Abstract
We have developed an electrochemical immunosensor for the detection of ultratrace amounts of aflatoxin M1 (AFM1) in food products. The sensor was based on a competitive immunoassay using horseradish peroxidase (HRP) as a tag. Magnetic nanoparticles coated with antibody (anti-AFM1) were used to separate the bound and unbound fractions. The samples containing AFM1 were incubated with a fixed amount of antibody and tracer [AFM1 linked to HRP (conjugate)] until the system reached equilibrium. Competition occurs between the antigen (AFM1) and the conjugate for the antibody. Then, the mixture was deposited on the surface of screen-printed carbon electrodes, and the mediator [5-methylphenazinium methyl sulphate (MPMS)] was added. The enzymatic response was measured amperometrically. A standard range (0, 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.3, 0.4 and 0.5 ppb) of AFM1-contaminated milk from the ELISA kit was used to obtain a standard curve for AFM1. To test the detection sensitivity of our sensor, samples of commercial milk were supplemented at 0.01, 0.025, 0.05 or 0.1 ppb with AFM1. Our immunosensor has a low detection limit (0.01 ppb), which is under the recommended level of AFM1 [0.05 μg L-1 (ppb)], and has good reproducibility.
Keywords: electrochemical immunosensor, aflatoxin M1, mycotoxin, milk, horseradish peroxidase (HRP), superparamagnetic nanoparticles
1. Introduction
Aflatoxins are a group of secondary metabolites produced by fungi. Different aflatoxins exist, including aflatoxins B1, B2, G1 and G2. Aflatoxin B1 is mainly produced by two fungi, Aspergillus flavus and Aspergillus parasiticus [1,2]. These fungi grow on a great variety of food commodities under a variety of temperature and humidity conditions, and contamination of animal feed materials, including corn, peanuts, cereal crops, either before or after harvest, is a common occurrence [1,3,4]. The optimal growth temperature of mycotoxin-producing moulds ranges between 24 and 35 °C. Crops that grow in warm, humid areas, principally subtropical and tropical countries [5], are contaminated the most often. This contamination results in important losses in terms of human and animal health and agricultural production [6]. Ecological and environmental conditions contribute to the production of mycotoxins in food or feed [7]. Mycotoxins exhibit a wide range of biological effects, and individual mycotoxins can be mutagenic, carcinogenic, embryo-toxic, teratogenic, oestrogenic or immunosuppressive [2].
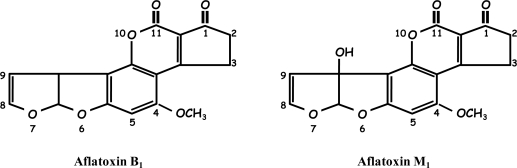
When aflatoxin B1 (AFB1), the most toxic aflatoxin, is ingested by cows through contaminated feed [2], it is transformed into aflatoxin M1 (AFM1) through enzymatic hydroxylation of AFB1 at the 9a-position (Scheme 1) and has an approximate overall conversion rate equal to 0.3 to 6.2% [1,8,9]. AFM1 is secreted in milk by the mammary gland of dairy cows [9,10]. Even though it is less toxic than its parent compound, AFM1 has hepatotoxic and carcinogenic effects [4,11]. This toxin, initially classified as a Group 2B agent [12], has now been reclassified as Group 1 by the International Agency for the Research on Cancer (IARC) [13].
Scheme 1.
The structures of aflatoxin B1 and aflatoxin M1. The only difference between the two compounds is the presence of the hydroxyl group at the 9a position of AFM1. Both molecules have the 8,9-double bond, which is the putative active site of the molecule [9].
AFM1 is relatively stable during the pasteurisation, storage and preparation of various dairy products [4,14], and therefore, AFM1 contamination poses a significant threat to human health, especially to children, who are the major consumers of milk.
The legal regulations concerning AFM1 levels in milk and dairy products vary from country to country. EU regulations allow a maximum level of 0.05 μg L−1 (ppb) AFM1 in milk [15]. The official methods of sampling and analysis are regulated by the European Commission directive [16]. High-performance liquid chromatography analysis with fluorometric detection (HPLC-FD) coupled with clean-up treatment by immunoaffinity columns (IC) is the reference method used for the determination of aflatoxin concentrations in milk [17]. This procedure, which is long and laborious, requires expensive equipment and well-trained personnel. Other methods for AFM1 concentration determination have also been proposed: thin layer chromatography [18], fluorescence detection after immunoaffinity clean-up [19], liquid chromatography coupled to mass spectrometry [20] and immunoenzymatic assays.
To minimise the occurrence of AFM1, it is essential to identify the sources of contamination using rapid, selective and sensitive assays. Immunochemical assays, which are rapid, simple, specific, sensitive and even portable, have become the most common quick methods for the routine analysis of mycotoxins in food and feed materials [21,22]. There is a need for more suitable methods, and rapid methods based on the use of biosensors or immunosensors have been proposed in the last decade [23,24]. The aim of our work was to develop a method for aflatoxin M1 (AFM1) detection and quantification in milk samples using an electrochemical immunosensor. A screen-printed carbon electrode is chosen as the transducer.
2. Materials and Methods
2.1. Safety notes
Aflatoxins are highly carcinogenic and should be handled with extreme care. Aflatoxin-contaminated labware should be decontaminated with an aqueous solution of sodium hypochlorite (5%). Aflatoxins are subject to light degradation; therefore, analytical work must be protected from daylight, and aflatoxin standard solutions are stored in amber vials. The use of non-acid-washed glassware for aqueous aflatoxin solutions may result in the loss of aflatoxin, and thus special attention should be paid to new glassware. Prior to use, glassware should be soaked in dilute acid (10% sulphuric acid) for several hours and then rinsed extensively with distilled water to remove all traces of acid [25].
2.2. Materials and apparatus
The I’Screen AFLA M1 milk test kit was from Tecna s.r.l. (Trieste, Italy). Milk samples were obtained from local supermarkets. Aflatoxin M1 from Aspergillus flavus, 5-methylphenazinium methyl sulphate (MPMS) and hydrogen peroxide (H2O2) were purchased from Sigma-Aldrich (Germany). Aflatoxin M1 linked to horseradish peroxidase (AFM1-HRP conjugate) from the I’Screen AFM1 milk test kit (Tecna s.r.l, Trieste, Italy) was used. An anti-AFM1 antibody (1 mg/mL) was purchased from Soft Flow Biotechnology (Hungary). Superparamagnetic nanoparticles (d = 300 nm), Bio-Adembeads Protein G (uniform-sized superparamagnetic nanoparticles conjugated with protein G), were from Ademtech SA (Pessac, France). Adem-Mag SV (single magnet position adapted for both 1.5/2 mL microfuge tubes or glass vials) were from Ademtech S.A. (Pessac, France). All solutions were stored in glass to limit adsorption. A horizontal shaker (IKA, vibrax, VXR) was also used for the coating step.
Chronoamperometric and cyclic voltammetric measurements were performed with an AUTOLAB PGSTAT12 potentiostat interfaced to a PC, and GPES (General Purpose Electrochemical System) software was used to collect and analyse the data (Utrecht, The Netherlands). DropSens 110 screen-printed carbon electrodes (DropSens, S.L., Spain) were used. We used a three-electrode system, with carbon working and counter electrodes and a silver reference electrode.
2.3. Reagents
Phosphate-buffered saline-Tween (PBS-T), 0.05 M, pH 7.4 (Tween-20, 0.05% v/v), and acetate buffer, 0.05 M, pH 5.2, were used.
2.4. Preparation of the AFM1 standard range and controls
The standard range (0, 0.005, 0.01, 0.025, 0.05, 0.1, and 0.25 ppb) of the AFM1 ELISA kit was used. To construct this standard range for AFM1, aliquots of the 0 ppb standard milk (blank) from the ELISA kit were spiked with the stock AFM1 solution to obtain final concentrations of 0.3, 0.4 or 0.5 ppb. The controls were prepared in PBS-T or in the 0 ppb blank from the ELISA kit. These controls were spiked with the stock AFM1 solution to obtain final concentrations of 0.01, 0.025, 0.05 or 0.1 ppb.
2.5. Preparation of milk samples
The sample was defatted by centrifugation for 15 min at 6,000 rpm. After centrifugation, the two phases were separated, the fatty cream was discarded, and the skimmed milk was recovered and used to carry out the experimental work. Aliquots of defatted AFM1-free milk samples were spiked with the stock solution of AFM1 to obtain final concentrations of 0.01, 0.025, 0.05 or 0.1 ppb.
2.6. Methods and instrumentation
All affinity reactions were performed off-line by mixing the sample with the tracer (AFM1-HRP) and antibody until equilibrium was reached.
2.7. Bead preparation
All steps (coating, competition and washing) were carried out with phosphate-buffered saline-Tween (PBS-T), 0.05 M, pH 7.4 (Tween 20 0.05% v/v). Prior to use, the suspended superparamagnetic nanoparticles conjugated with protein G were washed three times with working buffer (26 μL beads + 1374 μL PBS-T) to remove the ProClin 300 which acted as a preservative. The optimised procedure was as follows:
- Coating: the washed beads were collected using the Adem-Mag SV and the antibody solution (2 μg/ml) prepared in working buffer (2.8 μL antibody at 1 mg/mL + 1371 μL PBS-T) was added and allowed to react for 20 minutes. Then, the particles were collected using the Adem-Mag SV, washed three times with working buffer (1,400 μL) and resuspended in 1,400 μL of working buffer.
- A 101-μL aliquot of this dispersion was introduced into a glass vial, and the buffer was removed. Meanwhile, the nanoparticles were collected using the Adem-Mag SV.
- Competition: AFM1 (91 μL; from the liquid standard range from the ELISA kit or spiked with milk), AFM1-HRP solution (91 μL) prepared in working buffer (1:750 v/v) and acetate buffer (252 μL, 100 mM) were allowed to compete for antibody binding sites for 15–20 minutes. During the coating and competition steps, a horizontal shaker (200 rpm) was employed.
2.8. Immunosensor protocol
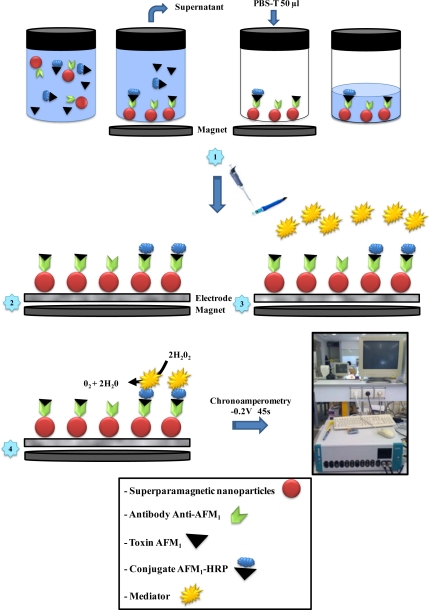
The construction of the immunosensor required the immobilisation of the antibodies on the electrodes via the superparamagnetic nanoparticles. To this end, the screen-printed carbon electrode was placed in a magnet support to collect the superparamagnetic nanoparticles at the electrode surface (Figure 1). Then, after the competition step the particles were collected using the Adem-Mag SV, the supernatant was discarded and 50 μL of PBS-T was added to resuspend the particles (Figure 1, illustration 1), which were then introduced via a Pipetman (Gilson, France) to the surface of the screen-printed carbon electrode. Only the superparamagnetic nanoparticles remain attached to the screen-printed carbon electrode (Figure 1, illustrations 1 and 2).
Figure 1.
Immunosensor protocol and principle of the electrochemical immunosensor for AFM1 detection.
Next, the electrode surface was washed with 100 μL of the mediator solution (1 mM MPMS; 10 mM H2O2; 100 mM acetate buffer) to remove all of the toxin or the conjugate that were not attached to the antibody. Before taking the measurements, 100 μL of the mediator solution was introduced to the surface of the electrode (Figure 1, illustrations 3 and 4). The measurements were carried out using a chronoamperometry method at a potential of −0.2 V vs. Ag/AgCl for 45 s. All of the experiments were carried out in triplicate in independent assays.
3. Results and Discussion
This immunoassay method is based on the use of an AFM1-horseradish peroxidase conjugate (AFM1-HRP) as a probe. HRP catalyses the oxidation of various hydrogen-donating substrates with hydrogen peroxide to produce oxidised substrate and water. MPMS and H2O2 were the substrates used to determine HRP activity.
First, the electrochemical behaviour of both MPMS and MPMSred were investigated to optimise the conditions for the determination of HRP activity by amperometry. A cyclic voltammetric investigation of MPMS was carried out using a carbon electrode (DropSens 110). The addition of HRP to a solution containing the two substrates (MPMS and H2O2) led to the consumption of MPMS and consequently to a decrease in the oxidation current and a increase in the reduction current. A working potential of −0.2 V (−200 mV) vs. Ag/AgCl for the measurement of HRP activity was chosen for this study [26]. At this potential, the current was near zero, and no substrate reduction occurred. These conditions were optimal for enzymatic activity determinations when a small amount of product (MPMSred) was measured in the presence of a high concentration of substrate.
Before testing the response of the spiked milk samples, a control assay was performed (Table 1) to verify that the AFM1 concentration could be detected accurately by the sensor and to determine the amount of interference from the milk matrix during the measurement. For this control, PBS-T and the 0 ppb blank from the ELISA kit were spiked with the AFM1 solution to obtain four different sample concentrations: 0.01, 0.025, 0.05 and 0.1 ppb. Electrochemical measurements of the calibration standard solutions prepared in buffer and in milk were made using the immunosensor (Table 1). The response curve for the standard series, the spiked buffer and the spiked milk were identical. Thus, the defatted milk did not affect the measurements.
Table 1.
Sensor calibration using standard solutions of AFM1 and results obtained using control samples and spiked milk.
| AFM1 standard range (ppb) |
Biosensor response (A) |
Control assays response (A) |
Spiked milk samples response (A) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean values | Standard deviation | Spiked PBS-T | Standard deviation | Spiked 0 ppb ELISA Kit Blank | Standard deviation | Mean values | Standard deviation | References | |
| 0 | −9.735E-06 | 2.46E-08 | |||||||
| 0.005 | −9.743E-06 | 2.35E-08 | |||||||
| 0.01 | −9.738E-06 | 4.93E-09 | −9.74E-06 | 8.60E-08 | −9.73E-06 | 8.35E-08 | −9.745E-06 | 1.617E-08 | a |
| 0.025 | −9.702E-06 | 3.06E-09 | −9.70E-06 | 4.00E-08 | −9.69E-06 | 3.91E-08 | −9.694E-06 | 1.528E-09 | b |
| 0.05 | −9.508E-06 | 1.48E-08 | −9.58E-06 | 2.98E-08 | −9.56E-06 | 4.68E-08 | −9.523E-06 | 2.442E-08 | c |
| 0.1 | −9.300E-06 | 3.61E-09 | −9.30E-06 | 1.89E-08 | −9.32E-06 | 6.64E-08 | −9.316E-06 | 1.106E-08 | d |
| 0.25 | −8.956E-06 | 1.53E-08 | |||||||
| 0.3 | −8.939E-06 | 7.90E-08 | |||||||
| 0.4 | −8.965E-06 | 3.11E-08 | |||||||
| 0.5 | −8.932E-06 | 2.28E-08 | |||||||
References:
0.01 ppb;
0.025 ppb;
0.05 ppb;
0.1 ppb
After this first step, which validated the immunosensor protocol, we performed the second step of our experiment with real milk samples. The milk used for the standard range came from the ELISA kit, as in the first experiment, and experimental milk samples were from commercial sources.
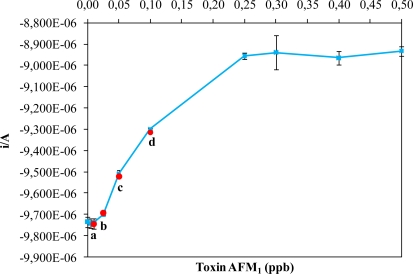
We constructed a standard curve to determine the relationship between the concentration of AFM1 in the sample and the measured intensity. With this standard curve (Figure 2, blue squares), we also calculated the upper and lower limits of detection of the immunosensor. The detection limits of AFM1 by the sensor were 0.25 μg L−1 (ppb) for the upper limit and 0.01 μg L−1 (ppb) for the lower limit (Figure 2 and Table 1).
Figure 2.
Curve of the AFM1 standard range (blue squares) and the spiked AFM1 milk samples a, b, c and d (red circles). Vertical bars represent standard errors (not shown when smaller than the symbols).
In the second part of the experiment, commercial milk samples contaminated with a known concentration of AFM1 (0.01, 0.025, 0.05 or 0.1 ppb; references a, b, c, d in Table 1 and Figure 2) were tested. The intensity responses for each concentration were measured. The values for the spiked milk samples were the similar to those values measured for the standard range (Table 1 and Figure 2, red circles). The analytical performance of our approach is better for the low concentrations of toxin in comparison with the other. For example, Badea et al. [23] realized an flow injection immunoassay system for aflatoxin M1 determination and with our approach we have the same limit of detection for the high concentration (0.5 ppb) but we have a higher sensitivity for the lower concentration (0.01 ppb), the same as the system developed by Carlson et al. [24].
Our immunosensor allows the detection and the quantification of AFM1 over a large range of concentrations. Our immunosensor allows the estimation the real contamination level of spiked milk samples.
4. Conclusions
This immunosensor has a working range that is comparable or better than that found for conventional methods. The detection range of 0.01 to 0.1 ppb obtained for milk samples allows the use of this method in dairy industry laboratories. The use of this immunosensor can ensure that the milk purchased by consumers is harmless. Our system allows the measurement of AFM1 directly in milk after a single centrifugation step without dilution or pretreatment steps. Another advantage of our method is that the analysis time is reduced and the sample preparation is very simple and fast in comparison with the conventional methods (HPLC and ELISA, for example).
The goal of developing a method using magnetic beads was to optimise this immunosensor by developing a protocol that will allow automation of the sanitary control of foodstuffs. Future work will investigate the development of this immunosensor using flux methods. If the optimisation of a flow-injection system immunoassay for AFM1 could be realised, then this assay system would be a good method for the rapid screening of raw milk samples for this toxin. This immunosensor is inexpensive, easy to operate and very suitable to automation.
Acknowledgments
The authors are grateful to EraSME (Food for better human health program) for financial support for the project Real Time Aflatoxin M1 Biosensor Development.
References
- 1.Creppy EE. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002;127:19–28. doi: 10.1016/s0378-4274(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 2.Waliyas F, Reddy SV. Aspergillus Flavus Seed Infection and Aflatoxin Estimation by ELISA and Aflatoxin Management Option in Ground Nut. ICRISAP International Crops Research Institue for the Semi-Arid Tropics; Andhra Pradesh, India: 2009. pp. 502–524. [Google Scholar]
- 3.Ellis JWO, Smith P, Simpson BK. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection and methods of control. Food Sci. Nutr. 1991;30:403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- 4.Radoi A, Targa M, Prieto-Simon B, Marty JL. Enzyme-Linked Immunosorbent Assay (ELISA) based on superparamagnetic nanoparticles for aflatoxin M1 detection. Talanta. 2008;77:138–143. doi: 10.1016/j.talanta.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 5.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal A. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Amer. J. Clin. Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 6.Shane SH, Groopman JD. The Toxicology of Aflatoxins: Human Health, Veterinary and Agricultural Significance. Academic Press; San Diego, CA, USA: 1994. pp. 513–527. [Google Scholar]
- 7.Hansmann T, Sanson B, Stojan J, Weik M, Marty JL, Fournier D. Kinetic insight into the mechanism of cholinesterasterase inhibition by aflatoxin B1 to develop biosensors. Biosens. Bioelectron. 2009;24:2119–2124. doi: 10.1016/j.bios.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Applebaum RS, Brachett RE, Wiseman DW, Marth EH. Aflatoxin: Toxicity to dairy cattle and occurence in milk and milk products: A review. J. Food Protection. 1982;45:752. doi: 10.4315/0362-028X-45.8.752. [DOI] [PubMed] [Google Scholar]
- 9.Cathey CG, Huang G, Sarr AB, Clement BA, Phillips TD. Development and evaluation of a minicolumn assay for the detection of aflatoxin MI in milk. J. Dairy Sci. 1994;77:1223–1231. doi: 10.3168/jds.S0022-0302(94)77061-2. [DOI] [PubMed] [Google Scholar]
- 10.Van Egmond HP. Introduction to Mycotoxins in Dairy Products. Applied Science Publishers; London, UK: 1989. pp. 11–55. [Google Scholar]
- 11.International Agency for Research on Cancer . Monograph on the Evaluation of Carcinogenic Risk for Humans. IARC; Lyon, France: 1993. [Google Scholar]
- 12.International Agency for Research on Cancer . Monograph on the Evaluation of Carcinogenic Risk to Human. Some Naturally Occuring Substances: Food Items and Constituent Heterocyclic Aromatic Amines and Mycotoxins. IARC; Lyon, France: 1993. [Google Scholar]
- 13.International Agency for Research on Cancer . Monograph on the Evaluation of Carcinogenic Risk to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphtalene and Styrene. IARC; Lyon, France: 2002. [Google Scholar]
- 14.Stubblefield RD, Shannon GM. Aflatoxine M1: Analysis in dairy products and distribution in dairy foods made from artificially contaminated milk. J. Assoc. Anal. Chem. 1974;57:847–851. [PubMed] [Google Scholar]
- 15.European Commission (EC) Commission Regulation No 1881/2006. Dec 19, 2006.
- 16.European DC. Laying down the sampling methods and the methods of analysis for the official levels of ochratoxin A in food stuffs. Offic. J. Eur. Communities L075. 2002;45:38–43. [Google Scholar]
- 17.International Standards Organisation (ISO) Clean-up by Immunoaffinity Chromatography and Determination by High-Performance Liquid Chromatography. ISO; Geneva, Switzerland: 1998. Milk and Milk Powder. Determination of Aflatoxin M1 Content. [Google Scholar]
- 18.Grosso F, Frenny JM, Bevis S, Dragacci S. Joint IDF-UPAC-IAEA (FAO) interlaboratory validation for determinz alfatoxin M1 in milk by using immunoaffinity clean-up before thin-layer chromatography. Food Additive Contam. 2004;21:348–357. doi: 10.1080/02652030410001662048. [DOI] [PubMed] [Google Scholar]
- 19.Chiavaro E, Cavicchioli C, Berni E, Spotti E. Immunoaffinity clean-up and direct fluorescence measurement of aflatoxins B1 and M1 in pig liver: Comparison with high-performance liquid chromatography determination. Food Additive Contam. 2005;22:1154–1161. doi: 10.1080/02652030500307115. [DOI] [PubMed] [Google Scholar]
- 20.Cavaliere C, Foglia P, Pastorini E, Samperi R, Laganà A. Liquid chromatography/tandem mass spectrometric confirmatory method for determining aflatoxin M1 in cow milk. Comparison between electrospray and atmospheric pressure photoionization sources. J. Chromat. A. 2006;1101:69–78. doi: 10.1016/j.chroma.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 21.Magliulo M, Mirasoli M, Simoni P, Lelli R, Portanti O, Roda A. Development and validation of an ultrasensitive chemiluminescent enzyme immunoassay for aflatoxin M1 in milk. J. Agr. Food Chem. 2005;53:3300–3305. doi: 10.1021/jf0479315. [DOI] [PubMed] [Google Scholar]
- 22.Devi KT, Mayo MA, Hall AJ, Craufurd PQ, Wheeler TR, Waliyar F, Subrahmanyam A, Reddy DVR. Development and applicationof an indirect competitive enzyme-linked immunoassay for aflatoxin M1 in milk and milk-based confectionery. J. Agr. Food Chem. 2002;50:933–937. doi: 10.1021/jf011139b. [DOI] [PubMed] [Google Scholar]
- 23.Badea M, Micheli L, Messia MC, Candigliota T, Marconi E, Mottram T, Velasco-Garcia M, Moscone D, Palleschi G. Aflatoxin M1 determination in raw milk using a flow-injection immunoassay system. Anal. Chim. Acta. 2004;520:141–148. [Google Scholar]
- 24.Carlson MA, Bargeron CB, Benson RC, Fraser AB, Phillips TE, Velky JT, Groopman JD, Strickland PT, Ko HW. An automated, handheld biosensor for aflatoxin. Biosens. Bioelectron. 2000;14:841–848. doi: 10.1016/s0956-5663(99)00057-3. [DOI] [PubMed] [Google Scholar]
- 25.Dragacci S, Grosso F. Immunoaffinity column cleanup with liquid chromatography for determination of aflatoxinm1 in liquid milk: Collaborative study. J. AOAC Int. 2001;84:437–443. [PubMed] [Google Scholar]
- 26.Campàs M, Marty JL. Highly sensitive amperometric immunosensors for microcystin detection in algae. Biosen. Bioelectron. 2007;22:1034–1040. doi: 10.1016/j.bios.2006.04.025. [DOI] [PubMed] [Google Scholar]