT, Sowers JR. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011 Aug 3; doi: 10.1152/ajpregu.00316.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumagai E, Adachi H, Jacobs DR, Hirai Y, Enomoto M, Fukami A, Otsuka M, Kumagae SI, Nanjo Y, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Kasahara A, Tsukagawa E, Ohbu-Murayama K, Imaizumi T. Plasma Aldosterone Levels and Development of Insulin Resistance-Prospective Study in the General Population. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.111.180521. in press. [DOI] [PubMed] [Google Scholar]
- 4.Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, Shamlaye C, Burnier M. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48:239–245. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 5.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrin Metab. 2006;91:454–459. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 6.Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25(1):177–186. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 7.Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, Favret G, Melis A, Cavarape A, Sechi LA. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–3463. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 8.Examination Committee of Criteria for 'Obesity Disease' in Japan; Japan Society for the Study of Obesity. New criteria for 'obesity disease' in Japan. Circ J. 2002;66:987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 9.Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D'Agostino RB, Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. doi: 10.1161/CIRCULATIONAHA.107.708537. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Thornton JC, Burastero S, Shen J, Tanenbaum S, Heymsfield SB, Pierson RN., Jr Comparisons for body mass index and body fat percent among Puerto Ricans, blacks, whites and Asians living in the New York City area. Obes Res. 1996;4:377–384. doi: 10.1002/j.1550-8528.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]