Abstract
Nursing in the rabbit is under circadian control, and pups have a daily anticipatory behavioral arousal synchronized to this unique event, but it is not known which signal is the main entraining cue. In the present study we hypothesized that food is the main entraining signal. Using mother-deprived pups we tested the effects of artificial feeding on the synchronization of locomotor behavior, plasma glucose, corticosterone, FOS and PER1 protein rhythms in suprachiasmatic, supraoptic, paraventricular and tuberomammillary nuclei. At postnatal day 1 an intragastric tube was placed by gastrostomy. The next day and for the rest of the experiment pups were fed with a milk formula through the cannula at either 02:00 or 10:00 h (feeding time = zeitgeber time (ZT) 0). At postnatal days 5–7 pups exhibited behavioral arousal with a significant increase in locomotor behavior 60 min before feeding. Glucose levels increased after feeding, peaking at ZT4–ZT12 and then declining. Corticosterone was highest around the time of feeding then decreased to trough concentrations at ZT12–ZT16, increasing again in anticipation of next feeding bout. In the brain, the suprachiasmatic nucleus had a rhythm of FOS and PER1 that was not significantly affected by the feeding schedule. Conversely, the supraoptic, paraventricular and tuberomammillary nuclei had rhythms of both FOS and PER1 induced by the time of scheduled feeding. We conclude that the nursing rabbit pup is a natural model of food entrainment, since food, in this case milk formula, is a strong synchronizing signal for behavioral, hormonal, metabolic and neural parameters.
Keywords: synchronization by food, anticipatory arousal, clock genes, corticosterone, FOS protein
Introduction
When food is restricted during certain hours of the day subjects develop food anticipatory activity (FAA), a phenomenon commonly studied under experimental manipulations in adult rodents (Mistlberger, 1994). The most salient event during FAA is an increase in locomotor behavior before food presentation; in addition, there is also an increase in hormone corticosterone (CORT) and several hormonal and metabolic variables (Díaz-Muñoz et al., 2000; Antle & Silver, 2009). In the brain, scheduled food restriction drives daily neuronal rhythms in hypothalamic and corticolimbic structures (Angeles-Castellanos et al., 2003; Verwey & Amir, 2009). It had been recognized that rabbit pups also show characteristics similar to FAA, but in contrast to adult rats, without excessive manipulations, since they are naturally exposed during lactation to a restricted feeding schedule, as they are nursed and thus ingest food, just once a day for few minutes, usually during the dark phase (Broekhuizen & Mulder, 1983; Jilge, 1993; Zarrow et al., 1965). Before the mother's arrival, pups show a circadian increase in core body temperature and locomotor behavior at postnatal day (PD) 7 (Jilge, 1993; Jilge et al., 2000), resembling the features observed in FAA in rodent species. In addition, pups also show an increase in CORT levels before and during daily nursing (Rovirosa et al., 2005). Moreover, rhythms in liver glycogen, free fatty acids and plasma ghrelin concentrations are also synchronized by timing of nursing (Morgado et al., 2008; 2010). Finally, similar to rodents under restricted feeding, the daily nursing event synchronizes neuronal activity in the hypothalamus as observed by detection of rhythms in FOS and the clock protein PER1 (Caba et al., 2008).
On the basis of this evidence, we recently proposed that the rabbit pup is a natural model of nursing-anticipatory activity (Caba & González-Mariscal, 2009). We used the word “nursing” for this entrainment process because it was not clear which cue was the main synchronizing signal, as during nursing pups are exposed to several stimuli from the mother (Hudson & Distel, 1989). During this brief nursing opportunity rabbit pups abruptly fill up their stomachs achieving up to 35% of their body weight in milk in less than five min. (Caba et al., 2003). In the present study we explored the role of food as the main synchronizing signal by the artificial feeding of mother-deprived rabbit pups. To this aim we infused milk formula through an intragastric cannula either at 02:00 or 10:00 h., which were considered as ZT 0, from PD 2 to PD 7 in pups maintained in the dark. At PD 7 locomotor behavior, glucose and CORT were determined every four hours. In addition, we used immunohistochemistry for FOS and PER1 protein to examine oscillations in the suprachiasmatic nucleus (SCN), the master circadian clock, as well as in the supraoptic (SON), paraventricular (PVN) and tuberomammillary (TMN) nuclei, based on their involvement in FAA and food ingestion in rodents under a restriction feeding schedule (Angeles-Castellanos et al., 2003; Verwey & Amir, 2009) and in rabbit pups synchronized by nursing (Allingham et al., 1998; Caba et al., 2003; Caba et al., 2008; Caldelas et al., 2009).
Materials and methods
Animals and housing conditions
Subjects were newborn rabbits born from New Zealand white female rabbits bred in our colony in Xalapa, México, which were housed under controlled light conditions (12-h/12-h light/dark cycle, lights on at 07:00 h), stable temperature conditions (23±2 °C) and provided with rabbit chow (Purina) and water ad libitum. Pregnant females were kept individually housed and monitored daily from day 28 of pregnancy until delivery. In the last week of pregnancy, each doe was provided with about 100g of straw for building of the nest; then, just before parturition, does covered the nest with fur from her belly to culminate the construction of the maternal nest. On the day of parturition (PD0), litters were adjusted to 6–8 pups and remained in the maternal nest, in constant darkness, until surgery time, without further contact with their mothers. Pups remained in constant darkness thereafter until the time of sacrifice at PD7.
Gastrostomy
On PD1 nine litters were assigned to one of two experimental groups: artificial feeding at 02:00 (AF02:00) or at 10:00 (AF10:00) h and, for each group, gastrostomy was performed at approximately those hours following a modification of the procedures described by Bierle et al. (2004). Pups were anesthetized with ketamine i.m. (3mg/100g) and then administered halothane by inhalation during surgery. The stomach was visualized through the skin; a small incision (0.5 cm approx) was made through the skin and a polyethylene tubing (PE-50) 15 cm long was passed over into the stomach with a wire inside to help guide the cannula. A small “V” shaped piece of plastic was glued in the external wall of the cannula close to the cannula tip to help maintain it inside the stomach. Finally the wire was removed, the stomach and skin were sutured around the cannula, and a local antibiotic cream was applied to prevent infection. The free tip of the cannula was sealed tightly with a small piece of plastic. Pups were maintained under warm temperature in a box until recovery from anesthesia, and then returned to a styrofoam cage lined with material from their maternal nest. Finally, the pups were placed inside the nest compartment of our maternal cages (Caba et al., 2008) and left undisturbed.
Milk formula infusion
The next day and for the following six days at 02:00 or 10:00 h pups were weighed and then fed through the cannula in the dark. The cannula was connected to a 23G needle attached to a syringe filled with milk formula with a capacity of 10 or 20 ml as required. The syringe was mounted on a timer and speed-controlled Harvard infusion pump (Harvard Apparatus Syringe Pumps, PHD 2000). Milk administration lasted from 3–5 min, simulating the time the mother takes to nurse their pups (Hudson & Distel, 1989; Jilge, 1995). Milk formula was prepared following the report of Messer et al. (1969). The volume of milk formula was adjusted every day based on data reported from our laboratory on the amount of milk ingested during first seven days postpartum in the rabbit (Caba et al., 2003). Milk formula was infused through the cannula then the tube was cleaned with physiological solution, and the tip was sealed again.
All procedures were approved by the Ethics Committee of Universidad Veracruzana, according to procedures of the National Guide for the Production, Care and Use of laboratory animals (Norma Oficial Mexicana NOM-062-ZOO-1999).
Experimental groups
48 artificially reared rabbit pups were distributed in two groups: AF02:00 and AF10:00. These schedules were assigned according to protocols previously established in our laboratory for rabbit pups fed by their mother (Caba et al., 2008). At PD7 pups were sacrificed starting just before their scheduled time of feeding (ZT0) and then at 4 h intervals (n=4 per time point) at ZT4, ZT8, ZT12, ZT 16 and ZT 20. We included no more than two pups from each litter at the same time point. Mean body weight of subjects at PD7 was AF02:00 = 78.26 ± 10.67 g and AF10:00 81.197 ± 5.42 g. The pup weight in both groups at PD7 was 80% in comparison to pups nursed by their mother (Caba et al., 2008).
Blood sampling and perfusion
Pups were euthanized with an overdose of sodium pentobarbital (50 mg per pup, intraperitoneal). A volume of 3 ml of blood was collected from the left ventricle of the heart with a 21G needle attached to a syringe and then transferred to microcentrifuge tubes. Samples rested 30 min at room temperature and then were centrifuged for 10 min at 2500 rpm at 4°C. The serum was transferred to labeled 1.5-ml tubes and stored at 20°C for further analysis of CORT and glucose. Immediately after blood sampling, pups were perfused transcardially with saline solution (0.9%), followed by 4% paraformaldehyde in phosphate buffer (PB, pH 7.4). The brains were removed immediately after perfusion, cryoprotected successively in 10, 20, and 30% sucrose in PB and then sectioned coronally at 50 μm with a cryostat (Microm).
Immunohistochemistry
Serial sections were collected in PB from the level of the diagonal band of Broca to the mammillary bodies. Two adjacent series of every 4 sections were used for labeling of FOS or PER1 as described below, following protocols previously established in rabbit brain (Caba et al., 2003; 2008, Meza et al., 2008). Tissue was washed in PB four times, 10 min each to remove excess of aldehydes and then exposed for 10 min in 0.5 % hydrogen peroxide solution to eliminate endogenous peroxidase activity. Free floating sections were incubated in PER1 antibody raised in goat (sc-7724, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or in FOS antibody raised in goat (sc-52G, Santa Cruz Biotechnology, Santa Cruz, CA, USA) both diluted at 1:2000, in 3% normal horse serum and 0.3% Triton X-100 (Sigma), for 48 h at 4°C. Tissue was incubated in biotinylated horse anti-goat antibody diluted at 1:200 (Vector Laboratories) in PB and 0.3% Triton X-100 for 1 h at room temperature. Then, sections were incubated in avidinbiotin-HRP complex diluted at 1:200 (Vector Laboratories) in PB and 0.3% Triton X-100 for 1 h at room temperature. Between incubations tissue was washed four times for 10 min in PB. Both FOS and PER1 antibody-peroxidase complex were stained with a solution of 0.05% diaminobenzidine (Polysciences) in the presence of nickel sulfate (10mg/ml, Fisher Scientific), cobalt chloride (10mg/ml, Fisher Scientific) and 0.01% hydrogen peroxide to obtain a black-purple precipitate. After 10 min, tissue was transferred to PB to stop the reaction. Sections were mounted onto gelatin-subbed slides, dehydrated and coverslipped with Permount. Antibodies had been well characterized and tested in the rabbit in previous studies (Caba et al., 2008).
Quantification of immunostaining
FOS and PER1 immunoreactivity (-ir) was identified as a black-purple precipitate from the DAB-nickel/cobalt reaction in the cell nucleus. Sections were coded and analyzed by two observers blind to the experimental conditions with an Olympus BX41 microscope. FOS- and PER1-ir was counted unilaterally in one section at the middle level of each nucleus according to a protocol for quantification previously established in our laboratory for rabbit pups (Caba et al., 2008). The identification of nuclei and brain structures was determined using the terminology of the rabbit brain of Gerhard (1968), Girgis and Shih-Chang (1981), and our previous experience in the brain of this species (Caba et al., 2003; 2008; Meza et al., 2008). The regions analyzed correspond to the levels NA1 for SCN, NAP0 for SON and PVN and NP3 for the TMN, according to the stereotaxic atlas of Girgis and Shi-Chang (1981).
Glucose and corticosterone assays
Serum aliquots were thawed and processed using spectrophotometric methods, as previously described for rabbit pups (Morgado et al., 2008; 2010). Glucose was estimated from 10 μl of serum sample using a commercial colorimetrical kit (Spinreact, Ref. 41012) based on an enzymatic glucose oxidase reaction and fenol-ampirona as chromogen, and measured at 500 nm. CORT was determined from 25 μl of serum sample by radioimmunoassay (RIA) with a commercial kit by MP Biomedicals (Costa Mesa, CA). The sensitivity of assay was 0.25 μg/dl and the intra-assay variation was 1.7%; the standards were run in triplicate and the samples in duplicate. Both glucose and CORT assay protocols were run exactly as recommended by the manufacturers.
Locomotor activity
From PD5 to PD7, at around feeding time, we recorded the locomotor activity of nine litters with 6–8 pups each. The locomotor activity was recorded following the method reported by Pongrácz and Altbäcker (2003). This method measures pup's activity of the litter in a nest cage on a balance, which detect the slightest movements of the pups. Activity score is equivalent with the variance of the weight of the whole litter in their styrofoam nest cage, measured on a digital, electric balance (Sartorius BL1500). Weight of the litter was recorded six times over 1 min, this is, once every 10 seconds at 120, 90, 60, 30 and 15 min before and 15, 30 and 60 min after feeding time.
Statistical analysis
Locomotor activity records, glucose and CORT concentrations and number of PER1-ir and FOS-ir cells of each nucleus studied, were statistically analyzed with oneway analysis of variance (ANOVA) in order to determine whether there were differences across different time points. This was followed by a post-hoc Fisher LSD test with significant values set at P<0.05. Statistical analysis was performed using SigmaStat 3.5. In order to not violate the assumptions of homogeneity of variance, in cases of SCN SON, PVN and TMN data were rank-transformed before ANOVA (Conover & Iman, 1981; Morgado et al., 2010). Data were expressed as the mean ± standard error and they were plotted without transformation.
Results
Locomotor activity
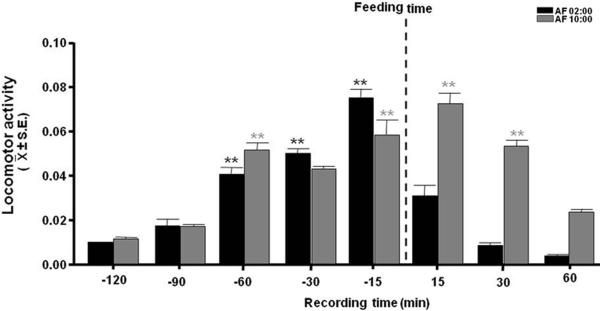
Both groups AF02:00 and AF10:00 developed anticipatory activity with significant high levels 60 min before milk formula infusion. Locomotor activity varied significantly with time in both AF02:00 (F(7,31)= 83.87; P<0.001) and AF10:00 (F(7,31)= 42.75; P<0.001) groups (Fig. 1). In the AF02:00 Group, locomotor activity started to increase 60 min before feeding, steadily increased to reach highest levels 15 min before feeding, then decreased to lowest levels 60 min after feeding. The highest level of activity, 15 min before feeding, was significantly different than values at all other time points before and after feeding (P<0.001), whereas activity levels at 30 and 60 min before feeding were significantly higher than values at 120 and 90 min before and 15, 30 and 60 min after feeding (P<0.001).
Figure 1.
Locomotor activity Both groups AF02:00 and AF10:00 developed anticipatory activity with significant high levels 60 min before milk formula infusion. Locomotor activity varied significantly with time in both AF02:00 (F(7,31)= 83.87; P<0.001) and AF10:00 (F(7,31)= 42.75; P<0.001) groups
In the AF10:00 Group, activity levels were lowest 120 min before feeding, increased to their highest levels 15 min before and after feeding, and then decreased 30 and 60 min after feeding. The highest activity levels 15 min before and after feeding were significantly higher than values at 120, 90 and 30 min before and 60 min after feeding (P<0.001), whereas the activity level at 30 min after feeding was significantly higher than values at 120, 90 (P<0.001) and 30 (P< 0.04) min before and 60 min after feeding.
Glucose
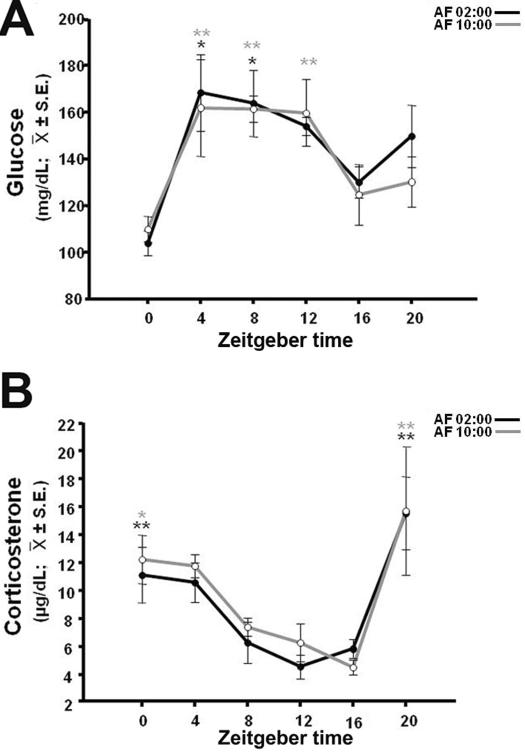
Daily milk formula infusion induced a daily rhythm of serum glucose in both groups (Fig. 2A). In the AF02:00 Group (F(5,23)= 4.76; P=0.006), maximum concentrations of glucose at ZT4 and ZT8 were significantly different than concentrations at ZT0 (P<0.001) and ZT16 (P<0.05). In AF10:00 Group (F(5,23)= 3.12; P=0.033), maximum concentrations at ZT4, ZT 8 and ZT12 were significantly different than the concentration at ZT0 (P=0.01).
Figure 2.
Temporal profiles of glucose (A) and corticosterone (B) in 7-day-old rabbits artificially fed at 02:00 (AF02:00) or 10:00 (AF10:00) h, from postnatal days 2–7. Significant differences between the highest and the lowest values within same group: * P<0.05, ** P< 0.001. 234×321mm (80 × 80 DPI)
Corticosterone
Serum values of CORT varied significantly with time, both in AF02:00 (F(5,23)=6.46; P=0.001) and AF10:00 (F(5,23)= 3.99; P=0.013) groups (Fig. 2B) with highest values seen four hours before and at the time of feeding. In the AF02:00 Group, CORT level at ZT0 was significantly higher than levels at ZT8 (P=0.05), ZT12 (P=0.011) and ZT16 (P=0.035), and level at ZT20 was also significantly higher than values at ZT8, ZT12 and ZT16 h after feeding (P<0.001 in all cases). In the AF10:00 Group, level at ZT0 was significantly higher than those at ZT16 (P=0.02), whereas level at ZT20 was significantly different from those at ZT8 (P=0.013), ZT12 (P=0.006) and ZT16 h (P=0.006).
PER1 in SCN
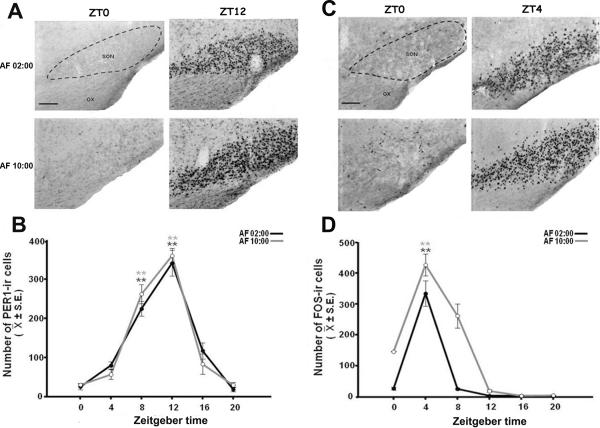
Figures 3A and 3B show PER1 protein at six different time points throughout a complete 24-h cycle at PD7 in pups fed at 02:00 and 10:00 h respectively. Quantitative analysis indicated that PER1 protein in SCN had a robust rhythm in both night and day fed pups, which was not affected by the time of feeding (Fig. 3E). PER1 expression varied significantly with time, both in AF02:00 (F(5,23)= 20.14; P<0.001) and AF10:00 (F(5,23)= 36.02; P<0.001) groups. Values at ZT0 and ZT20 for AF02:00 group and ZT12 and ZT16 for AF10:00 group were significantly different than remaining time point groups (P<0.001 in all cases).
Figure 3.
Rhythmic expression of PER1 and FOS in the middle portion of suprachiasmatic nucleus (SCN) in 7-day-old rabbits artificially fed from postnatal day 2 to 7. (A, B) Photomicrographs of representative sections illustrating PER1-ir cells of SCN at six temporal points throughout a 24-h cycle in pups fed at 02:00 (A) or 10:00 (B) h. (C, D) Photomicrographs of representative sections illustrating FOS-ir cells of SCN at six temporal points throughout a 24-h cycle in pups fed at 02:00 (C) or 10:00 h (D). Dotted line delimits SCN. 3V, third ventricle. OX, optic chiasma. Scale bar, 100 0m. (E, F) Comparison of PER1-ir (E) and FOS-ir (F) cells in the SCN in both AF02:00 and AF10:00 groups. Values are mean ± SE. Significant differences between the highest and the lowest values within same group: ** P< 0.001).
FOS in SCN
Figures 3C and 3D show FOS protein at six different time points throughout a complete 24-h cycle in PD7 in pups fed at 02:00 and 10:00 h respectively. Quantitative analysis indicated that FOS protein in SCN had a robust rhythm in both night and day fed pups, which was not affected by time of feeding (Fig. 3F). FOS expression varied significantly with time, both in AF02:00 (F(5,23)= 45.34; P<0.001) and AF10:00 (F(5,23)= 30.17; P<0.001) groups. In the AF02:00 Group, the highest value at ZT16 was significantly different than all remaining time point groups (P<0.001). In the AF10:00 Group, the highest value at ZT8 was significantly different than those at ZT4, ZT12, ZT16, ZT20 (P<0.001 in all cases) and ZT0 (P=0.03).
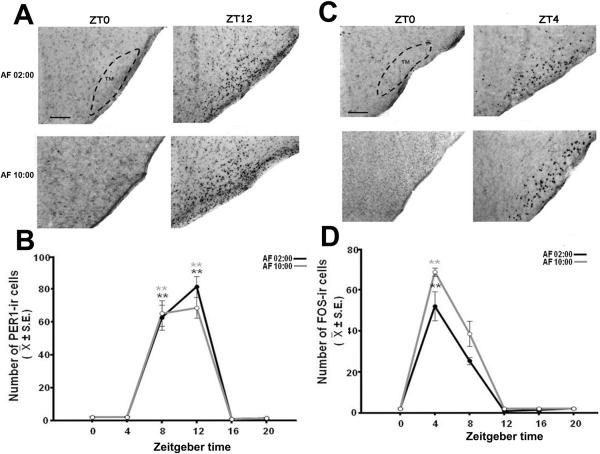
PER1 in SON
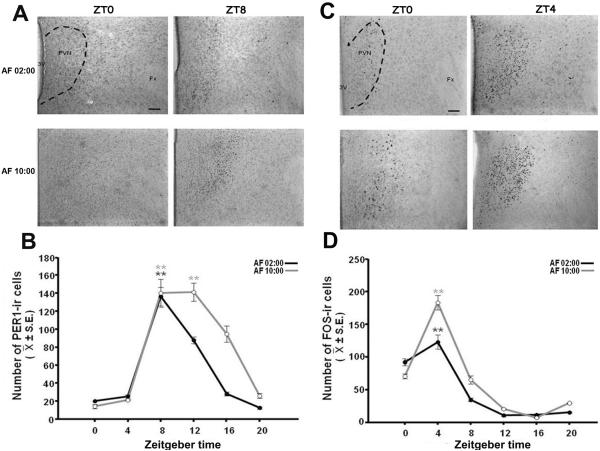
Figure 4A shows PER1 protein at ZT0 and ZT12 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of PER1-ir cells in the SON varied significantly with feeding time in both AF02:00 (F(5,23)= 47.00; P<0.001) and AF10:00 (F(5,23)= 65.43; P<0.001) groups (Fig. 4B). In both groups, PER1 levels were very low at ZT0, and then increased at ZT8 reaching a peak at ZT12. Values at ZT8 and ZT12 were significantly different than all remaining time point groups (P<0.001).
Figure 4.
Synchronization of PER1 and FOS expression in the supraoptic nucleus (SON) of pups artificially fed from postnatal days 2–7. (A) Photomicrographs of representative sections illustrating the rhythmic expression of PER1-ir cells in the middle portion of SON in AF02:00 and AF10:00 groups at ZT0 and ZT12. (B) Expression of PER1 in the SON in six temporal points throughout a 24-h cycle. (C) Photomicrographs of representative sections illustrating the expression of FOS-ir cells in the middle portion of SON in AF02:00 and AF10:00 groups at ZT0 and ZT4. (D) Expression of FOS-ir in the SON in six temporal points throughout a 24-h cycle. Dotted line delimits SON. OX, optic chiasma. Scale bar, 100 1m. Values are mean ± SE. Significant differences between the highest and the lowest values within same group
FOS in SON
Figure 4C shows FOS protein at ZT0 and ZT4 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of FOS-ir cells in the SON varied significantly with time in both AF02:00 (F(5,23)= 22.26; P<0.001) and AF10:00 (F(5,23)=96.45; P<0.001) groups (Fig. 4D). In both groups, FOS was lowest at ZT0 then increased to maximum level at ZT4, which was significantly different than all remaining time points (P<0.001).
PER1 in PVN
Figure 5A shows PER1 protein at ZT0 and ZT8 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of PER1-ir cells in the PVN varied significantly with time in both AF02:00 (F(5,23)= 57.93; P<0.001) and AF10:00 (F(5,23)= 49.56; P<0.001) groups (Fig. 5B). In both groups, values were very low at ZT0 then increased to highest level at ZT8, which was significantly different than values at ZT0, ZT4, ZT16 and ZT20. Additionally, the value at ZT12 in the AF10:00 Group was significantly higher than values at ZT0, ZT4, ZT16 and ZT20 (P<0.001 in all cases).
Figure 5.
Synchronization of PER1 and FOS expression in the paraventricular nucleus (PVN) of pups artificially fed from postnatal days 2–7. (A) Photomicrographs of representative sections illustrating the expression of PER1-ir cells in the middle portion of PVN in AF02:00 and AF10:00 groups at ZT0 and ZT8. (B) Expression of PER1-ir in the PVN in six temporal points throughout a 24-h cycle. (C) Photomicrographs of representative sections illustrating the expression of FOS-ir in the middle portion of PVN in AF02:00 and AF10:00 groups at ZT0 and ZT4. (D) Expression of FOS-ir in the PVN in six temporal points throughout a 24-h cycle. Dotted line delimits PVN. Fx, Fornix; 3V, third ventricle. Scale bar, 100 5m. Values are mean ± SE. Significant differences between the highest and the lowest values within same group
FOS in PVN
Figure 5C shows FOS protein at ZT0 and ZT4 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of FOS-ir cells in the PVN varied significantly with time in both AF02:00 (F(5,23)= 42.98; P<0.001) and AF10:00 (F(5,23)= 68.24; P<0.001) groups (Fig. 5D). In the AF02:00 Group, values were low at ZT0 then increases to highest level at ZT4. Maximal value at ZT4 was significantly different than those at ZT0 (P= 0.02), ZT8, ZT12, ZT16 and ZT20 (P<0.001). Similarly, in the AF10:00 Group, FOS cell numbers were low at ZT0 then increased to maximal value at ZT4 which was significantly different than remaining time points (P<0.001 in all cases).
PER1 in TMN
Figure 6A shows PER1 protein at ZT0 and ZT12 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of PER1-ir cells in the TMN varied significantly with time in both AF02:00 (F(5,23)= 12.92; P<0.001) and AF10:00 (F(5,23)=12.14; P<0.001) groups (Fig. 6B). In both groups values were very low at ZT0 and ZT4 then sharply increased to highest levels at ZT8 and ZT12. Also in both groups values at ZT8 and ZT12 were significantly different than all remaining time points (P<0.001 in all cases).
Figure 6.
Synchronization of PER1 and FOS expression in the tuberomammillary nucleus (TMN) of pups artificially fed from postnatal days 2–7. (A) Photomicrographs of representative sections illustrating the expression of PER1-ir in the middle portion of TMN in AF02:00 and AF10:00 groups at ZT0 and ZT12. (B) Expression of PER1-ir in the TMN in six temporal points throughout a 24-h cycle. (C) Photomicrographs of representative sections illustrating the expression of FOS-ir in the middle portion of TMN in AF02:00 and AF10:00 groups at ZT0 and ZT4. (D) Expression of FOS in the TMN in six temporal points throughout a 24-h cycle. Dotted line delimits TMN. Scale bar, 100 2m. Values are mean ± SE. Significant differences between the highest and the lowest values within same group:
FOS in TMN
Figure 6C shows FOS protein at ZT0 and ZT4 in AF02:00 and AF10:00 groups. Quantitative analysis indicated that the number of FOS-ir in the TMN varied significantly with time in both AF02:00 (F(5,23)= 14.36; P<0.001) and AF10:00 (F(5,23)= 9.73; P<0.001) groups (Fig. 6D). In both groups, cell numbers were very low at ZT0 then sharply increased to their highest value at ZT4, which was significantly different than all remaining time point values (P<0.001 in all cases).
Discussion
It has been well known that nursing is a strong synchronizer of circadian rhythms in rabbit pups, but the precise identity of the stimulus responsible for entrainment by nursing was unclear. The present study indicates that food serves as the primary synchronizing cue for entrainment of behavioral, hormonal, metabolic and neural rhythms associated with nursing in newborn rabbit pups. This assumption is supported by the fact that pups were deprived at birth of all stimulatory signals from the mother and were fed by an artificial formula.
During most of the day, newborn rabbits remain huddled, well covered with material from the nest and rather inactive. Occasionally, pups at the edge of the group crawl around the nest but they usually soon rejoin the group (Hudson & Distel, 1989). However as nursing approaches they show increased locomotor activity and rearing movements, reacting intensely to external stimuli (Hudson & Distel, 1989). Chronobiological studies indicate that indeed 3 h before nursing pups show an increase in locomotor behavior recorded by vibrations caused by major activity, which is more intense one hour prior to nursing between postnatal days 6–14 (Jilge, 1993). In addition, the increase in the alertness state of the pups is also evidenced by a rise in the number of vocalizations occurring during the hour prior to nursing (Schuh et al., 2004). In the present study, we found a significant increase in pup's activity beginning 60 min before nursing which reaches highest levels 15 min before and after feeding, similar to results by Pongrácz and Altbäcker (2003) who used a similar methodology to quantify pup activity before and after nursing. In addition, it is important to mention that locomotor activity after feeding of pups at 10:00 h is higher in comparison to that of pups fed at 02:00 h, similar to results from pups nursed by their mother (Caba et al., 2008). We do not know the reason for this difference but it may be related to a modulatory effect of the SCN on pup's locomotor behavior. Future studies should address this issue. The increase in alertness in rabbit pups is analogous to the increased activity shown by adult rodents under a restricted food schedule prior to food presentation (Stephan, 2002). Together with previous results from our laboratory (Caba et al., 2008) and others (Jilge 1993; 1995), our observations confirm that before food ingestion pups show a dramatic increase in activity when they are fed either by nursing or by milk formula, which, based on our present results, can now indeed be termed food anticipatory activity.
CORT rhythms in artificially fed pups, like activity rhythms, were synchronized by milk formula. CORT concentrations increased before and at the time of feeding, then levels dropped to increase again in advance of the next feeding bout, similar to those changes seen in pups nursed by their mother (Rovirosa et al., 2005; Morgado et al., 2008; 2010). In adult rats there is also a CORT elevation before food presentation under a restricted feeding paradigm (Krieger, 1974). Although the milk formula infused lacked some components of mother's milk, nutrients and volume of milk formula were sufficient to synchronize the CORT rhythm and induce an elevation of this hormone at the time of FAA. However composition of the milk formula for future studies may need to be improved as plasma glucose concentrations were slightly lower than in nursed pups (Morgado et al., 2008; 2010), but still well above the threshold of clinical hypoglycemia reported for adult rabbits (Suckow & Douglas, 1997).
On the other hand, it is important to note that in our previous experiments we reported a secondary CORT peak at around geographical hour 18:00 h, i.e., before the onset of night in pups nursed during the day (Morgado et al., 2008) or the night (Morgado et al., 2010). In adult rats under a restricted feeding schedule, two CORT peaks have also been reported, one at the time of food presentation and a secondary attenuated peak in the evening (Honma et al., 1992; Moberg et al., 1975). The presence of the secondary peak in newborn rabbits may be related to the nocturnal CORT rise observed in adult rabbits at 18:00 h (Szeto et al., 2004). We previously proposed that this secondary elevation in rabbit pups could be related to the development of the endogenous circadian rhythm of CORT, that will be need to be established in adult subjects, to which pups were exposed in utero (Morgado et al., 2010). The reason for this difference in CORT secretion in artificially fed pups is not clear, but perhaps is related to an unidentified cue received from the mother during normal nursing. In rat pups, separation from the mother during first days of life has an important and long-lasting impact on the responsiveness of the hypothalamic-pituitary-adrenal (HPA) axis in adulthood (Ladd et al., 2004). In rabbit pups as well, separation from their mothers for 48 h at postnatal days 9–11 results in a long lasting disruption of HPA axis responsiveness later in life (Brecchia et al., 2009). As discussed above, during nursing pups are exposed to several cues from the mother, among them milk perhaps being one of the most important. Although there are no reports of milk composition in rabbits, in the rat besides nutrients, maternal milk contains hormones, steroids, antibodies, enzymes and growth factors, among many other biologically active compounds (Grosvenor et al., 1993; Withworth & Grosvenor, 1978; Rowe & Kennaway, 2002). In contrast to rats, rabbit pups remain alone in the nest most of the time, and the lack of one or more factors in the milk (or from the mother, i.e., pheromonal, tactile, etc.) may have resulted in a disruption of the normal developmental pattern of the HPA axis as observed at PD7 in present results. In fact, in rat pups ingestion of CORT through mother's milk during early lactation affects development of HPA axis (Brumelette et al., 2010; Catalani et al., 1993). Moreover, in general absence of particular stimuli during maternal separation profoundly affects physiology, neural development and behavior in adult rats (Chatterjee et al., 2007; Melo et al., 2009). Future studies could explore the effects of absence of particular stimuli from the mother during lactation in the rabbit.
In the brain, SCN was not entrained by timing of feeding. Specifically, both FOS and PER1 protein rhythms in rabbit pup SCN did not shift as a consequence of scheduled nursing, similar to our previous results with pups nursed by their mother in which we observed only a small change in amplitude of the rhythm (Caba et al., 2008). In contrast, others have reported a shift in mRNA clock genes in the SCN as a consequence of a shift in nursing time (Caldelas et al., 2009), although this change may have been due to a developmental process of this nucleus (Caldelas et al., 2009, reviewed in Caba & González-Mariscal, 2009). In adult rats several reports indicate that clock genes and proteins in SCN do not shift in relation to feeding schedules (Damiola et al., 2000; Hara et al., 2001). The fact that subjects exhibit normal or enhanced FAA in the absence of the SCN has reinforced the idea that this nucleus is not necessary for food entrainment (Stephan et al., 1979; Acosta-Galvan et al., 2011). Moreover, similar to adult rats, bilateral SCN lesions in newborn rabbit pups indicate that this nucleus is not necessary for FAA (Hernández-Campos et al., 2011).
On the contrary other brain structures showed a clear synchronization by scheduled feeding. There was an immediate induction of FOS in the SON, PVN and TMN evidenced at 4 h after feeding. Previously we reported a large FOS induction in both the SON and PVN as a consequence of nursing and most of these cells were oxytocinergic (Caba et al., 2003). On this basis it is possible that the FOS induction in present contribution also is related to an activation of the oxytocinergic system by the gastric distention (Renaud et al., 1987) of milk formula, a release of oxytocin associated to satiety (Verbalis et al., 1995; Olszewski et al., 2010) or a modulation of the vagal digestive input in the brainstem (Flanagan et al., 1992). We also found activation in the TMN after feeding, similar to other studies in adult rats under a food restriction schedule (Angeles-Castellanos et al., 2003; Inzunza et al., 2000), although we did not find activation of this nucleus before feeding as reported by these authors. This suggests an important difference between FAA in rats and the rabbit. In rats, FOS is induced in the TMN of motivated, hungry rats enticed with food but not in the TMN of non-motivated rats presented with food (Valdés et al., 2005). Therefore, one explanation for the observed difference between rats and rabbit pups, is that while the rat is induced to FAA for several days and weeks, usually during their rest period, for rabbit pups, it is normal to eat once a day and entrain to this regime by 3–4 days after birth (Jilge et al., 1993; Caba et al., 2008).
In agreement with FOS results, 8–12 h after feeding there is strong PER1 induction in the same nuclei. We have reported a similar induction of PER1 in several hypothalamic areas 8–12 h after suckling in pups (Caba et al., 2008) and nursing in adult rabbits (Meza et al., 2008; 2011). The presence of clock genes in other brain areas, besides SCN, it is well documented in other species (Abe et al., 2002; Granados-Fuentes et al., 2006; Kriegsfeld et al., 2003; Wakamatsu et al., 2001). It has been suggested that clock genes/proteins provide temporal regulation to specific brain structures in order to organize appropriate responses to a particular stimulus (Kriegsfeld & Silver, 2006). Induction of PER1 in the SON and PVN may reflect a synchronization of neuroendocrine function, whereas in the TMN, PER1 induction may be related to the synchronization of FAA since this nucleus is part of the ascending arousal system (Valdés et al., 2005).
In conclusion, our findings suggest that, in nursing rabbit pups, food is the major signal responsible for synchronizing activity, glucose, and corticosterone rhythms, as well as rhythms in FOS and PER1 in the SON, PVN and TMN. By contrast, rhythms in FOS and PER1 in the SCN were not synchronized by artificial feeding of pups, paralleling the inability of food to shift SCN rhythms in adult rodents. It remains to be established whether induced rhythms persist in absence of artificial feeding in fasted pups. The results reinforce the usefulness of the rabbit pup as a natural model of food entrainment, with milk being able to serve as a strong synchronizing signal for behavioral, hormonal, metabolic and neural parameters. We conclude that the rabbit pup is a natural model of food anticipatory activity, as food synchronizes behavioral, hormonal, metabolic and neural parameters.
Acknowledgments
Acknowledgements
This research was partially supported by National Institutes of Health/Fogarty grant R01TW006636 (M. Caba). We gratefully acknowledge Biol. Mercedes Acosta for her invaluable help with maintaining and caring for the rabbit colony.
Abbreviations
- AF
artificial feeding
- CORT
corticosterone
- FAA
food anticipatory activity
- FOS
cfos protein
- FOS-ir
c-Fos immunoreactivity
- HPA
hypothalamic-pituitary-adrenal axis
- PB
phosphate buffer
- PER1
PERIOD1 protein
- PER1-ir
PERIOD1 immunoreactivity
- PD
postnatal day
- PVN
paraventricular nucleus
- SCN
suprachiasmatic nucleus
- SON
supraoptic nucleus
- TMN
tuberomammillary nucleus
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J. Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M, Basualdo M.d.C., Escobar C, Buijs RM. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl. Acad. Sci. USA. 2011;108:5813–5818. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allingham K, von Salderm C, Brennan PA, Distel H, Hudson R. Endogenous expression of c-Fos in hypothalamic nuclei of neonatal rabbits coincides with their circadian pattern of suckling-associated arousal. Brain Res. 1998;783:210–218. doi: 10.1016/s0006-8993(97)01379-6. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp. Physiol. 2003;286:R158–165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Neural basis of timing and anticipatory behaviors. Eur. J. Neurosci. 2009;30:1643–1649. doi: 10.1111/j.1460-9568.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierle EA, Chen MK, Hartwich JE, Iyengar M, Dai W, Li N, Demarco V, Neu J. Artificial rearing of mouse pups: development of a mouse pup in a cup model. Pediatric Research. 2004;56:250–255. doi: 10.1203/01.PDR.0000132753.81333.39. [DOI] [PubMed] [Google Scholar]
- Brecchia G, Bonanno A, Dall'Aglio C, Mercati F, Zerani M, DiGrigoli A, Boiti C. Neuroendocrine responses in neonatal mother-deprived rabbits. Brain Res. 2009;1304:105–112. doi: 10.1016/j.brainres.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Broekhuizen S, Mulder JL. Differences and similarities in nursing behaviour of hares and rabbits. Acta Zool. Fenn. 1983;174:61–63. [Google Scholar]
- Brummelte S, Schmidt KL, Taves MD, Soma KK, Galea LA. Elevated corticosterone levels in stomach milk, serum, and brain of male and female offspring after maternal corticosterone treatment in the rat. Dev. Neurobiol. 2010;70:714–725. doi: 10.1002/dneu.20805. [DOI] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expresion in hypothalamic oxytocinergic neurons of rabbit pups. Dev. Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Caba M, Tovar A, Silver R, Morgado E, Meza E, Zavaleta Y, Juárez C. Nature's food anticipatory experiment: entrainment of locomotor behavior, suprachiasmatic and dorsomedial hypothalamic nuclei by suckling in rabbit pups. Eur. J. Neurosci. 2008;27:432–443. doi: 10.1111/j.1460-9568.2008.06017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caba M, González-Mariscal G. The rabbit pup, a natural model of nursing anticipatory activity. Eur. J. Neurosci. 2009;30:1697–1706. doi: 10.1111/j.1460-9568.2009.06964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelas I, González B, Montúfar-Chaveznava R, Hudson R. Endogenous clock gene expression in the suprachiasmatic nuclei of previsual newborn rabbits is entrained by nursing. Develop. Neurobiol. 2009;69:47–59. doi: 10.1002/dneu.20687. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LA, Porcu A, Korányi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. Maternal isolation alters the expression of neural proteins during development: 'Stroking' stimulation reverses these effects. Brain. Res. 2007;1158:11–27. doi: 10.1016/j.brainres.2007.04.069. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Rank transformation as a bridge between parametric and nonparametric statistics. Am. Stat. 1981;35:132–133. [Google Scholar]
- Damiola F, Minh NL, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes and Devel. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Muñoz M, Vázquez-Martínez O, Aguilar-Roblero R, Escobar C. Anticipatory changes in liver metabolism and entrainment of insulin, glucagon and corticosterone in food-restricted rats. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2000;279:2048–2056. doi: 10.1152/ajpregu.2000.279.6.R2048. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain. Res. 1992;578:256–60. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- Gerhard L. Atlas of the Mes- and Diencephalon of the Rabbit. Springer-Verlag; Berlín: 1968. [Google Scholar]
- Girgis M, Shih-Chang W. A New Stereotaxic Atlas of the Rabbit Brain. W. H. Green; St. Louis: 1981. [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J. Neurosci. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosvenor CE, Picciano MF, Baumrucker CR. Hormones and growth factors in milk. Endoc. Rev. 1993;14:710–728. doi: 10.1210/edrv-14-6-710. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Hernández-Campos O, Montúfar-Chaveznava R, Caldelas I. 3rd World Congress of Chronobiology. Puebla; México: 2011. Do newborn rabbits bearing suprachiasmatic nuclei lesions anticipate to nursing? [Google Scholar]
- Honma K, Noe Y, Honma S, Katsuno Y, Hiroshighe T. Roles of paraventricular catecholamines in feeding-associated corticosterone rhythm in rats. Am. J. Physiol. Endocrinol. Metab. 1992;25:E948–E965. doi: 10.1152/ajpendo.1992.262.6.E948. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. Temporal pattern of suckling in rabbit pups: a model of circadian synchrony between mother and young. In: Reppert SM, editor. Development of circadian rhythmicity and photoperiodism in mammals. Perinatology Press; Ithaca, NY: 1989. pp. 83–102. [Google Scholar]
- Inzunza O, Serón-Ferré MJ, Bravo H, Torrealba F. Tuberoammillary nucleus activation anticipates feeding under a restricted schedule in rats. Neurosci. Lett. 2000;293:139–142. doi: 10.1016/s0304-3940(00)01516-0. [DOI] [PubMed] [Google Scholar]
- Jilge B. The ontogeny of circadian rhythms in the rabbit. J. Biol. Rhytms. 1993;8:247–260. doi: 10.1177/074873049300800307. [DOI] [PubMed] [Google Scholar]
- Jilge B. Ontogeny of the rabbit's rhythms without an external zeitgeber. Physiol. Behav. 1995;58:131–140. doi: 10.1016/0031-9384(95)00006-5. [DOI] [PubMed] [Google Scholar]
- Jilge B, Kuhnt B, Landerer W, Rest S. Circadian thermoregulation in suckling rabbit pups. J. Biol. Rhythms. 2000;15:329–35. doi: 10.1177/074873000129001431. [DOI] [PubMed] [Google Scholar]
- Krieger D. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: timing is everything. Horm. Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Korets R, Silver R. Expression of the circadian clock gene Period1 in neuroendocrine cells: an investigation using mice with a Per1∷GFP transgene. Eur. J. Neurosci. 2003;17:212–220. doi: 10.1046/j.1460-9568.2003.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol. Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Melo AI, Hernández-Curiel M, Hoffman KL. Maternal and peer contact during the postnatal period participate in the normal development of maternal aggression, maternal behavior, and the behavioral response to novelty. Behav. Brain. Res. 2009;201:14–21. doi: 10.1016/j.bbr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Messer M, Thoman EB, Galofre A, Dallman T, Dallman PR. Artificial feeding of infant rats by continuous infusion. Journal of Nutrition. 1969;98:404–410. doi: 10.1093/jn/98.4.404. [DOI] [PubMed] [Google Scholar]
- Meza E, Juárez C, Morgado E, Zavaleta Y, Caba M. Brief daily suckling shifts locomotor behavior and induces PER1 protein in paraventricular and supraoptic nuclei, but not in the suprachiasmatic nucleus, of Rabbit does. Eur. J. Neurosci. 2008;28:1394–1403. doi: 10.1111/j.1460-9568.2008.06408.x. [DOI] [PubMed] [Google Scholar]
- Meza E, Waliszewski SM, Caba M. Circadian nursing induces PER1 protein in neuroendocrine tyrosine hydroxylase neurons in the rabbit doe. J. Neuroendocrinol. 2011;23:472–480. doi: 10.1111/j.1365-2826.2011.02138.x. [DOI] [PubMed] [Google Scholar]
- Mistlbeger RE. Circadian Food-Anticipatory Activity: Formal Models and Physiological Mechanisma. Neuroci. Biobehav. Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moberg GP, Bellinger LL, Mendel VE. Effect of meal feeding on daily rhythms of plasma corticosterone and growth hormone in the rat. Neuroendocrinology. 1975;19:160–169. doi: 10.1159/000122436. [DOI] [PubMed] [Google Scholar]
- Morgado E, Gordon MK, Minana-Solis M.d.C., Meza E, Levine S, Escobar C, Caba M. Hormonal and metabolic rhythms associated with the daily scheduled nursing in rabbit pups. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R690–R695. doi: 10.1152/ajpregu.00162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado E, Meza E, Gordon MK, Pau KY, Juárez C, Caba M. Persistence of hormonal and metabolic rhythms during fasting in 7-to 9-day-old rabbits entrained by nursing during night. Hormons and Behavior. 2010;58:465–472. doi: 10.1016/j.yhbeh.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski PK, Klockars A, Schiöth HB, Levine AS. Oxytocin as feeding inhibitor: maintaining homeostasis in consumatory behavior. Pharmacol. Biochem. Behav. 2010;97:47–54. doi: 10.1016/j.pbb.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongrácz P, Altbäcker V. Arousal, but not nursing, is necessary to elicit a decreased fear reaction toward humans in rabbit pups. Developmental Psychobiology. 2003;43:192–199. doi: 10.1002/dev.10132. [DOI] [PubMed] [Google Scholar]
- Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am. J. Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- Rovirosa MJ, Levine S, Gordon MK, Caba M. Circadian rhythm of corticosterone secretion in the neonatal rabbit. Dev. Brain. Res. 2005;8:92–96. doi: 10.1016/j.devbrainres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Rowe SA, Kennaway DJ. Melatonin in rat milk and the likelihood of its role in postnatal maternal entrainment of rhythms. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2002;282:R797–R804. doi: 10.1152/ajpregu.00228.2001. [DOI] [PubMed] [Google Scholar]
- Schuh D, Hoy S, Selzer D. Vocalization of rabbit pups in the mother-young relationship; 8thWorld Rabbit Congress; 2004. pp. 1266–1270. [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24 hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav. Neural Biol. 1979;25:345–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a zeitgeber. J. Biol. Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Suckow MA, Douglas FA. The Laboratory Rabbit. 2nd ed. CRC Press; 1997. [Google Scholar]
- Szeto A, Gonzales JA, Spitzer SB, Levine JE, Zaias PG, Saab N, Schneiderman N, McCabe PM. Circulating levels of glucocorticoid hormones in WHHL and NZW rabbits: circadian cycle and response to repeated social encounter. Psychoneuroendocrinology. 2004;29:861–866. doi: 10.1016/S0306-4530(03)00153-7. [DOI] [PubMed] [Google Scholar]
- Valdés JL, Farías P, Ocampo-Garcés A, Cortés N, Serón-Ferré M, Torrealba F. Arousal and differential Fos expression in histaminergic neurons of the ascending arousal system during a feeding-related motivated behaviour. Eur. J. Neurosci. 2005;21:1931–1942. doi: 10.1111/j.1460-9568.2005.04013.x. [DOI] [PubMed] [Google Scholar]
- Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM. Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv. Exp. Med. Biol. 1995;395:209–225. [PubMed] [Google Scholar]
- Verwey M, Amir S. Food-entrainable circadian oscillators in the brain. Eur. J. Neurosci. 2009;30:1650–1657. doi: 10.1111/j.1460-9568.2009.06960.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur. J. Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- Withworth NS, Grosvenor CE. Transfer of milk prolactin to the plasma of neonatal rats by intestinal absorption. J. Endocrinol. 1978;79:191–199. doi: 10.1677/joe.0.0790191. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Denenberg VH, Anderson C. Rabbit: frequency of suckling in the pup. Science. 1965;150:1835–1836. doi: 10.1126/science.150.3705.1835. [DOI] [PubMed] [Google Scholar]