SUMMARY
Anatomic and physiologic studies have suggested a model in which neurons of the piriform cortex receive convergent input from random collections of glomeruli. In this model, odor representations can only be afforded behavioral significance upon experience. This property is consistent with the observation that the same odor can elicit appetitive or aversive responses dependent upon learning. We have devised an experimental strategy that permits us to ask whether the activation of an arbitrarily chosen subpopulation of neurons in piriform cortex can elicit different behavioral responses dependent upon experience. Activation of a small subpopulation of piriform neurons expressing channelrhodopsin at multiple loci, in the piriform cortex when paired with reward or shock elicits either appetitive or aversive behavior. Moreover, we demonstrate that different subpopulations of piriform neurons expressing ChR2 can be discriminated and independently entrained to elicit distinct behaviors. These observations demonstrate that the piriform cortex is sufficient to elicit learned behavioral outputs in the absence of sensory input. These data imply that the piriform does not use spatial order to map odorant identity or behavioral output.
INTRODUCTION
Olfactory sensory systems transmit information to the brain where it is processed to create an internal representation of odors in the external world. This internal representation must then be translated into appropriate behavioral output. Sensory systems do not passively represent the external world. Rather, they actively interpret features of the world that are combined in higher cortical centers to construct meaningful sensory representations. In vision, touch, and hearing, features central to perception are topographically ordered in the sense organ. These features, which include spatial location or sound frequency, are continuously variable in at least one dimension in the external world. The persistence of topographic order from the periphery to primary sensory cortices (Marshall, 1941; Talbot, 1941; Woolsey, 1942) has led to the view that early sensory processing is mediated by developmentally programmed neural circuits. In contrast, olfactory features cannot be meaningfully represented along continuous dimensions in the physical world and are not topographically organized in the olfactory sensory epithelium (Ressler et al., 1993; Vassar et al., 1993). Olfactory information from the nose is transmitted to the olfactory bulb and then to higher centers. The vast majority of odors drive behavior only after learning, but the brain regions responsible for these learned behaviors remain elusive. The piriform cortex receives extensive input from the bulb (Price and Powell, 1970) and projects to areas implicated in behavioral output (Schwabe et al., 2004), posing the question as to whether the piriform is a substrate for olfactory learning. We have developed an experimental strategy that permits us to ask whether exogenous activation of the same subpopulation of piriform neurons can be entrained to elicit distinct behavioral responses depending on the learning paradigm.
Olfactory perception is initiated by the recognition of odorants by a large repertoire of receptors in the sensory epithelium. Individual sensory neurons in mice express only one of 1500 different receptor genes (Buck and Axel, 1991; Godfrey et al., 2004; Zhang and Firestein, 2002). An odorant can interact with multiple distinct receptors resulting in the activation of an ensemble of sensory neurons (Araneda et al., 2004; Malnic et al., 1999; Oka et al., 2006). Discrimination among odorants then requires that the brain determine which of the sensory neurons have been activated by a given odorant. Neurons expressing a given receptor are distributed within zones of the epithelium but project with precision to two spatially invariant glomeruli in the olfactory bulb (Mombaerts et al., 1996; Ressler et al., 1993, 1994; Vassar et al., 1994; Vassar et al., 1993). Thus a transformation in the representation of olfactory information is apparent in the bulb where the dispersed population of active neurons in the sense organ is consolidated into a discrete spatial map of glomerular activity.
If an odorant activates a unique ensemble of glomeruli, then the recognition of an odor requires integration of information from multiple glomeruli by higher olfactory centers. The projection neurons of the olfactory bulb, mitral and tufted cells, extend an apical dendrite into a single glomerulus and send axons to several telencephalic areas, including a significant input to the piriform cortex (Price and Powell, 1970). Anatomic tracing reveals that axonal projections from individual glomeruli diffusely innervate the piriform and do not exhibit the segregated pattern of the bulb (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011). In accord with this anatomy, electrophysiological and optical imaging studies demonstrate that individual odorants activate subpopulations of neurons distributed across the piriform without apparent spatial preference (Illig and Haberly, 2003; Poo and Isaacson, 2009; Rennaker et al., 2007; Stettler and Axel, 2009; Sugai et al., 2005; Zhan and Luo). Moreover, individual piriform neurons respond to multiple structurally dissimilar odorants and no similarity in response properties is observed among neighboring neurons (Stettler and Axel, 2009). Therefore, piriform representations differs from those of other neocortical sensory areas where cells are tuned for stimulus features and show macroscopic spatial patterning (Hubel and Wiesel, 1959; Mountcastle et al., 1957).
These observations are consistent with a model in which individual piriform cells receive convergent input from random collections of glomeruli (Stettler and Axel, 2009). In this model, odor representations in piriform can only be afforded behavioral significance upon learning. Furthermore, the piriform cortex is anatomically poised to mediate learned olfactory behaviors. Piriform output to the amygdala, basal ganglia, and hippocampus (Schwabe et al., 2004) may link sensory representations to behavioral output. In this study, we demonstrate that exogenous activation of an arbitrarily chosen ensemble of piriform neurons can elicit multiple behaviors of contrasting valence dependent on learning. Thus the piriform cortex can mediate learned behaviors in the absence of sensory input. Ensembles composed of less than 500 piriform neurons can be entrained to elicit different behaviors. Moreover, aversive behavior can be elicited by these ensembles independent of their position across the piriform, indicating that the piriform does not use spatial order to map either olfactory input (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011; Stettler and Axel, 2009) or behavioral output.
RESULTS
Expression of Channelrhodopsin in an Ensemble of Piriform Neurons
We introduced channelrhodopsin (ChR2), a light-activated cation channel (Aravanis et al., 2007; Boyden et al., 2005), into a small subpopulation of neurons in the piriform cortex of the mouse. In these mice, light should activate the ensemble of ChR2-expressing neurons, independent of mitral cell input. Activation of the ensemble of piriform neurons with light served as a conditioned stimulus (CS) that was paired with either an aversive or appetitive unconditioned stimulus (US). We then asked whether subsequent exposure to the CS alone (light) would elicit a behavioral response consistent with the conditioning paradigm. This experimental strategy permitted us to ask whether the same random ensemble of neurons was capable of eliciting different behaviors depending on the nature of the unconditioned stimulus.
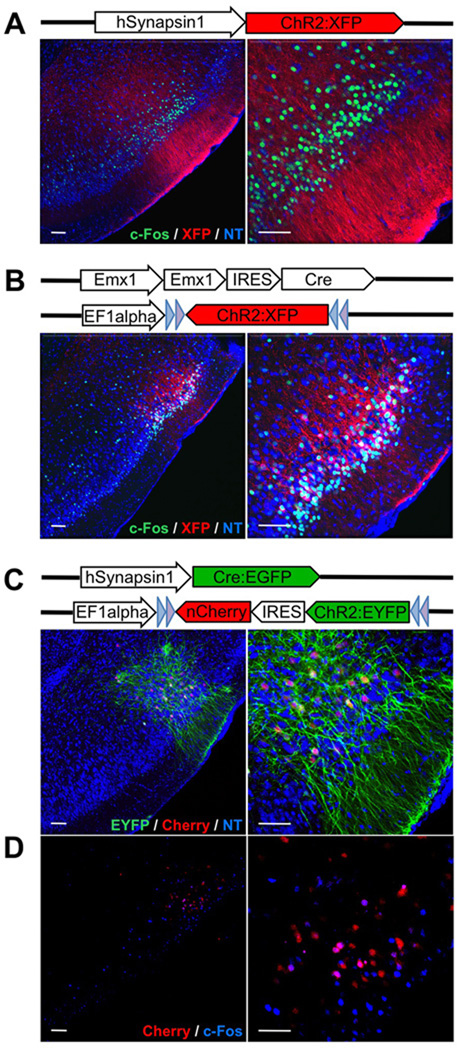
Channelrhodopsin was expressed in layer 2 and 3 piriform neurons by infection with three different genetic variants of lentivirus (Dittgen et al., 2004). Photostimulation via an optical fiber results in the activation of ChR2-expressing neurons with millisecond precision in awake, behaving animals (Aravanis et al., 2007; Boyden et al., 2005). In initial experiments, channelrhodopsin expression was driven by the human Synapsin1 promoter (Kugler et al., 2003) in both excitatory and inhibitory cells in the piriform cortex (Figure 1A). In a second set of experiments, a ChR2 gene in the reverse orientation was flanked by pairs of loxP sites, such that expression of ChR2 was dependent upon Cre recombinase (Atasoy et al., 2008). Injection of this virus into the piriform cortex of Emx1-IRES-Cre mice in which Cre recombinase is restricted to excitatory neurons (Gorski et al., 2002), resulted in ChR2 expression in pyramidal neurons but not inhibitory interneurons (Figure 1B). These two strategies for ChR2 delivery resulted in abundant expression of ChR2 in over 50% of the neurons in an injection site that ranged from 500 µm-1000 µm in diameter (Figure 1A and Figure 1B).
Figure 1. Expression of ChR2 in layer 2 and 3 of piriform cortex after injection with different variants of lentivirus encoding ChR2.
A. Lentivirus carrying ChR2 fused to a fluorescent reporter (XFP = Cherry or EYFP) under control of the hSynapsin1 promoter was stereotactically injected into the piriform. The hSynapsin1 promoter drives ChR2:XFP expression in both excitatory and inhibitory neurons. Coronal sections through the injection site reveal expression of ChR2:XFP (red) in dense populations of layer 2 and 3 piriform neurons. The labeled cells are shown at higher magnification on the right. c-Fos expression after in vivo photostimulation is shown in green. NT = Neurotrace (blue). Scale bars on left = 50 µm and on right =100 µm.
B. Lentivirus carrying ChR2:XFP flanked by loxP sites and under control of the EF1 alpha promoter was injected into the piriform of Emx1-IRES-Cre mice. ChR2:XFP (red) expression is restricted to dense populations of excitatory neurons in these mice.
C. Lentivirus carrying ChR2:EYFP-IRES-nCherry (nuclear Cherry) flanked by loxP sites and under control of the EF1alpha promoter was co-injected into piriform with a second lentivirus carrying the hSynapsin1 promoter driving Cre:EGFP. This dual virus strategy was used to generate sparse labeling of piriform neurons. nCherry (red) labels the cell bodies whereas EYFP (green) labels both cell bodies and processes.
D. c-Fos expression (blue) after in vivo photostimulation for the same animal shown in C.
We have achieved more sparse distribution of active neurons by co-injection of high-titer Cre-dependent ChR2 virus with a range of dilutions of lentivirus encoding Cre. Under these conditions (dual virus strategy), only a small subset of the cells were co-infected with both viruses and high-level ChR2 expression was therefore sparse, with about 10% [8.25±0.33%, N=3] of neurons expressing ChR2 at the injection site (Figure 1C). Analysis of c-Fos expression, a marker of neuronal activation (Morgan and Curran, 1991), revealed that all three genetic approaches to effect channelrhodopsin expression resulted in robust neural activation upon exposure to light (Figure 1A, Figure 1B and Figure 1D). We observed a threefold [3.06±0.46, N=4] increase in the number of c-Fos+ cells at injection sites using the first two expression strategies that produced dense populations of ChR2-expressing neurons. In the third expression strategy that generated sparse populations of ChR2-expressing neurons, the co-expression of nuclear Cherry (Figure 1C) allowed us to identify the incidence of c-Fos expression among ChR2+ cells. We observed that about 40% of cells expressing ChR2 also expressed c-Fos [37.76±11.7%, N=6], whereas ~5% of cells were positive for c-Fos expression in uninjected control hemispheres [6.09±0.36%, N=3].
An Ensemble of Neurons Trained to Elicit Aversive Behavior
In initial experiments, we asked whether photostimulation of ChR2-expressing neurons in the pirform cortex could serve as a conditioned stimulus, eliciting avoidance behavior in an aversive conditioning paradigm. We adopted a conditioning paradigm (Yan et al., 2008) to discern whether photostimulation of a subpopulation of piriform neurons could recapitulate the ability of odor to elicit aversive behavior. Training was carried out in a custom-designed rectangular arena in which the animal was allowed to move freely. Foot shock was applied only to the side where the animal was located at the time of CS presentation, allowing the animal to flee from the aversive stimulus by running toward the opposite side of the arena (Figure 2A). The CS-US presentation was randomly applied to either side. After two training sessions (one session = 10 pairings of the CS with US), the animals were returned to the same arena to determine whether the CS alone, photostimulation of ChR2-expressing neurons, was sufficient to elicit flight behavior. In mice expressing ChR2, we observed that photostimulation of piriform cortex served as an effective CS, resulting in robust flight behavior (Figure 2B). In at least 95% of the trials, mice exhibited flight behavior in response to photostimulation after two training sessions [% flight behavior: hSynapsin1=96.92±10.8%, N=7; Emx1=100±0%, N = 4; dual virus strategy with >300 ChR2+ cells=95.71±9.64%, N=10]. This conditioned response was retained 5 days after training [83.33±33.33%, N=4, data not shown].
Figure 2. Ensembles of ChR2-expressing piriform neurons entrained to elicit aversive behavior.
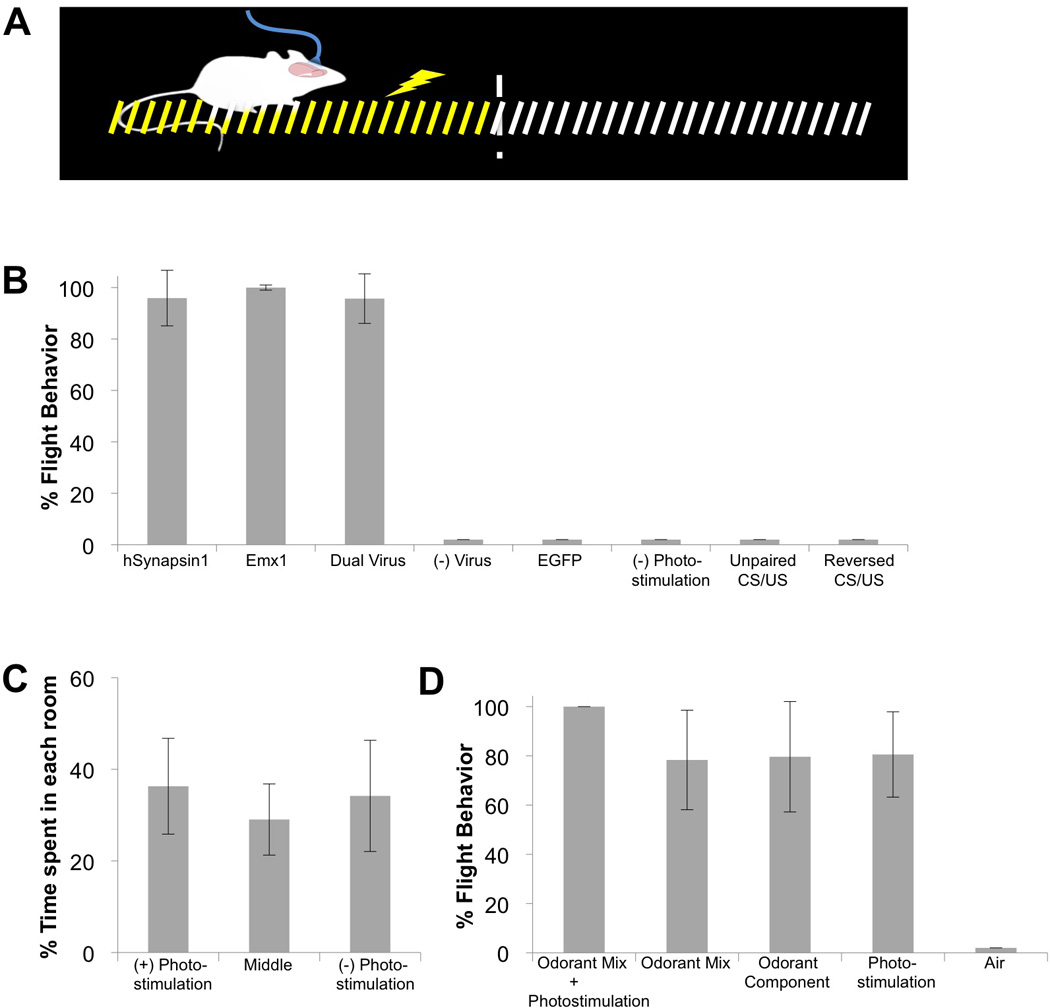
A. Schematic of the apparatus used for the aversive conditioning paradigm. During training, photostimulation of ChR2-expressing neurons in piriform, the conditioned stimulus (CS), was paired with foot shock, the unconditioned stimulus (US), applied only on the side of the arena where the animal was located at the time of photostimulation. The animals escaped foot shock by running to the opposite side.
B. The percentage of trials in which animals exhibited flight behavior in response to the CS alone during the testing phase. hSynapsin1 = ChR2 expression driven from the human Synapsin1 promoter (N=7); Emx1 = ChR2 expression driven by the Emx1 promoter (N=4); Dual Virus = ChR2 expression generated by co-infection of Cre and Cre-dependent ChR2 viruses (animals with > 300 ChR2+ neurons, N=10); (−) Virus = no viral injection (N=3); EGFP = virus encoding EGFP but not ChR2 was injected into piriform (N=6); (−) Photostimulation = ChR2 expression driven by the hSynapsin1 promoter without photostimulation during training (N=3); Unpaired CS/US = ChR2 expression was driven from the human Synapsin1 promoter but foot shock application was not contingent upon photostimulation (i.e., equal numbers of CSs and USs were presented in random order with delays always exceeding 1 minute, N=4); Reversed CS/US = ChR2 expression was generated by the dual virus strategy but foot shock application preceded photostimulation.
C. The percentage of time naïve ChR2-expressing animals spent in each chamber during a 5 minute (N=2) or 10 minute (N=8) testing period. One of the side chambers in a three-chambered arena was chosen as the (+) photostimulated compartment. Photostimulation was applied only when the animals entered the (+) photostimulated chamber. Training was not involved. ChR2 was densely expressed using the dual virus strategy.
D. The percentage of trials in which ChR2-expressing animals (N=3) exhibited flight behavior in response to a complete multi-component CS or its components after training in the aversive conditioning paradigm. The complete CS was an odorant mix (ethyl acetate + citronellol) co-delivered with photostimulation. Odorant component was either ethyl acetate or citronellol. ChR2 was densely expressed using the dual virus strategy.
Photostimulation of ChR2-expresing piriform neurons in CNG2A knock-out animals, which lack odor-evoked activity in the main olfactory epithelium (Brunet et al., 1996), could be entrained to elicit conditioned flight behaviors [100±0%, N=2, data not shown]. These experiments demonstrate that an ensemble of piriform neurons can elicit conditioned aversive behavior in the absence of olfactory sensory input.
Animals that were not infected with virus [0±0%, N=3] or animals infected with lentivirus encoding EGFP [0±0%, N=6] in the absence of ChR2 failed to exhibit an aversive behavioral response upon photostimulation (Figure 2B). In addition, mice expressing ChR2 in piriform neurons but not subjected to photostimulation during training [0±0%, N=3], mice exposed to unpaired CS and US presentation [0±0%, N=4] and mice exposed to CS and US in reverse order [0±0%, N=3] did not exhibit flight behavior (Figure 2B). We have also demonstrated that light activation of ChR2-expressing ensembles did not elicit a behavioral preference without conditioning. Animals expressing ChR2 were placed in the central compartment of a three-chambered arena and were photostimulated when the animal entered one side chamber. Animals spent the same amount of time in each chamber (Figure 2C), demonstrating that light activation of ChR2-expressing neurons did not elicit approach or avoidance behaviors [(+)Photostimulation = 36.3±10.46%, Middle = 29.04±7.77%, (−)Photostimulation = 34.19±12.14%, N=10].
We next asked whether photostimulation of ChR2+ ensembles exhibited the properties of an odorant component in a mix. Animals expressing ChR2 in piriform were trained using the aversive conditioning paradigm with a CS consisting of a mix of two odorants (ethyl acetate and citronellol) plus photostimulation. The simultaneous delivery of these three stimuli was paired with a foot shock and animals were subsequently tested with the complete CS or its components. Animals exhibited flight behavior in response to the mixture of two odors, either odor alone, as well as to light (Figure 2D) [Odorant mix + photostimulation = 100±0%, Odorant mix = 78.33±20.21%, Odorant component = 79.63±22.43, Photostimulation=80.57±17.33%, Air=0±0%, N=3]. These results indicate that light activation of an ensemble of ChR2-expressing neurons exhibit properties similar to a component of odorant mix. The inclusion of odorant during training does not interfere with an animal’s ability to generalize a conditioned response to the photostimulation component, nor does the inclusion of photostimulation interfere with generalized responses to odorant components.
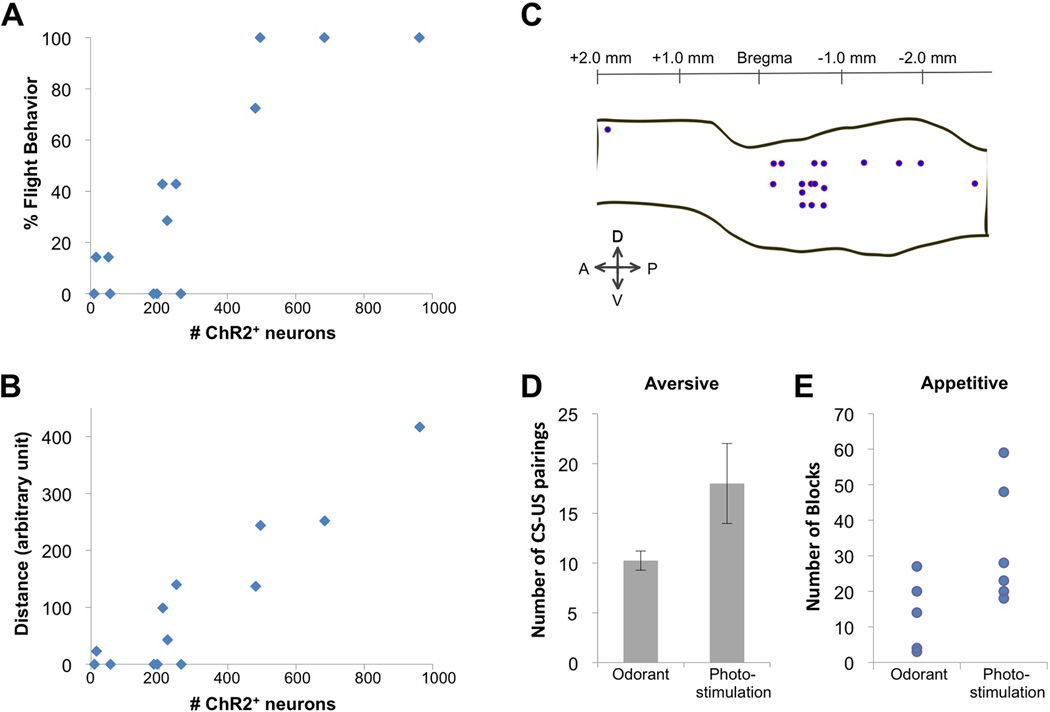
We have determined the number of ChR2-expressing cells required to elicit conditioned aversive behavior. It was possible to titrate the frequency of ChR2-expressing cells in mice in which the ChR2 expression was dependent upon dual infection with virus encoding Cre-dependent ChR2 and a second Cre-expressing virus. Animals with fewer than 200 piriform neurons expressing ChR2 failed to exhibit a behavioral response to the photostimulation after training [4.71±7.39%, N=6] (Figure 3A). Mice with about 300 ChR2-expressing cells exhibited flight behavior in 30% of the trials [28.56±20.19%, N=4]. Mice with more than 500 ChR2-expressing cells exhibited this behavior in 95% of the trials [93.1±13.79%, N=4]. This behavioral scaling with cell number was also observed when we scored the distance run in response to photostimulation (Figure 3B).
Figure 3. Efficiency of ChR2-expressing piriform neurons in eliciting conditioned behavior.
A. Relationship between the number of ChR2-expressing neurons and incidence of flight behavior in the aversive conditioning paradigm. The number of ChR2+ neurons was determined and correlated with the percentage of trials in which animals exhibited flight behavior for mice expressing ChR2 using the dual virus strategy.
B. Relationship between the number of ChR2-expressing neurons and distance traveled in the aversive conditioning paradigm. Same animals as in A.
C. Spatial distribution of conditioned ensembles. The centers of ChR2-expressing ensembles are mapped on a schematic showing the borders of the piriform cortex for mice expressing ChR2 using the dual virus strategy and trained in the aversive conditioning behavioral paradigm. The borders of the piriform were drawn by referring to the Paxinos atlas. Only animals with > 300 ChR2+ neurons are included. Percent flight behavior for each injection site is documented in Table S1.
D. Comparison of the number of CS-US pairings required for the onset of flight behavior in response to the CS alone in the aversive conditioning paradigm when the CS was either an odorant (N=4) or photostimulation of ChR2+ neurons (hSynapsin1: N=2, Emx1: N=2, Dual Virus with >300 ChR2+ neurons: N=7).
E. Comparison of the number of blocks of trials required to reach a fraction of correct licks (# of licks following CS+ / total # of licks) exceeding 0.7 for two consecutive blocks in the appetitive go/no go discrimination assay when the CS was either an odorant (N=6) or photostimulation of ChR2+ neurons (Dual Virus with >300 ChR2+ neurons: N=6).
Each odor activates about 100,000 neurons distributed across the piriform cortex without spatial preference (Stettler and Axel, 2009). ChR2-expressing neurons, however, localize to restricted domains that occupy less than 10% of the piriform (0.35 to 1.0 mm in diameter). We have demonstrated that each of 18 independent ensembles at different locations across about 30% of piriform is capable of eliciting aversive behavior (Figure 3C and Supplementary Table 1). The observation that aversive behavior can be entrained at multiple loci distributed across the piriform indicates that valence of behavioral output is not spatially segregated in the piriform cortex.
We have compared the efficiency with which odor and photostimulation served as a conditioned stimulus to elicit flight behavior. Pairing of odor exposure with foot shock resulted in a consistent aversive response to odor alone after 10 CS-US pairings [10.25±0.96 pairings, N=4]. Approximately twice as many pairing were required to elicit flight behavior in mice expressing ChR2 in a subpopulation of piriform neurons [18±4.02 pairings, N=11] (Figure 3D). Thus, the activation of an ensemble of 500 piriform neurons approaches the efficacy of odor activation of 100,000 neurons (Stettler and Axel, 2009), in eliciting conditioned aversion.
An Ensemble of Neurons Trained to Elicit Appetitive Behavior
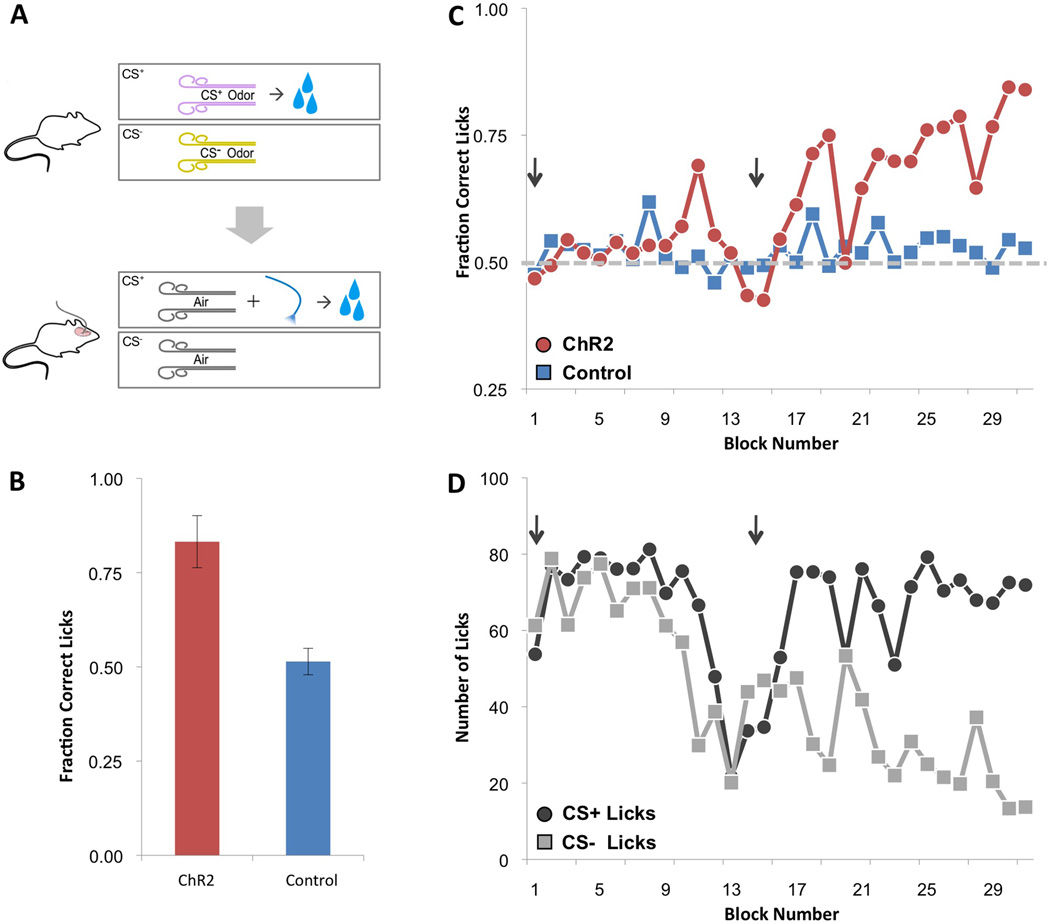
We next asked whether the photostimulation of an ensemble of piriform neurons expressing ChR2 could elicit appetitive behavioral responses if paired with a rewarding US. We modified a go/no-go odor discrimination assay (Bodyak and Slotnick, 1999) in which water-restricted mice were exposed to two odors, one of which was followed by a water reward (Figure 4A, top). During this pre-training period, mice learned to sample the odor stimuli and lick only in response to the rewarded odor (data not shown). This behavioral paradigm was then modified such that water-restricted mice received a water reward only after photostimulation of ChR2-expressing piriform neurons (CS+) but not in the absence of photostimulation (CS−) (Figure 4A, bottom). Both the CS+ and CS− were accompanied by a pulse of air to mimic the pre-training condition. After switching from odor to photostimulation, mice initially licked in anticipation of water reward upon presentation of either the CS+ or CS− (Figure 4C and Figure 4D). As the trials progressed, animals expressing ChR2 reliably learned to lick after photostimulation and suppressed licking in its absence [fraction correct licks (number of licks following CS+ / total number of licks) = 0.83±0.07, N=7] (Figures 4B–4D). Control mice that did not express ChR2 continued to perform at chance levels [fraction correct licks = 0.51±0.04, N=7] (Figure 4B and Figure 4C). When photostimulation served as the CS+, approximately twice as many blocks of trials were required to elicit robust conditioning compared with odor [odorant=14.67±9.58 blocks, N=6; photostimulation=32.67±16.85 blocks, N=6] (Figure 3E). Thus the activation of an arbitrarily chosen ensemble of piriform neurons could be entrained to drive appetitive as well as aversive behavioral responses.
Figure 4. Ensembles of ChR2-expressing piriform neurons entrained to elicit appetitive behavior.
A. Mice expressing ChR2 using the dual virus strategy were trained in an appetitive behavioral conditioning paradigm. Mice pre-trained to sample and lick only in response to a rewarded odor (CS+) subsequently received a water reward after photostimulation of ChR2-expressing neurons (CS+) but not in the absence of photostimulation (CS−). The CS+ and CS− were accompanied by a pulse of air to cue discrimination. Each training block consisted of 10 CS+ and 10 CS− trials.
B. The average fraction of correct licks over the last three training blocks for ChR2-expressing animals (Emx1: N=1, Dual Virus with >300 ChR2+ neurons: N=6) and control animals (in which EGFP but not ChR2 was expressed or a Cre-dependent ChR2 virus was injected without a second Cre-expressing virus) trained using the same paradigm that included photostimulation (N=7). Fraction correct licks = # of licks following CS+ / total # of licks.
C. Performance plotted as the fraction correct licks per block number for a ChR2-expressing mouse using the dual virus strategy and a control mouse in which EGFP but not ChR2 was expressed. Start of the training session on each day in C and D is marked with an arrow.
D. Same data for the ChR2-expressing mouse shown in C plotted as the number of licks following the CS+ and CS−. The decrease in licks at the end of the first training session is typical and is likely due to the animal reaching satiety.
We also demonstrated that an ensemble of ChR2-expressing neurons in piriform could be entrained to a socially rewarding unconditioned stimulus. We designed a behavioral paradigm in which males were exposed to odor in the presence or absence of a female (Figure 5A, left). Training was performed in a three-chambered arena housing a female and odor (CS+) on one side and odor alone (CS−) on the other side. A male was introduced to the middle chamber and allowed to freely explore the arena. As previously described, the male spent most of the training time exploring the female (Nadler et al., 2004). After training, the male was returned to the same arena that now contained only the CS+ and CS− odors. We observed that males spent two to threefold more time in the chamber with the CS+ odor than in the other chambers [CS+ chamber=50.76±5.31%; Middle chamber=22.87+4.84%; CS− chamber=26.37±3.17%, N=6] (Figure 5A, right). These data demonstrate that odor can be associated with a socially rewarding US to elicit conditioned approach behavior.
Figure 5. Ensembles of ChR2-expressing piriform neurons entrained to a socially rewarding stimulus.
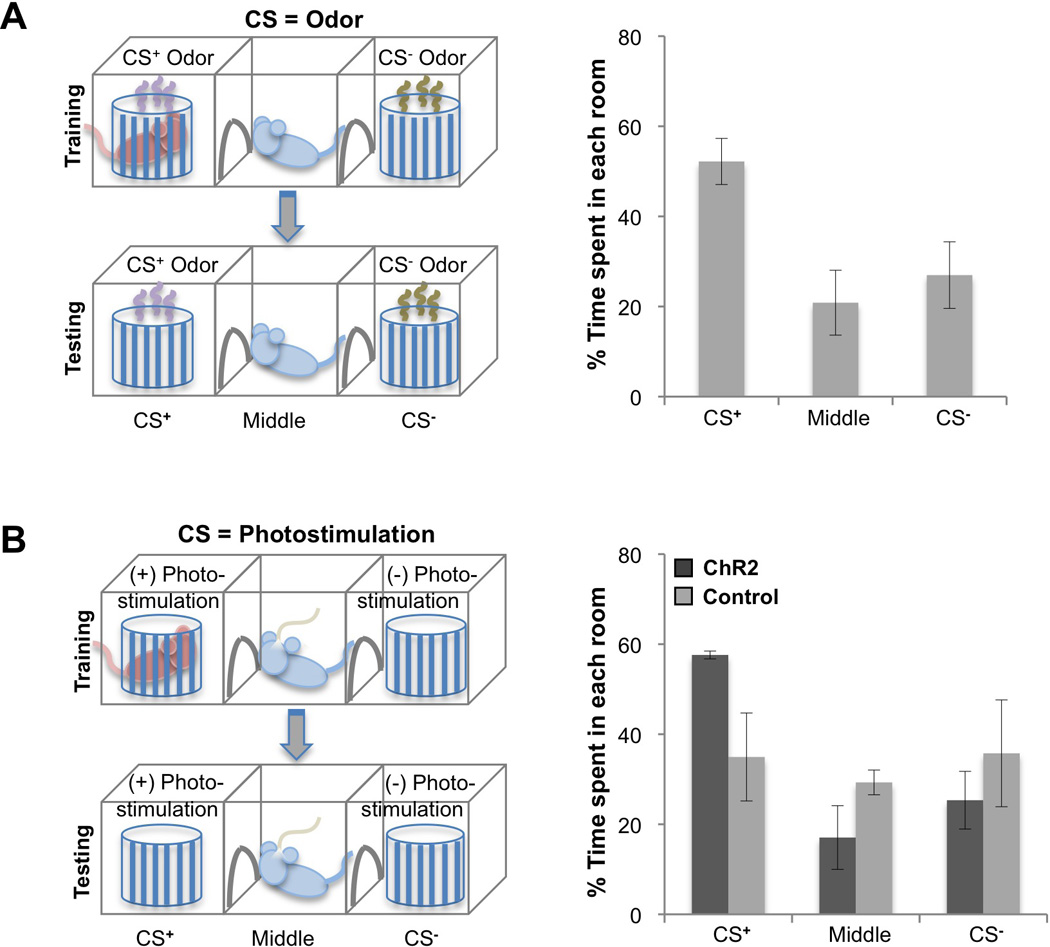
A. Entrainment of an odorant with a social reward. A male was trained in a three-chamber arena, in which the CS+ odor was paired with a female in a randomly selected side chamber (left). The other side chamber contained the CS− odor without a female. For testing, the animal was returned to the same arena with side chambers containing only the CS+ and CS− odors. On right, the percentage of time animals spent in each chamber during a 5 minute testing period is plotted for when CS+ and CS− were odors (N=6). CS+ = chamber with CS+ odor, Middle = middle chamber, CS− = chamber with CS- odor.
B. Entrainment of ChR2-expressing ensembles with a social reward. During training, photostimulation was applied when the males actively investigated the female in a randomly selected side chamber (left). Upon testing in the absence of a female, photostimulation was delivered in one of the side chambers (CS+ chamber) but not the other (CS− chamber). On right, the percentage of time animals spent in each chamber during a 5 minute testing period when the CS+ was photostimulation is plotted for animals expressing ChR2 (ChR2, N=3) and for control animals without ChR2 (N=3). ChR2 was densely expressed using the dual virus strategy.
We then asked whether an ensemble of ChR2-expressing piriform neurons could also be entrained to a socially rewarding US by replacing the CS+ odor with photostimulation during training (Figure 5B, left). In this paradigm, photostimulation was applied when males investigated the female. Testing was performed in the absence of a female and photostimulation was delivered in only one of the chambers (CS+ chamber). The percentage of time trained males spent in the CS+ chamber was threefold higher than in the other chambers (Figure 5B, right) [CS+ chamber=57.6±0.87%; Middle chamber=17.05±7.07%; CS− chamber=25.34+6.41%, N=3], demonstrating that activation of ChR2+ neurons can be associated with a socially rewarding US to elicit a learned approach behavior. This behavioral paradigm extends the repertoire of appetitive behaviors that can be elicited by an arbitrarily chosen ensemble of neurons in the piriform.
The Same Ensemble of Neurons Can Elicit Aversive and Appetitive Behavior
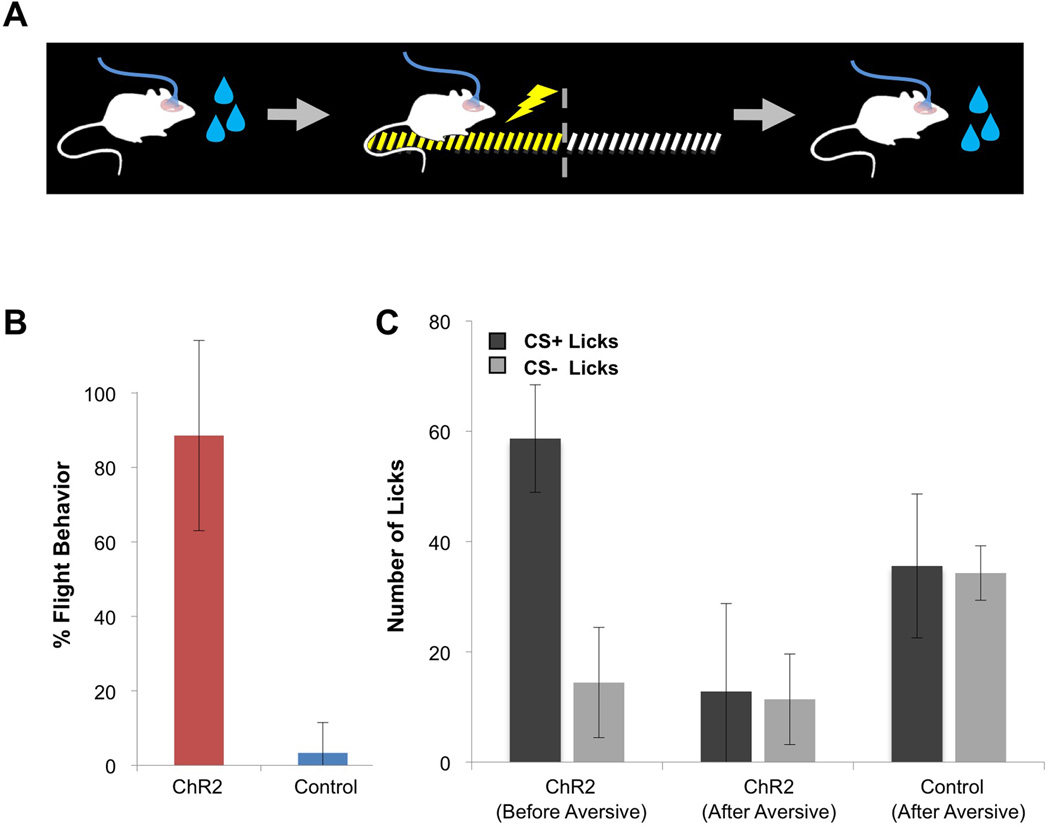
If the representation of odor in the piriform results from the random convergence of glomerular inputs, then its valence cannot be developmentally programmed and is likely to be imposed by experience. Consistent with this reasoning, the same odor can elicit appetitive or aversive responses dependent upon learning (Abraham et al., 2004; Yan et al., 2008). We therefore asked whether the same population of ChR2-expressing neurons in piriform could be entrained to sequentially elicit behaviors of different valence (Figure 6A). Mice expressing ChR2 in piriform were first trained in the appetitive paradigm and consistently exhibited conditioned licking responses upon photostimulation (Figure 4B). The animals were then conditioned in the aversive foot shock paradigm and now displayed robust flight behavior (Figure 6B). Sequentially trained animals acquired the aversive behavior as quickly as naïve animals (data not shown) and exhibited flight behavior in 90% of the trials [ChR2+ animals=88.57±25.55%, N=5; control animals=3.33±8.16%, N=6].
Figure 6. The same ensemble of ChR2-expressing neurons can be entrained to elicit appetitive and aversive behaviors.
A. A schematic of the sequential training of ChR2-expressing animals to produce appetitive and aversive behaviors.
B. A subset of mice shown in Figure 4B, which were trained in an appetitive water reward behavior, was subsequently trained in the aversive foot shock paradigm. The percentage of trials in which animals exhibited flight behavior in response to photostimulation alone during the testing phase is plotted for ChR2-expressing (Emx1: N=1, Dual Virus with >300 ChR2+ neurons: N=4) and control animals (N=6).
C. The average lick number over the last three training blocks of sequentially trained animals (from 4B and 6B) before and after aversive conditioning with the same ensemble. ChR2 Before Aversive: number of licks following CS+ and CS− for ChR2-expressing mice during initial appetitive conditioning (CS+=58.68+9.76 licks and CS−=14.43+9.99 licks, N=5). ChR2 After Aversive: number of licks following CS+ and CS−for these mice after sequential appetitive-aversive conditioning (CS+=12.81+15.96 licks and CS−=11.39+8.21 licks, N=5). Control After Aversive: number of licks following CS+ and CS− for control animals after sequential appetitive-aversive conditioning (CS+=35.58±13.05 licks and CS−=34.29±4.94 licks, N=5).
We next asked whether the response to photostimulation in these sequentially trained animals was context-dependent. Sequentially trained animals were returned to the appetitive conditioning context. Upon photostimulation, they no longer exhibited appetitive responses in anticipation of water reward. Rather, the level of licking to the CS+ approached that of the CS− (Figure 6C). Moreover, photostimulation occasionally elicited freezing, another form of aversive behavior, but failed to produce flight behavior (data not shown). These experiments demonstrate that a given ensemble of piriform neurons can be sequentially entrained to elicit appetitive and aversive behavioral responses. After the sequential training, the aversive behavior appears to dominate the response to photostimulation.
Distinct Ensembles of Active Neurons Can be Discriminated
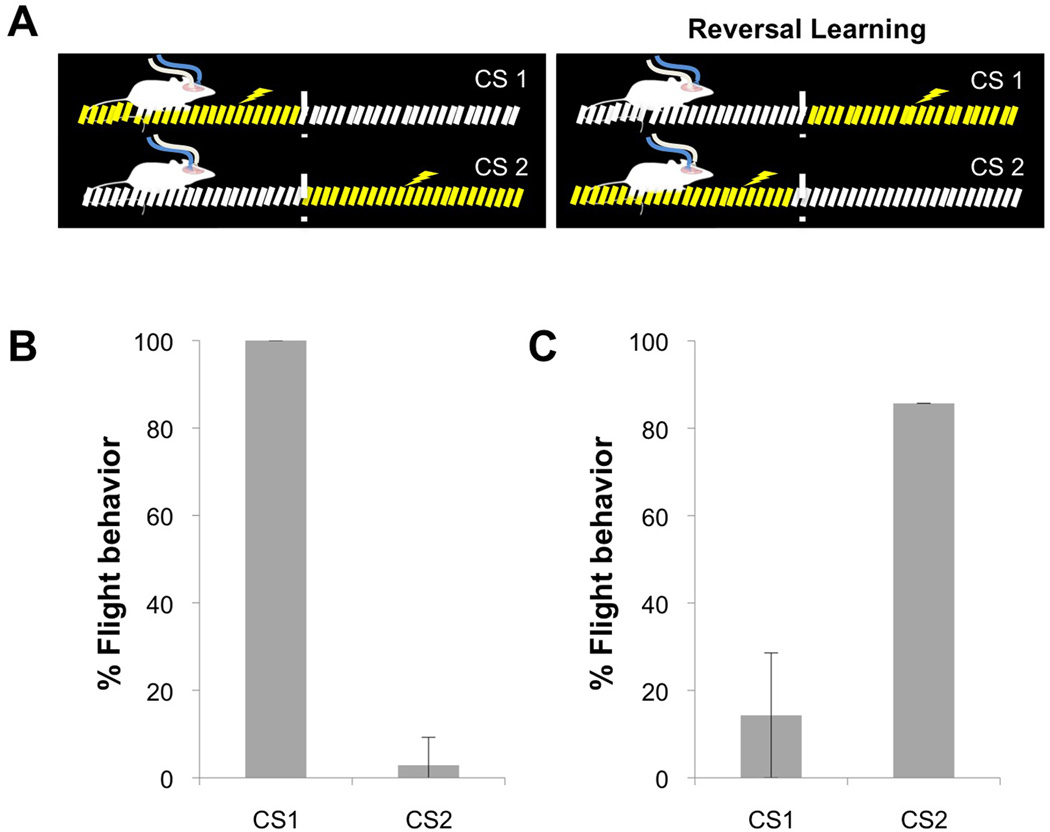
Distinct odors activate unique ensembles of neurons in the piriform cortex (Stettler and Axel, 2009) and can be linked by conditioning to different behavioral responses (Abraham et al., 2004; Yan et al., 2008). We have therefore asked whether different subpopulations of piriform neurons expressing ChR2 could be discriminated and independently entrained to elicit distinct behaviors. Lentivirus encoding ChR2 was injected into the piriform cortex of each hemisphere to generate two anatomically distinct populations of neurons that could be independently photostimulated by two optical fibers. Stimulation of one ensemble (CS1) was paired with shock on the side of the arena where the animal received the photostimulation. As described earlier, this resulted in robust flight to the opposite side of the training arena that was free of shock. Stimulation of the second ensemble (CS2) was paired with shock to the opposite side of the arena and the mice remained within the vicinity of the site of CS presentation (Figure 7A, left). Thus, two different ensembles of ChR2-expressing neurons could be behaviorally discriminated: CS1 elicited reliable flight whereas CS2 resulted in a stationary behavioral response [% flight behavior: CS1=100±0% and CS2=2.86±6.39%, N=5] (Figure 7B).
Figure 7. Distinct ensembles of ChR2-expressing piriform neurons can be entrained to elicit different behaviors.
A. A schematic of the apparatus used for the conditioning paradigm. Initially, stimulation of one ensemble (CS1) was paired with shock on the side of the arena where the animal received the photostimulation, and stimulation of the second ensemble (CS2) was paired with shock to the opposite side of the arena. For a reversal-learning paradigm, the shock contingency was switched between the two ensembles (Reversal Learning).
B. The percentage of trials in which animals exhibited flight behavior in response to CS1 and CS2 after training with the CS-shock contingencies described in A, left (hSynapsin1: N=1, Dual Virus: N=4).
C. The percentage of trials in which flight behavior was elicited by the CS1 and CS2 for a subset of animals shown in B after they were subsequently trained with reversed CS-shock contingencies described in A, right (Reversal Learning, hSynapsin1: N=1, Dual Virus: N=2).
We then asked whether each ensemble retained the potential to elicit both behavioral responses. We trained the mice in a reversal-learning paradigm in which the shock contingency was switched between the two ensembles (Figure 7A, right: Reversal Learning). The CS2, which initially elicited stationary behavior, resulted in flight after reversal learning indicating that both ChR2-expressing ensembles were capable of eliciting flight behavior after appropriate training [CS2=85.71±0%, N=3] (Figure 7C). Immediately upon reversal, the CS1 that initially elicited flight continued to produce aversive behavior. However, upon further training, animals learned that flight resulted in shock and CS1 ultimately elicited stationary behavior [% flight behavior: CS1=14.2+14.29%, N=3] (Figure 7C). These observations demonstrate that different ensembles of ChR2-expressing neurons in the piriform can be discriminated and can be entrained to elicit distinct behavioral outputs.
DISCUSSION
We have devised an experimental strategy that permits us to ask whether the activation of an arbitrarily chosen subpopulation of neurons in piriform cortex can elicit different behavioral responses dependent upon experience. Activation of a small subpopulation of as few as 300 neurons at multiple loci in the piriform cortex, when paired with different unconditioned stimuli elicits either appetitive or aversive behavior. Moreover, we demonstrate that different subpopulations of piriform neurons expressing ChR2 can be discriminated and independently entrained to elicit distinct behaviors. Thus, an experimentally generated network comprised of a small ensemble of piriform neurons, activated in the absence of sensory input, is sufficient to elicit one of multiple, learned behavioral outputs.
Each odorant activates 3–15% of the neurons distributed across the piriform cortex without spatial preference (Stettler and Axel, 2009). Photostimulation of as few as 300 spatially localized neurons in piriform, about 0.5% the number of neurons activated by odor, is capable of eliciting both appetitive and aversive behaviors. Moreover, we demonstrate that photostimulation of neuronal ensembles at several positions across the piriform are capable of eliciting aversive behavior, indicating that valence is not spatially segregated in the piriform. Taken together, these observations imply that the piriform cortex does not use spatial order to map olfactory input (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011; Stettler and Axel, 2009) or behavioral output.
How does the same ensemble of piriform neurons elicit behavioral outputs of different valence? In one model, each neuron within an ensemble connects with multiple, different behavioral outputs and learning reinforces only one of these outputs to assure an appropriate response. Alternatively, different subsets of neurons within the ensemble may be connected with distinct behavioral outputs and learning will enhance the output of only one subset of neurons. If the piriform is comprised of subsets of neurons dedicated to distinct behavioral outputs, our data indicate that these neurons do not reside within gross, spatially defined domains. We have not determined the brain regions responsible for these behavioral outputs. Piriform projects to multiple downstream areas, including amygdala, tubercle and prefrontal cortex that have been implicated in motivated behavior. Piriform also sends recurrent projections to the olfactory bulb that arborize in the granule cell layer (Shepherd, 2004). It remains possible that this feedback also participates in eliciting the observed behaviors.
This experimental scenario may provide insight into the neural processing that transforms olfactory sensory input into behavioral output. In the piriform, projections from individual glomeruli are distributed throughout the cortex without apparent topographic order (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011). Individual odors activate a distributed subpopulation of neurons across the cortex without spatial preference (Illig and Haberly, 2003; Poo and Isaacson, 2009; Rennaker et al., 2007; Stettler and Axel, 2009; Sugai et al., 2005; Zhan and Luo). One model of piriform processing consistent with these anatomic and physiologic observations invokes the random convergence of a combination of excitatory inputs from multiple mitral cells onto piriform neurons (Stettler and Axel, 2009). In this model, a given odor will activate a different ensemble of piriform neurons in different individuals. However, in an individual, a given odor will consistently activate the same ensemble and this representation will acquire coherence to dictate a specific behavioral output. This model is supported by recent experiments demonstrating a cell in piriform can be activated by stimulating a random combination of glomeruli (Davison and Ehlers, 2011). Despite this evidence, it remains possible that piriform odorant representations will reveal an undiscovered order.
If the connections from bulb to cortex are indeed random, then the quality of an odorant or its valence in the piriform must be imposed by experience. This experience-dependent relation between representation and valence is observed upon light activation of an arbitrarily chosen ensemble of piriform neurons: a given ensemble of neurons can elicit different behaviors dependent upon the conditioning paradigm. However, we cannot conclude that the ChR2 network we have generated recapitulates the neural processing of the circuit elicited by an odorant. An odorant representation comprises far more neurons in piriform than the ChR2 network we have generated. Moreover, odorants activate the bulb that in turn projects to several other brain areas in addition to piriform.
Earlier models have been elaborated in which features of the external world are transmitted from the sense organ to the cortex via ordered, genetically determined pathways (Changeux et al., 1973; Edelman, 1987). Topologic order is then reorganized at higher centers creating a degenerate network with variability in cortical connections among individuals. This degenerate network is then subject to selection by experience over the life of an organism. Selection reinforces connections from neurons that represent sensory objects of behavioral significance. The ability to entrain populations of piriform neurons to elicit specific behavioral responses is in accord with these models.
A similar conceptual organization in which the random convergence of entorhinal inputs creates a distributed ensemble may also be operative for hippocampal place cells. Place cells in the hippocampus, a three-layered cortical structure like piriform, exhibit no apparent relationship between their positions in brain space and their firing field in the external world (O'Keefe et al., 1998; Redish et al., 2001). As a consequence, the spatial map in the hippocampus is likely to differ in different individuals residing in the same environment. Moreover, place cells remap: they alter their firing properties in response to changes in spatial environment (Colgin et al., 2008). This suggests models in which inputs to individual place cells are randomly chosen during development such that a given location is represented by a distributed ensemble of active neurons.
The exogenous activation of neurons in other sensory cortices, either by microstimulation (Doty, 1969; Murphey and Maunsell, 2007; Yang et al., 2008) or photostimulation (Huber et al., 2008), has been shown to elicit behaviors following training. Microstimulation of loci within visual, auditory, and somatosensory cortex suggests that neuronal activation at many neocortical levels of sensory processing is capable of influencing perceptual tasks. In more recent experiments, photostimulation of sparse ensembles of ChR2-expressing neurons in somatosensory cortex was conditioned to drive appetitive behavior (Huber et al., 2008). These sensory neocortices maintain topographic order that represents stimulus features in at least one dimension across the cortex. As a consequence, microstimulation or photostimulation of a locus in these cortices results in the activation of a topographically constrained subpopulation of neurons that is likely to encode specific features of a sensory stimulus. In these experiments, focal activation may exploit the underlying topographic organization of sensory neocortices to elicit specific behavioral output. In the piriform, features of an olfactory stimulus are not topographically organized (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011; Stettler and Axel, 2009) and the ability of an arbitrarily chosen ensemble of ChR2-expressing neurons to elicit specific outputs cannot exploit an underlying spatial order. Rather, the behavioral entrainment of an ensemble of ChR2+ neurons may reflect the ability of piriform to elicit odor-evoked behavior by activating a distributed ensemble of neurons without regard to spatial order.
It may be argued that it is possible to elicit specific behaviors by the activation of any collection of neurons in cortex independent of the underlying neural organization. The postulated ability of any arbitrarily chosen ensemble to elicit learned behaviors would reflect a striking, inherent property of neural populations in the brain. Our data suggest that piriform cortex may exploit this property to translate olfactory input into learned behavioral output.
In vision, touch, and sound, features central to perception are topographically ordered in the sense organ and this representation is maintained in the primary sensory neocortices (Marshall, 1941; Talbot, 1941; Woolsey, 1942). Moreover, these features, such as spatial location and sound frequency, are continuously variable in at least one dimension in the external world. A meaningful representation of a sensory object, however, may require that these features are combined at higher cortical centers. It is difficult to conceive of a developmentally programmed strategy to encode the inordinately large number of complex stimuli that can be discriminated. A strategy involving selection from a random combination of features could, however, accommodate the complex problem of sensory discrimination. However, experience-dependent selection from randomness would be most apparent, not in primary sensory cortex but at higher processing centers. In contrast, olfactory features cannot be meaningfully represented along continuous dimensions in the physical world and are not topographically organized in the piriform cortex (Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011; Stettler and Axel, 2009). In the olfactory system, features of an odorant may be encoded by the receptors themselves such that the random combination of these features is already apparent in primary olfactory cortex.
If the connections from bulb to cortex are indeed random, then the representation of an odorant or its valence in the piriform must be imposed by experience. A small subset of odorants, however, elicit stereotyped behaviors that are likely to be mediated by genetically-determined projections from the olfactory bulb to other olfactory centers (Kobayakawa et al., 2007). Spatially invariant projections from the olfactory bulb to cortical amygdala implicate this structure in the generation of innate behaviors (Miyamichi et al., 2011; Sosulski et al., 2011). This bifurcation in the olfactory circuit in the mouse is analogous to the architecture of the olfactory system in Drosophila despite the six hundred million years of evolution that separates the two organisms. In Drosophila, information from the antennal lobe (olfactory bulb equivalent) bifurcates with one branch exhibiting spatially invariant projections to the lateral horn, a brain region mediating innate olfactory behavior. A second branch projects to the mushroom body, a structure that may receive random convergent input and is required for learned olfactory responses (Marin et al., 2002; Murthy et al., 2008; Wong et al., 2002). Thus, innate, olfactory-driven behaviors are likely to derive from determined neural circuits that result from Darwinian selection over evolutionary time whereas learned behaviors may be mediated by the selection and reinforcement of random ensembles over the life of an organism.
EXPERIMENTAL PROCEDURES
Histochemistry
Immunofluorescence was performed on coronal sections of brain perfused with 4% paraformaldehyde, following standard protocols. The prepared slices were labeled with the following antibodies: chicken anti-GFP (Abcam, ab5450), goat anti-c-Fos (Santa Cruz, sc-52-G), rabbit anti-c-Fos (Santa Cruz, sc-7270), or rabbit anti-DsRed (Clontech, 632496).
Stereotaxic injections
All procedures were carried out according to the approved protocols at Columbia University. Wild-type C57 BL6/J, heterozygous Emx1-IRES-Cre or homozygous CNG2A mice were injected with lentivirus carrying ChR2, Cre-dependent ChR2, or Cre-dependent ChR2 mixed with lentivirus carrying Cre.
Aversive behaviors
The conditioning paradigm consisted of 3–4 s of laser activation (photostimulation = 20 Hz with 25 ms pulses) followed immediately by a 0.7 mA foot-shock. Photostimulation/shock pairings were spaced 3–4 min apart. Each of the two training sessions consisted of 10 photosimulation/shock pairings, for a total of 20 pairings. The testing session was identical in set-up to the training sessions, but only photostimulation was applied when the animal was located in either end of the apparatus.
Appetitive go/no-go discrimination assay
Training and testing were performed using the Slotnick operant conditioning paradigm and a liquid-dilution, eight-channel olfactometer (Knosys, Lutz, FL). The animals were trained to discriminate between the photostimulation of ChR2+ piriform neurons as CS+ (photostimulation = 20 or 30 Hz with 25 ms pulses, for 3 seconds) and absence of photostimulation as CS−. Both the CS+ and CS− were accompanied by a pulse of air to mimic the pre-training condition. The fraction correct licks were calculated as number of licks following the CS+ / total number of licks
Social Approach Behavioral paradigm
Behavioral training and testing were carried out in a custom-built three-chambered arena. During training, a wire cage containing a female was placed in one side chamber while an empty wire cage was placed in the opposite side chamber. Placement of the female-containing cage was randomly selected for each trial. Photostimulation was applied when the male actively investigated the female and lasted for total of ~30 seconds per trial. A minimum of 10 trials was completed, with an inter-trial interval of ~3 minutes. During testing in the absence of a female, photostimulation was delivered when the male was in one of the arbitrarily chosen side chambers (CS+ chamber).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Karl Deisseroth for ChR2 reagents; Nadia Propp for setting up lentiviral production method; Meredith Glinka, Justin Schwarz and Dan Feng Mei for help with behavioral assays; Monica Mendelsohn, Jennifer Kirkland and Nataliya Zabello for help with the mice; Anmo Kim for help with laser and olfactometer machines; Phyllis Kisloff for assistance in preparation of the manuscript; David J. Anderson, Larry Abbott, Thomas Jessell, Eric R. Kandel and members of the Axel lab for critical reading of the manuscript and discussions; and Miriam Gutierrez for general laboratory support. This work was supported by the Howard Hughes Medical Institute and the Mathers Foundation. G.B.C. was supported by the Damon Runyon Cancer Research Foundation Postdoctoral Fellowship. A.F. was supported by long-term Postdoctoral fellowships from EMBO and the Human Frontiers Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Araneda RC, Peterlin Z, Zhang X, Chesler A, Firestein S. A pharmacological profile of the aldehyde receptor repertoire in rat olfactory epithelium. J Physiol. 2004;555:743–756. doi: 10.1113/jphysiol.2003.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Courrege P, Danchin A. A theory of the epigenesis of neuronal networks by selective stabilization of synapses. Proc Natl Acad Sci U S A. 1973;70:2974–2978. doi: 10.1073/pnas.70.10.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Davison IG, Ehlers MD. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RW. Electrical stimulation of the brain in behavioral context. Annu Rev Psychol. 1969;20:289–320. doi: 10.1146/annurev.ps.20.020169.001445. [DOI] [PubMed] [Google Scholar]
- Edelman G. The Theory of Neuronal Group Selection. New York: Basic Books; 1987. Neural Darwinism. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- Godfrey PA, Malnic B, Buck LB. The mouse olfactory receptor gene family. Proc Natl Acad Sci U S A. 2004;101:2156–2161. doi: 10.1073/pnas.0308051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat’s striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kugler S, Kilic E, Bahr M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003;10:337–347. doi: 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Marshall WH, Woolsey CN, Bard P. Observations on cortical somatic sensory mechanisms of cat and monkey. J Neurophysiol. 1941;4:1–24. [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Davies PW, Berman AL. Response properties of neurons of cat’s somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957;20:374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- Murphey DK, Maunsell JH. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353:1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Katada S, Omura M, Suwa M, Yoshihara Y, Touhara K. Odorant receptor map in the mouse olfactory bulb: in vivo sensitivity and specificity of receptor-defined glomeruli. Neuron. 2006;52:857–869. doi: 10.1016/j.neuron.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Powell TP. The mitral and short axon cells of the olfactory bulb. J Cell Sci. 1970;7:631–651. doi: 10.1242/jcs.7.3.631. [DOI] [PubMed] [Google Scholar]
- Redish AD, Battaglia FP, Chawla MK, Ekstrom AD, Gerrard JL, Lipa P, Rosenzweig ES, Worley PF, Guzowski JF, McNaughton BL, et al. Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-05-j0004.2001. RC134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Ebert U, Loscher W. The central piriform cortex: anatomical connections and anticonvulsant effect of GABA elevation in the kindling model. Neuroscience. 2004;126:727–741. doi: 10.1016/j.neuroscience.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The Synaptic Organization of the Brain. New York: Oxford University Press; 2004. [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Sugai T, Miyazawa T, Fukuda M, Yoshimura H, Onoda N. Odor-concentration coding in the guinea-pig piriform cortex. Neuroscience. 2005;130:769–781. doi: 10.1016/j.neuroscience.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Talbot SA, Marshall WH. Physiological studies on neural mechanisms of visual localization and discrimination. Am J Ophthalmol. 1941;24:1255–1263. [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Woolsey CN, Walzl EM. Topical projection of nerve fibers from local regions of the cochlea to the cerebral cortex of the cat. Bull Johns Hopkins Hosp. 1942;71:315–344. [Google Scholar]
- Yan Z, Tan J, Qin C, Lu Y, Ding C, Luo M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58:613–624. doi: 10.1016/j.neuron.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Yang Y, DeWeese MR, Otazu GH, Zador AM. Millisecond-scale differences in neural activity in auditory cortex can drive decisions. Nat Neurosci. 2008;11:1262–1263. doi: 10.1038/nn.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, Luo M. Diverse patterns of odor representation by neurons in the anterior piriform cortex of awake mice. J Neurosci. 2011;30:16662–16672. doi: 10.1523/JNEUROSCI.4400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124–133. doi: 10.1038/nn800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.