Abstract
Background
Iron-deficiency anemia (IDA) is associated with alterations in infant behavior and development that may not be corrected with iron therapy.
Objective
To determine if a home-based intervention to foster child development improves behavior and development of infants with IDA.
Methods
Infants with IDA and nonanemic infants aged 6 and 12 months were treated with oral iron and randomly assigned to a year of surveillance or intervention. Infants in the surveillance group were visited weekly, and information on iron intake, feeding, and health were recorded. Infants in the intervention were visited weekly, and the home visits included an hour-long program to foster child development by providing support to the mother-infant relationship. The number of infants enrolled was 128 (66 who received intervention) and 149 (70 intervention) at 6 and 12 months, respectively. Psychologists who were unaware of iron status and intervention assignment assessed infants' cognitive, motor, and social-emotional development (Bayley Scales) at the beginning, midpoint, and end of the year; 116 6-month-olds and 134 12-month-olds had at least 2 assessments. Hierarchical linear modeling was used to analyze change over time.
Results
Infants with IDA, regardless of enrollment age, were rated as less positive in social-emotional behavior at baseline. There were significant interactions between iron status and intervention associated with change in cognitive performance and positive social-emotional behavior. Infants with IDA who received intervention had developmental trajectories comparable to those of nonanemic infants in the intervention and surveillance groups, but these infants did not catch up in social-emotional behavior. Infants with IDA who received surveillance showed less increase in cognitive scores and had declines in positive social-emotional ratings.
Conclusions
Home-based intervention to foster child development improved cognitive and social-emotional scores in infants with IDA, but social-emotional differences remained between infants with IDA and those without IDA.
Keywords: anemia, iron deficiency, home-visiting, infants, development
Results of numerous studies have demonstrated that infants with iron-deficiency anemia (IDA) show poorer cognitive, motor, social-emotional, and neurophysiologic functioning than those without.1–5 Studies that included reassessments after a full course of iron therapy have had mixed results. In the majority of these studies, alterations were found to persist in infants with IDA despite therapy,6–10 but complete correction of low cognitive and motor scores has also been reported.11,12 Results of follow-up studies at ages from later infancy to young adulthood have shown poorer outcomes in the cognitive, motor, social-emotional, and neurophysiologic domains in individuals treated in infancy for IDA or chronic iron deficiency. These results indicate long-term risk.3,13
These findings are similar to those reported for studies of undernourished infants, especially in developing countries. In these infants, results for developmental recovery have been more promising when developmental support was provided in addition to improved nutrition.14–23 To our knowledge, the use of this approach in infants with iron deficiency has not been reported. In our study we sought to determine if a home-based intervention program designed to foster child development, provided in combination with iron therapy, could improve behavior and development in infants with IDA.
Subjects and Methods
Study Design
This intervention study was conducted in conjunction with a randomized trial of developmental/behavioral effects of prevention of IDA in infancy. Infants from working-class communities near Santiago, Chile, who received well-child care in community clinics were considered for study participation. Entrance criteria included birth weight ≥3.0 kg, uncomplicated singleton term birth, and no acute or chronic health problems. Exclusion criteria included illiterate or mentally impaired caregiver, infant in day care, and residence outside the neighborhoods. Sample details have been published previously.24–29
The early-intervention component of the study, with enrollment conducted from 1991 to 1995, involved mothers and their infants who were identified as having IDA or who were clearly non-anemic at either 6 or 12 months.24–29 When the hematology laboratory identified infants with IDA, they also identified the next nonanemic infant on the basis of date and time of venipuncture, a random selection process. The laboratory provided the project coordinator with the identification numbers of infants who were identified for possible study inclusion during periods of 1 to 2 weeks and did not indicate iron status. Anemia was defined as a venous hemoglobin concentration of ≤100 g/L at 6 months and <110 g/L at 12 months; clearly nonanemic as a hemoglobin concentration of ≥115 g/L. Iron deficiency was defined as 2 or more abnormal iron measures (a mean corpuscular volume of <70 fL, free erythrocyte protoporphyrin concentration of ≥100 μg/dL red blood cells [1.77 μmol/L], serum ferritin concentration of <12 μg/L), and/or an increase in hemoglobin concentration of ≥10 g/L after 6 months of iron therapy. Infants with IDA and randomly selected infants without IDA at 6 months were invited to participate in early intervention and neuromaturation components of the project (6-month cohort) and were treated for 1 year with 15 mg per day of elemental iron as oral ferrous sulfate.30 The preventive trial ended when the infants were 12 months old, and then infants with IDA and randomly selected infants without IDA were also invited to join the early-intervention study (12-month cohort) and treated with oral iron (30 mg per day) for at least 6 months.
The institutional review boards of the University of Michigan Medical Center and the Instituto de Nutrición y Tec-nología de los Alimentos, University of Chile, approved the protocol. Signed informed consent was obtained from the parent(s) or guardians of all participating infants.
Intervention
All infants received weekly home visits during the year of intervention study participation, beginning when the infants were 6 or 12 months old for the 6- and 12-month cohorts, respectively. For surveillance-only visits, project personnel recorded data on the infant's iron intake, feeding, and health. Intervention visits included implementation of a program to support child development, and surveillance data were also recorded. The frequency and duration of the intervention program were determined on the basis of research data available at the time.31,32 After considering several models, we focused the intervention on the mother-child relationship,33,34 because we anticipated that this focus would foster cognitive and social-emotional development, both of which were consistently found to be poorer in infants with IDA compared with infants without IDA.1–5 Consideration of the family context was also an integral part of the program.
Intervention visits, which lasted about an hour, were conducted by monitors, who were professional educators devoted to encouraging the development of individual abilities. The monitors were trained and supervised by project psychologists. Monitors worked half time and visited ñ6 families per week and had weekly group and individual supervision. Three manuals that covered the diagnostic phase and intervention in the first and second year of life were developed to guide training and ongoing work with families (available on request, in Spanish only).
The program consisted of 3 phases: introduction (14% of visits), intervention (70% of visits), and termination (16% of visits). The main introduction-phase objectives were to familiarize the mother with the program and have the monitor assess the strengths and weaknesses of the family and mother/child dyad. The monitor interviewed the mother, observed the mother and infant at home, and reached agreement with the mother about areas of the mother-infant relationship to be addressed. The main intervention-phase objective was to support the mother/infant dyad within the family context according to the agreed-on goals. Monitors showed mothers enjoyable verbal and nonverbal activities with the infants, demonstrated positive feedback, discussed child development issues, provided mothers with related written material, and supported them in their interactions with their infants. Monitors also considered with the mother ways to address problems or concerns related to the behavior and development of the infant and relationships in the family. The main termination-phase objectives were to reinforce the mother's independent abilities to support her child's development, resolve problems, and address ending the program and the year-long relationship with the monitor.
Randomization, Masking, and Sample Size
The study was a randomized single-blind clinical trial. Infants were randomly assigned to intervention or surveillance within IDA and nonanemic groups on the basis of a predetermined algorithm. All outcome measures were obtained by psychologists who had no role in the intervention program and were unaware if the infant had IDA or received intervention. Of necessity, parents and home visitors knew if a given infant received intervention.
To have >80% power to detect an intervention impact of 0.50 SD or more on cognitive or motor test scores, the desired sample size was 50 infants per group at each age. Infants were enrolled on the basis of openings in the schedule of the monitors, until a shortage of funds necessitated a halt in enrollment in August 1995. Cell sizes ended up being approximately two thirds of the desired n in the 6-month cohort, because IDA was uncommon at this young age. After study completion, we also noted that infants with IDA were over represented in the 12-month cohort. This result was likely attributable to the study design and limitations in the number of families that monitors could accommodate at a given time. The imbalance was not recognized until the study was completed and the code was broken.
Outcomes
Outcomes were assessed at 3 time points for a period of 1 year: at ages 6, 12, and 18 months for the 6-month cohort and 12,18, and 24 months for the 12-month cohort. Testers were trained to ≥85% agreement before starting the study, with random reliability checks thereafter. Overall cognitive and motor development were measured by use of the original Bayley Scales of Infant Development,35 the version available at the time. To have a single metric to assess change over time, we analyzed raw mental and motor scores. Behavioral measures were derived from a prerelease version of the Behavior Rating Scale36 and based on a previously reported factor analysis conducted for more than 1600 infants who completed the preventive trial at 12 months.24 The factors measured were (1) object orientation, (2) motor quality, (3) negative affect, and (4) positive social responsiveness. To permit analysis of behavioral change over time, we used factor score weights from the preventive trial analysis to construct the 4 factors at each time point.
Statistics
Background/baseline characteristics at enrollment were compared by use of intention to treat (analysis of variance and χ2, SPSS 16.0 for Windows [SPSS Inc, Chicago, IL]). Effects of intervention, which required at least 2 data points to estimate change over time and thus could not be analyzed by intention to treat, were examined by use of 2-stage hierarchical linear modeling (HLM) of measures within individuals (HLM 6: SSI Scientific Software, Chicago, IL). For the within-person stage (level 1), we modeled 2 changes over time (start to midpoint and midpoint to end) with the intercept fixed and the 2 slopes allowed to vary. Individual age at testing, measured in months, was the time parameter. For the between-person stage (level 2), we modeled the impact of intervention group, iron status group, and the interaction between intervention and iron status during each half-year period. We first examined the 3-way interaction between iron group and intervention group over time for each cohort. When a significant interaction was identified, we examined the impact of the intervention on change in outcome separately according to iron status. Because there were no group differences in background characteristics at the level of randomization (see below), we identified potential covariates on the basis of significant correlations with cognitive or motor scores.
Results
Subjects and Baseline Characteristics
Enrollment included 128 infants at 6 months (66 intervention) and 149 infants at 12 months (70 intervention). Background characteristic are shown in Table 1 and Table 2. Intervention and surveillance groups within IDA and nonanemic groups (the levels of randomization) were similar in background in each cohort. As often observed,37 infants with IDA had lower gestational age or birth weight, and their mothers had less education (and lower IQ in the 6-month cohort); a higher proportion of infants with IDA were male. Maternal education and IQ did not correlate with cognitive test scores in these young infants. Gender and birth weight were the only background characteristics significantly related to developmental test scores; these variables were controlled in all analyses. There were no differences in temperament at 6 months38 (data not shown). Table 3 shows differences between infants with IDA and nonanemic infants in iron status measures.
Table 1. Characteristics of the 6-Month Cohort at Enrollment.
| IDA | Nonanemic | |||
|---|---|---|---|---|
| Surveillance (N = 36) |
Intervention (N = 33) |
Surveillance (N = 26) |
Intervention (N = 33) |
|
| Family characteristics | ||||
| Maternal age, y | 27.6 ± 1.5 | 27.1 ± 1.2 | 26.6 ± 1.0 | 27.3 ± 1.2 |
| Maternal education, ya | 7.9 ± 0.5 | 9.2 ± 0.6 | 9.5 ± 0.6 | 9.9 ± 0.4 |
| Maternal IQa,b | 79.5 ± 1.6 | 81.8 ± 2.0 | 86.4 ± 2.0 | 84.9 ± 2.0 |
| Maternal depressionc | 16.9 ± 2.1 | 14.1 ± 2.4 | 15.3 ± 2.4 | 9.9 ± 1.6 |
| Father present, n (%) | 26 (72.2) | 26 (78.8) | 22 (84.6) | 25 (75.8) |
| Household head education, y | 8.0 ± 0.5 | 9.0 ± 0.6 | 9.2 ± 0.6 | 8.7 ± 0.7 |
| No. of people in household | 5.0 ± 0.2 | 5.2 ± 0.4 | 5.2 ± 0.4 | 6.0 ± 0.6 |
| Social class indexd | 27.5 ± 1.2 | 29.9 ± 1.4 | 28.2 ± 1.6 | 27.4 ± 1.2 |
| Life stressa,e | 4.9 ± 0.4 | 4.9 ± 0.5 | 4.0 ± 0.4 | 3.6 ± 0.4 |
| Home environmentf | 28.1 ± 0.8 | 27.9 ± 1.1 | 29.7 ± 0.9 | 29.5 ± 0.9 |
| Child characteristics | ||||
| Male, n (%)g | 23 (63.9) | 28 (84.8) | 14 (53.8) | 17 (51.5) |
| Birth weight, kga | 3.38 ± 0.05 | 3.47 ± 0.06 | 3.57 ± 0.08 | 3.60 ± 0.07 |
| Gestational age, wk | 39.2 ± 0.2 | 39.1 ± 0.2 | 39.4 ± 0.2 | 39.5 ± 0.2 |
| Weight-for-age z score | − 0.26 ± 0.14 | − 0.04 ± 0.19 | 0.19 ± 0.15 | 0.12 ± 0.17 |
| Length-for-age z score | − 0.23 ± 0.12 | 0.15 ± 0.16 | −0.07 ± 0.14 | − 0.04 ± 0.17 |
| Age at first bottle, d | 101.7 ± 14.9 | 145.7 ± 24.2 | 103.1 ± 20.4 | 110.9 ± 18.9 |
| Intake of milk/formula, mL/d | 451.3 ± 58.9 | 366.5 ± 63.2 | 451.1 ± 73.5 | 391.7 ± 70.0 |
| Baseline developmental status | ||||
| Age at first test, mo | 7.5 ± 0.1 | 7.5 ± 0.1 | 7.5 ± 0.1 | 7.3 ± 0.2 |
| Mental raw score, points | 78.5 ± 0.9 | 76.9 ± 0.9 | 76.1 ± 0.7 | 75.7 ± 0.6 |
| Mental Development Index | 102.9 ± 3.2 | 101.6 ± 2.7 | 97.0 ± 2.5 | 97.7 ± 2.4 |
| Motor raw score, points | 29.9 ± 0.6 | 29.1 ± 0.7 | 28.9 ± 0.5 | 28.8 ± 0.6 |
| Motor Development Index | 94.7 ± 2.1 | 96.5 ± 2.5 | 94.7 ± 2.2 | 96.7 ± 2.3 |
| Behavior-rating scaleh | ||||
| Factor 1 | 0.01 ± 0.02 | 0.01 ± 0.03 | −0.02 ± 0.02 | − 0.01 ± 0.02 |
| Factor 2 | − 0.09 ± 0.04 | − 0.10 ± 0.05 | −0.12 ± 0.04 | − 0.11 ± 0.05 |
| Factor 3 | 0.11 ± 0.02 | 0.13 ± 0.02 | 0.09 ± 0.02 | 0.01 ± 0.03 |
| Factor 4a | − 0.11 ± 0.06 | − 0.11 ± 0.06 | 0.09 ± 0.06 | 0.12 ± 0.05 |
| No. of weekly visits before infant's last assessmenti | 29.6 ± 1.9 | 30.6 ± 1.4 | 30.1 ± 1.9 | 30.1 ± 1.9 |
Values are mean ± SE unless otherwise indicated. Behavior Rating Scale factor scores are expressed in SD units. Values for n are at enrollment (intention to treat); 1 infant with IDA and 2 nonanemic infants were removed before the baseline developmental assessment.
Values for the IDA group were significantly lower than those for the nonanemic group (P< .05), as tested by 2-way analysis of variance.
Measured by use of a short form of the Wechsler Adult Intelligence Scale, Revised.49
Measured by use of the Center for Epidemiologic Studies Depression Scale.50
Measured by use of the Graffar Scale,51 designed to differentiate families at the lower end of the socioeconomic spectrum.
Measured by use of a scale modified from Weinraub et al (1987).52
Measured by use of the Home Observation for Measurement of the Environment, Revised.53
More infants with IDA than infants without IDA were male (P = .01).
Behavior-Rating Scale factors consisted of the following scales: factor 1, energy, interest, initiative, exploration, attention, persistence, enthusiasm, and fearfulness; factor 2, gross-motor, fine-motor, movement control, hypotonicity, and slow movement; factor 3, negative affect, hypersensitivity, adaptation, frustration, orientation, and cooperation; and factor 4, positive affect, social referencing, and social engagement.
The number of visits before the second assessment was used if there was no third assessment.
Table 2. Characteristics of the 12-Month Cohort at Enrollment.
| IDA | Nonanemic | |||
|---|---|---|---|---|
| Surveillance (N = 44) |
Intervention (N = 44) |
Surveillance (N = 35) |
Intervention (N = 36) |
|
| Family characteristics | ||||
| Maternal age, y | 25.5 ± 1.0 | 25.8 ± 1.1 | 26.5 ± 1.2 | 27.0 ± 1.2 |
| Maternal education, ya | 8.4 ± 0.4 | 9.0 ± 0.3 | 9.7 ± 0.4 | 10.1 ± 0.5 |
| Maternal IQb | 83.8 ± 1.3 | 82.5 ± 1.2 | 83.9 ± 1.6 | 86.7 ± 2.3 |
| Maternal depressionc | 14.5 ± 1.6 | 19.9 ± 2.0 | 16.0 ± 1.7 | 16.0 ± 1.9 |
| Father present, n (%) | 31 (72.1) | 37 (88.1) | 29 (82.9) | 19 (73.1) |
| Household head education, y | 8.3 ± 0.5 | 8.1 ± 0.4 | 7.7 ± 0.6 | 7.4 ± 0.8 |
| No. of people in householda | 5.4 ± 0.4 | 4.8 ± 0.2 | 5.4 ± 0.4 | 5.6 ± 0.5 |
| Social class indexd | 29.0 ± 1.0 | 28.6 ± 1.0 | 26.8 ± 1.1 | 27.3 ± 1.4 |
| Life stresse | 4.9 ± 0.4 | 5.6 ± 0.4 | 5.0 ± 0.5 | 5.8 ± 0.6 |
| Home environmentf | 31.5 ± 0.8 | 30.2 ± 0.8 | 30.1 ± 1.0 | 31.1 ± 0.8 |
| Child characteristics | ||||
| Male, n (%)g | 28 (65.1) | 32 (76.2) | 13 (37.1) | 10 (38.5) |
| Birth weight, kg | 3.50 ± 0.06 | 3.47 ± 0.05 | 3.61 ± 0.06 | 3.58 ± 0.08 |
| Gestational age, wka | 39.3 ± 0.2 | 39.2 ± 0.2 | 39.7 ± 0.2 | 39.7 ± 0.2 |
| Weight-for-age z score | − 0.05 ± 0.14 | − 0.18 ± 0.16 | −0.18 ± 0.16 | − 0.13 ± 0.20 |
| Length-for-age z score | −0.003 ± 0.11 | − 0.23 ± 0.15 | −0.03 ± 0.13 | − 0.08 ± 0.16 |
| Age at first bottle, da | 111.1 ±14.2 | 100.4 ± 13.9 | 105.9 ± 9.9 | 72.6 ± 17.9 |
| Intake of milk/formula, mL/d | 449.5 ± 35.3 | 496.3 ± 36.5 | 486.2 ± 40.8 | 551.5 ± 39.3 |
| Baseline developmental status | ||||
| Age at first test, mo | 12.3 ± 0.05 | 12.3 ± 0.05 | 12.3 ± 0.06 | 12.2 ± 0.05 |
| Mental raw score, points | 104.1 ± 0.8 | 104.2 ± 0.7 | 104.2 ± 0.5 | 103.8 ± 1.1 |
| Mental Development Index | 103.3 ± 2.0 | 103.6 ± 1.8 | 104.4 ± 1.9 | 102.7 ± 2.7 |
| Motor raw score, points | 45.8 ± 0.4 | 45.6 ± 0.4 | 45.7 ± 0.4 | 45.9 ± 0.5 |
| Motor development index | 96.8 ± 2.3 | 96.1 ± 2.3 | 96.1 ± 2.3 | 97.4 ± 3.1 |
| Behavior rating scaleh | ||||
| Factor 1 | 0.01 ± 0.02 | − 0.003 ± 0.02 | −0.03 ± 0.02 | 0.01 ± 0.02 |
| Factor 2 | − 0.01 ± 0.03 | − 0.03 ± 0.04 | 0.03 ± 0.03 | 0.02 ± 0.04 |
| Factor 3 | 0.004 ± 0.03 | 0.02 ± 0.03 | −0.02 ± 0.04 | 0.01 ± 0.02 |
| Factor 4a | − 0.11 ± 0.04 | − 0.12 ± 0.04 | −0.07 ± 0.05 | 0.02 ± 0.05 |
| No. of weekly visits before infant's last assessmenti | 31.3 ± 1.8 | 28.1 ± 2.1 | 28.7 ± 1.3 | 28.7 ± 1.8 |
Values are mean ± SE unless otherwise indicated. Values for n shown are at enrollment (intention to treat); 1 nonanemic infant was removed from the study before the baseline developmental assessment.
Results for the IDA group were significantly lower than those for the nonanemic group (P< .05) as tested by 2-way analysis of variance, except for age at first bottle, which was higher.
Measured by use of a short form of the Wechsler Adult Intelligence Scale, revised.49
Measured by use of the Center for Epidemiologic Studies Depression Scale.50
Measured by use of the Graffar Scale,51 designed to differentiate families at the lower end of the socioeconomic spectrum.
Measured by use of a scale modified from Weinraub et al (1987).52
Measured by use of the Home Observation for Measurement of the Environment, revised.53
More infants with IDA than infants without IDA were male (P < .0001).
See Table 1 footnote for the scales comprising each factor.
The number of visits before the second assessment was used if there was no third assessment.
Table 3. Iron Status of Infants With and Without IDA During the Course of Iron Therapy.
| 6-mo Cohort | 12-mo Cohort | |||
|---|---|---|---|---|
| IDA | Nonanemic | IDA | Nonanemic | |
| Hemoglobin, g/L | ||||
| Pretreatmenta | 95.0 ± 0.8 | 121.4 ± 0.6 | 104.1 ± 0.6 | 125.4 ± 0.6 |
| After 6 mo of irona,b | 118.0 ± 1.2 | 127.1 ± 0.9 | 120.4 ± 0.9 | 129.2 ± 1.1 |
| After 12 mo of iron | 122.1 ± 1.5 | 126.5 ± 1.8 | 124.4 ± 1.2a,b | 130.5 ± 1.4a,b |
| Mean corpuscular volume, fL | ||||
| Pretreatmenta | 66.1 ± 0.6 | 73.8 ± 0.4 | 66.9 ± 0.5 | 76.4 ± 0.4 |
| After 6 mo of irona,b | 72.7 ± 0.5 | 76.6 ± 0.4 | 73.7 ± 0.5 | 78.2 ± 0.5 |
| Free erythrocyte protoporphyrin, μg/dL RBC | ||||
| Pretreatmenta | 185.7 ± 12.3 | 95.0 ± 3.1 | 163.4 ± 6.9 | 80.5 ± 1.7 |
| After 6 mo of irona,b | 97.5 ± 3.5 | 76.6 ± 2.3 | 92.7 ± 4.1 | 69.1 ± 2.3 |
| Ferritin, μg/L | ||||
| Pretreatmenta | 10.9 ± 1.3 | 17.8 ± 1.9 | 6.2 ± 0.8 | 14.5 ± 1.1 |
| After 6 mo of irona,b | 18.8 ± 1.8 | 25.0 ± 2.3 | 15.7 ± 1.3 | 23.7 ± 2.1 |
Values are mean ± SE. RBC indicates red blood cells.
Difference between IDA and nonanemic groups
change within group from previous time point significant at P< .05 for both cohorts. The only exception was hemoglobin after 12 months of iron, for which the difference between IDA and nonanemic groups and the within-group increase from 6 to 12 months of iron treatment were not significant in the 6-month cohort.
Baseline Test Results
For infants with IDA in both cohorts, ratings in positive social-emotional responsiveness (fourth behavioral rating factor) were lower than for infants without IDA, with differences of −0.22 SD in the 6-month cohort (95% CI: −0.28 to −0.16; P< .001) and −0.09 SD in the 12-month cohort (95% CI: −0.14 to −0.05; P = .002). Groups were similar in baseline mental and motor raw scores and other behavior-rating factors.
Exposure to Intervention or Surveillance
Results for exposure measured by attrition or number of visits seemed comparable. Figs 1 and 2 show the number of participants at each time point. In the 6-month cohort, there were no statistically significant differences in the drop-out rates between the first and second assessments or the second and third assessments. In the 12-month cohort, there was no difference in drop-out rate between the second and third assessments, but an indication that more participants dropped out from the intervention group between the first and second assessments (intervention versus surveillance:χ2 = 3.09; P = .08 for IDA and χ2 = 1.57; P = .21 for nonanemic). The increase in drop-out rates between the first and second assessments for intervention versus surveillance was statistically significant for infants who were dropped from the study because of lack of funding χ2 = 5.85; P = .02) but not for those who were removed for any other reason. Within each cohort, there were no significant differences between groups (iron status by intervention) in the mean number of home visits (Tables 1 and 2).
Figure 1.
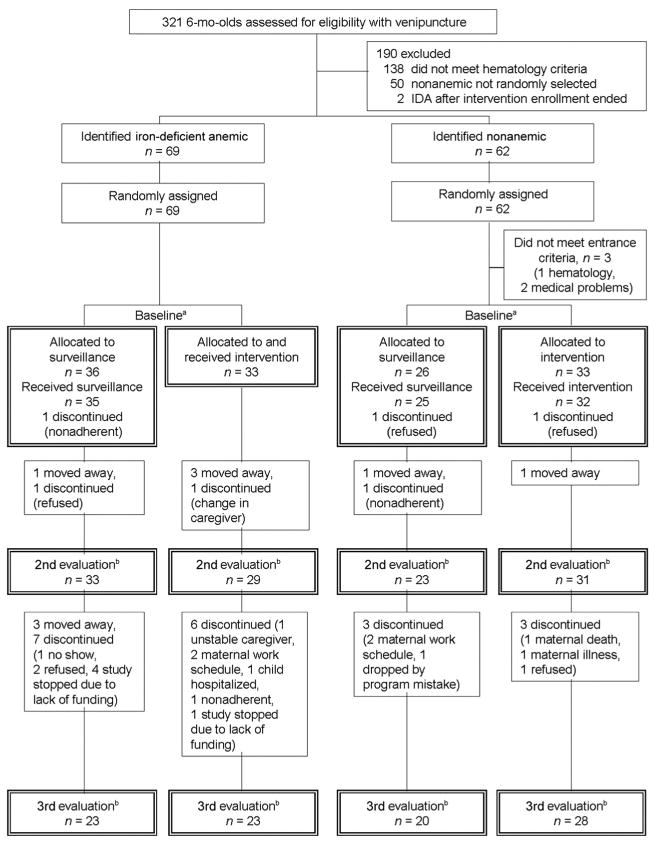
Flowchart of subject participation for the 6-month cohort. a Corresponds to Table 1 and intention-to-treat (baseline) analyses; b corresponds to change-over-time analyses; n values for the second evaluation are those in Fig 3 and Table 4.
Figure 2.
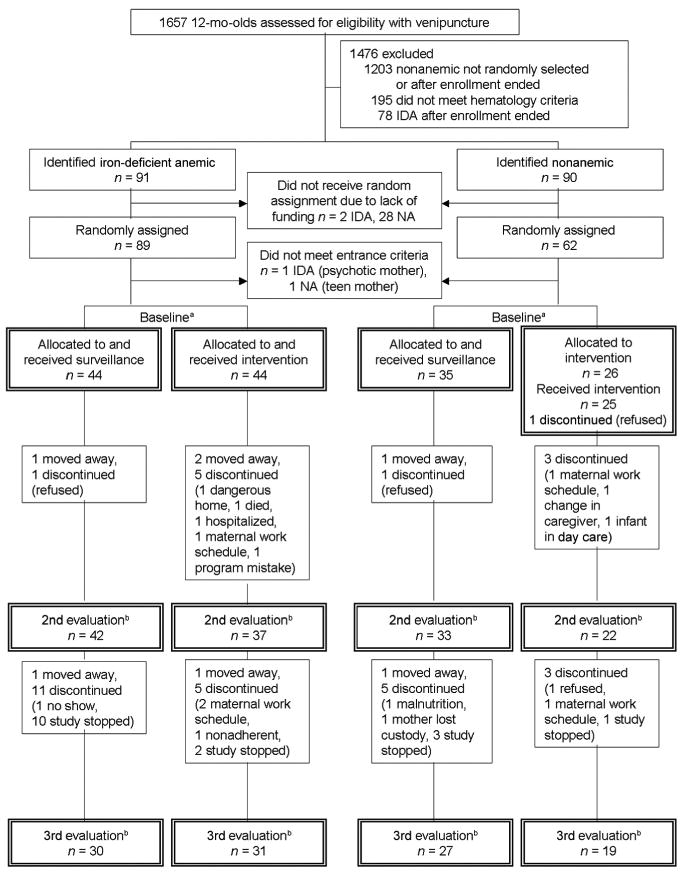
Flowchart of subject participation for the 12-month cohort. NA indicates nonanemic. a Corresponds to Table 2 and intention-to-treat (baseline) analyses; b corresponds to change-over-time analyses; n values for the second evaluation are those in Fig 4 and Table 4.
Iron Status
Infants with IDA showed an excellent response to iron (Table 3); only 5 infants did not have a clear response. However, iron status measures remained lower in infants with IDA after 6 months of treatment, compared with nonanemic infants. Hemoglobin was the only measure obtained after 12 months of iron therapy; levels remained lower in infants with IDA in the 12-month cohort.
Cognitive and Motor Scores
The longitudinal analyses included 116 infants enrolled at 6 months (60 intervention) and 134 infants enrolled at 12 months (59 intervention). As expected, all groups showed gains in mental and motor raw scores overtime. Intervention did not affect gain in motor scores (data available on request). For cognitive scores, each cohort showed a significant 3-way interaction (intervention group according to iron status by monthly change in score: P = .03 and P = .04 for the 6- and 12-month cohorts, respectively). Change overtime was similar for nonanemic intervention, nonanemic surveillance, and IDA intervention groups and lower for the IDA surveillance group, as shown in Figs 3 and 4 for the 6- and 12-month cohorts. Table 4 provides details of the HLM parameters and statistics. Changes that occurred during the 1-year period are summarized here.
Figure 3.
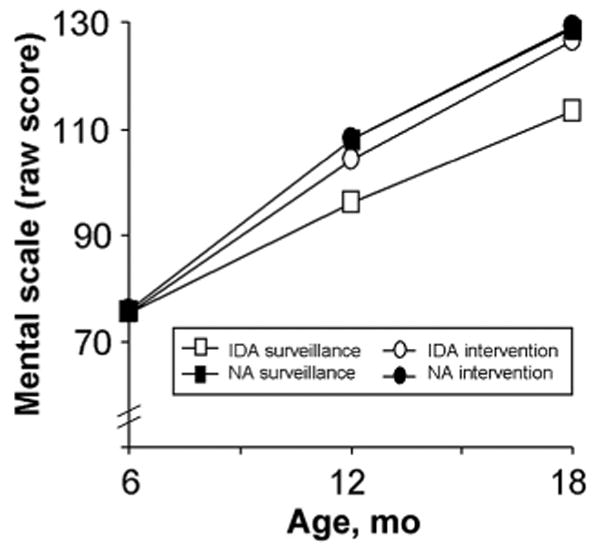
Change over time in raw mental development scores in the 6-month cohort. All groups had comparable scores at baseline. The groups showed similar rates of increase in raw mental scores over time, except for infants with DA randomly assigned to surveillance, who showed a lower rate of change (3-way interaction: intervention group according to iron status according to monthly change in score; P = .03). Table 4 gives the HLM parameters NA indicates nonanemic.
Figure 4.
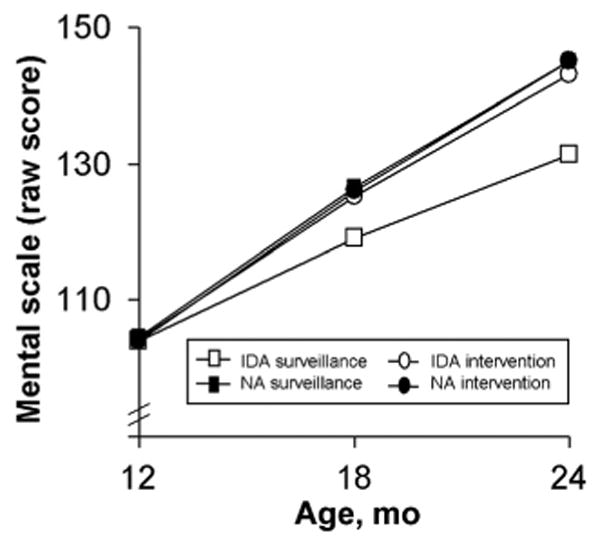
Change over time in raw mental development scores in the 12-month cohort. All groups had comparable scores at baseline. The groups showed similar rates of increase in raw mental scores over time, except for infants with DA randomly assigned to surveillance, who showed a lower rate of change (3-way interaction: intervention group according to iron status according to monthly change in score; P = .04). Table 4 gives the HLM parameters NA indicates nonanemic.
Table 4. HLM Results for Iron Group Differences in Monthly Change in Scores According to Intervention.
| 6-mo Cohort | 12-mo Cohort | |||
|---|---|---|---|---|
| IDA (N = 62) | Nonanemic (N = 54) | IDA (N = 79) | Nonanemic (N = 55) | |
| Mental scale, raw score | ||||
| Baseline for surveillance groups | 75.5 (74.8 to 76.2) | 75.9 (75.2 to 76.6) | 103.9 (103.1 to 104.7) | 104.5 (103.7 to 104.6) |
| Baseline for intervention groups | 75.5 (74.7 to 75.9) | 75.7 (74.8 to 76.6) | 104.2 (103.1 to 105.3) | 103.9 (102.8 to 105.0) |
| Monthly change first half for surveillance | 3.4b (3.0 to 3.8) | 5.4 (4.9 to 5.9) | 2.7b (2.6 to 2.8) | 3.7 (3.5 to 3.9) |
| Monthly change first half for intervention | 4.7a (4.6 to 5.1) | 5.4 (4.8 to 6.0) | 3.7a (3.5 to 3.9) | 3.7 (3.4 to 4.0) |
| Monthly change second half for surveillance | 2.9b (2.4 to 3.3) | 3.4 (3.1 to 3.7) | 2.1b (1.9 to 2.3) | 3.1 (2.9 to 3.3) |
| Monthly change second half for intervention | 3.6a (3.3 to 4.0) | 3.5 (3.1 to 3.9) | 3.0a (2.7 to 3.3) | 3.0 (2.6 to 3.4) |
| Bayley social interaction factor | ||||
| Baseline surveillance | −0.19b (−0.24 to −0.14) | − 0.07 (−0.09 to −0.05) | −0.04b (−0.06 to −0.02) | 0.12 (0.09 to 0.15) |
| Baseline intervention | −0.16b (−0.19 to −0.13) | − 0.06 (−0.08 to −0.04) | −0.05b (−0.08 to −0.02) | 0.12 (0.10 to 0.14) |
| Monthly change first half for surveillance | −0.01b (−0.02 to 0.0) | .03 (0.02 to 0.04) | −0.01b (−0.03 to 0.01) | 0.02 (0.01 to 0.03) |
| Monthly change first half for intervention | 0.02a (0.0 to 0.04) | 0.02 (0.01 to 0.03) | 0.02a (0.01 to 0.03) | 0.02 (0.0 to 0.04) |
| Monthly change second half for surveillance | −0.01b (−0.02 to 0.0) | 0.02 (0.01 to 0.03) | −0.01b (−0.02 to 0.0) | 0.02 (0.0 to 0.04) |
| Monthly change second half for intervention | 0.02a (0.01 to 0.03) | 0.02 (0.01 to 0.03) | 0.01a (−0.01 to 0.03) | 0.01 (0.0 to 0.02) |
Values are parameter estimates (95% CI).
Scores for the intervention group increased significantly more than for the surveillance group, at P < .05.
Scores for the IDA group increased significantly less or declined compared with the nonanemic group, at P < .05.
Raw mental scores in the 6-month cohort increased during the 1-year period by 54.0 points for the nonanemic intervention group (95% CI: 53.0–55.0), 52.6 points for the nonanemic surveillance group (95% CI: 51.5–53.7), and 51 points for the IDA intervention group (95% CI: 49.7–52.3). The increase for the IDA surveillance group was 37.7 points (95% CI: 36.1–39.3), significantly lower than the other groups (P = .01 and P = .03 for first and second half-years). The lower rate of change resulted in significantly lower scores after 6 and 12 months: 12.0 points (95% CI: 11.0–13.0) and 16.0 points (95% CI: 15.0–17.0) lower, respectively.
In the 12-month cohort, raw mental scores increased during the year by 41.1 points for the nonanemic intervention group (95% CI: 39.4–42.8), 40.5 points for the nonanemic surveillance group (95% CI: 39.0–42.0), and 38.7 points for the IDA intervention group (95% CI: 37.2–40.2). The increase for the IDA surveillance group was 27.3 points (95% CI: 25.7–28.9), significantly lower than for the other groups (P = .02 and .03 for first and second half-years, respectively). The lower rate of change resulted in significantly lower scores after 6 and 12 months in the study: 8.3 points (95% CI: 7.3–9.3) and 13.2 points (95% CI: 11.6–14.8) lower, respectively.
Behavior Ratings
As with cognitive scores, changes in social responsiveness scores were similar for the nonanemic intervention, nonanemic surveillance, and IDA intervention groups and lower for infants with IDA who were randomly as-signed to the surveillance group. There was no evidence of catch up in positive social-emotional ratings of infants with IDA who were randomly assigned to the intervention group. The 3-way interaction was statistically significant in each cohort (intervention according to iron status according to monthly change in factor score: P = .002 and .01 in the 6- and 12-month cohorts, respectively; rates of change were similar in the first and second half-years).
Change in positive social-emotional responsiveness over time is shown in Figs 5 and 6. Briefly, social-emotional responsiveness factor scores in the 6-month cohort increased during the 1-year period by 0.24 SD for the nonanemic intervention group (95% CI: 0.22–0.26), 0.26 SD for the nonanemic surveillance group (95% CI: 0.22–0.30), and 0.22 SD for the IDA intervention group (95% CI: 0.19–0.25). The IDA surveillance group decreased 0.09 SD (95% CI: −0.06 to −0.12), a result that was significantly lower than the other groups (P = .01 and .02 for the first and second half-years). The decrease resulted in significantly lower scores after 6 and 12 months: 0.20 SD (95% CI: 0.18–0.22) and 0.33 SD (95% CI: 0.30– 0.36) lower, respectively.
Figure 5.
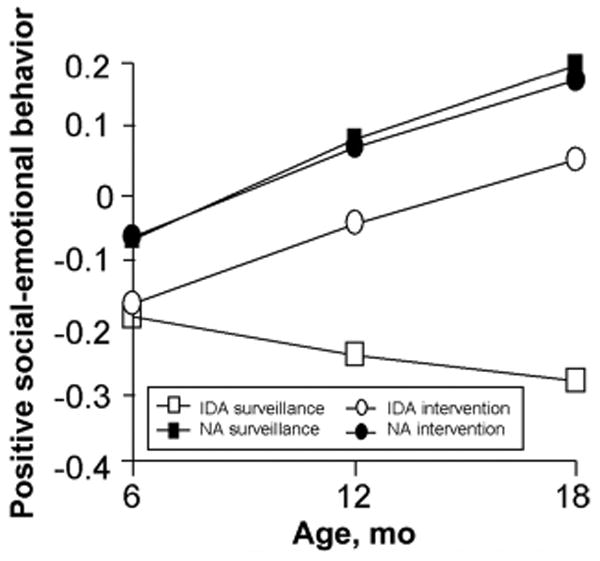
Change over time in positive social-emotiona scores in the 6-month cohort. Infants with IDA had lower positive social-emotional scores at baseline (factor scores expressed in SD units) The groups showed similar rates of increase in scores over time, except for infants with IDA randomly assigned to surveillance, who showed a decline in scores overtime (3-way interaction intervention group according to iron status according to monthly change in score; P = .002) Table 4 gives the HLM parameters. NA indicates nonanemic.
Figure 6.
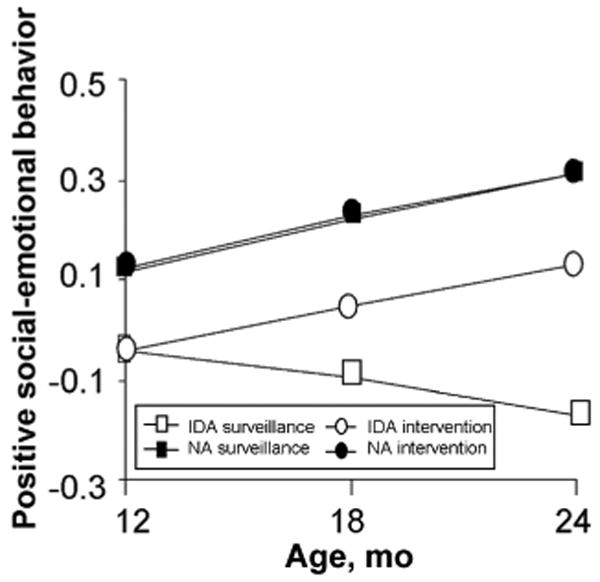
Change over time in positive social-emotional scores in the 12-month cohort. Infants with IDA had lower positive social-emotional scores at baseline (factor scores expressed in SD units) The groups showed similar rates of increase in scores over time, except for infants with IDA randomly assigned to surveillance, who showed a decline in scores overtime (3-way interaction ntervention group according to iron status according to monthly change in score; P = .01) Table 4 gives the HLM parameters. NA indicates nonanemic.
In the 12-month cohort, social-emotional responsiveness factor scores increased during the year by 0.19 SD for the nonanemic intervention group (95% CI: 0.17–0.21), 0.19 SD for the nonanemic surveillance group (95% CI: 0.16–0.22), and 0.17 SD for the IDA intervention group (95% CI: 0.13– 0.21). The IDA surveillance group decreased 0.13 SD (95% CI: −0.17 to − 0.09), which was significantly lower than the other groups (P= .02 for both half-year intervals). The decrease resulted in significantly lower scores after 6 and 12 months in the study: 0.14 (95% CI: 0.12–0.16) and 0.30 SD (95% CI: 0.27–0.33) lower, respectively.
Discussion
The results of this study showed that a home-based intervention in infants with IDA resulted in gains in cognitive and positive social-emotional scores that were similar to those observed in nonanemic infants regardless of intervention. Infants with IDA who were randomly assigned to the surveillance group showed smaller gains in cognitive performance and worsening positive social-emotional responsiveness. However, infants with IDA who received intervention did not catch up to non-anemic infants in social-emotional ratings; the similar rates of change indicate that the initial difference was maintained. Because infants were randomly assigned to groups, the benefits to infants with IDA seem to be attributable to the intervention program.
Comparisons of our findings with those from previous research are not possible, because no such intervention study has been published with respect to IDA. However, our findings are similar to those from studies of undernutrition (or zinc deficiency).14-–23,39 Such studies have shown that development improves when infants with generalized undernutrition or a specific micronutrient deficiency are offered early intervention in addition to health monitoring and nutritional rehabilitation.
The benefits that we observed corresponded to the nature of the intervention, which focused on the mother-infant relationship and aspects of maternal behavior thought to foster cognitive and social-emotional development.40 These are the areas in which infants with IDA who received intervention showed developmental improvements compared with those who received surveillance without intervention. Note that all infants were visited weekly to maintain comparability in the frequency of contact with study personnel. Thus, all infants could be considered to have received some kind of intervention. The impact of the early-intervention program may have been even greater compared with routine health care alone.
In contrast to previous investigators,1,3 we did not observe lower initial cognitive and motor scores in infants with IDA. In our study population, however, infants with IDA showed less positive social-emotional responsiveness and were observed to have delayed development, as assessed on the basis of more brain-based measures.26,29 This pattern of results suggests that in the first year of life global cognitive and motor scores are not as sensitive to IDA effects as are social-emotional or more brain-based measures. This interpretation is supported by our recent study of 9-month-old infants in an inner-city US population, in whom social-emotional measures showed more marked differences related to iron status than overall cognitive and motor scores.41–43 Positive social-emotional behavior was also the area most improved by iron supplementation in the preventive trial component of the Chile project.24 Together such results point to the following conclusions: positive social-emotional behavior may be fostered by iron supplementation as a preventive measure but is not fully corrected once IDA occurs, even with additional intervention. Social-emotional behavior may get worse without intervention, even when IDA is treated with iron.
Another possible reason why we did not observe lower initial cognitive and motor scores is that IDA was not present long enough to have an impact on global scores. No infant had IDA postnatally for >6 months, because IDA was identified and treated at 6 and 12 months. In previous studies, infants were older and probably had IDA longer.2–5 Even in the present study, however, infants with IDA who did not receive intervention showed lower cognitive scores as the year went on, despite iron therapy.
Our study could not be double-blind. Although infants were randomly assigned to surveillance and intervention groups and outcomes were measured by masked testers, mothers and families knew which group their infant was in. In regard to generalizability, it is unclear whether such an intervention program would be equally effective if IDA was more chronic or severe or families more or less advantaged than those in our sample. The labor-intensive nature of the intervention and our use of skilled professionals also limit broad application. Concerted efforts are underway to identify developmentally supportive interventions that are feasible and sustainable on a large scale.20,44 For instance, approaches involving less skilled personnel and homemade toys have shown good results in several contexts.15–18,21–23,39 The optimal age for intervention remains unclear, although there was no differential effect associated with infant age in our study. Processes by which early-intervention programs produce benefits are also not well understood and warrant additional research.
Conclusions
Developmental interventions hold promise to benefit infants with IDA, whereas iron supplementation alone seems to be insufficient. However, the lack of catch up in positive social-emotional behavior that we observed points to the need to prevent IDA from occurring in the first place. Because ˜25% of infants in developing countries have IDA,45,46 and infants everywhere whose families are poor or are members of minority groups are at increased risk,47,48 new avenues to improve the developmental outcomes of these infants are sorely needed.
What's Known on this Subject
Infants with IDA, despite iron therapy, show poorer cognitive, motor, social-emotional, and neurophysiologic function than infants without IDA. In malnourished infants, who also show poorer long-term outcome, early-intervention programs have improved developmental recovery.
What this Study Adds
Developmental interventions have not been reported for IDA, which affects ˜25% of infants worldwide. The results of this study showed that home-based intervention to foster child development improved mental scores and social responsiveness in infants with IDA.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HD14122 and HD33487) and the Gerber Companies Foundation.
We thank Candice Percansky, MA, formerly of the Ounce of Prevention Fund and Erikson Institute in Chicago, Illinois, for consultation on the early-intervention program and Susan McDonough, PhD, for recommendations about parent-focused early intervention. We also thank the monitors, weekly visitors, psychologists, and supervisors for their dedicated efforts and professionalism and the study families for their participation.
Funded by the National Institutes of Health (NIH).
Abbreviations
- IDA
iron-deficiency anemia
- HLM
hierarchical linear modeling
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Gerber Companies Foundation.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00998998).
Financial Disclosure: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369(9556):145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 2.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(2S-2):649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 3.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28(4 suppl):S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 4.McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931–945. doi: 10.1093/ajcn/85.4.931. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 2005;8(2):117–132. doi: 10.1079/phn2004677. [DOI] [PubMed] [Google Scholar]
- 6.Hasanbegović E, Sabanovic S. Effects of iron therapy on motor and mental development of infants and small children suffering from iron deficiency anaemia [in Bosnian] Medicinski Arhiv. 2004;58(4):227–229. [PubMed] [Google Scholar]
- 7.Antunes H. Iron Deficiency Anaemia in Infants: A Prospective Neurodevelopment Evaluation. Porto, Portugal: Faculty of Medicine, University of Portugal; 2004. [Google Scholar]
- 8.Lozoff B, Brittenham GM, Wolf AW, et al. Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics. 1987;79(6):981–995. [PubMed] [Google Scholar]
- 9.Lozoff B, Wolf AW, Jimenez E. Iron deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996;129(3):382–389. doi: 10.1016/s0022-3476(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 10.Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics. 1989;84(1):7–17. [PubMed] [Google Scholar]
- 11.Akman M, Cebeci D, Okur V, Angin H, Abah O, Akman AC. The effects of iron deficiency on infants' developmental test performance. Acta Paediatr. 2004;93(10):1391–1396. [PubMed] [Google Scholar]
- 12.Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet. 1993;341(8836):1–4. doi: 10.1016/0140-6736(93)92477-b. [DOI] [PubMed] [Google Scholar]
- 13.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64(5 pt 2):S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Super CM, Herrera GM, Mora JO. Long-term effects of food suplementation and psychosocial intervention on the physical growth of Columbian infants at risk of malnutrition. Child Dev. 1990;61(1):29–49. [PubMed] [Google Scholar]
- 15.Grantham-McGregor S, Stewart ME, Schofield WN. Effect of long-term psychosocial stimulation on mental development of severely malnourished children. Lancet. 1980;2(8198):785–789. doi: 10.1016/s0140-6736(80)90395-5. [DOI] [PubMed] [Google Scholar]
- 16.Grantham-McGregor S, Schofield W, Harris L. Effect of psychosocial stimulation on mental development of severely malnourished children: an interim report. Pediatrics. 1983;72(2):239–243. [PubMed] [Google Scholar]
- 17.Grantham-McGregor SM, Schofield W, Powell C. Development of severely malnourished children who received psychosocial stimulation: six year follow-up. Pediatrics. 1987;79(2):247–254. [PubMed] [Google Scholar]
- 18.Grantham-McGregor S, Powell C, Walker S, Chang S, Fletcher P. The long-term follow-up of severely malnourished children who participated in an intervention program. Child Dev. 1994;65(2 spec No):428–439. [PubMed] [Google Scholar]
- 19.Black M, Dubowitz H, Hutcheson J, Berenson-Howard J, Starr RH., Jr A randomized clinical trial of home intervention for children with failure to thrive. Pediatrics. 1995;95(6):807–814. [PubMed] [Google Scholar]
- 20.Engle PL, Black MM, Behrman JR, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369(9557):229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 21.Walker SP, Chang SM, Powell CA, Grantham-McGregor S. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-retarded Jamaican children: prospective cohort study. Lancet. 2005;366(9499):1804–1807. doi: 10.1016/S0140-6736(05)67574-5. [DOI] [PubMed] [Google Scholar]
- 22.Powell C, Baker-Henningham H, Walker S, Gernay J, Grantham-McGregor S. Feasibility of integrating early stimulation into primary care for undernourished Jamaican children: cluster randomised controlled trial. BMJ. 2004;329(7457):89. doi: 10.1136/bmj.38132.503472.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamadani JD, Huda SN, Khatun F, Grantham-McGregor SM. Psychosocial stimulation improves the development of undernourished children in rural Bangladesh. J Nutr. 2006;136(10):2645–2652. doi: 10.1093/jn/136.10.2645. [DOI] [PubMed] [Google Scholar]
- 24.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846–854. [PubMed] [Google Scholar]
- 25.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr. 1998;132(4):635–640. doi: 10.1016/s0022-3476(98)70352-x. [DOI] [PubMed] [Google Scholar]
- 26.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brain stem responses. Am J Clin Nutr. 1998;68(3):683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 27.Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum Dev. 2002;70(1–2):85–101. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 28.Angulo-Kinzler RM, Peirano P, Lin E, Garrido M, Lozoff B. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66(2):67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 29.Peirano P, Algarin C, Garrido M, Algarin D, Lozoff B. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res. 2007;32(10):1665–1672. doi: 10.1007/s11064-007-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dallman PR, Reeves JD, Driggers DA, Lo EYT. Diagnosis of iron deficiency: the limitations of laboratory tests in predicting response to iron treatment in 1-year-old infants. J Pediatr. 1981;99(3):376–381. doi: 10.1016/s0022-3476(81)80321-6. [DOI] [PubMed] [Google Scholar]
- 31.Powell C, Grantham-McGregor S. Home visiting of varying frequency and child development. Pediatrics. 1989;84(1):157–164. [PubMed] [Google Scholar]
- 32.Chamberlin RW. Home visiting: a necessary but not in itself sufficient program component for promoting the health and development of families and children. Pediatrics. 1989;84(1):178–180. [PubMed] [Google Scholar]
- 33.Hans SL, Bernstein VJ. Planning programmes for high-risk infants: a facet analysis of parent-infant communication. Appl Psychol. 1990;39(4):457–478. [Google Scholar]
- 34.Bernstein VJ, Hans SL, Percansky C. Advocating for the young child in need through strengthening the parent-child relationship. J Clin Child Psychol. 1991;20(1):28–41. [Google Scholar]
- 35.Bayley N. Bayley Scales of Infant Development. New York, NY: Psychological Corporation; 1969. [Google Scholar]
- 36.Bayley N. Bayley Scales of Infant Development. San Antonio: Psychological Corporation; 1993. [Google Scholar]
- 37.Lozoff B, Walter T, Kaciroti N. Iron deficiency in infancy: applying a physiologic framework for prediction. Am J Clin Nutr. 2006;84(6):1412–1421. doi: 10.1093/ajcn/84.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bates JE, Freeland CA, Lounsbury ML. Measurement of infant difficultness. Child Dev. 1979;50(3):794–803. [PubMed] [Google Scholar]
- 39.Gardner JM, Powell CA, Baker-Henningham H, Walker SP, Cole TJ, Grantham-McGregor S. Zinc supplementation and psychosocial stimulation: effects on the development of undernourished Jamaican children. Am J Clin Nutr. 2005;82(2):399–405. doi: 10.1093/ajcn.82.2.399. [DOI] [PubMed] [Google Scholar]
- 40.Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Dev Psychol. 2006;42(4):627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- 41.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152(5):696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shafir T, Angulo-Barroso R, Jing Y, Jacobson S, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev. 2008;84(7):479–485. doi: 10.1016/j.earlhumdev.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter RC, Jacobson JL, Burden MJ, et al. Iron deficiency anemia and cognitive function in infants. Pediatrics. 2010;126(2) doi: 10.1542/peds.2009-2097. Available at: www.pediatrics.org/cgi/content/full/126/2/e427. [DOI] [PMC free article] [PubMed]
- 44.Bakermans-Kranenburg MJ, van Ijzendoorn MH, Juffer F. Less is more: meta-analyses of sensitivity and attachment interventions in early childhood. Psychol Bull. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- 45.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 46.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 47.Brotanek JM, Halterman J, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159(11):1038–1042. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- 48.Brotanek JM, Gosz J, Weitzman M, Flores G. Secular trends in the prevalence of iron deficiency among US toddlers, 1976–2002. Arch Pediatr Adolesc Med. 2008;162(4):374–381. doi: 10.1001/archpedi.162.4.374. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 50.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 51.Alvarez M, Muzzo S, Ivanovic D. Scale for measurement of socioeconomic level, in the health area [in Spanish] Rev Med Chil. 1985;113(3):243–249. [PubMed] [Google Scholar]
- 52.Weinraub M, Wolf B. Stress, social supports and parent-child interactions: similarities and differences in single-parent and two-parent families. In: Boukydis I, Zachariah CF, editors. Research on Support for Parents and Infants in the Postnatal Period. Norwood, NJ: Ablex Publishing Corporation; 1987. pp. 114–135. [Google Scholar]
- 53.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment— Revised Edition. Little Rock, AR: University of Arkansas; 1984. [Google Scholar]


