Abstract
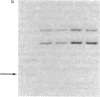
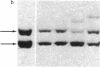
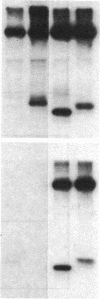
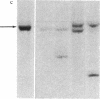
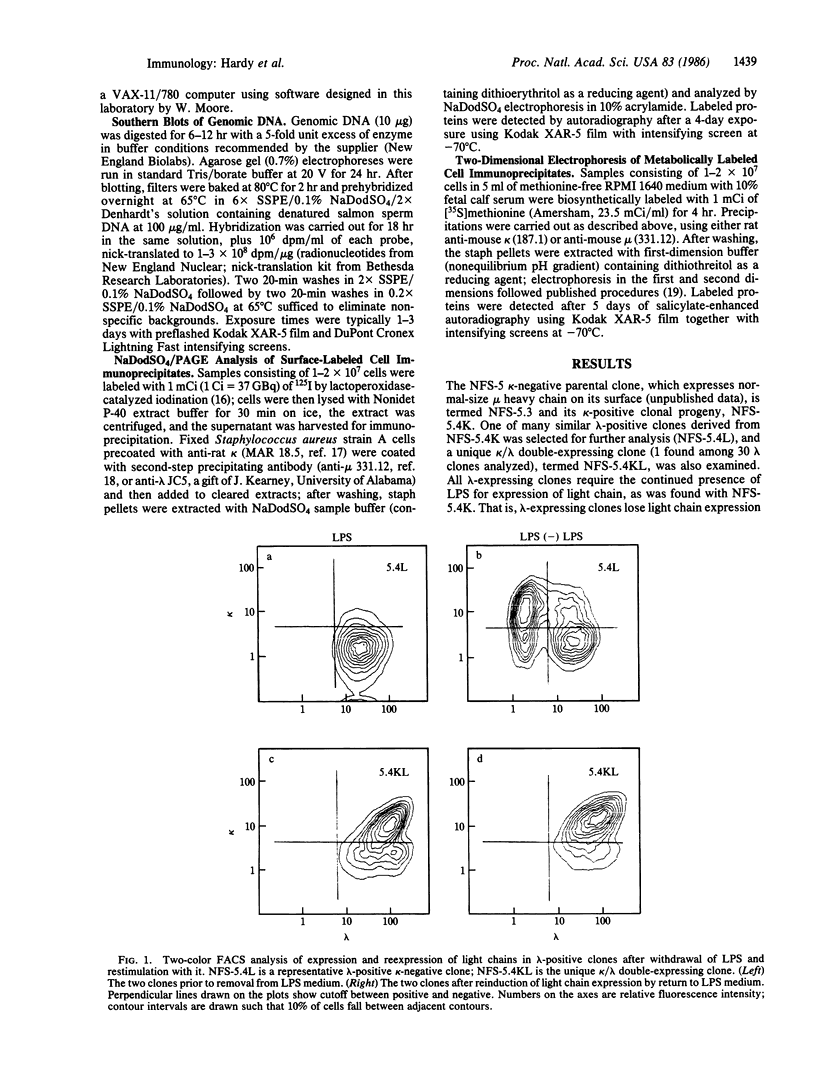
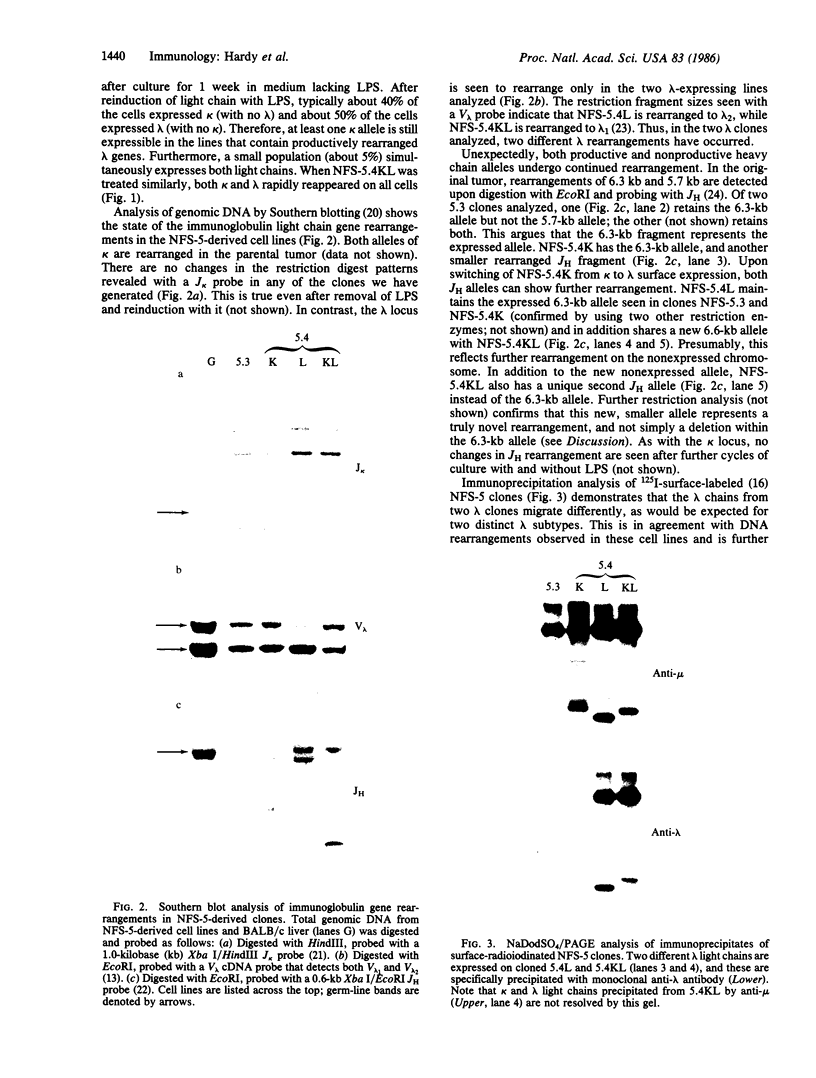
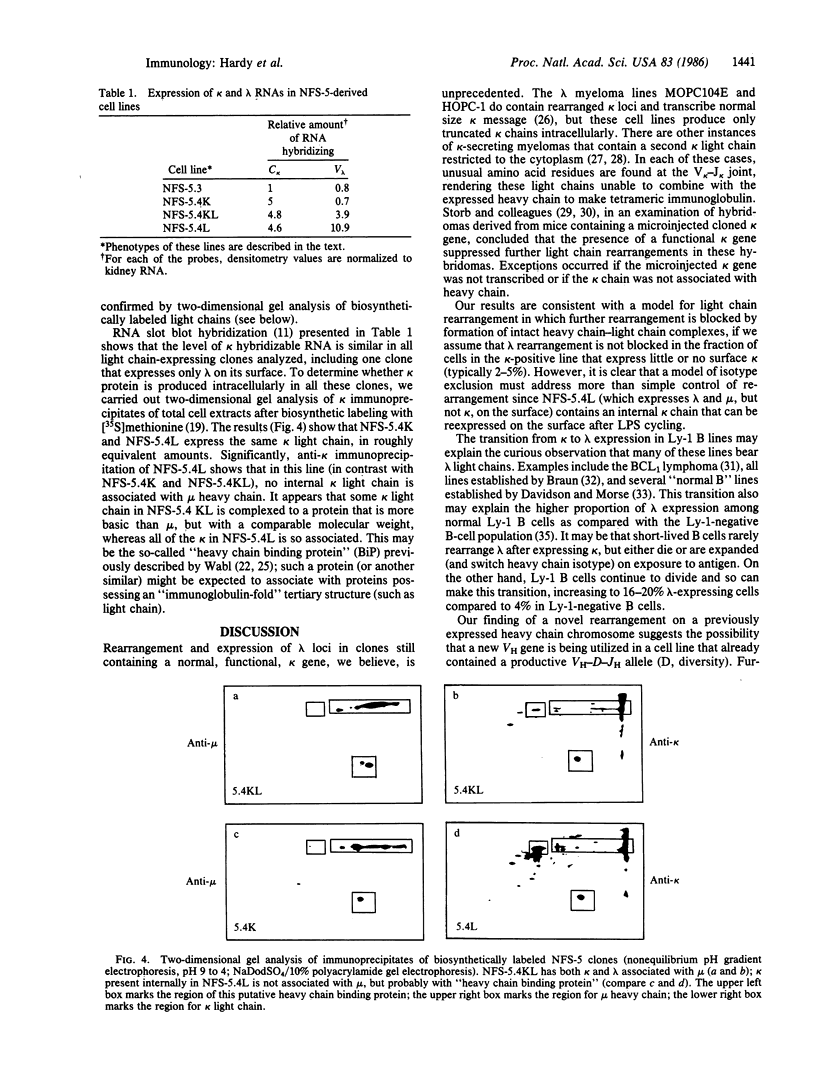
We describe here a murine Ly-1-bearing pre-B-cell tumor that, when induced for kappa light chain expression with bacterial lipopolysaccharide, also gives rise spontaneously to a few percent of cells expressing surface lambda light chains. These lambda-positive cells have undergone DNA rearrangements involving either V lambda 1 or V lambda 2 genes. Nearly all clones of lambda-bearing cells express mu and lambda on their surface (but not kappa). However, all these lambda-positive clones continue to transcribe kappa mRNA and synthesize internal kappa chains. Further, surface lambda-positive clones show JH rearrangements on one or both heavy chain chromosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Bernard O., Gough N. M., Adams J. M. Plasmacytomas with more than one immunoglobulin kappa mRNA: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5812–5816. doi: 10.1073/pnas.78.9.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B., Traunecker A., Eisen H., Tonegawa S. Organization of four mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3765–3769. doi: 10.1073/pnas.78.6.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. Spontaneous in vitro occurrence and long-term culture of murine B lymphoblast cell lines. J Immunol. 1983 May;130(5):2113–2116. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman R. L. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1982;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Fredrickson T. N., Rudikoff E. K., Coffman R. L., Hartley J. W., Morse H. C., 3rd A unique series of lymphomas related to the Ly-1+ lineage of B lymphocyte differentiation. J Immunol. 1984 Aug;133(2):744–753. [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Haaijman J., Herzenberg L. A. B-cell subpopulations identified by two-colour fluorescence analysis. Nature. 1982 Jun 17;297(5867):589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Herzenberg L. A., Morse H. C., 3rd, Davidson W. F., Herzenberg L. A. Ly-1 as a differentiation antigen on normal and neoplastic B cells. Curr Top Microbiol Immunol. 1984;113:231–236. doi: 10.1007/978-3-642-69860-6_39. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Parks D. R., Herzenberg L. A., Herzenberg L. A. Murine B cell differentiation lineages. J Exp Med. 1984 Apr 1;159(4):1169–1188. doi: 10.1084/jem.159.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Knapp M. R., Jones P. P., Black S. J., Vitetta E. S., Slavin S., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). I. Cell surface expression of IgM, IgD, Ia, and FcR. J Immunol. 1979 Sep;123(3):992–999. [PubMed] [Google Scholar]
- Kwan S. P., Max E. E., Seidman J. G., Leder P., Scharff M. D. Two kappa immunoglobulin genes are expressed in the myeloma S107. Cell. 1981 Oct;26(1 Pt 1):57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Warner N. L., Ledbetter J. A., Herzenberg L. A. Expression of Lyt-1 antigen on certain murine B cell lymphomas. J Exp Med. 1981 Apr 1;153(4):998–1003. doi: 10.1084/jem.153.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Bothwell A., Storb U. Physical linkage of the constant region genes for immunoglobulins lambda I and lambda III. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3829–3833. doi: 10.1073/pnas.78.6.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Lamers M. C., Rudikoff E. K., Davidson W. F. Characteristics of continuous in vitro lines of BALB/c and NZB B cells. Curr Top Microbiol Immunol. 1984;113:210–216. doi: 10.1007/978-3-642-69860-6_36. [DOI] [PubMed] [Google Scholar]
- Newell N., Richards J. E., Tucker P. W., Blattner F. R. J genes for heavy chain immunoglobulins of mouse. Science. 1980 Sep 5;209(4461):1128–1132. doi: 10.1126/science.6250219. [DOI] [PubMed] [Google Scholar]
- Ritchie K. A., Brinster R. L., Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984 Dec 6;312(5994):517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Storb U., O'Brien R. L., McMullen M. D., Gollahon K. A., Brinster R. L. High expression of cloned immunoglobulin kappa gene in transgenic mice is restricted to B lymphocytes. Nature. 1984 Jul 19;310(5974):238–241. doi: 10.1038/310238a0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wabl M., Steinberg C. A theory of allelic and isotypic exclusion for immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6976–6978. doi: 10.1073/pnas.79.22.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]