Abstract
Considerable evidence implicates the basolateral amygdala (BLA) in the formation of outcome representations that link cues to the incentive properties of reinforcers. Animals with BLA damage show impaired performance in reinforcer devaluation tasks, in which the value of the food reinforcer is reduced by satiation or food–toxin pairings after the completion of cue or response training. Although intact animals spontaneously reduce their conditioned responding after such reinforcer devaluation procedures, animals with BLA lesions made before training typically do not, as evidenced across a range of species, training contingencies, and devaluation procedures. In contrast, the role of the BLA in devaluation task performance once such outcome representations are established is unclear. Whereas Pickens et al. (2003) found normal devaluation performance in rats when BLA lesions were made after pavlovian light–food pairings but before devaluation by food–toxin pairings, Ostlund and Balleine (2008) found impaired devaluation performance when BLA lesions were made after instrumental training with multiple instrumental responses and food reinforcers but before devaluation of one reinforcer by selective satiation. Those studies differed in their use of pavlovian or operant training contingencies, single or multiple reinforcers, and associative or motivational devaluation procedures. Here we found that, when multiple reinforcers were used, posttraining BLA lesions disrupted the expression of devaluation performance in rats, using either pavlovian or instrumental training procedures and either conditioned taste aversion or satiation devaluation procedures. Thus, BLA apparently plays a critical role in maintaining or using sensory associations of reinforcer value when multiple outcomes must be coded but not under single-outcome conditions.
Keywords: reward, learning, memory, inactivation, motivation, incentive, limbic
Introduction
Outcome-mediated behavioral control enables animals to adapt to changing environmental conditions. The amygdala, particularly its basolateral nuclei (BLA), has long been implicated in motivational learning (Klüver and Bucy, 1937; LeDoux et al., 1986). Recently, many studies using the reinforcer devaluation paradigm identify a critical role for the BLA in forming or using outcome representations that link cues with the incentive properties of outcomes (Hatfield et al., 1996; Málková et al., 1997; Balleine et al., 2003; Pickens et al., 2003; Wellman et al., 2005; Ostlund and Balleine, 2008). In that paradigm, animals are trained to associate either a neutral stimulus (Hatfield et al., 1996) or a response (Balleine et al., 2003) with a particular reinforcer. After training, the reinforcer is devalued by either motivational (e.g., prefeeding the reinforcer) or associative (e.g., pairing the reinforcer with illness) manipulations. Finally, cue or response performance is assessed, usually in the absence of the reinforcer. Normal animals show spontaneous reductions in performance, whereas animals with pretraining BLA lesions typically do not.
Pickens et al. (2003) reported that BLA is required only for the acquisition of such outcome representations but not for maintaining them, modifying them, or using them to guide subsequent behavior. Although rats that received BLA lesions before all training failed to show devaluation effects, rats that were lesioned after pavlovian cue–food pairings, but before devaluation of the food by food–illness pairings, showed normal devaluation effects. In contrast, recent studies by Wellman et al. (2005), using monkeys, and Ostlund and Balleine (2008), using rats, suggest a broader role for BLA in devaluation tasks. In those experiments, either BLA lesions (Ostlund and Balleine, 2008) or disruption of BLA function by muscimol infusions (Wellman et al., 2005) made after initial training but before the devaluation manipulation impaired devaluation performance. Thus, unlike the study by Pickens et al. (2003), intact BLA function was required for integrating changes in reinforcer value with previously acquired reinforcer representations to guide performance.
Notably, the studies by Ostlund and Balleine (2008) and Wellman et al. (2005) differed from that of Pickens et al. (2003) in the use of instrumental rather than pavlovian training contingencies, multiple rather than single reinforcers, and selective satiation rather than taste aversion devaluation procedures. Any of those variables may have contributed to the different outcomes reported. The current series of experiments systematically evaluated the role of training contingency and devaluation procedure in determining the effects of posttraining BLA lesions on reinforcer devaluation performance in rats trained with multiple reinforcers. We first examined the effects of devaluation by selective satiation (experiment 1) or food–illness pairings (experiment 2) after multiple-outcome instrumental training. As in the studies by Ostlund and Balleine (2008) and Wellman et al. (2005), posttraining disruption of BLA function impaired devaluation test performance. In experiments 3 and 4, we made contact with our previous studies (Pickens et al., 2003) by examining the effects of BLA lesions made after multiple-outcome pavlovian training but before reinforcer devaluation by either food–illness pairings or selective satiation.
Materials and Methods
Subjects
All behavioral testing was conducted with male Long–Evans rats (Charles River Laboratories), which weighed 300–325 g on arrival to the Psychological and Brain Sciences Department, Johns Hopkins University. Each rat was housed individually in the animal vivarium, which was climate controlled and illuminated from 6.00 A.M. to 8.00 P.M. Food deprivation began 5 d before the start of behavioral training and continued throughout training and testing, with rats returning to ad libitum food and water access for 12–15 d while recovering from surgery.
Surgical procedures
All surgical procedures were performed under aseptic conditions using isoflurane anesthesia (Henry Schein). Surgeries were conducted after either instrumental (experiments 1 and 2) or pavlovian (experiments 3 and 4) training but before either sensory-specific satiation (experiments 1 and 4) or LiCl taste aversion conditioning (experiments 2 and 3). Neurotoxic BLA lesions were made at two injection sites in each hemisphere using NMDA (Sigma) at a concentration of 17.5 mg/ml in PBS. Injections were made 2.7 mm posterior to bregma and 4.8 mm from the midline, at 8.4 mm (0.1 μl/min for 1.5 min) and 8.7 mm (0.1 μl/min for 2.5 min) ventral from the skull surface and the injection site. For sham-lesioned control rats, the micropipette was lowered and PBS was infused into each injection site in a similar manner. In experiment 1, BLA lesions were made to eight rats, and eight rats received sham lesions. In experiment 2, nine rats received BLA lesions, and 10 received sham lesions. In each of experiments 3 and 4, eight rats received BLA lesions, and eight rats received sham lesions.
Histological procedures
For all experiments, after completion of behavioral testing, rats were anesthetized with sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline, followed by 4% Formalin in 0.1 m PBS with 20% (w/v) sucrose at 4°C for 24–48 h. Sections (40 μm) were taken from each brain throughout the BLA, mounted on slides, and Nissl stained to verify lesions.
Apparatus
The behavioral training apparatus consisted of eight individual chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides, and a floor made of 0.48 cm stainless steel rods spaced 1.9 cm apart. An illuminated clear acrylic food cup was placed behind a square opening in the center of the front wall. A photocell in the food cup was used to detect entries and time spent in the cup. Levers were available on the left and right sides of the food cup in experiments 1 and 2, and, when necessary, aluminum boxes (3.0 × 2.0 × 3.0 cm) covered each lever. A speaker that delivered either a 1500 kHz tone or a white noise (each ∼80 dB) was placed on the back wall of a double-walled sound-attenuating shell, which enclosed each experimental chamber. The chambers were illuminated for television viewing by a panel of infrared light-emitting diodes mounted on the top of the chamber. An IBM-compatible computer controlled and recorded all stimuli and responses.
Behavioral training procedures (experiment 1)
Food-cup training.
After food deprivation to 85% of their baseline body weights, all rats were initially preexposed for a 2 h period to each of the two reinforcers used throughout behavioral training; solutions of orange or grape Kool-Aid flavoring (1 g/L) in 0.2 m sucrose. After preexposure, rats received a 64 min food-cup training session on each of 2 consecutive days. In one session, the reinforcer was a 0.1 ml delivery of the orange solution and, in the other session, a 0.1 ml delivery of grape solution. Each session included 16 deliveries of a specific reinforcer. The order in which the two flavors were presented was counterbalanced.
Instrumental training.
Rats then received two instrumental training sessions per day, separated by ∼2 h, one with only the left lever present and one with only the right lever present, with the order of the two sessions alternating daily. The response–outcome contingencies were fully counterbalanced, such that for half the rats left lever responses resulted in delivery of grape and responses on the right lever produced delivery of orange, whereas the remaining rats were assigned the opposite contingencies. For the first 3 d, rats received 30 min sessions in which each response was reinforced on a fixed-interval schedule. Next, the session duration was reduced to 20 min, and reinforcer delivery was switched to a random ratio (RR) schedule of reinforcement for a total of 14 sessions. Initially, reinforcer delivery was available on a RR-5 schedule, in which on average every five responses resulted in reinforcer delivery. After three sessions of instrumental training under the RR-5 schedule, the schedule was changed to RR-10 for three sessions and then to RR-15 for an additional three sessions. For the final five sessions, the schedule was switched to RR-20. After instrumental training, the rats underwent surgery, followed by 12–15 d of recovery. In experiment 1, during left lever training on the RR-15 schedule, the lever was mistakenly covered in one session. This session was excluded and rats were given an extra training session on each lever.
Instrumental reinforcer devaluation: sensory-specific satiety, extinction, and choice test.
After recovery from surgery, the rats in each lesion condition received sensory-specific selective satiation devaluation treatment, by prefeeding with one of the two outcomes. A drinking bottle, filled with 50 ml of either grape or orange solution, was placed into each home cage for a 2 h period. The identity of the solution was counterbalanced across the previous response–outcome contingencies. Immediately after satiety treatment, rats were given a 20 min extinction test session in the experimental chamber, during which responses were not reinforced with reinforcer delivery. Unlike in training, both levers were available in this test session. By testing in the absence of the reinforcers themselves, one ensures that test performance reflects an interaction of response–outcome information acquired during initial training with some internal representation of the status of the outcome as a goal after satiety treatment. To the extent that responding was controlled by the current value of the reinforcer anticipated after each of the two responses (left and right lever presses), rats would preferentially perform the response that had been reinforced previously with the reinforcer that had not been prefed (i.e., the non-devalued response).
Finally, the effectiveness of the prefeeding devaluation treatment in altering the rats preference for the reinforcers themselves was assessed. On completion of prefeeding identical to that used previously, each rat was given access to two drinking bottles in its home cage, one containing 25 ml of the prefed reinforcer and other containing 25 ml of the other reinforcer. Rats were given 30 min to consume each reinforcer, with the expectation that consumption would be greater for the non-prefed reinforcer.
Behavioral training procedures (experiment 2)
Instrumental training.
The rats received food-cup and instrumental training procedures identical to those of experiment 1.
Instrumental reinforcer devaluation: conditioned taste aversion, extinction, and choice test.
After 12–15 d recovery from surgery, rats in each lesion condition were assigned a particular reinforcer (i.e., orange or grape) that was subsequently paired with LiCl, whereas the alternate reinforcer was unpaired. Taste aversion training took place in the rats home cages. On days 1, 3, and 5, all rats received 50 ml of the paired reinforcer for 15 min, followed by an intraperitoneal injection of 0.3 m LiCl at 5 ml/kg. On days 2, 4, and 6, all rats received 50 ml of the unpaired reinforcer for 15 min. Because of generalization of the taste aversion to the unpaired reinforcer, unpaired trials were subsequently extended through days 7–8 and 10–12. To confirm that the taste aversion was maintained to the paired reinforcer, all rats received a paired trial on day 9.
After taste aversion training, the instrumental extinction test proceeded as in experiment 1. Finally, to confirm that the taste aversion readily transferred to the operant chambers, a 15 min consumption choice test was performed with rats given 25 ml of simultaneous access to both reinforcers in metal cups attached to the chamber floors.
Behavioral training procedures (experiment 3)
Pavlovian training.
After food-cup training like that in the previous experiments, the rats received two pavlovian training sessions per day (separated by an interval of ∼2 h), one with the tone stimulus and another with the white noise stimulus. Each session consisted of five 10 s presentations of the stimulus, followed by delivery of 0.1 ml of either grape or orange solution, with a variable intertrial interval that averaged 4 min. For half the rats, presentations of the tone stimulus were paired with grape, whereas white noise presentations were reinforced with orange. For the remaining rats, these stimulus–outcome contingencies were reversed. Rats received a total of 10 sessions of pavlovian training, with the order of the two sessions alternating daily. On completion, half the rats underwent neurotoxic BLA surgeries, whereas the remaining rats were given sham lesions.
Pavlovian reinforcer devaluation: conditioned taste aversion, extinction, and choice test.
After 12–14 d recovery from surgery, all rats received taste aversion conditioning as described in Experiment 2. On completion, rats were given a pavlovian extinction test. In this test, rats received 4 10 s presentations of each stimulus (tone and noise), with a 4 min fixed interval between stimulus presentations. The order of stimulus presentation was randomized with the criterion that the same cue would not be repeated more than twice consecutively. Finally, to confirm that the taste aversion readily transferred to the operant chambers, a 15 min consumption choice test was performed as in experiment 2.
Behavioral training procedures (experiment 4)
This experiment was identical to experiment 3 except that sensory-specific satiety procedures identical to those used in experiment 1 were used to devalue one reinforcer before the extinction and reinforcer choice tests.
Results
Histology (experiments 1–4)
Lesions were similar across all experiments. Typically, BLA lesions were large, including on average 90% damage to the lateral, basal, and accessory basal nuclei and some damage (∼50%) to the anterior and posterior basomedial nuclei. In addition, unilateral damage to the dorsal and ventral endopiriform areas and/or the lateral central nucleus was observed in some subjects. Of the eight BLA-lesioned rats, one rat was excluded from experiment 1 for failing to acquire conditioning before surgery. In experiment 2, three BLA-lesioned rats were excluded, and, in experiment 4, two BLA-lesioned rats were excluded because of unilateral damage to the BLA. Figure 1 provides photomicrographs of representative lesioned and sham-lesioned brains and drawings of the smallest and largest lesions at several anteroposterior planes.
Figure 1.
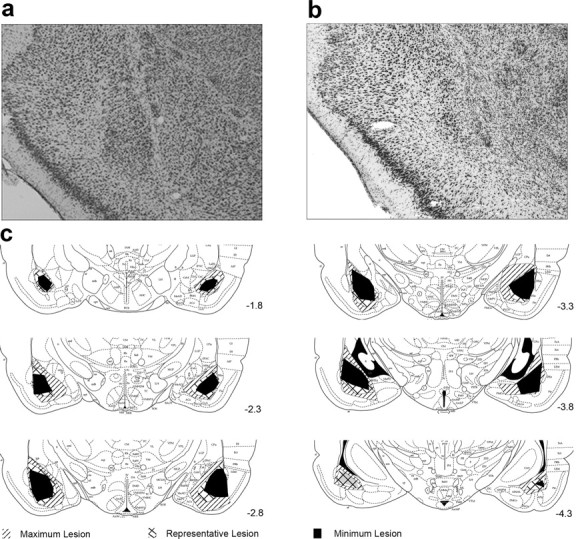
BLA lesion histology. Photomicrographs of coronal sections of the amygdala from the brains of representative sham-lesioned (a) and BLA-lesioned (b) rats. c, Extents of minimum, maximum, and representative BLA lesions at various distances posterior to bregma.
Behavior (experiment 1)
Instrumental training
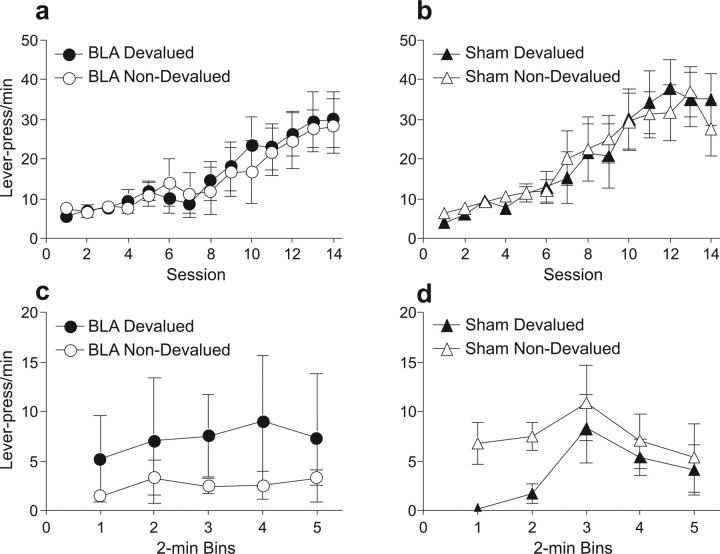
Before surgery, all rats displayed similar rates of responding for both reinforcers (one to be devalued later and one not) and increased their response rates after increments in the response-reinforcer schedule (Fig. 2A,B). A group (to-be-lesioned or sham-lesioned) × response type (to-be-devalued vs non-devalued) × session ANOVA found no significant effect of group (F(1,13) = 0.60, p = 0.44) or response type (F(1,13) = 0.15, p = 0.7) but showed a significant effect of session (F(13,169) = 15.13, p < 0.0001). There were no significant interactions among the variables (largest F value; response × session, F(13,169) = 0.85, p = 0.60). Thus, before surgery and test, the to-be-devalued and non-devalued responses did not differ in frequency in either group.
Figure 2.
Experiment 1 behavioral results. Mean lever presses per minute during instrumental training for BLA-lesioned (a) and sham-lesioned (b) rats for the response in training that delivered the outcome subsequently devalued by sensory-specific satiety (devalued response; filled symbols) and for the response that subsequently remained valued (non-devalued response; open symbols). Mean lever presses per minute during choice extinction test for BLA-lesioned (c) and sham-lesioned (d) rats for the response associated with the devalued (filled symbols) and non-devalued (open symbols) outcome. Error bars indicate SEM.
Extinction test
The data of primary interest are depicted in Figure 2, C and D, which shows responding throughout the extinction test session after devaluation induced via sensory-specific satiation. In sham-lesioned rats (Fig. 2D), prefeeding of one reinforcer resulted in a suppression of responding to the lever previously associated with that reinforcer compared with responding on the alternate (non-devalued) lever. In contrast, BLA-lesioned rats (Fig. 2C) displayed a small preference for the lever associated with the devalued reinforcer. A three-way ANOVA, with variables of lesion, response (devalued or non-devalued), and time bin, revealed no main effects of any of these variables (F values <0.76, p > 0.54). Importantly, however, the analysis revealed a lesion × response interaction (F(1,13) = 5.31, p < 0.05). Tests of simple main effects revealed a main effect of response type for sham-lesioned (F(1,13) = 11.34, p < 0.01) but not BLA-lesioned (F(1,13) = 1.81, p = 0.22) rats with non-devalued (F(1,13) = 12.99, p = 0.003) but not devalued (F(1,13) = 1.01, p = 0.33) responding, differing significantly between the two groups. These results indicate that posttraining lesions of the BLA disrupted the performance of instrumental reinforcer devaluation at test.
Reinforcer choice test
The lack of devaluation effect noted in the BLA-lesioned group could not be attributable to a simple failure of the devaluation treatment to alter preferences for the two reinforcers, because both groups of rats readily consumed less of the devalued reinforcer when presented with a choice between it and the non-devalued reinforcer. Sham-lesioned rats consumed (mean ± SEM volumes) 2.5 ± 0.73 ml of the devalued reinforcer and 10.88 ± 2.97 ml of the non-devalued reinforcer, and the BLA-lesioned rats consumed 3.57 ± 1.48 ml of the devalued reinforcer and 10.28 ± 1.53 ml of the non-devalued reinforcer. A lesion × reinforcer type (devalued vs non-devalued) ANOVA confirmed a main effect of reinforcer type (F(1,13) = 23.5, p < 0.001) but no effect of lesion (F(1,13) = 0.01, p = 0.91) or lesion × reinforcer type interaction (F(1,13) = 0.28, p = 0.60). Collectively, these results suggest that BLA lesions made after training do not affect the ability of rats to discriminate between the reinforcers or to alter their food preferences after satiation. Instead, these rats were unable to incorporate the changed reinforcer value into previously established response–outcome associations to guide instrumental behavior.
Behavior (experiment 2)
Instrumental training
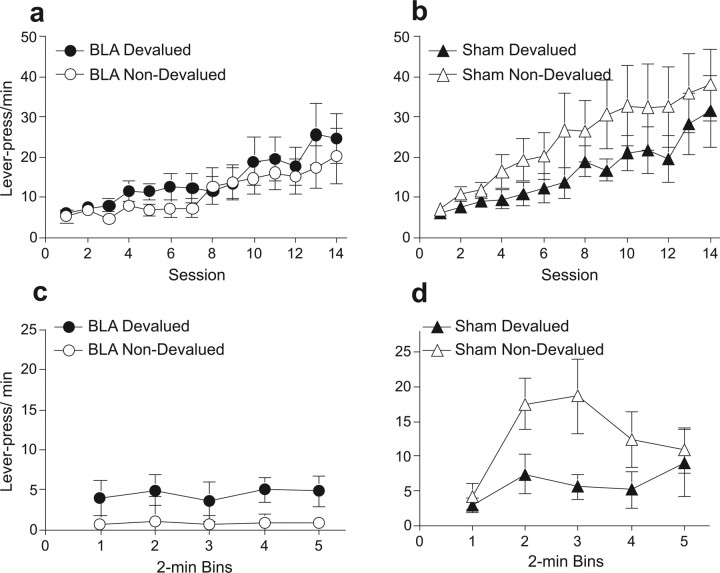
As in the previous experiment, both groups of rats showed similar rates of responding before surgery (Fig. 3A,B). A three-way ANOVA revealed no effect of group (F (1,14) = 1.80, p = 0.20) or response (F (1,14) = 0.26, p = 0.61) but a significant main effect of session (F(13,182) = 14.59, p < 0.0001). Additionally, no interactions were significant (largest F value; group × response, F(1,14) = 1.38, p = 0.25).
Figure 3.
Experiment 2 behavioral results. Mean lever presses per minute during instrumental training for BLA-lesioned (a) and sham-lesioned (b) rats for the response in training that delivered the outcome subsequently devalued by conditioned taste aversion (devalued response; filled symbols) and for the response that subsequently remained valued (non-devalued response; open symbols). Mean lever presses per minute during choice extinction test for BLA-lesioned (c) and sham-lesioned (d) rats for the response associated with the devalued (filled symbols) and non-devalued (open symbols) outcome. Error bars indicate SEM.
Conditioned taste aversion
Taste aversion training produced an equivalent reduction of consumption in both groups. Consumption of the paired outcome declined in sham-lesioned rats from 17.2 ± 1.2 to 3.2 ± 0.7 ml, whereas consumption of the unpaired reinforcer increased from 7.5 ± 0.9 to 12.0 ± 2.5 ml. Similarly, in BLA-lesioned rats, consumption of the paired outcome decreased from 18.6 ± 1.1 to 4.2 ± 1.1 ml, whereas consumption of the unpaired reinforcer increased from 5.9 ± 0.3 to 10.8 ± 1.6 ml. Note that, because the first presentation of the unpaired reinforcer occurred after the first pairing of the other reinforcer with LiCl, the low level of consumption shown on the first unpaired reinforcer trial probably reflects a generalization of the taste aversion. Importantly, consumption of the unpaired reinforcer recovered to the same extent in both groups after additional discrimination trials. A lesion × taste aversion contingency (paired or unpaired) × trial ANOVA revealed main effects of taste aversion contingency (F(1,14) = 3.00, p = 0.01) and trial (F(1,14) = 14.89, p < 0.01) and a significant interaction between these two factors (F(1,14) = 64.68, p < 0.001) but no effect of lesion (F(1,14) = 0.004, p = 0.94) or any of its interactions (largest F = 1.71, p = 0.21). Thus, after repeated pairings with LiCl, both groups of rats suppressed consumption of the paired reinforcer compared with the unpaired reinforcer.
Extinction test
Figure 3, C and D, depicts the data of primary interest for this experiment, those from the reinforcer devaluation extinction test. The sham-lesioned rats displayed a clear preference for pressing the lever associated with the non-devalued (unpaired) reinforcer. In contrast, BLA-lesioned rats showed similar response rates to each lever. A lesion × response type × time bin ANOVA revealed no main effect of response type (F(1,14) = 0.43, p = 0.52) but significant main effects of lesion (F(1,14) = 7.22, p < 0.05) and time bin (F(4,56) = 2.61, p < 0.05) and, most importantly, a lesion × response type interaction (F (1,14) = 4.63, p < 0.05). Tests of simple main effects revealed a significant main effect of response type for sham (F(1,14) = 5.25, p = 0.03) but not BLA-lesioned rats (F(1,14) = 0.89, p = 0.36), indicating a devaluation effect in the sham controls but not in the lesioned rats. In addition, responding to the devalued lever was equivalent between the two groups (F(1,14) = 0.25, p = 0.62), whereas sham-lesioned rats showed significantly more responses to the non-devalued lever (F(1,14) = 10.03, p < 0.01).
Reinforcer choice test
The taste aversion transferred readily from the home cage (where it was established) to the experimental chamber. Lesions of the BLA did not affect this transfer, with both groups of rats consuming significantly less of the devalued (1.83 ± 0.38 ml in the BLA-lesioned rats and 1.0 ± 0.24 ml in the sham-lesioned rats) than of the non-devalued reinforcer (5.16 ± 0.53 and 8.5 ± 2.1 ml, respectively). This impression was confirmed by a lesion × reinforcer ANOVA, which showed a main effect of reinforcer (F(1,14) = 13.59, p < 0.01) but not of lesion (F(1,14) = 0.83, p = 0.38) and no significant interaction between those two variables (F(1,14) = 2.01, p = 0.18). Thus, previous taste aversion training served to devalue the reinforcer in both groups of rats, altering food preference toward the non-devalued reinforcer. Critically, this reinforcer devaluation was ineffective in directing subsequent instrumental behavior in BLA-lesioned rats. Collectively, the results from experiments 1 and 2 show that posttraining lesions of the BLA disrupt the expression of instrumental devaluation performance with both motivational and associative manipulations of reinforcer value, suggesting that this procedural difference was not critical to the difference between the outcomes reported by Pickens et al. (2003) and those reported by Ostlund and Balleine (2008) and Wellman et al. (2005).
Behavior (experiment 3)
Pavlovian training
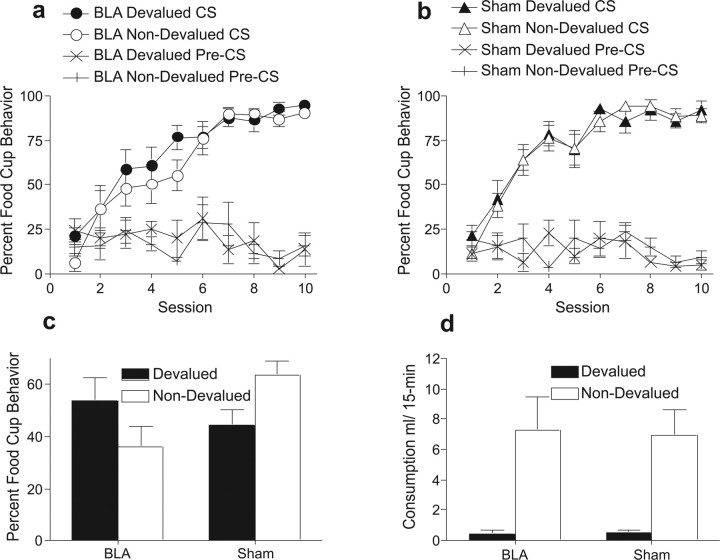
Over the course of training, both to-be-lesioned and to-be-sham groups increased the time spent in the food cup in the 5 s periods immediately before reinforcer delivery (Fig. 4A,B). This impression was confirmed by a three-way ANOVA with the variables group, cue (to be devalued later or not), and session, which showed a main effect of session (F(9,117) = 61.1, p < 0.0001) but no effect of group (F(1,13) = 1.27, p = 0.28), cue (F(1,13) = 3.12, p = 0.10), or interactions among the variables (F values <1.49, p values >0.23), confirming no behavioral differences before surgery. Responding prior to the conditioned stimulus (pre-CS) also did not differ among the groups: a group × session ANOVA showed a main effect of session only (F(9,117) = 2.51, p < 0.05), and neither group nor its interaction with sessions was significant (F values <1.5, p values >0.15).
Figure 4.
Experiment 3 behavioral results. Percentages of time spent in the food cup during pavlovian training of the cue paired with the subsequently devalued outcome (filled symbols) and the cue paired with the outcome that subsequently remained valued (open symbols) in BLA-lesioned (a) and sham-lesioned (b) rats. c, Percentages of time spent in food cup during pavlovian extinction test in BLA- and sham-lesioned rats for the cue associated with the devalued outcome (filled bars) and the cue associated with the non-devalued outcome (open bars). d, Consumption (in milliliters) of devalued (filled bars) and non-devalued (open bars) reward during reinforcer choice test for BLA- and sham-lesioned rats.
Conditioned taste aversion
After surgery, taste aversion proceeded similarly in both groups of rats. Sham-lesioned rats reduced their consumption of the lithium-paired reinforcer from 12.4 ± 1.3 to 2.5 ± 0.3 ml, whereas consumption of the unpaired reinforcer was maintained at 5.8 ± 0.9 to 6.2 ± 0.9 ml. Rats with lesions of the BLA displayed an equivalent reduction in consumption of the paired reinforcer, from 13.0 ± 1.0 to 3.1 ± 0.5 ml, whereas no such reduction was noted with the unpaired reinforcer (5.0 ± 1.0 to 9.4 ± 2.5 ml). As noted in experiment 2, the low initial consumption of the unpaired reinforcer was likely attributable to generalization from the pairing of the other reinforcer with LiCl on the first trial of this phase. A group × taste aversion contingency (paired or unpaired) × trial ANOVA revealed no differences between the groups (F(1,13) = 0.70, p = 0.41) and no main effect of taste aversion contingency (F(1,13) = 2.15, p = 0.16) but a significant main effect of trial (F(1,13) = 28.9, p < 0.001) and interaction between taste aversion contingency and trial (F(1,13) = 104.18, p < 0.00001).
Extinction test
Although both groups of rats performed similarly during taste aversion conditioning, their performance during the pavlovian extinction test differed drastically (Fig. 4C). Sham-lesioned rats spent significantly less time in the food cup during presentations of the cue associated with the devalued reinforcer than during presentations of the non-devalued cue. The opposite pattern was observed in BLA-lesioned rats. ANOVA confirmed this impression, revealing no main effect of lesion (F(1,13) = 1.01, p = 0.33) or cue (F(2,13) = 0.03, p = 0.86) but a significant lesion × cue interaction (F(1,13) = 17.48, p = 0.001). Tests of simple main effects revealed a main effect of cue for both sham-lesioned (F(1,13) = 10.17, p = 0.007) and BLA-lesioned (F(1,13) = 7.52, p = 0.016) rats, but the effects were in the opposite directions. In addition, both groups of rats showed similar levels of food-cup entry during the devalued cue (F(1,13) = 0.75, p = 0.39), whereas the sham-lesioned rats showed significantly more responding than BLA-lesioned rats during the non-devalued cue (F(1,13) = 8.77, p = 0.011). Thus, in sham-lesioned rats, taste aversion training served to reduce food-cup responding during presentations of the devalued cue compared with non-devalued cue responding. In contrast, after taste aversion training, rats with posttraining BLA lesions showed lower responding to the non-devalued cue.
Choice test
Consistent with the results of experiment 2, taste aversion learning transferred readily from the home cage to the testing chambers. Both groups of rats consumed less of the devalued reinforcer than of the non-devalued reinforcer (Fig. 4D). This assertion was confirmed by a lesion × reinforcer (devalued or not) ANOVA, which revealed a main effect of reinforcer (F(2,13) = 25.51, p < 0.001) but no main effect of group (F(1,12) = 0.007, p = 0.93) or group × reinforcer interaction (F(1,13) = 0.03, p = 0.86).
Behavior (experiment 4)
Pavlovian training
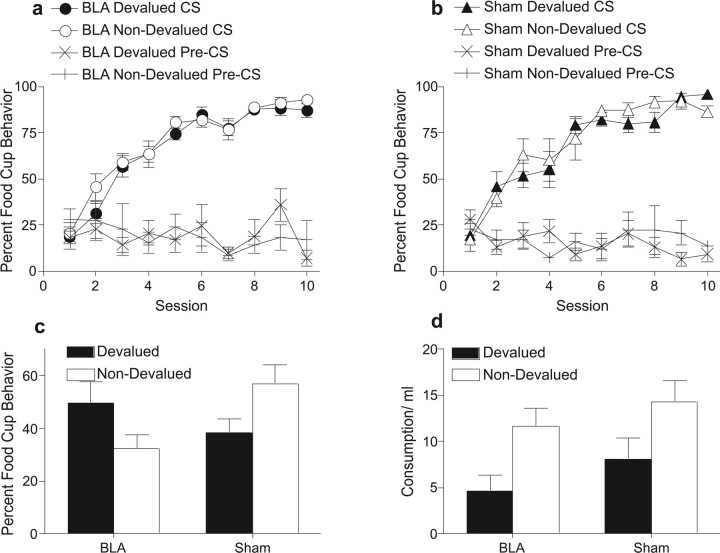
As in experiment 3, all rats showed similar levels of pavlovian acquisition of food-cup responding during the training stage (Fig. 5A,B). A group × cue × session ANOVA showed only a significant main effect of session (F(9,99) = 70.64, p < 0.0001; largest other F = 0.72, p = 0.41). Similarly, pre-CS responding did not differ among the groups; a group × session ANOVA showed no significant effects (largest F = 1.91, p = 0.09).
Figure 5.
Experiment 4 behavioral results. Percentages of time spent in the food cup during pavlovian training of the cue paired with the subsequently devalued outcome (filled symbols) and the cue paired with the outcome that subsequently remained valued (open symbols) in BLA-lesioned (a) and sham-lesioned (b) rats. c, Percentages of time spent in food cup during pavlovian extinction test in BLA- and sham-lesioned rats for the cue associated with the devalued outcome (filled bars) and the cue associated with the non-devalued outcome (open bars). d, Consumption (in milliliters) of devalued (filled bars) and non-devalued reward (open bars) during reinforcer choice test for BLA- and sham-lesioned rats
Extinction test
The data of primary interest for this experiment, those from the extinction test, are displayed in Figure 5C. In the sham-lesioned rats, food-cup responding was lower during the cue that signaled the prefed reinforcer than during the cue that signaled the other reinforcer. As in the previous experiment, the opposite pattern was observed in BLA-lesioned rats. These impressions were confirmed by an ANOVA, which showed no main effect of lesion (F(1,11) = 0.66, p = 0.43) or cue (F(1,11) = 0.12, p = 0.73) but a significant lesion × cue interaction (F(1,11) = 9.97, p < 0.01). Tests of simple main effects revealed a significant effect of cue for sham (F(1,11) = 6.63, p = 0.03) but not BLA-lesioned (F(1,11) = 3.67, p = 0.08) rats, with both groups showing similar levels of food-cup responding to the devalued cue (F(1,11) = 1.60, p = 0.23) but not the non-devalued cue (F(1,11) = 8.92, p = 0.01). Thus, as in experiment 3, posttraining BLA lesions interfered with the rats' ability to selectively reduce responding during presentations of the cue previously associated with the devalued (i.e., prefed reinforcer). Collectively, the results of experiments 3 and 4 indicate that BLA plays a critical role in devaluation performance after multiple-reinforcer pavlovian conditioning, as well as after multiple-reinforcer instrumental training (experiments 1 and 2).
Reinforcer choice test
The impairment in pavlovian devaluation test performance seen in the BLA-lesioned rats was not attributable to a simple failure of the prefeeding devaluation treatment in those rats. In the reinforcer choice test, both sham- and BLA-lesioned rats consumed less of the prefed reinforcer (Fig. 5D). ANOVA of choice test consumption revealed a main effect of reinforcer (F(1,11) = 6.57, p = 0.02) but no effect of lesion (F(1,11) = 4.41, p = 0.06) or lesion × reinforcer interaction (F < 1).
Discussion
In four experiments, rats that received lesions of BLA after either pavlovian or instrumental training with two reinforcers, but before devaluation of one of those reinforcers by either selective satiation or taste aversion training, failed to selectively reduce responding associated with the devalued reinforcer. In contrast, sham-lesioned rats showed highly selective reinforcer-specific reductions in learned responding in all cases. At the same time, the devaluation procedures were equally effective at reducing consumption of the reinforcers themselves in BLA- and sham-lesioned rats.
Previous studies that examined the effects of posttraining disruptions in BLA function on devaluation performance produced contrasting results. Pickens et al. (2003) found that rats trained with single-reinforcer pavlovian procedures showed normal devaluation effects in testing if BLA function was disrupted by neurotoxic lesions made after that training but before reinforcer devaluation by taste aversion training. Similarly, we recently replicated the results of Pickens et al. (2003), using procedures identical to those of the present experiment 3, except that a single reinforcer was delivered after each cue in training, and fewer trials were required to establish nondiscriminative taste aversion. Rats with posttraining lesions of BLA showed normal devaluation effects (our unpublished findings). In contrast, Ostlund and Balleine (2008) found that rats trained with multiple-reinforcer instrumental procedures failed to show devaluation effects in testing if BLA lesions were made after training but before reinforcer devaluation by selective satiation. Similar to the findings of Ostlund and Balleine (2008), Wellman et al. (2005) found that monkeys trained with a multiple-reinforcer instrumental object discrimination procedure failed to show devaluation effects in testing if BLA function was depressed by muscimol before the selective satiation treatment used to devalue one of the reinforcers.
The present results indicate that those earlier conflicting results are not attributable to differences in species (rats or monkeys), method of disrupting BLA function (lesions or transient inactivation), training contingency (pavlovian or operant), or devaluation procedure (associative or motivational). Thus, some previously suggested accounts for these discrepancies can be rejected. For example, Ostlund and Balleine (2008) suggested that BLA might only be required for the expression of stimulus–outcome learning when these associations are needed to guide instrumental action selection. However, experiments 3 and 4 showed deficits in devaluation performance with posttraining BLA lesions made after pavlovian training procedures. Similarly, Wellman et al. (2005) suggested that the BLA serves an “amplification function” necessary to register and encode changes in sensory-specific incentive value in other brain regions, such as orbitofrontal cortex (OFC), during selective satiation, whereas when the reinforcer value is made negative, as in taste aversion training, this amplification function is bypassed. However, experiments 2 and 3 showed deficits in devaluation performance with posttraining BLA lesions made before taste aversion training procedures.
Ostlund and Balleine (2008) suggested that successful devaluation performance after multiple- but not single-reinforcer training might depend on the ability to generate outcome representations detailed enough to be discriminated from those of other available outcomes at the time of action. Such an ability may require intact BLA function, whereas the ability to retrieve or modify less-detailed representations may not (Blundell et al., 2001; Balleine and Killcross, 2006). For example, some theorists have conceptualized outcome representations as involving multiple parallel associations that can separately encode motivational and sensory properties of the outcome (Konorski, 1967; Wagner and Brandon, 1989). Within this account, variations in training conditions may differentially encourage coding of these outcome properties. For example, the use of multiple reinforcers might especially encourage the formation of associations between cues or responses and detailed sensory properties of reinforcers. Such sensory representations may be maintained and processed further in the BLA, whereas less-detailed motivational representations, once established, may be processed elsewhere, for example in the OFC. Notably, lesions of OFC made after the completion of even single-outcome pavlovian training prevent the expression of normal pavlovian devaluation performance (Pickens et al., 2003, 2005).
The pattern of test responding of BLA-lesioned rats deserves additional comment. The devaluation impairment was revealed as either lower responding to the non-devalued CS (experiments 3 and 4) or a general reduction in responding on both levers (experiments 1 and 2), as if the postlesion reinforcer devaluation procedure successfully altered the motivational value of the reinforcer representation but left the rats unable to correctly distinguish between the devalued and non-devalued representations. Notably, Ostlund and Balleine (2008) observed the same pattern of general reductions in instrumental test responding in their study. Thus, consistent with the suggestions of Pickens et al. (2003), BLA function may be unnecessary for updating previously established representations of reinforcer value after food-illness or satiation procedures, but, as suggested by Ostlund and Balleine (2008), it may be critical for the maintenance of more detailed sensory-specific reinforcer representations that would permit integrating new information about reinforcer value selectively into existing associative structures. Even from this perspective, the significantly greater responding to the devalued cues than to the non-devalued cues in the lesioned rats in experiment 3 remains puzzling. However, it is notable that, in a pavlovian devaluation experiment, Kerfoot et al. (2007) found that presentation of a cue for a devalued reinforcer produced greater Fos expression in BLA, OFC, gustatory cortex, and portions of the accumbens shell than presentation of a non-devalued cue. Perhaps in the absence of moderating influences from BLA (Baxter and Murray, 2002; Arana et al., 2003), these greater neural responses in other brain regions may be reflected in more vigorous conditioned responding.
Additional study is needed to further refine our understanding of the posttraining role of BLA in multiple-reinforcer devaluation experiments. For example, is BLA required simply to maintain sensory-specific reinforcer representations, or is its function more proscribed, for example, to integrate new information about reinforcer value into those representations, or to use that information in guiding behavior (or both)? Notably, Wellman et al. (2005) found that, although inactivation of BLA throughout both selective satiation procedures and response testing eliminated accurate devaluation performance, BLA inactivation only at the time of response testing left performance intact. Thus, Wellman et al. (2005) concluded that BLA was necessary for registering the changed reinforcer value but not for expressing that devaluation in choice performance. However, the training and testing procedures of Wellman et al. (2005) were considerably different from those used here, including presentation of both reinforcers during response testing. It remains to be seen whether similar outcomes would be observed in rats after training and testing procedures more like those used in the present studies.
Regardless of the precise nature of the BLA lesion deficit in devaluation noted after multiple-reinforcer training, it is notable that data from other experimental paradigms also support the assertion that the roles of BLA differ depending on whether task performance involves detailed sensory representations. For example, considerable data, beyond the results of single-reinforcer devaluation studies already discussed, also indicate that BLA function is critical to the acquisition of associations with more generic motivational information about the reinforcer but not to the maintenance or subsequent use of that information in guiding behavior. For example, whereas intact BLA function is needed for a first-order CS paired with food to acquire the ability to serve as a reinforcer for subsequent second-order conditioning of another cue (Hatfield et al., 1996; Setlow et al., 2002), once the first-order CS has acquired its conditioned reinforcement power as a result of CS–food pairings, BLA lesions have no effect on its ability to establish second-order conditioning (Setlow et al., 2002; Lindgren et al., 2003). It would be of interest to determine whether BLA function is required for expression of reinforcer-selective conditioned reinforcement (Burke et al., 2008). Similarly, although BLA function is not critical to the acquisition or display of single-outcome pavlovian-instrumental transfer (Hall et al., 2001; Holland and Gallagher, 2003; Corbit and Balleine, 2005), it is needed for transfer when multiple outcomes are involved (Blundell et al., 2001; Corbit and Balleine, 2005).
Finally, it is notable that, even in intact animals, the extent to which learned responding is ultimately governed by sensory-specific reinforcer representations may vary as a function of the use of single or multiple reinforcers (Adams, 1982; Colwill and Rescorla, 1985; Holland, 2004). After extensive single-reinforcer instrumental training, responding often loses its sensitivity to changes in reinforcer value (Adams, 1982; Dickinson et al., 1998), whereas performance under multiple-reinforcer conditions does not appear to be susceptible to such a transition to more habitual modes of responding (Colwill and Rescorla, 1985; Holland, 2004).
The use of outcome representations to guide behavior provides the flexibility needed to adapt efficiently to changing environmental conditions. Patients with damage to the amygdala and other, especially prefrontal, brain regions often have difficulty adjusting their behavior according to the consequences of their actions (Tranel and Hyman, 1990; Adolphs et al., 1998; Bechara et al., 1999; Weller et al., 2007). A better understanding of the conditions under which various sorts of outcome representations are formed, maintained, and used to guide behavior and the brain mechanisms underlying those capacities may contribute to the understanding and treatment of such pathologies.
Footnotes
This work was supported by National Institutes of Health Grant MH60179. We thank Megan Dinenna, Stephanie Towe, and Heather Lasseter for their assistance in running of experiments.
References
- Adams, 1982.Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol. 1982;34B:77–98. [Google Scholar]
- Adolphs et al., 1998.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Arana et al., 2003.Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23:9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine and Killcross, 2006.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Balleine et al., 2003.Balleine BW, Killcross AS, Dickinson A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J Neurosci. 2003;23:666–675. doi: 10.1523/JNEUROSCI.23-02-00666.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter and Murray, 2002.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bechara et al., 1999.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell et al., 2001.Blundell P, Hall G, Killcross S. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke et al., 2008.Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. doi: 10.1038/nature06993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill and Rescorla, 1985.Colwill RM, Rescorla RA. Instrumental responding remains sensitive to reinforcer devaluation after extended training. J Exp Psychol. 1985;11:520–526. [Google Scholar]
- Corbit and Balleine, 2005.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson et al., 1998.Dickinson A, Squire S, Varga Z, Smith JW. Omission learning after instrumental pretraining. Q J Exp Psychol. 1998;51:271–286. [Google Scholar]
- Hall et al., 2001.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hatfield et al., 1996.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, 2004.Holland PC. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J Exp Psychol Anim Behav Process. 2004;30:104–117. doi: 10.1037/0097-7403.30.2.104. [DOI] [PubMed] [Google Scholar]
- Holland and Gallagher, 2003.Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Kerfoot et al., 2007.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learn Mem. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver and Bucy, 1937.Klüver H, Bucy PC. “Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. Am J Physiol. 1937;119:352–353. [Google Scholar]
- Konorski, 1967.Konorski J. Integrative activity of the brain. Chicago: The University of Chicago; 1967. [Google Scholar]
- LeDoux et al., 1986.LeDoux JE, Iwata J, Pearl D, Reis DJ. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Res. 1986;371:395–399. doi: 10.1016/0006-8993(86)90383-5. [DOI] [PubMed] [Google Scholar]
- Lindgren et al., 2003.Lindgren JL, Gallagher M, Holland PC. Lesions of basolateral amygdala impair extinction of CS motivational value, but not of explicit conditioned responses, in Pavlovian appetitive second-order conditioning. Eur J Neurosci. 2003;17:160–166. doi: 10.1046/j.1460-9568.2003.02421.x. [DOI] [PubMed] [Google Scholar]
- Málková et al., 1997.Málková L, Gaffan D, Murray EA. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J Neurosci. 1997;17:6011–6020. doi: 10.1523/JNEUROSCI.17-15-06011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund and Balleine, 2008.Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008;28:4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens et al., 2003.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens et al., 2005.Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow et al., 2002.Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Tranel and Hyman, 1990.Tranel D, Hyman BT. Neuropsychological correlates of bilateral amygdala damage. Arch Neurol. 1990;47:349–355. doi: 10.1001/archneur.1990.00530030131029. [DOI] [PubMed] [Google Scholar]
- Wagner and Brandon, 1989.Wagner AR, Brandon SE. Evolution of a structured connectionist model of Pavlovian conditioning (AESOP) In: Klein SB, Mowrer RR, editors. Contemporary learning theories: pavlovian conditioning and the status of traditional learning theory. Hillsdale, NJ: Erlbaum; 1989. pp. 149–189. [Google Scholar]
- Weller et al., 2007.Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychol Sci. 2007;18:958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Wellman et al., 2005.Wellman LL, Gale K, Malkova L. GABAA-mediated inhibition of basolateral amygdala blocks reward devaluation in macaques. J Neurosci. 2005;25:4577–4586. doi: 10.1523/JNEUROSCI.2257-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]