Abstract
Implant-associated fibrotic capsule formation presents a major challenge for the development of long term drug release microspheres and implantable sensors. Since material properties have been shown to affect in vitro cellular responses and also to influence short term in vivo tissue responses, we have thus assumed that the type and density of surface chemical groups would affect the degree of tissue responses to microsphere implants. To test this hypothesis, polypropylene particles with different surface densities of -OH and –COOH groups, along with the polypropylene control (-CH2 groups) were utilized. The influence of functional groups and their surface densities on tissue reactions were analyzed using a mice subcutaneous implantation model. Our comparative studies included determination and correlation of the extents of fibrotic capsule formation, cell infiltration into the particles and recruitment of CD11b+ inflammatory cells for all of the substrates employed. We have observed major differences among microspheres coated with different surface functionalities. Surfaces with –OH surface groups trigger the strongest responses while –COOH rich surfaces prompt the least tissue reactions. However, variation of the surface density of either functional group has a relatively minor influence on the extent of fibrotic tissue reactions. The present results show that surface functionality can be used as a powerful tool to alter implant-associated fibrotic reactions and, potentially, to improve the efficacy and function of drug delivery microspheres, implantable sensors and tissue engineering scaffolds.
Keywords: Surface modification, inflammation, microspheres, subcutaneous implantation model
INTRODUCTION
Implantable medical devices play an increasingly important role in contemporary medicine. Specifically, implantable drug delivery devices are used increasingly for a number of drugs that cannot be delivered orally. For example, subcutaneous implants delivering contraceptives, chemotherapeutic drugs and insulin were widely used in the past.1–8 In addition to having a simple implantation procedure, they also relieve the patient from being hospitalized and/or receiving frequent painful injections.9 However, many of these medical devices fail to produce long term therapeutic effect raising serious concerns over the efficiency of these implants.10–17 For example, the chemotherapeutic doxorubicin delivering millirods has been reported to lose efficacy 8 days post subcutaneous implantation.8. Many recent evidences have supported that dense avascular fibrotic tissues serve as diffusion barrier for the released drugs to enter into circulation and for blood components and nutrition from the tissue fluid to interact with implanted sensor.11,18–20 The enormity of this complication is underlined by previous studies which have found that even low molecular weight substances cannot cross the barrier.21–24 Therefore, it is generally accepted that, by reducing implant-mediated fibrotic tissue, the life span, function, and efficacy of drug releasing devices, sensors, and tissue engineering scaffolds could be substantially improved.20,25–26
Many methods have been developed to alter fibrotic reactions. For example, dexamethasone releasing rods have been developed to reduce fibrotic reactions in implant surrounding tissue.27 Some studies have proposed coating implantable medical devices with anti inflammatory drug releasing systems.25,28 However to ensure that the implanted drug delivery device lasts for a long duration, a different approach to locally affect tissue responses is urgently needed.
Since the physical and chemical properties of biomaterials has long been shown to affect protein adsorption and subsequent cellular reactions,29–33 significant investigations have been placed on modifying material surface properties to generate surfaces with better tissue compatibility and/or reactivity. As an outcome, a plethora of techniques including physical modifications, chemical modifications, and radiation have been developed to effectively modify material surface characteristics.34–37 The modification of many material surface properties (including chemistry, wettability, domain composition and morphology) has been found to influence protein adsorption and subsequent cellular responses to biomaterials in vitro. Some studies have shown how surface chemistry and IgG and serum protein adsorption modulated monocytes and macrophage adhesion in vitro.29,38 A few other studies showed how fibrinogen and plasma adsorption on to material surface correlated positively and linearly to the monocytes adhesion in vitro.30,31,39,40 However, in animal models, these surface modifications, mostly on film or membrane form of biomaterials, exert insignificant influence on tissue responses and biocompatibility of the implants.31,41–43
The cause for the discrepancy of the in vitro and in vivo influence of surface functionality is not clear. Based on several lines of evidence, we have hypothesized that the ineffectiveness of surface functionality on in vivo tissue compatibility may simply reflect insufficient cell: functionality interaction. Specifically, in an in vitro culture system, cells are seeded on top of the functional group, thus, exerting maximal influence on cellular responses. However, in an in vivo environment, the functional groups on implants can only interact with the first layer of the cells, mostly inflammatory cells, and have limited or no contact with abundant outer fibrotic cells. In fact, our studies have found that surface functionality has significant influence on inflammatory cell accumulation (or inflammatory responses), but limited effect on fibrotic tissue reactions.42,43,55 To test this hypothesis, it is essential to increase the interactions between functional groups and cells by increasing the surface area and/or density of functional groups. Spherical particles were chosen for this purpose to maximize the surface to volume ratio of the implants and also to mimic drug release particles and highly porous tissue scaffolds.
There are very limited surface modification techniques which can covalently link various densities of functional groups on particles. By modifying Radio Frequency Glow Discharge (RFGD) technique, our laboratory has recently developed a novel method to functionalize a wide variety of micro,44 and more recently, nanosized, spheres.45 Additionally, using a variable duty cycle pulsed plasma technique, our previous studies have shown that controlled film chemistry can be effectively produced from a given monomer undergoing polymerization. In particular, when operated under relatively low plasma duty cycles, the film chemistry control permits retention of normally reactive functional groups in monomers polymerized under plasma conditions.46,47 This technology has been successfully employed in variety of biomaterial applications.44,48–51
To investigate the influence of types and densities of surface functional groups on foreign body reactions, polypropylene microspheres were coated with varying densities of –OH and –COOH functionalities. After subcutaneous implantation in mice for two weeks, the extent of inflammatory and fibrotic responses to the implants was then evaluated histologically. The results clearly demonstrate that surface functionality has a dramatic effect on tissue responses, as reflected by capsule thickness and cell infiltration around the implants. The densities of functional groups, on the other hand, have relatively little or no influence on modulating the degree of foreign body reactions.
MATERIALS AND METHODS
Materials
Polypropylene microspheres, of uniform 35 micron diameter, were obtained from Polysciences, Inc (Warrington, Pa). Di (ethylene glycol) vinyl ether [CH2=CH (OCH2CH2)2OH], (hereafter EO2V), and vinyl acetic acid, CH2=CHCH2COOH, (hereafter VAA), were obtained from Sigma Chemical Company (St. Louis, MO) and were of the highest purities available. Each monomer was out-gassed repeatedly, using freeze thaw cycles, prior to use. Primary antibody against CD11b and peroxidase labeled-secondary antibody were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA).
Modification of the polymeric surfaces
The micron-sized polypropylene microspheres were surface functionalized using plasma polymerization of the monomers. The two monomers were selected to provide uncharged hydrophilic (EO2V) and anionic (VAA) surfaces. A home-built, 360 degree rotatable, reactor was employed to provide constant agitation during the actual coating step. As shown previously, this approach provides effective uniform polymer film coverage of the micron sized substrates by reducing the tendency for particle aggregation.45 In fact, as shown by high resolution TEM studies, the rotating plasma reactor approach results in uniform, conformal polymer coatings on particles as small as 25 nm, as shown in coating studies of TiO2 nanoparticles.45 Since the micron sized polypropylene particles exhibited significantly less tendency to aggregate than the nano-TiO2 particles, we are confident that the plasma polymers were deposited as uniform films on the particle substrates. Furthermore, XPS examination of VAA coated particles produced essentially the same spectra as that obtained from the deposition of VAA on a flat Si substrate, for depositions carried out under identical plasma conditions. An uneven, or patch-like, coating of the particles would have resulted in a lower -COOH percent composition in comparing the particles with the Si sample. One gram samples of the particles were coated in each run. The polypropylene microspheres were cleaned and sterilized in ethanol, followed by prolonged vacuum drying prior to the plasma treatment. A portion of these ethanol sterilized untreated polypropylene microspheres were used as control samples.
Pulsed plasmas were employed during the polymer film coating process. Preliminary studies were carried out to vary the polymer functional group densities by altering plasma duty cycle and peak power input. The stabilities of the polymer films produced were carefully examined. These evaluations involved soaking of the coated substrates in PBS buffer solution (pH = 7.2) for periods extending up to two weeks, followed by vacuum drying and XPS analysis. It was observed that films produced under relatively low pulsed plasma conditions were not stable under the soaking conditions employed. For example, although it was possible to synthesize VAA films having –COOH functional group concentrations as high as 20% of the total surface carbons, only those films having –COOH content less than 10% were stable to the PBS exposure. The higher –COOH content films were simply not sufficiently cross-linked to resist dissolution in these aqueous solutions. All substrate particles employed in this work had films strongly grafted to the microspheres and they were sufficiently cross-linked to resist dissolution or delaminating when immersed in aqueous solutions. It is assumed that the films remained intact on the particles during the two week implant period. In light of extensive cell infiltration and biomolecule adsorptions, we are unable to check this assumption spectroscopically.
It should be noted that all conditions used in this study provided films strongly grafted to the microspheres and sufficiently cross-linked to resist dissolution when immersed in aqueous solutions. After coating and before implantation, the microspheres were placed in a vacuum oven, set at 500C, and pumped for 72 hours to remove any monomers and/or low molecular weight oligomers adsorbed on these particles from the plasma process. The functionalized microspheres were kept in a sterilized environment and containers at all times prior to implantation.
XPS and FT-IR spectroscopy were used to characterize the molecular structure of the polymer films. The XPS analyses were carried out using a Perkin-Elmer model PHI5000 instrument, using Al Kα radiation at 1486.6 eV, with a pass energy of 17.90 eV giving a resolution of 0.60 eV for the Ag(3d5/2) standard. A neutralizer was used to eliminate charging effects on these insulator films. The binding energy of the C(1s) component representing carbon atoms not bonded to oxygen atoms was adjusted to a value of 284.6 eV.47 A Bi-Rad, Model FTS-40, operated at 8 cm−1 resolution and transmission mode, was used for the FT-IR characterizations. Wettability of the surfaces was measured using a static angle Rame-Hart sessile drop goniometer, as described in our previous work.42,44,48,49,51 To obtain accurate surface property assessments, these film characterization measurements were made on flat substrates coated in the plasma reactor under identical conditions to those employed with the polypropylene particles. Polished Si substrates were employed for the XPS and water contact angle measurements and KBr discs for the FT-IR determinations. We have previously shown that the polymer film compositions are independent of the nature of the polymer substrate, once the film thickness exceeds 5 to 10 nm. In the present study, 100nm thick films were employed with all samples. The key surface functionalities obtained from these monomers were hydroxyl (-OH) groups from the EO2V and carboxylic acid (-COOH) groups from the VAA as described below. The relative surface density of these groups increased with decreasing average power input employed during the film formation. The average power input is defined as: Average power (W) = (Duty cycle) x Peak Power (W), where the duty cycle represents the ratio of the plasma on time divided by the plasma (on +off) time.
Animal implantation model
Animals were cared for in compliance with protocols approved by the Institutional Animal Care and Use (IACUC) at the University of Texas at Arlington. The surface functionalized microspheres and un-treated controls were implanted subcutaneously in Balb/C mice (25 grams body weight) from Taconic Farms (Germantown, NY, USA). Briefly, part of mice dorsal skins was shaved and microsphere solution (100 mg particles mixed with 0.5 ml of sterilized saline) was then injected into the subcutaneous spaces using 18 gauge needles. Implant-bearing mice were sacrificed two weeks after implantation. The microsphere implants and the surrounding tissues were excised and then frozen in OCT embedding media (Polysciences Inc., Warrington, PA)at −80°C for histological and immunohistochemical analyses.
Histological and immunohistochemical analyses
Ten μm thick sections were sliced from frozen tissues using a Leica Cryostat (CM1850) and placed on poly-L-lysine coated slides. To assess the extent of tissue responses to different implants, some of these tissue slices were stained with haematoxylin and eosin (H&E). The collagen deposition was determined by Masson Trichrome staining. To quantify the degree of biomaterial-mediated inflammatory responses, immunohistological analyses for CD11b+ inflammatory cells were carried out on some tissue samples. Specifically, these slides were rinsed in washing buffer and then blocked with 1% BSA solution for 40min at 37 °C. After brief rinsing with PBS, tissue sections were then incubated with the CD11b primary antibody (1:200 dilution) for 2 hrs at 37 °C. This was followed by rinsing in PBS and then incubating with peroxidase-conjugated rabbit anti-mouse CD11b (1:500 dilution) for 1hr at 37 °C. The tissue sections were then exposed to DAB liquid substrate for 15 minutes at 37 °C. Following PBS rinse, the tissue sections were ready for analysis.
Microscopy and Image Analyses
After staining, tissue sections were analyzed using a Leica fluorescence microscope (Leica Microsystems, Wetzlar, GmbH) equipped with a Nikon E500 Camera (8.4V, 0.9A, Nikon Corp., Japan). Microscopic images of H&E stained slides at a magnification of 20X (field of view 1.1mm, viewing area 3.8 mm2) were used to assess the extent of implant-mediated fibrotic responses by measuring the numbers of implant-associated cells and the distance of cell infiltration into particle implants. The numbers of implant-associated cells were calculated based on blue stained nuclei seen in the fibrotic capsule surrounding the implant. The distance of cell infiltration was determined based on the penetration of the cells into the polymeric implant. Immunohistochemistry stained slides observed at a magnification of 20X (field of view 1.1mm, viewing area 3.8 mm2) were used to quantify the densities of recruited CD11b+ inflammatory cells to reflect the degree of inflammatory responses to implants.
Statistical analyses
Statistical comparison (achieved on the basis of variable cell number, infiltration depth and thickness of capsule) within the groups with varying density of functionalization was carried out using ANOVA. Differences were considered statistically significant when p ≤0.05.
RESULTS
RFGD Surface Modification of the Particles
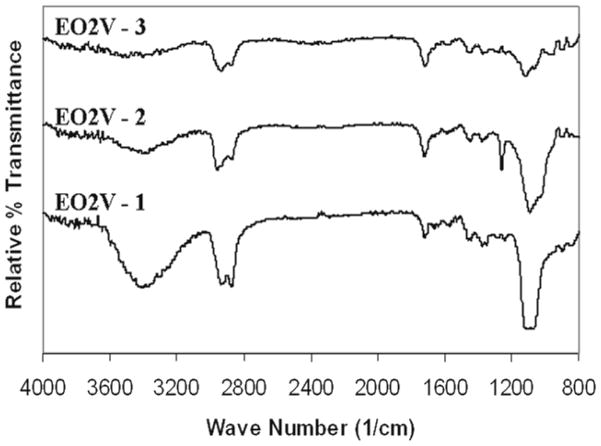
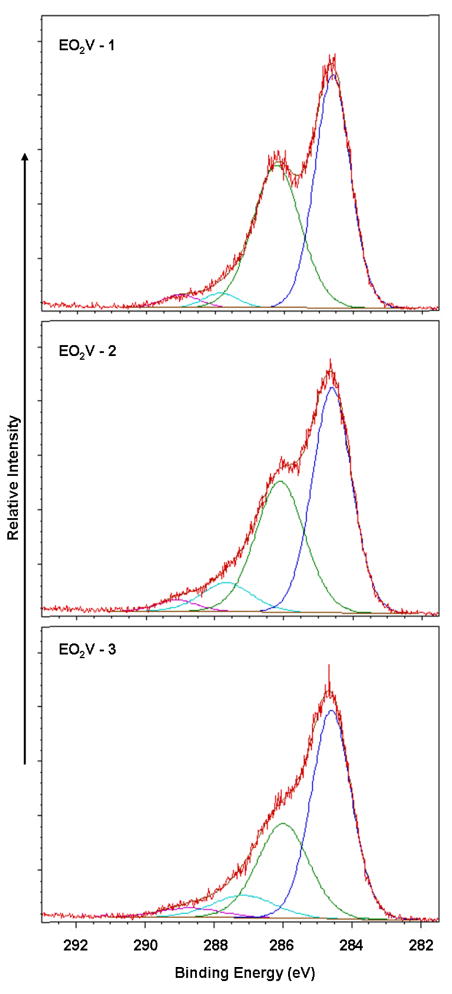
The surfaces of the PP particles were modified using pulsed plasmas to vary the chemical composition of the plasma generated thin polymeric films. Under the pulsed condition, the extent of retention of the functional group in a given monomer increases as the duty cycle employed during the plasma polymerization is decreased.52 In the present case, this technique was successfully employed to vary the surface densities of the –OH and -COOH groups obtained from the EO2V and VAA monomers, respectively. Both FT-IR and XPS characterization of the films confirmed the progressive change in film compositions with sequential changes in the plasma duty cycles, all other factors held constant. FT-IR transmission spectra of 3 separate films produced from the EO2V monomer are shown in Figure 1(A). These spectra are arranged in order of decreasing average power input (top to bottom) with the exact duty cycles, peak powers and monomer flow pressures provided in Table 1. Inspection of Figure 1 clearly reveals a sharp increase in the relative intensity of the –OH groups (at 3400 cm−1) compared to the intensity –(CH)X functionalities (at 2900 cm−1) as the average power input was decreased. Additional evidence for retention of monomer functionality in the polymer films, with decreasing average power input, is documented by the absorption band at 1150 cm−1, characteristic of the C-O stretch, which increases sharply with decreasing power input. This increase is consistent with increased retention of both the OH and ether groups, originally present in the monomer, in the polymer film as the average power input is decreased. The progressive film compositional changes, with average power input variations, was further confirmed by high resolution XPS analyses of the C(1s) peaks of the three films, which are shown in Figure 2. The C(1s) photoelectron was resolved into 4 peaks, corresponding to carboxyl (289.0 eV); carbonyl plus O-C-O groups (287.5 eV); hydroxyl plus ether groups (at 286.0 eV); and C atoms not bonded to O atoms (284.6 eV). These peak assignments are in accord with recommended values for these groups.53 The relative intensity of each of these peaks, as obtained from integration of the deconvoluted spectrum, is shown in Table 1. Clearly, as shown in Figure 2 and listed in Table 1, there is a substantial increase in the intensity of the peak at 286.0 eV, representing the -OH + ether groups, relative to the three other peaks, as the average power input was decreased. This result, of course, is consistent with the FT-IR analyses of these same films.
Figure 1.
FT-IR transmission spectra of EO2V polymer films produced as a function of the average power input during the pulsed plasma depositions. Spectra are arranged in order of decreasing power input, reading top to bottom. The exact plasma conditions employed are shown in Table 1.
Table 1.
Plasma conditions employed, functional group distribution and water contact angle of the poly EO2V films used in this study.
| Plasma Conditions (Duty cycle, Power, Pressure) | C – C, C – H ~ 284.6 eV |
C – O ~ 286.0 eV |
C=O, O-C-O ~ 287.5 eV |
- O – C = O ~ 289.0 eV |
Static water contact angle |
|---|---|---|---|---|---|
| 20/300, 33W, 60 mTorr EO2V – 1 |
51.6 | 42.4 | 3.1 | 2.9 | 45 ° |
| 25/300, 33W, 60 mTorr EO2V – 2 |
51.7 | 37.5 | 8.3 | 2.5 | 50 ° |
| 20/300, 50W, 60 mTorr EO2V – 3 |
55.3 | 32.1 | 8.9 | 3.7 | 55 ° |
Figure 2.
C(1s) high resolution XPS spectra of EO2V polymer films arranged in order of decreasing power input (top to bottom) employed during the deposition process.
Finally, the increased polarity of these films, anticipated by an increased content of -OH groups with decrease in average power input, was confirmed by static water contact angle measurements as shown in Table 1. In particular, one notes the increased surface wettability with increase C-O content which is consistent with increased retention of the polar C-O-H and C-O-C groups of the EO2V monomer with decreased average power input during polymer formation. Overall the film compositional control and compositions obtained from the EO2V monomer are in excellent agreement with prior studies involving the plasma polymerization of this monomer.48
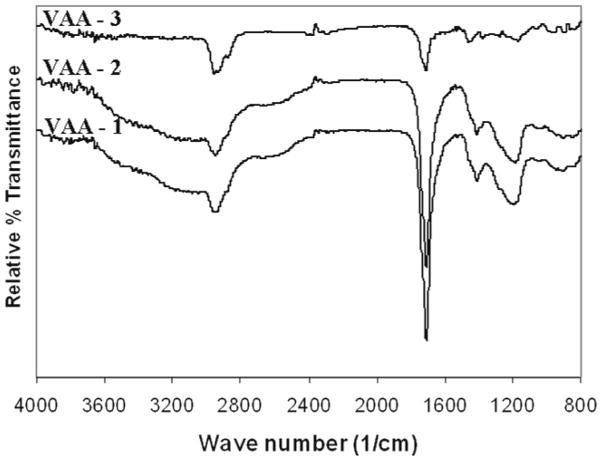
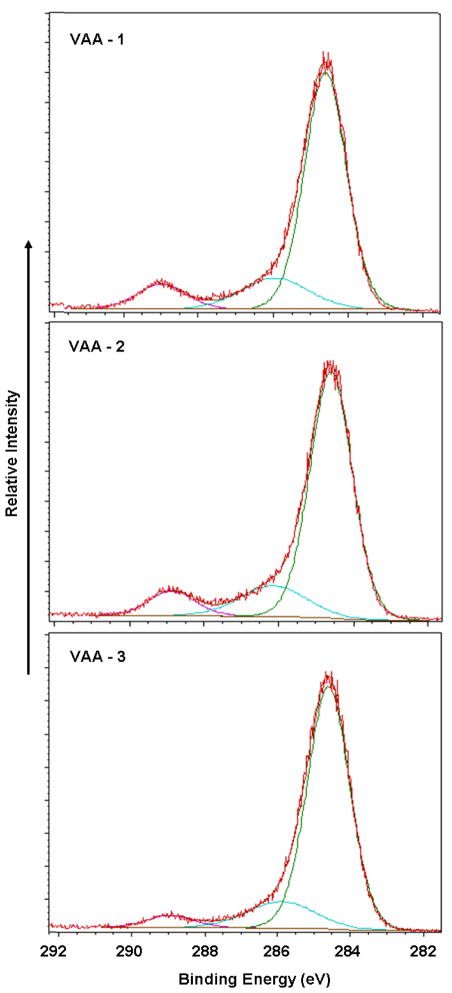
The results obtained with the VAA monomer parallel those obtained with EO2V as to increased retention of monomer functionality with decreasing average power input. The FT-IR and high resolution C(1s) spectra of films produced from VAA, as functions of average power input, are shown in Figures 3 and 4, respectively. The IR results showed a strong increased retention of the carbonyl group (1700 cm−1) with decreased average power input. Although more difficult to quantify, the increased presence of the –COOH groups, with decreasing average power input, is also revealed with the very broad band absorption extending from around 3500 to approximately 2700 cm−1. This broad band, characteristic of –COOH groups in solid samples,54 is essentially absent in the highest power input run but is clearly very prominent in the two samples produced at the lower power inputs. The increased retention of –COOH groups, with decreasing power input, was further confirmed by the XPS results which shown a clear increase in the highest binding energy component of the C(1s) at 289.0 eV with decreasing power input. As noted above, this peak represents the –COOH functionality. The relative contributions of the three separate components of the C(1s) spectra are shown in Table 2. Finally, as also provided in Table 2, the wettability of the samples increased as the power input decreases, revealing an increased polarity of the films, a result consistent with increased retention of the –COOH group in the films.
Figure 3.
FT-IR transmissionspectra of VAA polymer films produced at different average power input conditions during the pulsed plasma depositions. Spectra are arranged in order of decreasing power input, reading top to bottom. The exact plasma conditions employed are shown in Table 2.
Figure 4.
C(1s) high resolution XPS spectra of VAA films arranged in order of decreasing average power input (topto bottom) employed during the deposition process.
Table 2.
Plasma conditions employed, functional group distribution and water contact angle of the poly VAA films used in this study
| Plasma Conditions (Duty cycle, Power, Pressure) | C – C, C – H ~ 284.6 eV |
C – O ~ 286.0 eV |
- COOH ~ 289.0 eV |
Static water contact angle |
|---|---|---|---|---|
| 0.75/20, 200W, 80 mTorr VAA – 1 (High) |
75.0 | 15.7 | 9.3 | 40 ° |
| 1/15, 150W, 80mTorr VAA – 2 (Medium) |
76.7 | 14.9 | 8.4 | 45 ° |
| 2/15, 150W, 80mTorr VAA – 3 (Low) |
81.3 | 14.2 | 4.5 | 55 ° |
Overall, the above results document the compositional controllability of the polymer films produced under pulsed plasma conditions, in this case permitting variation of the surface functionalities of interest for the implant studies described below.
Surface functionality influences fibrotic capsule formation
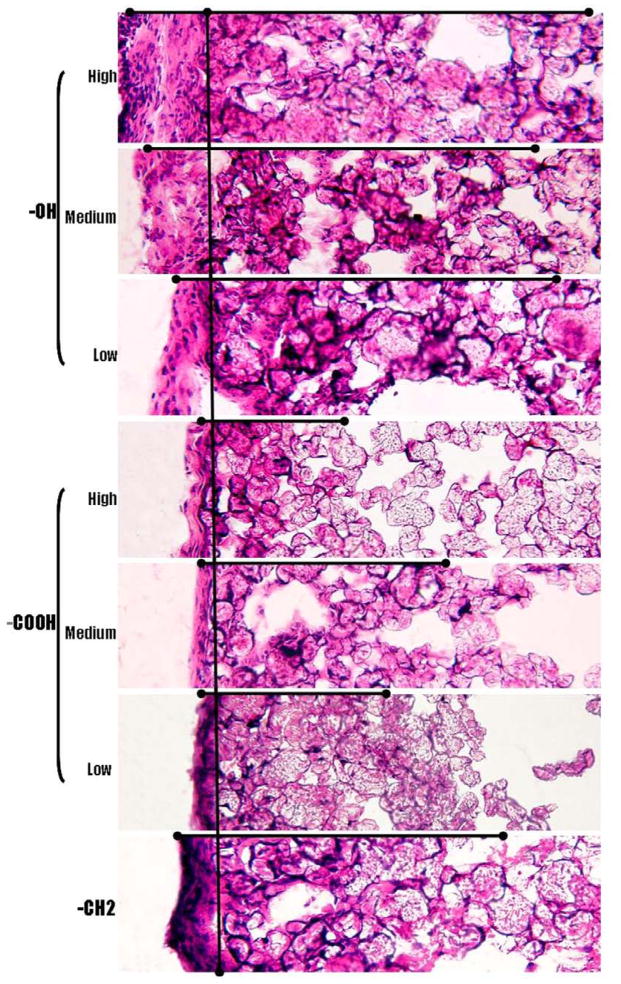
A 2 week implant study was carried out to study the impact of varying coating thickness on the host tissue response. H&E staining of the skin tissue 2 weeks post implantation revealed the effect of the coating thickness on the fibrotic capsule formation around the implants (Figure 5). The capsule thickness and the extent of the cell infiltration into the particle implants characterize the degree of fibrotic reaction.
Figure 5.
Implant-induced tissue responses were assessed based on capsule thickness and cellular infiltration. Polypropylene microspheres with –OH and –COOH functionalities at high, medium and low densities along with control –CH2 functionality were subcutaneously injected in to Balb/C mice. H&E stain of subcutaneous tissue 2 weeks post implantation shows the tissue reaction to different surface functionalities. (
 ) shows the trend followed by capsule thickness and the extent of cell infiltration in to the implant across functional groups (Magnification 20x).
) shows the trend followed by capsule thickness and the extent of cell infiltration in to the implant across functional groups (Magnification 20x).
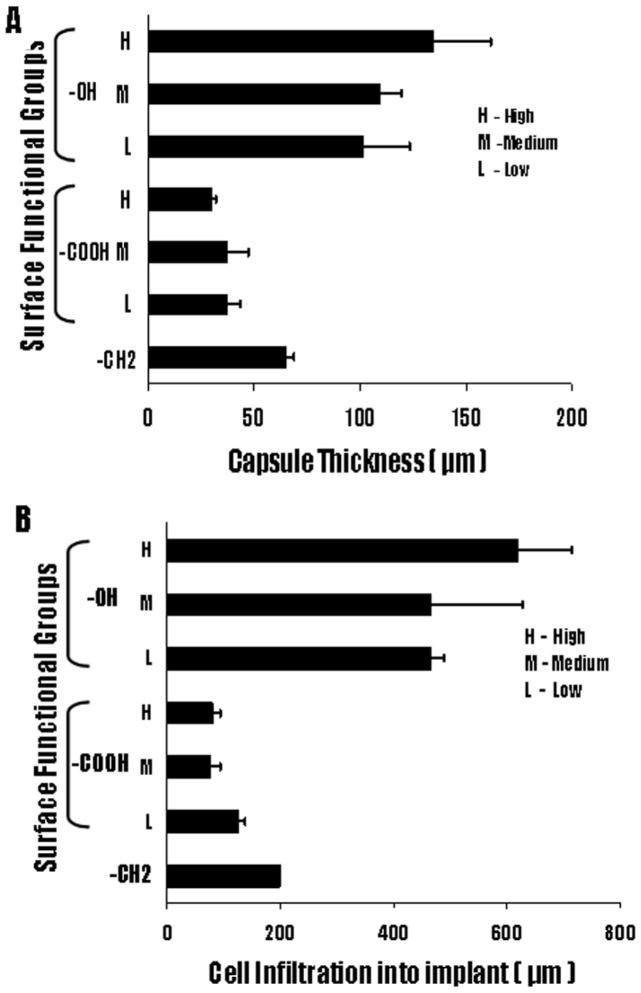
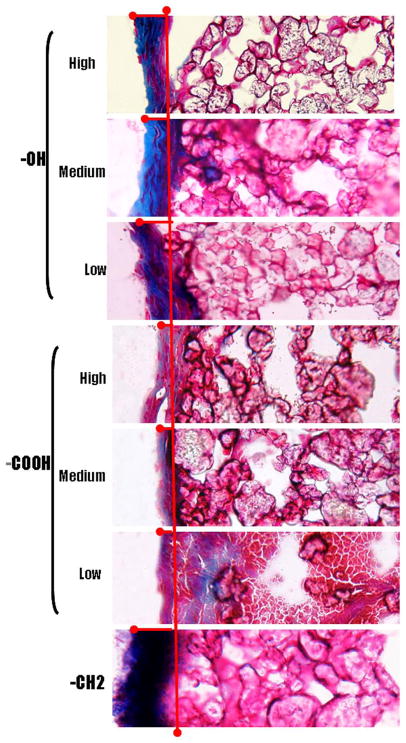
Consistent with our previous study,55 the fibrous capsule around the implants varied significantly among the three different functional groups represented. In general, the particles containing the –OH groups exhibited the thickest capsule formation, followed by the polypropylene control particles, which consist predominately of the -CH2 functionality. The polypropylene particles with –COOH groups showed the least capsule formation. However, within the –OH and –COOH functionalized particles only minor changes in capsule thickness was noted with variations in the surface density of these two groups. As shown in Figure 6, the capsule thickness for the –OH containing particles varied from 101.8 ± 21.4 μm to 109.2 ± 10.0 μm and 134.4 ± 27.5 μm as the surface density of this group was increased. The polypropylene particles exhibited a capsule thickness of 65.1 ± 10.3 μm, while the particles coated with the lowest density of the –COOH group resulted in a capsule thickness of 37.4 ± 6.1 μm. Subsequent increases in the –COOH concentrations increase marginally reduced the capsule formation to 37.0 ± 10.2 μm and 30.0 ± 2.2 μm respectively (Fig 6A). Clearly, these results indicate that whereas different surface chemistries promote very different tissue reactions, subsequent progressive changes in the surface density of key surface functional groups promote only relatively minor changes in capsule sizes. The importance of the different surface functionalities in modulating tissue reactions is further emphasized by the deposition of collagen along the capsule (Fig 7). The control and the –OH functionalities have a higher collagen deposition along the capsule. In contrast, the capsule around the implants with –COOH functionalities shows minimal collagen deposition.
Figure 6.
Extent of tissue responses to polypropylene microspheres with –OH, -COOH and control –CH2 functionality were assessed based on (A) capsule thickness and (B) cell infiltration distance into particle implants. Polypropylene (PP) microspheres coated with high, medium and low densities of –OH and –COOH groups along with control –CH2 were subcutaneously implanted in Balb/C mice. The animals were sacrificed at 2 weeks post implantation. Values shown reflect the average thickness of the capsule ± SD (n= 3 for -OH, -COOH and –CH2).
Figure 7.
The deposition of collagen along the implant surrounding capsule was observed by Masson Trichrome stain. Polypropylene microspheres with –OH and –COOH functionalities at high, medium and low densities along with control –CH2 functionality were subcutaneously injected in to Balb/C mice. Masson Trichrome stain of subcutaneous tissue 2 weeks post implantation shows the tissue reaction to different surface functionalities which leads to deposition of collagen. (
 ) shows the extent of collagen deposition. (Magnification 20x).
) shows the extent of collagen deposition. (Magnification 20x).
Surface functionality affects cell infiltration in toparticle implants
The analysis of cell infiltration into the particles, shown in Figure 5, demonstrates that surface chemistry exerts a strong effect on the extent of the infiltration. However, in common with the fibrotic capsule results, variations of surface densities within the –OH and –COOH groups resulted in relatively small changes in infiltration. Similar to the fibrotic data, the infiltration increases slightly with increasing surface density of –OH groups and decreases slightly with increasing –COOH concentrations. The cell infiltration for the polypropylene control particles was intermediate between the –OH and -COOH functionalized particles. The –OH samples produced cell infiltrations ranging from a depth of 467.0 ± 22.6 μm to a value of 619.6 ± 94.6 μm, while the –COOH surfaces exhibited depths ranging from 127.4 ± 11.3. The polypropylene control particles showed cells penetration to a depth of 199.6 ± 1.6 μm (Figure 6B). Overall, the cell penetration results parallel the fibrotic capsule measurements as to the importance of surface chemistry and, at the same time, revealing that the surface densities of the particular functional groups employed exert a minor influence on the extent of cell migration.
Surface functionality modulates CD11b+ inflammatory cell recruitment
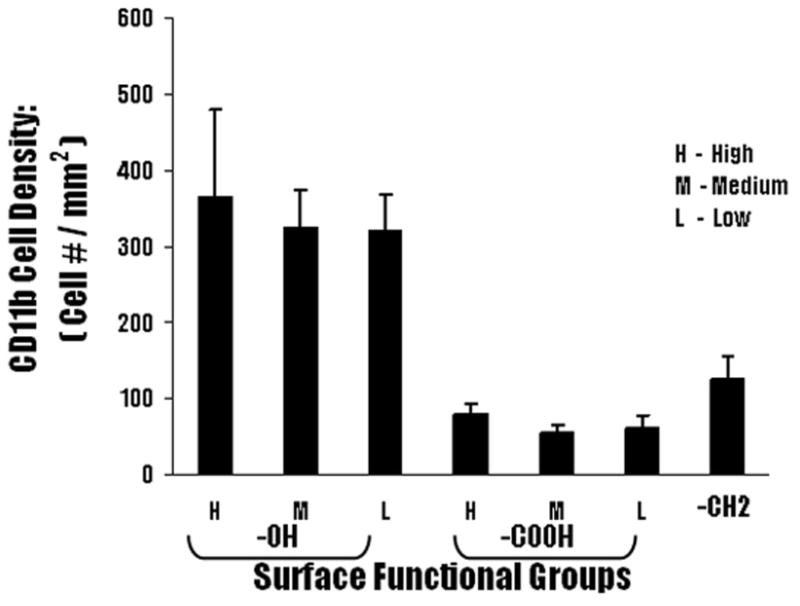
The CD11b+ inflammatory cells were quantified to determine the distribution of the inflammatory cells around the fibrotic tissue. Analysis of immunohistochemical staining of particle implants and surrounding tissues, showed that the –OH functionality triggered the highest recruitment of the CD11b+ cells around the implant. The lowest –OH surface density particles recruited 322.6 ± 44.9 CD11b+ cells per field of view, which was observed to increase to values of 326.3 ± 48.5 and 366.3 ± 113.6 per field of view for the particles having higher concentrations of this group (Figure 8). The number of inflammatory cells around the control – CH2 implants was 125.3 ± 31.2, while the –COOH functional group showed the least number of inflammatory cells with values of 62.1 ± 16.4, 56.2 ± 10.7 and 79.0 ± 14.0 CD11b+ cells, per field of view, observed for the sequence of increasing concentrations of the –COOH surface density (Figure 7).
Figure 8.
Immunohistochemical analyses of the recruitment of CD11b+ inflammatory cells to particle implants. Polypropylene particles coated with different surface functionalities were subcutaneously implanted in Balb/C mice. The animals were sacrificed at 2 weeks post implantations. Accumulation of CD11b + cells at the capsule highest for particles implant with –OH group. Values shown reflect the average number of CD11b+ cells seen at the capsule for different surface functionalities. ± SD (n= 4 for -OH, -COOH and –CH2).
Correlation between fibrotic capsule thickness and cell infiltration depth
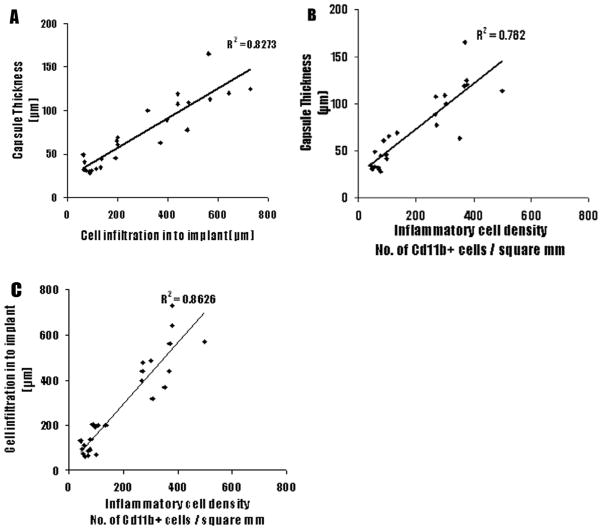
In order to validate the relationship between the nature of surface functionality and fibrotic reactions, we carried out linear regression between capsule thickness and depth of infiltration, as well as between the depth of infiltration and number of CD11b+ cells. We found a very strong correlation between capsule thickness and cell infiltration depth (Figure 9A) and between the CD11b+ cells and capsule thickness (Figure 9B). We also found a strong correlation between depth of infiltration and number of CD11b+ cells(Figure 9C).
Figure 9.
The linear correlations among capsule thickness, CD11b+ cell number, and cell infiltration depth. (A) The capsule thicknesses are graphed against cell infiltration depth. (B) The capsule thicknesses are graphed against CD11b+ cell numbers. (C) The cell infiltration depths are graphed against CD11b+ cell numbers.
DISCUSSION
Increasing evidence has now linked fibrotic tissue formation to the failure of many implantable devices, including drug release devices, encapsulated cells, sensors, and tissue engineering scaffolds.11,13,19,20,56–58 The collagenous capsule acts as a barrier to the diffusion of drugs and also blocks the supply of nutrients to the encapsulated cells in cell delivery systems. 11,18–20,59 By diminishing biomaterial-mediated fibrotic reactions and accompanied collagenous diffusion barrier formation, the drug release properties, life-span and sensor sensitivity could be significantly enhanced.11,18–20,23,57,58,60 Surface chemical properties of implant materials have been shown to affect protein adsorption and acute cellular responses.33,34,42,61–69 In the present study, we examined the hypothesis that biomaterial-mediated fibrotic reactions could be substantially reduced by altering both the type and/ordensity of surface functionality
Our results show that the type of surface functional groups has significant influence on biomaterial-mediated fibrotic reactions (reflected by capsule thickness and cell infiltration into microsphere implants). The influence of microsphere functionality in tissue reactions is much greater than previous published works on functionality of material films and membranes. 31,41,42,70 Although the cause for such differential responses has yet to be determined, we believe that the effect of surface functionality on tissue responses may be greatly enhanced by increasing the available functionalized surface area using microspheres. In terms of the biological effect of specific surface functionality, we find that the –OH group prompted the strongest capsule formation while the control –CH2 and, particularly, –COOH elicited both moderate and mild capsule formation and cell infiltration respectively. The capsule surrounding the implant bearing the –CH2 and –OH functionalities show high deposition of collagen in comparison to the –COOH bearing implants. Considering the time period for which the study was conducted, the high deposition of collagen along the –CH2 and –OH implant suggests that the initial tissue reaction could very well materialize in to a fibrotic reaction. The fibrotic capsule is marked by the presence of a high number of CD11b+ cells. CD11b, also known as Mac-1 integrin molecule, is seen when there is aggregation of inflammatory cells.14,43,67,71 We found a large number of CD11b+ cells near the capsule around –OH rich implants which elicited the strongest inflammatory response. Lower numbers of CD11b+ cells were found around the –COOH coated implants which elicited a weak inflammatory response. The strong tissue responses elicited by the hydrophilic –OH group are consistent with many earlier works. In those reports, -OH bearing surfaces were found to possess poor biocompatibility.67,70,72–75 A possible explanation for this phenomenon could be the activation of complement proteins, particularly C3b [Tang 1997]. C3b covalently links with the nucleophilic –OH group activating the alternative pathway in the complement system [McNally 1994;Hirata 2003]. Complement activation enhances the chemotaxis of inflammatory cells towards the surface with –OH group. In vitro assays have shown that the –OH groups are able to activate the alternative pathway of the complement cascade to a greater extent than –COOH groups [Barbosa 2004; Tengvall 1996; Liu 1996]. We can correlate our observation of a high number of Cd11b+ cells around the capsule in the –OH functionalized implants with the fact that Cd11b is the receptor for C3bi, the stable form of complement protein C3b [McNally 1994]. We observed very few Cd11b+ cells in the capsule surrounding the implants functionalized with –COOH surface functionality. This suggests that the deposition of complement protein C3bi is very low and thereby the complement system is not very active on the –COOH functionalized surface. It has also been shown that implants bearing the anionic –COOH group are well tolerated by the immune system,76 and has resulted in reduced fibrinogen adsorption. 77 The overall negative charge of the –COOH group repels plasma protein and reduces interactions with fibrotic cells. This observation can be correlated with previous studies which have linked the negatively charged surface functionality inhibiting the migration of negatively charged cells towards the functionalized surface.43,68,78–80
Rather unexpectedly, we find that the density of surface functionality has little or no influence on tissuereactions to microsphere implants. These results contradict many in vitro observations which found cell adhesion to be directly correlated to the density of functionalization.79,81 Furthermore, the density of surface functional groups has also been shown to increase the adhesion of cells to tissue culture plate surfaces. In an earlier study, the density of negatively charged surface functionality was varied along the length of the implant and then cultured with hamster ovary cells. An increase in cell adhesion was observed as the density of functionalization increased followed by a decrease in adhesion at higher density. This behavior was attributed more to the hydrophilicity than the effect of the functional group.79,81 The reason for relatively insignificant effects of functional group density on in vivo tissue responses, as observed in the present study, is not clear at this moment. It is possible that once a threshold density of a particular functional group is made available, further increases in these groups are relatively ineffective in that an essentially similar initial biomolecule surface-interaction, ultimately responsible for the capsule formation, cell infiltration, etc., dominates under in vivo conditions, independent of functional group concentrations, independent on the surface chemistry. Obviously, additional studies are indicated to explore this fundamental topic, both from the standpoint of helping to understand and control inflammatory response to implants, as well as to help reconcile the apparently different responses observed under in vivo and in vitro conditions.
Our results have shown good correlation between CD11b+ cell numbers, capsule thicknesses, and cell infiltration. This implies that the thickness of the surface functional group coating influences recruitment of the inflammatory cells and the fibrotic capsule formation. There are several implications from these very interesting relationships. First, CD11b+ is one of the common markers for inflammatory cells. It is well established that the numbers of CD11b+ cells reflect the extent of inflammatory responses. 15,43,71,82 The strong correlation between capsule thickness and inflammatory cell accumulation suggests that biomaterial-mediated inflammatory responses dictate the subsequent fibrotic tissue formation surrounding biomaterial implants. This suggestion is supported by observations from studies that showed the presence of a large number of inflammatory cells around untreated PET disks implanted in rabbits.83,84 Second, inflammatory responses (reflected by CD11b+ cell numbers) have good correlation with cell infiltration suggesting that inflammatory responses influence the extent of cell infiltration into microsphere implants. Third, there is a good relationship between capsule thickness and cell infiltration. This suggests that tissue reactions may lead to both fibrotic tissue thickening and inflammatory/fibrotic cells immigration into microsphere implants.85
In summary, this study represents an attempt to systematically investigate the influence of types and density of surface functional groups on host tissue responses. Interestingly, we found that the type, but not the density of the functional group coating has a significant effect on the inflammatory cell recruitment and tissue reactions. In time, such information may assist in the development of safe and biocompatible drug delivery devices and sensors.
Acknowledgments
This work was supported by NIH grant RO1 GM074021, and a Texas Higher Education Coordinating Board’s Advance Technology Program grant 003594-0003-2006.
References
- 1.Benagiano G, Gabelnick HL. J Steroid Biochem. 1979;11:449–455. doi: 10.1016/0022-4731(79)90066-9. [DOI] [PubMed] [Google Scholar]
- 2.Benagiano G, Ermini M, Gabelnick HL. Hormonal factors in fertlity, infertility and contraception. In: Vander Molen HJ, Klopper A, Lunefeld BL, Neves e Castro M, Sciarro F, Vermeulen A, editors. Excerpta Medica: Amsterdam. 1982. p. 141. [Google Scholar]
- 3.Ory SG, Hammond CB, Yancy SG, Hendern RW, Pitt CG. Am J Obstet Gynecol. 1983;145:600–605. doi: 10.1016/0002-9378(83)91204-8. [DOI] [PubMed] [Google Scholar]
- 4.Qian F, Nasongkla N, Gao J. J Biomed Mater Res. 2002;61:203–211. doi: 10.1002/jbm.10156. [DOI] [PubMed] [Google Scholar]
- 5.Qian F, Stowe N, Liu EH, Saidel GM, Gao J. J Cont Rel. 2003;91:157–166. doi: 10.1016/s0168-3659(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 6.Qian F, Stowe N, Saidel GM, Gao J. Pharm Res. 2004;21:394–399. doi: 10.1023/B:PHAM.0000019290.70358.30. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Qian F, Szymanski-Exner A, Stowe N, Haaga J. J Biomed Mater Res. 2002;62:308–314. doi: 10.1002/jbm.10292. [DOI] [PubMed] [Google Scholar]
- 8.Blanco E, Qian F, Weinberg B, Stowe N, Anderson JM, Gao J. J Biomed Mater Res. 2004;69:398–406. doi: 10.1002/jbm.a.30001. [DOI] [PubMed] [Google Scholar]
- 9.Blackshear PJ. Sci Am. 1979;241:66–73. doi: 10.1038/scientificamerican1279-66. [DOI] [PubMed] [Google Scholar]
- 10.Carlson AN, Stewart WC, Tso PC. Surv Opthalmol. 1998;42:417–440. doi: 10.1016/s0039-6257(97)00140-9. [DOI] [PubMed] [Google Scholar]
- 11.Fournier E, Passirani C, Montero-Menei CN, Benoit JP. Biomaterials. 2003;24:3311–3331. doi: 10.1016/s0142-9612(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 12.Tang L, Eaton JW. Cells and Materials. 1994;4:429–436. [Google Scholar]
- 13.Tang L, Eaton JW. Am J Clin Pathol. 1995;103:466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, Eaton JW. Mol Med. 1999;5:351–358. [PMC free article] [PubMed] [Google Scholar]
- 15.Hu WJ, Eaton JW, Tang L. Blood. 2001;98:1231–1238. doi: 10.1182/blood.v98.4.1231. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JM. Transactions -7th World Biomaterials Congress; Sydney. 2004. [Google Scholar]
- 17.Gallagher WM, Lynch I, Allen LT, Miller I, Penney SC, O’Connor DP, Pennington S, Keenan AK, Dawson KA. Biomaterials. 2006;27:5871–5882. doi: 10.1016/j.biomaterials.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 18.Sharkawy AA, Klitzmann B, Truskey GA, Reichert WM. J Biomed Mater Res. 1997;37:401–412. doi: 10.1002/(sici)1097-4636(19971205)37:3<401::aid-jbm11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 19.Rihova B. Adv Drug Del Rev. 2000;42:65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 20.Ratner BD. J Cont Rel. 2002;78:211–218. doi: 10.1016/s0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- 21.Woodward SC. Diabetes Care. 1982;5:278–281. doi: 10.2337/diacare.5.3.278. [DOI] [PubMed] [Google Scholar]
- 22.Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Diabetes Care. 1994;7:882–887. doi: 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 23.Wood RC, LeCluyse EL, Fix JA. Biomaterials. 1995;16:957–959. doi: 10.1016/0142-9612(95)93122-t. [DOI] [PubMed] [Google Scholar]
- 24.Sharkawy AA, Klitzmann B, Truskey GA, Reichert WM. Transactions 5th World Biomaterials Congress; Toronto. 1996. [Google Scholar]
- 25.Hickey T, Kreutzer D, Burgess DJ, Moussy F. J Biomed Mater Res. 2002;61:180–187. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 26.Giavaresi G, Tschon M, Borsari V, Daly JH, Liggat JJ, Fini M, Bonazzi V, Nicolini A, Carpi A, Morra M, Cassinelli C, Giardino R. Biomed Pharmacotherapy. 2004;58:411–417. doi: 10.1016/j.biopha.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Blanco E, Weinberg B, Stowe N, Anderson JM, Gao J. J Biomed Mater Res. 2006;76A:174–182. doi: 10.1002/jbm.a.30516. [DOI] [PubMed] [Google Scholar]
- 28.Patil SD, Papadimitrakopoulos F, Burgess DJ. Diab Technol Ther. 2004;6:887–897. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JM, Bonfield TL, Ziats NP. Int J Artif Org. 1990;13:375–382. [PubMed] [Google Scholar]
- 30.Pankowsky DA, Ziats NP, Topham NS, Ratnoff OD, Anderson JM. J Vasc Surg. 1990;11:599–606. [PubMed] [Google Scholar]
- 31.Shen M, Martinson L, Wagner MS, Castner DG, Ratner BD, Horbett TA. J Biomater Sci Polymer Ed. 2002;13:367–390. doi: 10.1163/156856202320253910. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Menges B, Timmons RB, Knoll W, Forch R. Langmuir. 2003;19:4765–4770. [Google Scholar]
- 33.Keselowsky BG, Collard DM, Garcia AJ. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 34.Sheu MS, Hudson DM, Loh IH. In Encyclopedic handbook of biomaterials and bioengineering. In: Wise DL, Trantolo DJ, Altobelli DE, Yaszemski MJ, Gresser JD, Schwartz ER, editors. Materials. Pt.A. Marcel Dekker, Inc; New York: 1995. p. 865. [Google Scholar]
- 35.Poncin-Epaillard F, Legeay G. J Biomater Sci Polymer Ed. 2003;14:1005–1028. doi: 10.1163/156856203769231538. [DOI] [PubMed] [Google Scholar]
- 36.Mao C, Qiu Y, Sang H, Mei H, Zhu A, Shen J, Lin S. Adv Colloid Interf Sci. 2004;110:5–17. doi: 10.1016/j.cis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Khorasani MT, Mirzadeh H. J Biomater Sci Polymer Ed. 2004;15:59–72. doi: 10.1163/156856204322752237. [DOI] [PubMed] [Google Scholar]
- 38.Collier TO, Anderson JM. J Biomed Mater Res. 2002;60:487–496. doi: 10.1002/jbm.10043. [DOI] [PubMed] [Google Scholar]
- 39.Shen M, Horbett TA. J Biomed Mater Res. 2001;57:336–345. doi: 10.1002/1097-4636(20011205)57:3<336::aid-jbm1176>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 40.Shen M, Pan YV, Wagner M, Hauch KD, Castner DG, Ratner BD, Horbett TA. J Biomater Sci Polymer Ed. 2001;12:961–978. doi: 10.1163/156856201753252507. [DOI] [PubMed] [Google Scholar]
- 41.Ratner BD, Bryant SJ. Ann Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 42.Tang L, Wu Y, Timmons RB. J Biomed Mater Res. 1998;42:156–63. doi: 10.1002/(sici)1097-4636(199810)42:1<156::aid-jbm19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 43.Barbosa JN, Madureira P, Barbosa MA, Aguas AP. Biomaterials. 2005;26:3021–3027. doi: 10.1016/j.biomaterials.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Susut C, Timmons RB. Int J Pharmaceutics. 2005;288:253–261. doi: 10.1016/j.ijpharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Cho J, Denes FS, Timmons RB. Chem Mater. 2006;18:2989–2996. [Google Scholar]
- 46.Savage CR, Lin JW, Timmons RB. Chem Mater. 1991;3:575–581. [Google Scholar]
- 47.Rinsch CL, Chen X, Panchalingam V, Eberhart RC, Wang JH, Timmons RB. Langmuir. 1996;12:2995–3002. [Google Scholar]
- 48.Wu Y, Timmons RB, Jen JS, Molock FE. Colloids Surfaces B: Biointerfaces. 2000;18:235–248. doi: 10.1016/s0927-7765(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 49.Harsch A, Calderon J, Timmons RB, Gross GW. J Neurosci Methods. 2000;98:135–144. doi: 10.1016/s0165-0270(00)00196-5. [DOI] [PubMed] [Google Scholar]
- 50.Friedrich J, Mix R, Kühn G, Retzko I, Schönhals A, Unger W. Composite Interfaces. 2003;10:173–223. [Google Scholar]
- 51.Li M, Timmons RB, Kinsel GR. Anal Chem. 2005;77:350–353. doi: 10.1021/ac0488107. [DOI] [PubMed] [Google Scholar]
- 52.Timmons RB, Griggs AJ. In: Plasma Polymer Films. Biederman H, editor. Imperial College Press; London: 2004. p. 217. [Google Scholar]
- 53.Beamson G, Briggs D. High Resolution XPS of Organic Polymers. John Wiley & Sons; New York: 1992. [Google Scholar]
- 54.Socrates G. Infrared Characteristic Group Frequencies. John Wiley & Sons; New York: 1994. [Google Scholar]
- 55.Kamath S, Bhattacharyya D, Padukudru C, Timmons RB, Tang L. J Biomed Mater Res. doi: 10.1002/jbm.a.31649. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider BL, Schwenter F, Pralong WF, Aebischer P. Mol Therapy. 2003;7:506–514. doi: 10.1016/s1525-0016(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 57.Soon-Shiong P, Otterlie M, Skjak-Braek G, Smidsrod O, Heintz R, Lanza RP, Espevik T. Transplant Proc. 1991;23:758–759. [PubMed] [Google Scholar]
- 58.Colton CK. Cell Transplant. 1995;4:415–436. doi: 10.1177/096368979500400413. [DOI] [PubMed] [Google Scholar]
- 59.Werkmeister JA, Tebb TA, White JF, Ramshaw JAM. Curr Opin Solid State Mat Sci. 2001;5:185–191. [Google Scholar]
- 60.Anderson JM, Niven H, Pelagalli J, Olanoff LS, Jones RD. J Biomed Mater Res. 1981;15:889–902. doi: 10.1002/jbm.820150613. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Q, Topham N, Anderson JM, Hiltner A, Lodoen G, Payet CR. J Biomed Mater Res. 1991;25:177–183. doi: 10.1002/jbm.820250205. [DOI] [PubMed] [Google Scholar]
- 62.Lee JH, Jung HW, Kang IK, Lee HB. Biomaterials. 1994;12:705–711. doi: 10.1016/0142-9612(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 63.Panchalingam V, Poon B, Huo HH, Savage CR, Timmons RB, Eberhart RC. J Biomater Sci Polymer Ed. 1993;5:131–145. doi: 10.1163/156856294x00707. [DOI] [PubMed] [Google Scholar]
- 64.Sevastianov VI. Crit Rev Biocomp. 1988;4:109–154. [Google Scholar]
- 65.Sperling C, Schweiss RB, Streller U, Werner C. Biomaterials. 2005;26:6547–6557. doi: 10.1016/j.biomaterials.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 66.Pompe T, Keller K, Mothes G, Nitschke M, Teese M, Zimmermann R, Werner C. Biomaterials. 2007;28:28–37. doi: 10.1016/j.biomaterials.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 67.Barbosa JN, Barbosa MA, Aguas AP. Biomaterials. 2004;25:2557–2563. doi: 10.1016/j.biomaterials.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Khang G, Lee JW, Lee HB. J Biomed Mater Res. 1998;40:180–186. doi: 10.1002/(sici)1097-4636(199805)40:2<180::aid-jbm2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 69.Sato Y, Kobayashi N, Murahara M. Mat Res Soc Symp Proc. 2005;843:227–232. [Google Scholar]
- 70.Barbosa JN, Madureira P, Barbosa MA, Aguas AP. J Biomed Mater Res. 2006;26A:737–743. doi: 10.1002/jbm.a.30602. [DOI] [PubMed] [Google Scholar]
- 71.Murphy RT, Foley JB, Crean P, Walsh MJ. Int J Cardiol. 2003;90:247–252. doi: 10.1016/s0167-5273(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 72.Gorbet MS, Sefton MV. J Lab Clin Med. 2001;137:345–355. doi: 10.1067/mlc.2001.114677. [DOI] [PubMed] [Google Scholar]
- 73.Gorbet MS, Sefton MV. J Biomed Mater Res. 2003;67:792–800. doi: 10.1002/jbm.a.10155. [DOI] [PubMed] [Google Scholar]
- 74.Barbosa JN, Barbosa MA, Aguas AP. J Biomed Mater Res. 2003;65A:429–434. doi: 10.1002/jbm.a.10488. [DOI] [PubMed] [Google Scholar]
- 75.Klltorp M, Oblogina S, Jacobsson S, Karlsson A, Tengvall P, Thomsen P. Biomaterials. 1999;20:2123–2137. doi: 10.1016/s0142-9612(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 76.Smetana K, Jr, Vacík J, Dvořánková B. Macromolecular Symposia. 1996;109:145–153. [Google Scholar]
- 77.Wamser CC, Gilbert MI. Langmuir. 1992;8:1608–1614. [Google Scholar]
- 78.Griesser HJ, Chatelier RC, Gengenbach TR, Johnson G, Steele JG. J Biomater Sci Polymer Ed. 1994;5:531–554. doi: 10.1163/156856294x00194. [DOI] [PubMed] [Google Scholar]
- 79.Lee JH, Lee JW, Khang G, Lee HB. Biomaterials. 1997;18:351–358. doi: 10.1016/s0142-9612(96)00128-7. [DOI] [PubMed] [Google Scholar]
- 80.Mirenghi L, Ramires PA, Pentassuglia RE, Rotolo P, Romito A. J Mat Sci: Mat in Med. 2000;11:327–331. doi: 10.1023/a:1008929902205. [DOI] [PubMed] [Google Scholar]
- 81.van Wachem PB, Beugeling T, Feijen J, Bantjes A, Detmers JP, van Aken WG. Biomaterials. 1985;6:403–408. doi: 10.1016/0142-9612(85)90101-2. [DOI] [PubMed] [Google Scholar]
- 82.Tang L, Ugarova TP, Plow EF, Eaton JW. J Clin Invest. 1996;97:1329–1334. doi: 10.1172/JCI118549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goreish HH, Lewis AL, Rose S, Lloyd AW. J Biomed Mater Res. 2004;68A:1–9. doi: 10.1002/jbm.a.10141. [DOI] [PubMed] [Google Scholar]
- 84.Li D-J, Ohsaki K, Ii K, Cui PC, Ye Q, Baba K, Wang QC, Tenshin S, Takano-Yamamoto T. J Biomed Mater Res. 1999;45:322–326. doi: 10.1002/(sici)1097-4636(19990615)45:4<322::aid-jbm6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 85.Zhang H, Sun L, Wang W, Ma X. J Biomed Mater Res. 2006;76A:120–125. doi: 10.1002/jbm.a.30491. [DOI] [PubMed] [Google Scholar]