Abnormalities in retinal mitochondria biogenesis continue to progress even after hyperglycemic insult is terminated, suggesting their major role in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy.
Abstract
Purpose.
Termination of hyperglycemia does not arrest the progression of diabetic retinopathy, and retinal mitochondrial DNA (mtDNA) remains damaged, resulting in a continuous cycle of mitochondrial dysfunction. This study is to investigate the role of mitochondria biogenesis (regulated by nuclear mitochondrial signaling) in the metabolic memory phenomenon.
Methods.
Mitochondria DNA copy number, functional integrity, and biogenesis (peroxisome proliferator-activated receptor-γ coactivator-1α [PGC1], nuclear respiratory factor 1 [NRF1], mitochondrial transcriptional factor [TFAM]) were analyzed in the retina from streptozotocin-diabetic rats maintained in poor or good control for 12 months (PC and GC respectively), or in PC for 6 months followed by 6 months of GC (Rev). The effect of direct inhibition of superoxide on prior insult was investigated by supplementing lipoic acid (LA) during their 6 months of GC (R+LA). Binding of TFAM with chaperones (heat shock proteins 70 and 60, Hsp70 and Hsp60 respectively) was quantified by coimmunoprecipitation. The key parameters and the number of mitochondria (by transmission electron microscopy and fluorescence microscopy) were confirmed in isolated retinal endothelial cells.
Results.
Six months of GC in the rats in Rev group did not provide any benefit to diabetes-induced decreased mtDNA copy number, increased gene transcripts of PGC1, NRF1, and TFAM, and decreased mitochondrial TFAM. The binding of TFAM with the chaperones remained subnormal. Supplementation of LA (R+LA), however, had a significant beneficial effect on the impaired mitochondria biogenesis, and also on the continued progression of diabetic retinopathy. Similar results of reversal of high glucose insult were observed in isolated retinal endothelial cells.
Conclusions.
Dysregulated mitochondria biogenesis contributes to the metabolic memory, and supplementation of GC with therapies targeted in modulating mitochondria homeostasis has potential in helping diabetic patients retard progression of retinopathy.
The landmark Diabetes Control and Complications Trial (DCCT) and the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study have shown that hyperglycemia has long-lasting detrimental consequences on diabetic complications. These trials have suggested a ‘metabolic memory’ of the prior glycemic exposure, and have demonstrated the importance of early glycemic control in the progression of diabetic retinopathy.1–3 Animal models have duplicated the metabolic memory phenomenon; retinopathy continues to progress for a considerable period even after hyperglycemia is corrected in chemically-induced diabetic dogs and rats.4,5 In addition to continued retinal capillary cell apoptosis and histopathology, we have shown that the mitochondria continue to be dysfunctional with its DNA (mtDNA) damaged after hyperglycemia is terminated in rats.6–10 However, the exact mechanism responsible for this metabolic memory phenomenon associated with the progression of diabetic retinopathy remains elusive.
Mitochondrial DNA is a small fraction of the total DNA, and it encodes only 13 proteins, all of which are essential for normal mitochondrial function,11,12 and transcription of mtDNA is controlled by a complex nucleus-mitochondria signaling cascade.13–15 Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1), a member of a family of transcription coactivators, is the master regulator of mitochondrial biogenesis. It integrates and coordinates the activity of multiple transcription factors, such as nuclear respiratory factors 1 and 2 (NRF1 and 2). These nuclear respiratory factors control the transcription of key mitochondrial proteins including nuclear-encoded respiratory complex proteins and mitochondrial transcriptional factor, TFAM.13,14 TFAM is essential for mtDNA transcription and helps maintain mitochondrial copy number.13,15 Abnormalities in mitochondria biogenesis are associated with mitochondrial function, and with mitochondrial number and size,16 and our recent studies have shown that in the pathogenesis of diabetic retinopathy, retinal mitochondria biogenesis is impaired and the number of mitochondria is decreased, possibly due to decreased transport of TFAM to the mitochondria.17 How mitochondria biogenesis contributes to the continued progression of diabetic retinopathy remains to be explored.
The aim of our study is to investigate the role of retinal mitochondria biogenesis in the resistance of retinopathy arrest after re-establishment of good glycemic control in rats. Because mtDNA is prone to increased oxidative damage,7 we have investigated the effect of supplementation during good control with lipoic acid, an antioxidant that prevents increase in retinal superoxide and the development of retinopathy,18 on mitochondria biogenesis. The effect of reversal of high glucose to normal glucose on the mitochondria biogenesis is also investigated in isolated retinal endothelial cells, the site of histopathology associated with diabetic retinopathy.
Methods
Animals
Diabetes was induced in Wistar rats (male, 200 g body weight, Harlan Laboratories, Indianapolis, IN) by streptozotocin (55 mg/kg). Diabetic rats were randomly divided either in poor glycemic (PC) or in good glycemic (GC) control for the entire 12 months, or were maintained in poor control for 6 months, followed by good control for 6 additional months (Rev). Lipoic acid (LA; 400 mg/kg) was administered to a group of diabetic rats soon after induction of diabetes as described previously,18 and in another group, rats were maintained in poor control for 6 months followed by good control, but this 6 months of good control was supplemented with lipoic acid (R+LA). Each group had 10 or more rats. The rats in poor control received 1 to 2 IU insulin four to five times a week to allow for slow weight gain and prevent ketosis, and the rats in good control received insulin twice daily (6–7 IU total) to maintain a steady gain in body weight, as routinely performed in our laboratory.6–9 Rats were housed in metabolic cages and their 24-hour urine samples were collected and tested for glycosuria (Keto-Diastix; Bayer Corporation, Elkhart, IN). Blood glucose was measured once every 7 to 10 days (Glucometer Elite, Bayer Corporation), and glycated hemoglobin (GHb, measured using kit 442-B, Sigma Chemicals, St. Louis, MO) every 2 months. Their body weights were measured two to three times every week. At the end of the desired duration of glycemic control, the animals were euthanized, and one eye was fixed in 10% formalin solution, and the retina was immediately isolated from the other eye. The treatment of the animals conformed to the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Research, and Institutional Guidelines.
Retinal Endothelial Cells
Retinal endothelial cells prepared from bovine retina (BRECs), from fourth through sixth passage were incubated in 20 mM glucose for 4 days followed by 5 mM glucose for four additional days (4d-4d). Parallel controls included cells incubated in continuous 5 mM glucose (5mM) or in 20 mM glucose (20mM) for the entire duration of the experiment. The cells received fresh media every 48 hours. In the 4d-4d group, at the end of the initial 4 days of 20mM glucose, the cells were rinsed with Dulbecco's Modified Eagle Medium (DMEM) before changing to 5mM glucose medium.8,10,19 The cells incubated in 20 mM mannitol served as osmotic control.
The effect of direct inhibition of superoxide radicals on the metabolic memory was determined by incubating the cells in 20 mM glucose for 4 days, followed by 5 mM glucose containing 250 μM MnTBAP20 (Mn(III)tetrakis(4-benzoic acid)porphyrin chloride; a cell-permeable mimetic of manganese superoxide dismutase, MnSOD, from Biomol, Plymouth Meeting, PA), for 4 days (R+MnT). Additional control included the cells exposed to continuous 20mM glucose for 8 days in the presence of MnTBAP (MnT). At the end of the incubation, the cells were trypsinized to prepare mitochondria, or harvested for whole homogenate, genomic DNA (gDNA), or RNA separation.
Sample Preparation
Retina or cells were sonicated in 30 mM Tris-HCl (pH 7.4) buffer containing 10 mM EGTA, 5 mM EDTA, 1% Triton X-100, 250 mM sucrose, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, and protease inhibitors, and centrifuged at 700g for 5 minutes to remove the cell debris. Protein concentration was measured using bicinchoninic acid solution (Sigma Aldrich, St. Louis, MO). Mitochondria were isolated using a kit from Pierce (Rockford, IL) as described previously.21
Mitochondria Copy Number
Mitochondria DNA copy number was quantified by real time quantitative RT-PCR. The genomic DNA (gDNA) was extracted by a blood and tissue kit according to the manufacturer's protocol (DNeasy; Invitrogen, Carlsbad, CA). Primers were designed to detect cytochrome b (Cytb: forward 5′-TGACCTTCCCGCCCCATCCA-3′ and reverse 5′-AGCCGTAGTTTACGTCTCGGCA-3′) and cytochrome oxidase subunit II (COII: forward 5′-TGAGCCATCCCTTCACTAGG-3′ and reverse 5′-TGAGCCGCAAATTTCAGAG-3′) for mtDNA, and β-actin (forward 5′-AGCGAGCCGGAGCCAATCAG-3′ and reverse 5′-TGCGCCGCCGGGTTTTATAGG-3′) for nuclear DNA (nDNA). PCR reaction was carried out using 15 ng of gDNA, 200 nM of each primer and SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). Amplification conditions were: 10 minutes at 95°C followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. To assess the specificity of the amplified PCR products, a sixfold gDNA concentration curve analysis was performed and the reaction end products were subjected to a 2% agarose gel electrophoresis. Relative values for mtDNA products Cytb and COII, and β-actin within each sample were used to obtain a ratio of mtDNA to nDNA.
Mitochondria number and their size were quantified using transmission electron microscopy (TEM). The cells were fixed in 2% glutaraldehyde in 100 mM cacodylate, washed with 7% sucrose and embedded in 2% agar. They were incubated in 1% osmium oxide (prepared in 100 mM cacodylate), and dehydrated with graded ethanol solutions and embedded in 812 resin (Electron Microscope Science, Hatfield, PA). Ultrathin sections were stained with uranyl acetate and lead citrate, and observed with a transmission electron microscope (model 400; Phillips, Eindhoven, The Netherlands). At least 8 to 10 images were recorded from each independent preparation, and analyzed using software available in the public domain (ImageJ, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Mitochondria number was confirmed using stain (MitoTracker Green; Molecular Probes, Carlsbad, CA). BRECs grown on cover slips were incubated in 20mM glucose medium with and without MnTBAP. At the end of the incubation, the cells were washed with PBS, and incubated with 200 mM stain (MitoTracker Green; Molecular Probes) for 30 minutes. The cells were rinsed with PBS and mounted on slides (Vecta Shield; Vector Laboratories, Burlingame, CA). The slides were examined under a fluorescence microscope (Zeiss ApoTome; Carl Zeiss Inc., Chicago, IL) using magnification ×40. The fluorescence was quantified using software (ImageJ, National Institutes of Health).15,17
Mitochondrial content was also assessed by quantifying the relative expression of a mitochondria-specific protein, cytochrome oxidase IV (Cox IV), adjusted to that of β-actin, as described by others.17,22
Mitochondria Function
For functional integrity of the mitochondria, the activity of citrate synthase was measured in retinal mitochondria (15 μg) using a reaction mixture containing 100 μM 5, 5-Dithiobis (2-nitrobenzoic acid) (DTNB) and 300 μM acetyl coenzyme A in 100 mM Tris-HCl buffer (pH 8.0). The reaction was initiated by the addition of 500 μM oxaloacetate, and DTNB reduction rate was measured for 3 minutes at 412 nm (extinction coefficient E = 13.6 mM/cm).17,23
Gene Expression
Total RNA was extracted from the retina or BRECs with reagent (Trizol; Invitrogen), and cDNA was synthesized with a kit (High Capacity cDNA Reverse Transcription Kit; Applied Biosystems).8,10,21 Gene specific assay (TaqMan; Applied Biosystems) was performed using 90 ng cDNA to assess mRNA abundance of PGC1, NRF1, and TFAM. Internal controls were β-actin for rats, and 18s rRNA for BRECs. The primers (TaqMan; Applied Biosystems) used were: rat retina, PGC1-NM_031347.1; NRF1- NM_001100708.1; TFAM, NM_031326.1; β-actin, NM_031144.2, and bovine retinal endothelial cells: NRF1, NM_001098002.1; TFAM, NM_001034016.2; and 18s rRNA, X03205.1.
Western Blot Analysis
Protein (30–80 μg) was separated on a 4%–16% SDS-PAGE, transferred to a nitrocellulose membrane and blocked with 5% nonfat milk for 1 hour. The membranes were incubated with antibody against the target protein (PGC1, Abcam, Cambridge, MA; NRF1 or TFAM, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. β-actin (Sigma Chemicals) and Cox IV (Molecular Probes) were used as loading controls for homogenate and mitochondria respectively.
Interactions between TFAM and Chaperone Proteins
Retinal homogenate (200 μg protein) was incubated overnight at 4°C with antibody against Hsp70 or TFAM (Santa Cruz Biotechnology), followed by 1 hour incubation with 20 μL protein A/G plus agarose beads reagent (prewashed and suspended in the lysis buffer).21 The beads were washed four times with the lysis buffer, and proteins were separated by Western blot technique using antibodies against TFAM or Hsp60 respectively.
Histopathology in Retinal Microvessels
To evaluate histopathology, after rinsing the retina from the formalin-fixed eyes overnight with running water, the microvasculature was isolated using 3% crude trypsin (Invitrogen-Gibco, Grand Island, NY) containing 200 mM sodium fluoride.5,21,24 The neuroretinal tissue was gently brushed away, the microvessels were stained with periodic acid-Schiff-hematoxylin, and examined by light microscopy.
Statistical Analysis
Data are expressed as mean ± SD. Statistical analysis was carried out using commercially-available software (SPSS 12.0 for Windows; IBM, Armonk, NY). Shapiro-Wilk tests were used to test for normal distribution of the data. For variables with normal distribution ANOVA followed by Bonferroni test were applied, while Kruskal-Wallis test followed by Mann-Whitney U test were performed for data which did not present normal distribution, and P < 0.05 was considered statistically significant.
Results
Animal Model
Severity of Hyperglycemia.
Rats maintained in PC and GC groups had their GHb values >11% and approximately 6% respectively, throughout the experiment (12 months duration). In reversal groups, (Rev and R+LA), glycemic control before initiation of good control was comparable to that in PC group (GHb >11%), but the values became significantly different (GHb approximately 6%) at 2 months after initiation of good control (the first measurement made after initiation of good control), and remained unchanged for the entire 6 months of good control. Supplementation of lipoic acid, either throughout the poor control period, or during reversal period, did not influence the severity of hyperglycemia (Table 1).
Table 1.
Severity of Hyperglycemia in Rats Maintained in Various Glycemic Controls
| Body Weight (g) | Glycemic Hemoglobin (%) | |
|---|---|---|
| Normal | 437 ± 51 | 6.3 ± 1.0 |
| PC (12 mo of poor control) | 337 ± 5* | 11.8 ± 1.6* |
| Rev (6 mo PC → 6 mo GC) | 310 ± 47* → 430 ± 88† | 12.2 ± 1.0* → 6.0 ± 1.0† |
| GC (12 mo good control) | 396 ± 13.8† | 5.9 ± 0.47† |
| Rev+LA (6 mo PC → 6 mo GC + LA) | 270 ± 27* → 388 ± 31† | 11.1 ± 0.3* → 5.9 ± 0.5† |
| LA (12 mo poor control + LA) | 308 ± 38* | 12.3 ± 1.1* |
The values are presented as mean ± SD of five or more rats in each group.
P < 0.05 compared with normal.
P < 0.05 compared with PC.
Effect of the Diabetes on Mitochondria Biogenesis.
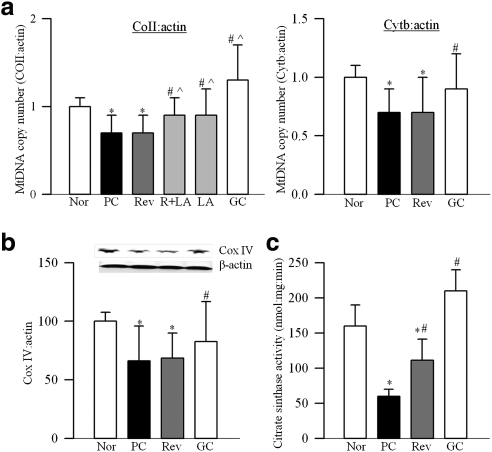
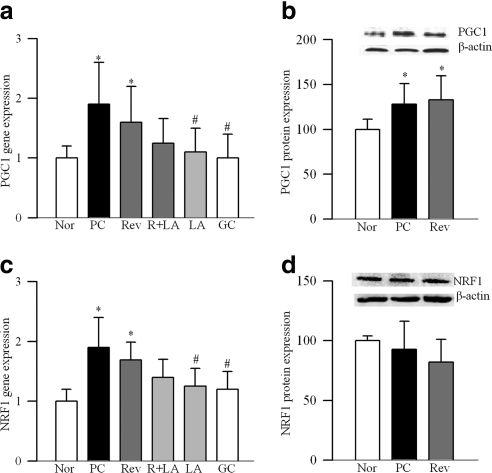
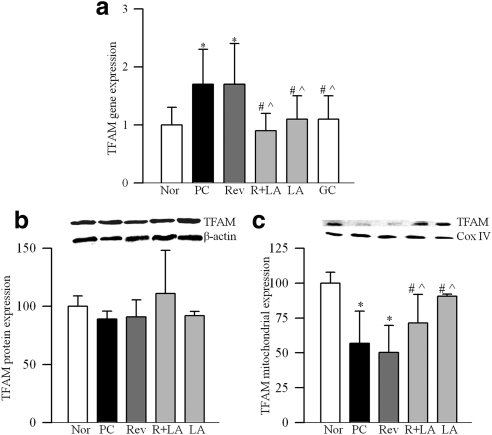
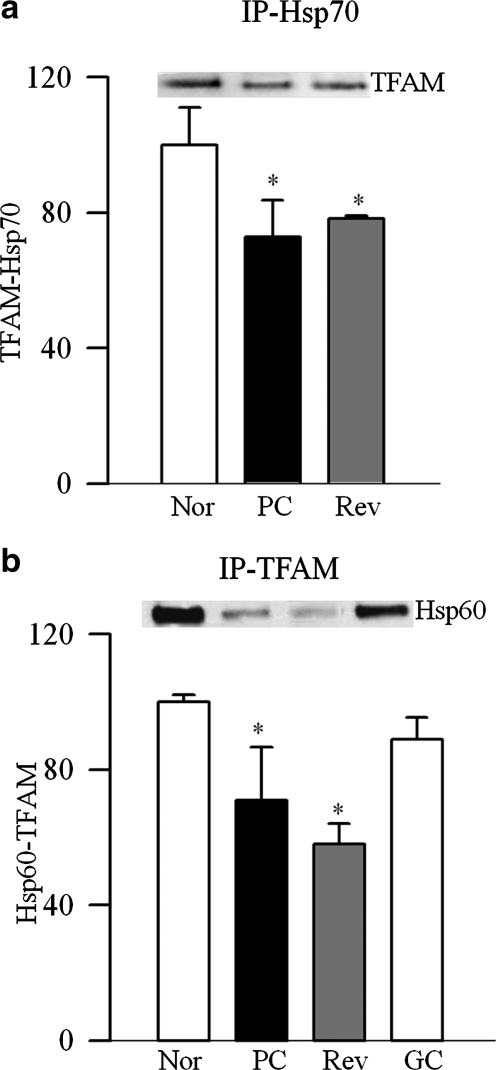
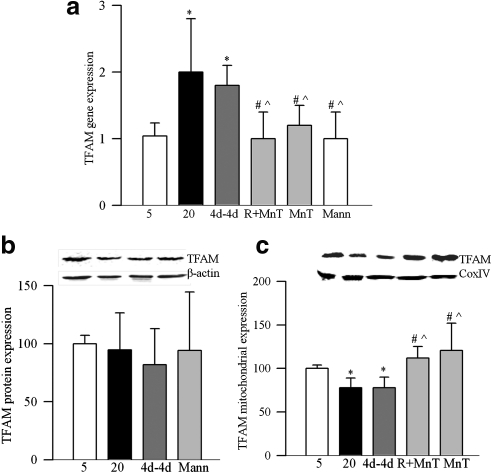
Retinal mitochondria copy number, quantified by the ratio of gene transcripts of COII and β-actin, or Cytb and β-actin, was decreased by approximately 30% in PC rats (Fig. 1a). Expression of Cox IV, relative to β-actin (Fig. 1b), and the activity of citrate synthase (Fig. 1c) were decreased by approximately 40% compared with the values from normal rats. Gene expression of PGC1 was increased by approximately twofold in PC rats (Fig. 2a), and this was accompanied by a 35% increase in its protein expressions (Fig. 2b). Similarly, gene transcript of NRF1 was elevated by almost twofold (Fig. 2c), but, its protein expression remained unchanged (Fig. 2d). In the same rats, gene transcript of TFAM was increased by 70% (Fig. 3a), and as with NRF1, its total protein expression remained unchanged (Fig. 3b). Because TFAM translocates to the mitochondria to initiate transcription and replication, its mitochondrial accumulation was quantified. The accumulation of TFAM in the mitochondria was significantly decreased compared with the values from normal rats (Fig. 3c). The translocation of TFAM to the mitochondria requires binding with chaperone proteins,14,25 and as shown in Figure 4a, the binding of TFAM with Hsp70 was decreased by 25%–30% in the PC group compared with the normal rats. Similar decrease was observed in the binding of TFAM with Hsp60 (Fig. 4b).
Figure 1.
Effect of reversal of hyperglycemia on mtDNA copy number and functional integrity. (a) Mitochondrial DNA copy number was assessed by real time RT-PCR using mitochondrial (COII and Cytb) or nuclear specific (β-actin) rat primers. Ratios of COII and β-actin, and Cytb and β-actin were calculated within each sample. (b) Mitochondria concentration was estimated by quantifying the protein expressions of Cox IV (mitochondrial protein) and β-actin (total protein) in the retinal homogenate by Western blot technique. (c) Citrate synthase activity was quantified in the mitochondrial fraction by following the reduction of DTNB. Values are represented as mean ± SD obtained from 6 to 10 rats in each of the six experimental groups. *P < 0.05 compared with normal; #P < 0.05 compared with PC; and P̂ < 0.05 compared with Rev. Nor, normal.
Figure 2.
Effect of reversal of hyperglycemia on mitochondrial biogenesis. Gene expression of (a) PGC1 and (c) NRF1 were quantified in rat retina by real-time RT-PCR using custom primers (TaqMan; Applied Biosystems). The values were normalized to β-actin in each sample. Protein expression of (b) PGC1 and (d) NRF1 were quantified in the retina by Western blot analysis. The accompanying histograms show the expression of PGC1 and of NRF1, adjusted to the expression of β-actin. Values are represented as mean ± SD obtained from five to seven animals in each group.
Figure 3.
Effect of cessation of hyperglycemia on the expression of mitochondrial transcription factors, TFAM (a) RNA isolated from rat retina was assessed by real-time RT-PCR for gene expression of TFAM using custom primers (TaqMan; Applied Biosystems), and β-actin was used as the housekeeping gene. Protein expression of TFAM was determined in the (b) homogenate and (c) in the mitochondria fraction by Western blot technique using β-actin as a loading control for homogenate, and Cox IV for mitochondrial fraction. The accompanying histograms represent protein expression of TFAM adjusted to that of β-actin (homogenate) or to Cox IV (mitochondria) in each sample. *P < 0.05, #P < 0.05, and P̂ < 0.05 compared with normal, PC, and Rev groups, respectively.
Figure 4.
Effect of reversal of hyperglycemia on the binding of TFAM with the chaperone proteins. Binding of TFAM with (a) Hsp70 was performed by immunoprecipitating Hsp70, followed by Western blot analysis for TFAM, and (b) with Hsp60 by immunoprecipitating TFAM and quantifying Hsp60. The membranes were stripped to quantify the expression of IgG (gel-loading control) in each sample. The values are represented as mean ± SD from four to seven animals in each group. The histograms represent TFAM bound to Hsp70 or Hsp60, and adjusted to IgG expression. *P < 0.05 compared with normal, and #P < 0.05 compared with PC.
Effect of the Reversal of Hyperglycemia on Mitochondria Biogenesis.
Reinstitution of good control after 6 months of poor control failed to reverse diabetes-induced reduction in retinal mitochondrial copy number as evidenced by decreased ratios of COII and β-actin, or Cytb and β-actin in the Rev group (Fig. 1a). Similarly, mitochondria content and citrate synthase activity were subnormal, suggesting the compromised functional integrity of the mitochondria (Figs. 1b, 1c). Six months of good control had no effect on the gene transcripts of PGC1 and NRF1, and the values in the Rev group were not different from those obtained from the PC group (Fig. 2). Reversal of hyperglycemia also had no beneficial effect on the gene transcript of TFAM, and its mitochondrial accumulation remained compromised (Fig. 3). The binding of TFAM with Hsp70 and Hsp60 was subnormal and the values in the Rev and PC groups were similar, and were significantly different from those from normal rats (Fig. 4).
However, when the rats were maintained in good control for the entire 12 months (GC group), mitochondrial copy number, content, and citrate synthase activity remained normal (Figs. 1a–c), and the gene transcripts of PGC1, NRF1, and TFAM were significantly different from those obtained from rats in the PC or Rev groups (Figs. 2, 3). The binding of TFAM with Hsp60 was also higher compared with the values from the rats in the PC or Rev groups (Fig. 4b).
Effect of Lipoic Acid on Mitochondrial Biogenesis.
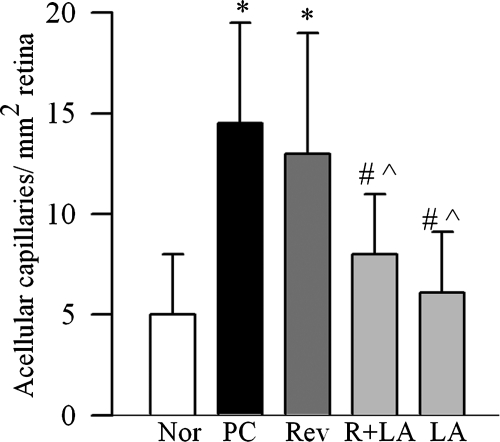
To investigate if direct inhibition of oxidative stress during good control that had followed poor control can help reverse abnormalities in mitochondria biogenesis, lipoic acid was administered during the good glycemic control phase. Supplementation of good control with lipoic acid had beneficial effects on mitochondria copy number (Fig. 1a), and on the gene transcripts of PGC1 (Fig. 2a) and NRF1 (Fig. 2c). The values in R+LA group were not different from those from normal rats. Similarly, lipoic acid supplementation during good control helped reduce alterations in TFAM, its gene expression was significantly decreased, and its mitochondrial accumulation elevated compared with the values from the Rev group (Figs. 3a–c). In the same animals, lipoic acid had beneficial effect on the continued progression of diabetic retinopathy; the number of acellular capillaries in the retinal microvasculature in the R+LA group was significantly decreased compared with the PC group and Rev group (Fig. 5).
Figure 5.
Effect of supplementation of good glycemic period with lipoic acid on diabetic retinopathy. Trypsin-digested retinal microvasculature was stained with periodic acid-Schiff-hematoxylin and examined by light microscopy for the basement membrane tubes lacking cell nuclei and maintaining at least one-fourth the normal capillary caliber over their length. Values are represented as mean ± SD obtained from five to seven animals in each group. *P < 0.05, #P < 0.05, and P̂ < 0.05 compared with normal, PC, and Rev groups, respectively.
Administration of lipoic acid soon after induction of diabetes (LA group), as expected, prevented decrease in retinal mitochondrial copy number (Fig. 1a), and increase in gene transcripts of PGC1, NRF1, and TFAM (Figs. 2, 3). In addition, the number of acellular retinal capillaries was significantly decreased (Fig. 5), and the values in the LA group were different from those obtained from the PC group, but were not significantly different from the R+LA group.
Retinal Endothelial Cells
Effect of High Glucose on Mitochondrial Number and Biogenesis.
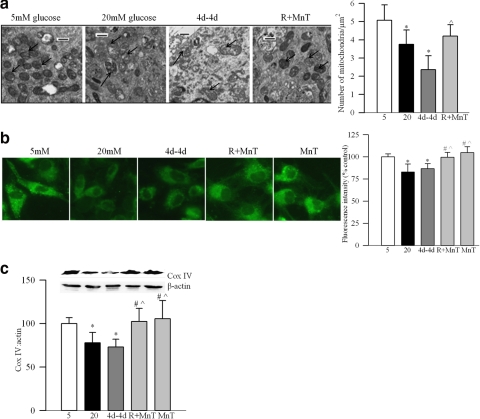
As shown in the electron micrograph (Fig. 6a), BRECs exposed to high glucose had approximately 20% less mitochondria, and these mitochondria were swollen with a 40% increase in their area compared with the cells exposed to normal glucose. The fluorescence intensity (MitoTracker Green; Molecular Probes) was approximately 20% less (Fig. 6b), and Cox IV was also significantly decreased (Fig. 6c), compared with the cells incubated in normal glucose.
Figure 6.
Reversal of high glucose on mitochondria mass. (a) Mitochondria number was quantified by electron microscopy. TEM was performed on ultrathin sections of the cells embedded in 812 resin. Mitochondria number was counted manually in a blind manner by calculating the number of mitochondria over total area of the cell in at least 8 to 10 images per sample. Each group had two to three different samples. Arrows indicate the mitochondria. Bars, 500 nm. (b) Mitochondria number was also quantified by using stain (MitoTracker Green; Molecular Probes). The cells were stained and examined under a fluorescence microscope (Zeiss ApoTome; Carl Zeiss Inc.) using magnification × 40, and the fluorescence was quantified using software (ImageJ, National Institutes of Health). Each measurement was made in triplicate using three to five different cell preparations. (c) Western blot analysis was performed to quantify the expression of Cox IV and β-actin. *P < 0.05 compared with 5mM glucose, #P < 0.05 compared with 20mM glucose and P̂ < 0.05 compared with 4d–4d. 5, 5 mM glucose; 20, 20 mM glucose.
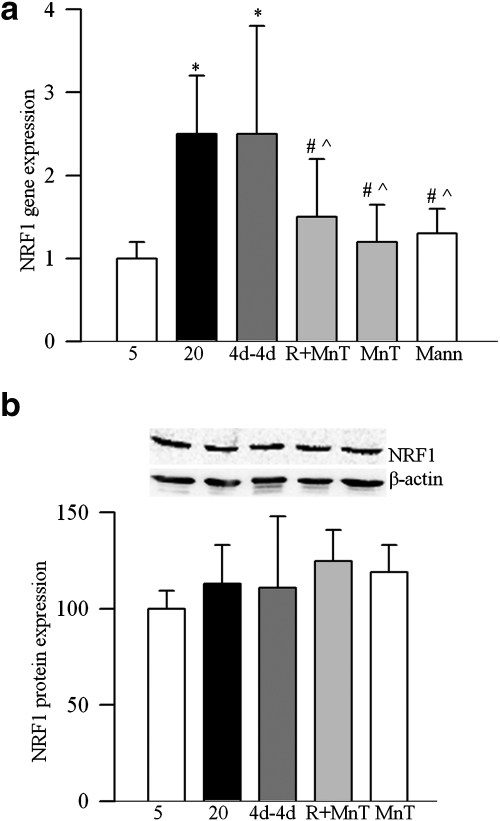
As with the whole retina, exposure of BRECs to high glucose increased the gene expression of NRF1 by over twofold compared with the cells incubated in normal glucose, but its protein expression remained unchanged (Figs. 7a, 7b). The gene expression of TFAM was also elevated by twofold with no change in its total protein expression, but its mitochondrial accumulation was compromised (Fig. 8a–c). These glucose-induced alterations were not influenced by the duration of high glucose exposure, and the values obtained after 4 or 8 days of 20 mM glucose exposure were comparable. Incubation of cells in 20 mM mannitol (instead of 20 mM glucose) did not have any effect on mitochondrial biogenesis, suggesting that the effects observed under high glucose conditions were not induced by increased osmolarity.
Figure 7.
Effect of reversal of high glucose on NRF1 in endothelial cells. (a) Gene transcript of NRF1 was quantified in the RNA isolated from BRECs by real-time RT-PCR using custom primers (TaqMan; Applied Biosystems) and 18s rRNA was used as a housekeeping gene. Fold change relative to 5mM glucose treatment was calculated using the ddCt method. (b) Protein expression of NRF1 was determined by Western blot technique using 4%–20% gradient polyacrylamide gel and β-actin as loading control. The histogram represents NRF1 protein expression adjusted to that of β-actin in each sample. Values obtained from cells incubated in continuous 5mM glucose are considered as 100%, and are represented as mean ± SD obtained from three or more different cell preparations. *P < 0.05, #P < 0.05, and P̂ < 0.05 compared with 5mM glucose, 20mM glucose, and 4d–4d respectively. Mann, 20 mM mannitol.
Figure 8.
Reversal of high glucose insult on TFAM in retinal endothelial cells. (a) Gene transcript of TFAM was quantified by real time PCR using 18s rRNA as internal control. TFAM expression was determined in the (b) cell homogenate and (c) in mitochondria by Western blot technique using β-actin (homogenate) and Cox IV (mitochondria) as loading controls. Values are represented as mean ± SD obtained from at least three cell preparations; each preparation analyzed in duplicate. *P < 0.05 compared with 5mM glucose, #P < 0.05 compared with 20mM glucose, and P̂ < 0.05 compared with 4d–4d.
Effect of Reversal of High Glucose Insult on Mitochondria Biogenesis.
Reversal of 4 days of high glucose by 4 days of normal glucose did not produce beneficial effects; the number and mass of mitochondria remained compromised as demonstrated by TEM micrographs and fluorescence intensity (MitoTracker Green; Molecular Probes) (Figs. 6a, 6b), and also by decreased expression of Cox IV (Fig. 6c). The gene expressions of NRF1 and TFAM continued to be elevated (Figs. 7a, 8a), and mitochondrial accumulation of TFAM remained subnormal (Fig. 8c). The values obtained from the 4d-4d group were not different from cells in continuous 20 mM glucose, but were significantly different from cells exposed to 5 mM glucose.
Effect of Direct Inhibition of Superoxide Accumulation on Mitochondria Biogenesis.
Inclusion of MnTBAP during the normal glucose period that had followed 4 days of high glucose exposure (R+MnT) resulted in higher mitochondria number and content compared with the 4d-4d group suggesting that MnTBAP helps reverse glucose-induced decrease in mitochondria mass induced by the prior high glucose insults (Figs. 6a–c). In addition, MnTBAP significantly decreased the gene transcripts of NRF1 and TFAM, and increased the accumulation of TFAM in the mitochondria compared with the values obtained from continuous 20mM glucose or 4d-4d group (Figs. 7a, 8a). These values were not different from those obtained from the cells incubated in high glucose supplemented with MnTBAP for the entire 8 days of the experiment (MnT group). Thus, we show ‘in principle’ that the direct inhibition of superoxide radicals during the reversal phase have better effect than just the reversal of high glucose insult.
Discussion
Retinopathy progresses in diabetic patients for a considerable period even after the hyperglycemic insult is terminated, and the same phenomenon is observed in animal models of diabetic retinopathy.1–5 The retina continues to experience increased oxidative and nitrative stress, and mitochondria remain compromised with mtDNA damaged and the transcripts of the mtDNA encoded proteins attenuated for at least 6 months after reinstitution of good glycemic control in rats that has followed 6 months of poor glycemic control. Apoptosis of retinal capillary cells and histopathology characteristic of diabetic retinopathy continue to progress.5–10 Here, our exciting data clearly demonstrate that the dysregulated retinal mitochondria biogenesis, which is implicated in diabetic complications, including diabetic retinopathy,16,17,26 also resists reversal for at least 6 months after reinstitution of good glycemic control. Similarly, in isolated retinal endothelial cells, reversal of high glucose insult with normal glucose fails to prevent dysregulation of mitochondria biogenesis. However, supplementation of lipoic acid during the 6 months of good control period in rats, which has followed 6 months of poor control, provides significant benefit to the impaired mitochondria biogenesis, and also on the continued progression of diabetic retinopathy. Similarly, in retinal endothelial cells inclusion of a superoxide dismutase mimetic during the normal glucose exposure, which has followed high glucose exposure, helps prevent dysregulation in mitochondria biogenesis. This is the first animal study demonstrating that the supplementation of a therapy during good glycemic control can reduce the detrimental effects of the prior poor glycemic control. The results clearly implicate the role of mitochondria biogenesis in the metabolic memory associated with continued progression of diabetic retinopathy, and demonstrate that the combination of good glycemic control and a therapy targeting mitochondrial damage has better effect on the outcome of good control which has followed a period of poor control, than the good control alone.
Mitochondrial damage plays a central role in the development of diabetic retinopathy.7,19–21,24,27 Retinal mtDNA is damaged and the electron transport system is dysfunctional in diabetes; this damaged DNA remains accumulated even after hyperglycemic insult is terminated, and MnSOD continues to be epigenetically modified.7,10 Consistent with this, here we show that the reversal of hyperglycemia fails to provide any benefit to the copy number of the mitochondria, and citrate synthase continues to be inactivated suggesting that the effects of prior poor control on mitochondrial copy number persist even after the hyperglycemic insult has been terminated.
Mitochondria DNA encodes only 13 proteins and > 95% of mitochondrial proteins are encoded in the nucleus, synthesized in the cytosol, and imported into the mitochondria.13,25 Nuclear-mitochondrial interactions regulate mitochondria biogenesis via PGC1-NRF1/2-TFAM.13–15,28 In the pathogenesis of diabetic retinopathy, retinal mitochondria biogenesis is impaired and the number of mitochondria is decreased, and this biogenesis machinery is under the control of superoxide;17 here we show that this nuclear-mitochondrial machinery also carries the memory of the past glycemic control, and it continues to be compromised, suggesting that the dysregulation of mitochondria biogenesis could have a significant role in the persistent dysfunctional mitochondria and accumulation of damaged mtDNA despite termination of the hyperglycemic insult.
Mitochondrial levels of TFAM directly affect mtDNA copy number.13,20,29 Our results show that TFAM transcript remains elevated and its mitochondrial accumulation subnormal after termination of hyperglycemic insult. For proper action, this nuclear encoded TFAM has to reach to the mitochondria, and Hsp70 is considered to aid in transmembrane transport of proteins.30,31 Once the proteins are imported into the mitochondria, Hsp60 helps fold key proteins.32 Subnormal interactions of TFAM with Hsp70 and with Hsp60 even after 6 months of good control suggest that the failure of decreased mitochondria biogenesis to recover could be, in part, due to continued subnormal transport and accumulation of TFAM in the mitochondria.
Reinstitution of good glycemic control soon after the induction of diabetes in rats, however, prevented alterations in mitochondria biogenesis in the retina, mitochondria copy number, gene expressions of PGC1, NRF1, and TFAM, and mitochondria TFAM levels remain unchanged. This strengthens the importance of early and sustained good glycemic control for diabetic patients.
Lipoic acid can directly scavenge superoxide and improve mitochondrial function.18,33 Long-term administration of lipoic acid to diabetic rats prevents retinal metabolic abnormalities including decrease in MnSOD enzyme activity, accumulation of oxidatively modified DNA, and the development of retinopathy.18 Furthermore our recent work has shown that retinal mitochondria biogenesis in diabetes is under the control of superoxide radicals.17 Now we show that lipoic acid supplementation during the good control, which has followed poor control, has beneficial effect on the dysregulation of retinal mitochondria biogenesis. This is accompanied by a decrease in the number of degenerative capillaries, a hallmark of diabetic retinopathy, further supporting the role of mitochondria biogenesis in the metabolic memory phenomenon. Because the presence of a basal level of reactive oxygen species are necessary to support normal cellular functions, the possibility that supplementation with lipoic acid during the good control phase could have some unwarranted detrimental effects on cellular metabolism, however, cannot be ruled out.
Retinal endothelial cells, the site of histopathology of diabetic retinopathy, undergo accelerated apoptosis before histopathology can be observed in diabetes.6,34,35 Our studies, and those of others, have shown that retinal capillary cells can mimic the metabolic memory phenomenon.8,10,19,36 Recent work from our laboratory has shown that epigenetic modifications of MnSOD gene in retinal endothelial cells fail to reverse after termination of high glucose exposure.10,19 Here we show that mitochondrial copy number and biogenesis remains compromised even after termination of high glucose insult, and these results support the role of mitochondria biogenesis in the metabolic memory associated with continued progression of diabetic retinopathy.
Mitochondrial superoxide dismutase mimic, MnTBAP, prevents glucose-induced abnormalities in mitochondrial dysfunction and accelerated apoptosis of retinal capillary cells.19,20 Our results with supplementation with MnTBAP during the normal glucose exposure (reversal period), and the in vivo results using lipoic acid in diabetic rodents, strongly imply that supplementation with agents that can target mitochondrial damage during good glycemic control should provide greater benefits of glycemic control in preventing continued progression of diabetic retinopathy than the good glycemic control itself. This is of immense clinical significance because for diabetic patients, along with the maintenance of good glycemic control, supplementation with therapies targeting mitochondrial damage could have potential to retard further progression of retinopathy.
In conclusion, our novel results demonstrate that dysregulated mitochondrial biogenesis is one of the major contributors in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Direct inhibition of mitochondrial damage during the good glycemic control helps ameliorate dysregulation of biogenesis. Thus, supplementation of good glycemic control with therapies targeted toward modulation of mitochondria homeostasis should help diabetic patients arrest further progression of retinopathy.
Acknowledgments
The authors thank Andrew F.X. Goldberg and Loan Dang from Eye Research Institute, Oakland University, Rochester, Michigan, for help with TEM, and Doug Putt and Yakov Shamailov for their technical assistance.
Footnotes
Supported in part by National Institutes of Health Grants EY014370 and EY017313, Juvenile Diabetes Research Foundation Grant EY017313, The Thomas Foundation, Research to Prevent Blindness, and the Michigan Eye Banks and Transplantation Center.
Disclosure: J.M. dos Santos, None; R.A. Kowluru, None
References
- 1. Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White NH, Sun W, Cleary PA, et al. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes. 2010;59:1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–812 [DOI] [PubMed] [Google Scholar]
- 5. Kowluru RA, Kanwar M, Kennedy A. Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries. Exp Diabetes Res. 2007;21976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kowluru RA. Effect of re-institution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52:818–823 [DOI] [PubMed] [Google Scholar]
- 7. Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010;13:797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowluru RA, Chan PS. Metabolic memory in diabetes - from in vitro oddity to in vivo problem: role of apoptosis. Brain Res Bull. 2010;87:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kowluru RA, Zhong Q, Kanwar M. Metabolic memory and diabetic retinopathy: role of inflammatory mediators in retinal pericytes. Exp Eye Res. 2010;90:617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stumpf JD, Copeland WC. Mitochondrial DNA replication and disease: insights from DNA polymerase mutations. Cell Mol Life Sci. 2010;68:219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JQ, Cammarata PR, Baines CP, Yager JD. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta. 2010;1793:1540–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–683 [DOI] [PubMed] [Google Scholar]
- 14. Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638 [DOI] [PubMed] [Google Scholar]
- 15. Uguccioni G, Hood D. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab. 2011;300:E361–E371 [DOI] [PubMed] [Google Scholar]
- 16. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010;88:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santos JM, Tewari S, Goldnerg AFX, Kowluru RA. Mitochondria biogenesis in the development of diabetic retinopathy. Free Radic Biol Med. 2011;51:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238 [DOI] [PubMed] [Google Scholar]
- 19. Madsen-Bouterse S, Zhong Q, Mohammad G, Ho YS, Kowluru RA. Oxidative damage of mitochondrial DNA in diabetes, and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334 [DOI] [PubMed] [Google Scholar]
- 21. Mohammad G, Kowluru RA. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3832–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutherland L, Capozzi L, Turchinsky J, Bell R, Wright D. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am J Physiol Endocrinol Metab. 2008;295:E1076–E1083 [DOI] [PubMed] [Google Scholar]
- 23. Williams AJ, Coakley J, Christodoulou J. Automated analysis of mitochondrial enzymes in cultured skin fibroblasts. Anal Biochem. 1998;259:176–180 [DOI] [PubMed] [Google Scholar]
- 24. Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007;48:3805–3811 [DOI] [PubMed] [Google Scholar]
- 25. Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci. 2008;1147:321–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards JL, Quattrini A, Lentz SI, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia. 2010;160:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 28. Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333 [DOI] [PubMed] [Google Scholar]
- 29. Ekstrand MI, Falkenberg M, Rantanen A, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944 [DOI] [PubMed] [Google Scholar]
- 30. Mihara K, Omura T. Cytoplasmic chaperones in precursor targeting to mitochondria: the role of MSF and hsp 70. Trends Cell Biol. 1996;6:104–108 [DOI] [PubMed] [Google Scholar]
- 31. Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50 [DOI] [PubMed] [Google Scholar]
- 32. Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. J Cell Mol Med. 2005;9:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–895 [DOI] [PubMed] [Google Scholar]
- 34. Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kern TS, Tang J, Mizutani M, Kowluru RA, Nagraj R, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: Comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978 [PubMed] [Google Scholar]
- 36. Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990;87:404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]