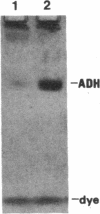
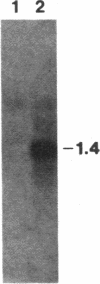
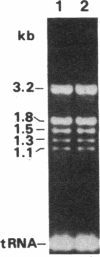
Abstract
Arabidopsis thaliana provides an excellent experimental plant system for molecular genetics because of its remarkably small genome size, near absence of dispersed middle repetitive DNA, and short life cycle. We have cloned and determined the nucleotide sequence of a single-copy gene from A. thaliana likely to be the gene encoding alcohol dehydrogenase (ADH; alcohol:NAD+ oxidoreductase, EC 1.1.1.1). The gene was isolated from a random recombinant library by cross-hybridization with a maize Adh1 gene probe. The DNA sequence contains an open reading frame capable of encoding a polypeptide the same length as maize ADH1 and ADH2 (379 amino acids) and having approximately equal to 80% homology with both maize enzymes. This open reading frame is interrupted by six introns whose positions are conserved with six of the nine intron positions present in both maize genes. The 5' and 3' untranslated regions are, respectively, 58 and 204 base pairs long. Sequences important for eukaryotic gene expression such as the TATA box, polyadenylylation signal, and intron splicesite sequences are found in the expected locations. The gene hybridizes to a specific anaerobically induced RNA in Arabidopsis whose appearance correlates with the anaerobic induction of Arabidopsis ADH protein.
Full text
PDF
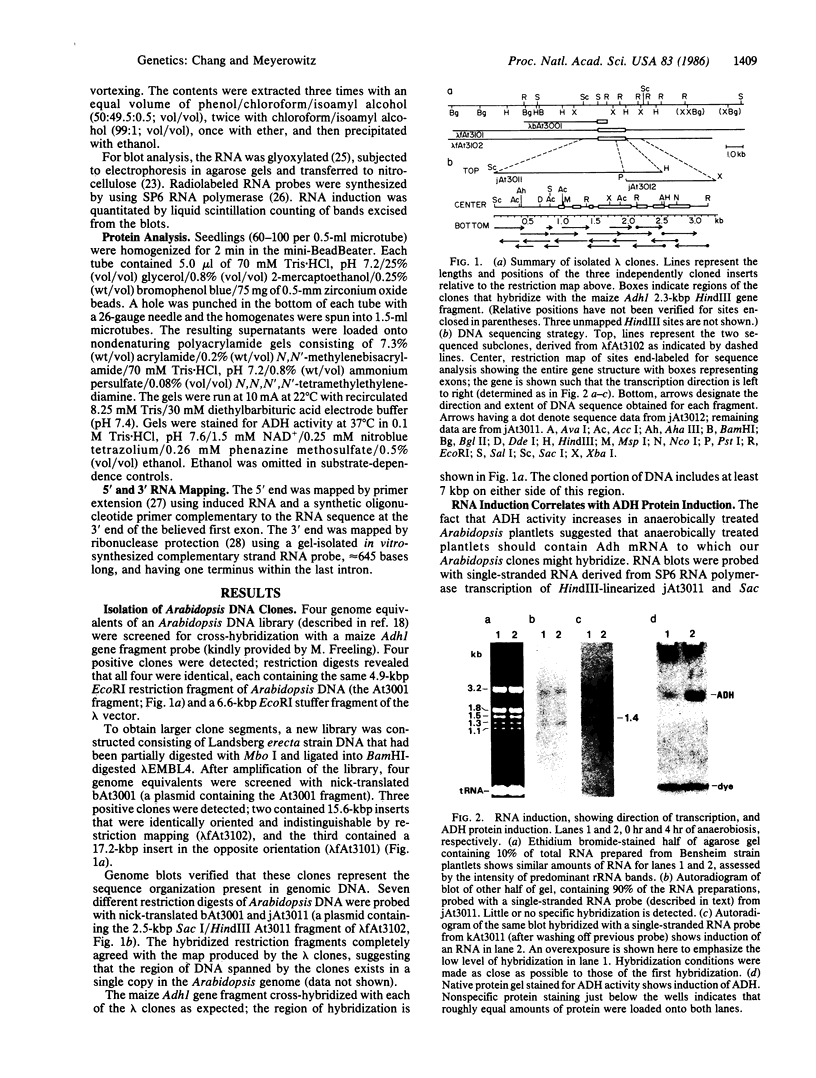



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APP A. A., MEISS A. N. Effect of aeration on rice alcohol dehydrogenase. Arch Biochem Biophys. 1958 Sep;77(1):181–190. doi: 10.1016/0003-9861(58)90054-7. [DOI] [PubMed] [Google Scholar]
- Banuett-Bourrillon F., Hague D. R. Genetic analysis of alcohol dehydrogenase isozymes in pearl millet (Pennisetum typhoides). Biochem Genet. 1979 Jun;17(5-6):537–552. doi: 10.1007/BF00498889. [DOI] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Sachs M. M., Gerlach W. L., Finnegan E. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985 Feb 11;13(3):727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Gielen J., Seurinck L., Van Montagu M., Schell J. Identification of sequences involved in the polyadenylation of higher plant nuclear transcripts using Agrobacterium T-DNA genes as models. EMBO J. 1983;2(3):419–426. doi: 10.1002/j.1460-2075.1983.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R., Jacobs M. Polymorphism of alcohol dehydrogenase in Arabidopsis thaliana (L.) Heynh.: genetical and biochemical characterization. Biochem Genet. 1984 Oct;22(9-10):817–838. doi: 10.1007/BF00499475. [DOI] [PubMed] [Google Scholar]
- Eklund H., Nordström B., Zeppezauer E., Söderlund G., Ohlsson I., Boiwe T., Söderberg B. O., Tapia O., Brändén C. I., Akeson A. Three-dimensional structure of horse liver alcohol dehydrogenase at 2-4 A resolution. J Mol Biol. 1976 Mar 25;102(1):27–59. doi: 10.1016/0022-2836(76)90072-3. [DOI] [PubMed] [Google Scholar]
- Ferl R. J., Brennan M. D., Schwartz D. In vitro translation of maize ADH: evidence for the anaerobic induction of mRNA. Biochem Genet. 1980 Aug;18(7-8):681–691. doi: 10.1007/BF00484585. [DOI] [PubMed] [Google Scholar]
- Freeling M., Schwartz D. Genetic relationships between the multiple alcohol dehydrogenases of maize. Biochem Genet. 1973 Jan;8(1):27–36. doi: 10.1007/BF00485554. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Piatak M., Lebowitz P., Weissman S. M. Determination of RNA sequences by primer directed synthesis and sequencing of their cDNA transcripts. Methods Enzymol. 1980;65(1):580–595. doi: 10.1016/s0076-6879(80)65061-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb L. D. Conservation and duplication of isozymes in plants. Science. 1982 Apr 23;216(4544):373–380. doi: 10.1126/science.216.4544.373. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford C. J., Gallwitz D. Evidence for an intron-contained sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983 Jun;33(2):519–527. doi: 10.1016/0092-8674(83)90433-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Guild G. M., Prestidge L. S., Hogness D. S. A new high-capacity cosmid vector and its use. Gene. 1980 Nov;11(3-4):271–282. doi: 10.1016/0378-1119(80)90067-0. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Martin C. H. Adjacent chromosomal regions can evolve at very different rates: evolution of the Drosophila 68C glue gene cluster. J Mol Evol. 1984;20(3-4):251–264. doi: 10.1007/BF02104731. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Pruitt R. E. Arabidopsis thaliana and Plant Molecular Genetics. Science. 1985 Sep 20;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]