Abstract
Molecular profiling of exhaled volatile organic compounds (VOC) by electronic nose technology provides breathprints that discriminate between patients with different inflammatory airway diseases, such as asthma and COPD. However, it is unknown whether this is determined by differences in airway caliber. We hypothesized that breathprints obtained by electronic nose are independent of acute changes in airway caliber in asthma. Ten patients with stable asthma underwent methacholine provocation (Visit 1) and sham challenge with isotonic saline (Visit 2). At Visit 1, exhaled air was repetitively collected pre-challenge, after reaching the provocative concentration (PC20) causing 20% fall in forced expiratory volume in 1 second (FEV1) and after subsequent salbutamol inhalation. At Visit 2, breath was collected pre-challenge, post-saline and post-salbutamol. At each occasion, an expiratory vital capacity was collected after 5 min of tidal breathing through an inspiratory VOC-filter in a Tedlar bag and sampled by electronic nose (Cyranose 320). Breathprints were analyzed with principal component analysis and individual factors were compared with mixed model analysis followed by pairwise comparisons. Inhalation of methacholine led to a 30.8 ± 3.3% fall in FEV1 and was followed by a significant change in breathprint (p = 0.04). Saline inhalation did not induce a significant change in FEV1, but altered the breathprint (p = 0.01). However, the breathprint obtained after the methacholine provocation was not significantly different from that after saline challenge (p = 0.27). The molecular profile of exhaled air in patients with asthma is altered by nebulized aerosols, but is not affected by acute changes in airway caliber. Our data demonstrate that breathprints by electronic nose are not confounded by the level of airway obstruction.
Keywords: volatile organic compounds, exhaled breathprint, electronic nose, pattern recognition, airway caliber, bronchial asthma, bronchial provocation
1. Introduction
Asthma is a chronic inflammatory disorder of the airways characterized by recurrent episodes of wheezing and chest tightness that are associated with variable airway obstruction. Asthma diagnosis is established based on symptoms, measurement of lung function and assessment of airway responsiveness [1]. In addition, the associated airway inflammation can be evaluated by validated non-invasive techniques such as sputum eosinophil counts [2] and exhaled nitric oxide level (NO) [3], which have shown to be useful in monitoring asthma.
Exhaled air is a mixture of thousands of volatile organic compounds (VOCs) [4], which are generated via metabolic pathways that may be altered by lung diseases. Identification and quantification of individual VOCs require laboratory methodologies employing gas chromatography coupled to mass spectrometry (GC-MS). Indeed, panels of VOCs enable the distinction of smoking and non-smoking healthy subjects [5], and can identify lung cancer with sufficient sensitivity and specificity [6]. Less laborious peak pattern analysis after spectrometry without direct VOC characterization has recently been successful in recognizing lung cancer patients [7]. Nevertheless, the procedure still requires skilled personnel and advanced technical facilities, limiting potential widespread medical applicability.
Electronic nose (eNose) technology provides a cost-effective on-site alternative for breath analysis. eNoses exploit arrays of broadly cross-reactive sensors responding to a variety of VOCs in a highly sensitive and reversible manner within a short response time [8,9]. eNoses generate a molecular profile of the VOC mixture in exhaled breath also called the breathprint, and allow analysis by pattern recognition algorithms for discrimination between individual breathprints without identifying the individual analytes. An eNose with carbon black polymer was able to predict pneumonia in ventilated patients [10], distinguish patients with lung cancer from COPD [11], discriminate asthma patients from controls [12] and from COPD patients [13]. In addition, an array of sensors based on gold nanoparticles was able to distinguish lung cancer patients from controls [14] and an electronic nose with quartz microbalance gas sensors could also discriminate asthma patients from healthy subjects [15]. Due to easy sampling procedures, portability and relatively low cost, eNose technology might be useful in medical decision making [16], which requires strict procedures to assess diagnostic accuracy (www.stard-statement.org).
Although the discriminative potential by eNoses is encouraging, patient-related and methodological issues have been raised concerning breath collection and analysis [15,17]. Expiratory volume and flow need to be standardized [12], but it is unknown whether the breathprints can be modulated by airway caliber. For exhaled NO it has been reported that airway narrowing leads to a reduction of exhaled NO in asthma [18,19], although this could not be confirmed in another study [20]. A salbutamol-induced acute increase in airway caliber may elevate exhaled NO level [21], although this is not a consistent finding [19,22]. Thus, it cannot be excluded that other exhaled components, such as VOCs, are also affected by acute changes in airway caliber. If so, this might complicate the interpretation of breathprints in general and particularly in asthma.
Therefore, the null-hypothesis of this study was that breathprints assessed by electronic nose technology are not affected by airway caliber. To investigate this we recorded breathprints before and at acute changes in airway caliber during methacholine provocation in asthmatic patients. A control challenge with nebulised isotonic saline was performed to examine any confounding effects of the challenge procedure on the breathprint. Finally, we assessed the between-day variability in asthmatics by comparing pre-challenge baseline breathprints.
2. Experimental Section
2.1. Study design
The effect of bronchoconstriction on exhaled breathprints was examined in a cross-over study performed on asthma patients (n = 10) attending two visits with a mean time between visits of 7.5 days (range 4–14 days) at a similar time of the day (± 2 h). At Visit 1, methacholine (MCh) provocation and at Visit 2, a sham challenge with isotonic saline was performed. At Visit 1 exhaled breath was collected before MCh provocation (baseline), when at least a 20% drop in FEV1 was achieved (post-methacholine) and after salbutamol inhalation (post-salbutamol). At Visit 2, exhaled air was collected before sham challenge (baseline), after the last inhalation of saline (post-saline) and after salbutamol inhalation (post-salbutamol). Subjects were not blinded to the procedures and visits were not randomized because of the need to match the number of inhaled doses between the visits.
The effect of methodological drifts in eNose signals was investigated in a control study performed in 10 volunteers (seven healthy, three asthmatics: see below). Breath sampling was performed three times following the same course as during the challenge procedures in the main study but without any intervention (0 min, 60 min and 90 min). Subjects did not eat and drank only water during and 3 hours prior to that period.
2.2. Subjects
Ten adult patients with previously diagnosed asthma agreed to participate in the main study. The patients were never-smokers and had episodic chest tightness or wheezing with a pre-bronchodilator FEV1 >65% predicted and documented airway responsiveness (PC20 methacholine <8 mg/mL) or reversibility in FEV1 predicted >12% after 400 μg inhaled salbutamol as established within 12 months prior to the study. Subjects with concurrent pulmonary disorders, diabetes mellitus, hypo- or hyper-thyroidism, severe cardiovascular disease, renal insufficiency, present cancer or cancer in the past 5 years, oral corticosteroid use, present parodontitis or recent dental treatment were excluded. Patients on inhaled medications other than short-acting or long-acting β2-agonists and/or inhaled steroids or those who had a history of upper or lower respiratory tract infection in the four weeks before the measurements were excluded from the study.
Ten volunteers, including seven healthy non-smoking subjects with no previous history of airway diseases or other chronic diseases, and three asthmatics but otherwise healthy patients, without an upper or lower respiratory tract infection in the four weeks before the measurements, were recruited to participate in the control study for eNose drift analysis. The protocol was approved by the local medical ethics committee. Written informed consent was obtained from all subjects.
2.3. Methacholine and sham challenges
MCh challenge test was performed according to the 2-min tidal breathing method [23]. The patients inhaled methacholine with tidal breathing for 2 min, and FEV1 was recorded 30 s and 90 s after the exposure (MasterscreenPneumo; Jaeger; Würzburg, Germany). Doubling doses of methacholine bromide ranging from 0.04 to 19.6 mg/mL were applied in 5-min intervals until PC20 was achieved. PC20 was calculated with linear interpolation. Subsequently, the patients inhaled 400 μg salbutamol per metered dose inhaler with a spacer, and after 10 min FEV1 was measured. FEV1 was considered to be restored if higher than 90% of baseline.
Sham challenge with 0.9% isotonic saline solution (154 mM NaCl) was performed in identical fashion with identical numbers of aerosol inhalations and spirometric maneuvers as performed when achieving PC20.
Patients withheld long-acting β2 agonists and antihistamines for 48 hours, and short-acting β2 agonists and inhaled corticosteroids >8 hours before both challenge tests.
2.4. Exhaled breath collection and electronic nose sampling
To reduce possible confounding effects, patients were asked not to eat and drink anything but water 3 h prior to breath collection and refrain from caffeine-containing beverages and peppermint exposure for 6 hours before the visits. Breath collection and sampling were performed using the 5-min tidal washin method [12]. Briefly, patients inhaled VOC-filtered air (A2, North Safety, Middelburg, Netherlands) and exhaled via a silica reservoir in a 2-way non-rebreathing valve (Hans Rudolph 2700, Hans Rudolph, Kansas City, MI, US). Following 5 min of equilibration with tidal breathing, patients performed a maximal inspiratory capacity maneuver and the full expiratory capacity volume was collected into a 10-L Tedlar bag. Bags were sampled within ten minutes followed by the parallel sampling of another Tedlar bag containing VOC-filtered room air as a reference.
Exhaled breath samples were analyzed at room temperature by the same handheld electronic nose (Cyranose 320; Smiths Detection, Pasadena, CA, US) with an array of 32 carbon black polymer sensors [8,9]. VOCs binding to a sensor cause a change in the electrical resistance of the sensor. The raw data of a breathprint compose of 32 values each corresponding to the relative change in electrical resistance of a sensor. According to the manufacturer’s instruction, the first measurement was disregarded at each session (first sniff effect).
2.5. Data analysis
Sensor data of the eNose were processed through Savitzky-Golay filtering and baseline correction. Offline analysis of raw data was performed using SPSS software (version 16.0). Principle component analysis was used to redistribute the variance of the original 32 sensors into a set of factors (four factors captured 95.6% of the variance within the data set in the main study and 97.3% of the variance at the control visit). The two factors (denoted as Factor 2 and Factor 3) which showed a significant response to methacholine and/or salbutamol inhalation were selected for further analysis and are being referred to as “the breathprint”.
A mixed model analysis followed by pairwise comparisons on factors was used to evaluate any change in breathprints when recorded repetitively during the visits. To compare the changes (deltas) in breathprints caused by nebulisation with methacholine and isotonic saline, corresponding baseline factors were subtracted from post-methacholine or post-saline factors, and these changes were compared with paired t-tests for each factor. To assess between-day variability of a breathprint, baseline factors at visits were compared with paired t-tests.
There are no previous data for calculation of the statistical power of studies with serial eNose measurements. However, previous parallel studies have shown adequate power at similar samples sizes [11,12]. Therefore, the current sample size was considered to be adequate for within-subject analysis.
FEV1 values recorded within a visit were compared with mixed model linear followed by pairwise comparison, the percentage change in FEV1 at the visits and baseline FEV1 values between visits were analyzed with paired t-tests. Data were normally distributed (Kolmogorov-Smirnov test) and are expressed as mean ± SEM in the figures and as mean ± SD in the table and in the text. The level of significance was considered as p < 0.05.
3. Results and Discussion
3.1. Study population
Patient characteristics and baseline lung function parameters are presented in Table 1. The population was well-characterized, but we acknowledge that the number of subjects in this study was limited. Even though data for formal power calculations of serial eNose measurements are lacking, similar numbers of patients were successfully used in previous eNose studies [11,12] as well as in former studies on the effects of airway caliber on exhaled NO [18,20] based on adequate power calculations.
Table 1.
Patient characteristics. Spirometric data are pre-challenge baseline values at Visit 2. FVC: forced vital capacity, FEV1: forced expiratory volume in 1 s, ICS: inhaled corticosteroids, Bud Eq: budesonide equivalent, PC20: provocative concentration of methacholine causing 20% fall in FEV1, MCh: methacholine, Post-salb: post-salbutamol inhalation.
| Patient Number | Sex | Age, years | Daily ICS (bud eq, μg) | Baseline FVC, % predicted | Baseline FEV1, % predicted | Baseline FEV1/FVC | PC20 MCh, mg/mL | FEV1 change at Visit1& | FEV1 change at Visit2& | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PC20 MCh | Post-salb | Post-saline | Post-salb | ||||||||
| 1 | F | 33 | 400 | 102 | 89 | 0.76 | 12.93 | −26 | 0 | −4 | +6 |
| 2 | F | 27 | 800 | 119 | 104 | 0.77 | 5.18 | −54 | −7 | −1 | +13 |
| 3 | M | 29 | 400 | 99 | 82 | 0.71 | 0.22 | −24 | 0 | −6 | 0 |
| 4 | M | 35 | 200 | 129 | 106 | 0.67 | 6.93 | −24 | +7 | −11 | +2 |
| 5 | M | 47 | 200 | 128 | 84 | 0.53 | 0.76 | −28 | +7 | −8 | +11 |
| 6 | F | 33 | 0 | 108 | 98 | 0.80 | 1.33 | −39 | 0 | −1 | +10 |
| 7 | F | 23 | 200 | 103 | 101 | 0.85 | 3.13 | −36 | −1 | 0 | −3 |
| 8 | F | 30 | 0 | 106 | 92 | 0.85 | 0.31 | −20 | +7 | −1 | +11 |
| 9 | M | 30 | 800 | 88 | 72 | 0.67 | 0.58 | −21 | −1 | −4 | +11 |
| 10 | F | 45 | 200 | 87 | 79 | 0.78 | 1.39 | −26 | −4 | −3 | +11 |
| 33 ± 8 | 400 ± 262# | 107 ± 15 | 91 ± 12 | 0.74 ± 0.01 | 1.55 (0.59–4.10) | −31 ± 11 | 1 ± 5 | −4 ± 4 | 7 ± 6 | ||
Change in percentage compared to corresponding baseline FEV1 values.
Only ICS users considered. Data are expressed as mean ± SD or geometric mean (95% confidence interval).
3.2. Methacholine and sham challenges modify breathprints
To investigate the effect of airway caliber on exhaled breathprints, we chose a controlled, cross-over challenge model with methacholine as well as sham (saline) provocation. This allowed the distinction in outcomes as produced by methacholine and inhaled aerosols or the procedure as such.
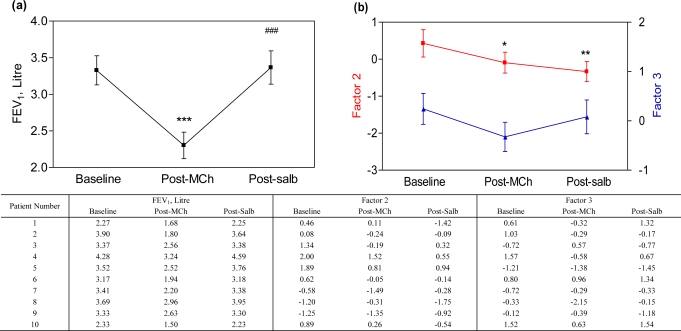
As expected, methacholine inhalation induced significant bronchoconstriction (baseline FEV1 3.33 ± 0.63 L vs. 2.30 ± 0.57 L post-methacholine, p < 0.001), and subsequently, airway caliber was restored by salbutamol inhalation [post-salbutamol FEV1 3.37 ± 0.72 L, p < 0.001, Figure 1(a)]. Breathprints, as demonstrated by Factor 2, were significantly changed after methacholine (p = 0.04) and also post-salbutamol (p = 0.006) when compared to the breathprints at baseline; however, the breathprints after salbutamol were not significantly different from the ones after methacholine [p = 0.34, Figure 1(b)].
Figure 1.
(a) FEV1 measurements at baseline, after methacholine (Post-MCh) inhalation and post-salbutamol (Post-salb). (b) Breathprints at baseline, after methacholine inhalation and post-salbutamol are presented by plotting Factor 2 (red line) and 3 (blue line). *p < 0.05, **p < 0.01, *** p < 0.001 vs. baseline; ###p < 0.001 vs. post-methacholine. The data are shown in the table below the figure.
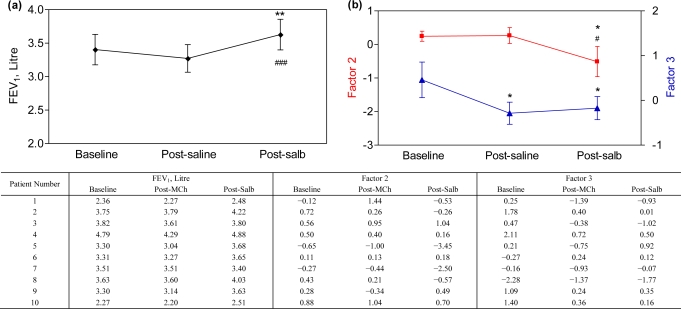
Inhalation of isotonic saline did not change FEV1 (baseline 3.40 ± 0.72 L vs. post-saline FEV1 3.27 ± 0.65 L, p = 0.13), but FEV1 increased to 3.63 ± 0.73 L after salbutamol inhalation [post-salbutamol vs. baseline and post-saline: p = 0.008 and p < 0.001, Figure 2(a)]. Breathprints were significantly changed post-saline (Factor 3: p = 0.01) and post-salbutamol (Factor 2: p = 0.02, Factor 3: p = 0.03) when compared to the breathprints at baseline. The post-salbutamol breathprint was also altered as compared to post-saline [Factor 2: p = 0.02, Figure 2(b)].
Figure 2.
(a) FEV1 measurements at baseline, after saline inhalation and post-salbutamol (post-salb). (b) Breathprints at baseline, after saline inhalation and post-salbutamol are presented by plotting Factor 2 (red line) and 3 (blue line). *p < 0.05, **p < 0.01 vs. baseline; #p < 0.05, ###p < 0.001 vs. post-saline. The data are shown in the table below the figure.
Our primary aim was to study the effect of bronchoconstriction on breathprints, but we additionally analyzed the effect of acute bronchodilation induced by salbutamol after the provocation test. Our data show that an acute and marked increase in airway caliber after methacholine challenge does not modify exhaled breathprints. Nevertheless, we cannot exclude any carry-over effect of inhaled methacholine on the post-salbutamol breathprints. Notably, the small but significant increase in airway caliber by salbutamol following sham provocation unexpectedly altered the breathprint. We cannot explain this observation and its inconsistency with regard to giving salbutamol after methacholine challenge. It may suggest that salbutamol inhalation in the absence of methacholine challenge as such provided a signal on the breathprint similar to the sham challenge with inhaled saline.
As temporal drift in polymer sensors due to incomplete desorption of molecules from sensor surfaces has been reported [24], a control study was done, in which non-smoking volunteers followed the same course of three repeated eNose analyses without any intervention. We found no significant temporal change in the four factors analyzed (0 min vs. 60 min vs. 90 min; Factor 1: 0.12 ± 0.94 vs. −0.50 ± 0.97 vs. 0.38 ± 0.97, p = 1.00; Factor 2: 0.61 ± 0.91 vs. −0.07 ± 0.99 vs. −0.54 ± 0.82, p = 0.66; Factor 3: 0.52 ± 1.22 vs. −0.17 ± 0.84 vs. −0.34 ± 0.75, p = 0.16; Factor 4: −0.23 ± 1.22 vs. 0.01 ± 0.86 vs. 0.23 ± 0.93, p = 1.00). This suggests that the change in breathprints observed during the challenge procedures is not the result of sensor drifts in the eNose.
3.3. Changes in breathprints are not related to bronchoconstriction
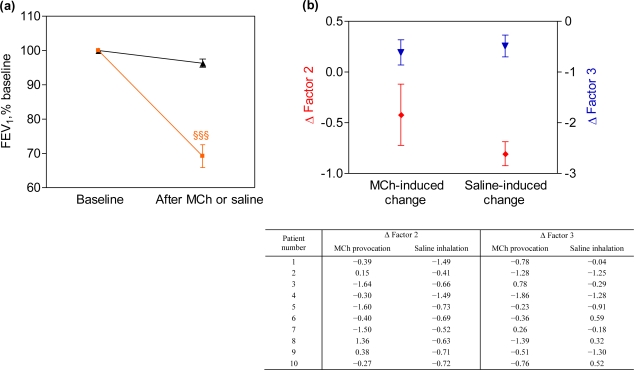
As expected, the decrease in FEV1 by MCh provocation was more marked than after saline [−30.8 ± 10.5% vs. −3.7 ± 3.8%, p < 0.001, Figure 3(a)]. However, there was no significant difference between the changes (deltas) in breathprints as induced by MCh provocation and sham challenge [delta Factor 2: p = 0.27, delta Factor 3: p = 0.66, Figure 3(b)].
Figure 3.
(a) Changes in FEV1 after the inhalation of methacholine (MCh; orange line) or isotonic saline (black line). (b) Changes in breathprints induced by methacholine or saline inhalation as the change (delta) in Factor 2 (red line) and 3 (blue line); for all deltas: p > 0.05. §§§p < 0.001 post-methacholine vs. post-saline.
How can these results be explained? The similar changes in breathprint after challenges with methacholine and isotonic saline indicate that alterations in breathprint might be due to the nebulisation procedure itself rather than to the change in airway caliber. We used a previously validated sampling technique with filtering of VOCs in inspired air and drying of exhaled air [12] in order to minimize the influence of humidity, expiratory flow rate and environmental VOCs on the exhaled VOC mixture. Although exhaled air is fully saturated, even under physiological conditions, we cannot exclude an effect of humidity on the breathprints after inhaling nebulized solutions. The current findings suggest that the drying step in the breath collection procedure may need to be optimized when used after aqueous nebulization. This issue might be particularly relevant in future studies where patients are subjected to nebulized therapies. An alternative explanation is that isotonic saline (alone or as a diluent for MCh), having a chloride concentration of 154 mM that is higher than that of the epithelial lining fluid (115 mM), induces metabolic changes in the bronchial epithelium [25]. If so, this could affect exhaled VOC profile thereby modifying exhaled breathprints. This possibility might be examined by local bronchoscopic fluid installation and air sampling.
3.4. Breathprints do not show between-day variability in asthma patients
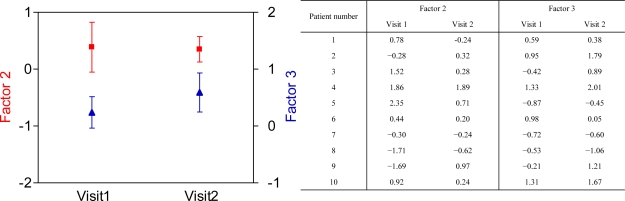
Baseline FEV1 values were similar between the two visits (Visit 1: 3.33 ± 0.63 L vs. Visit 2: 3.40 ± 0.72 L, p = 0.34), and neither was there a significant difference in baseline breathprints (Factor 2: p = 0.92, Factor 3: p = 0.18, Figure 4). These findings demonstrate that breathprints do not show between-day variability in non-smoking asthma patients. This extends a previous study reporting no differences in breathprints in healthy smoking and non-smoking control subjects when recorded at two separate days [13]. As variability of breathprints is an important issue in the standardization of breath analysis, further large-scale studies should confirm these findings.
Figure 4.
Baseline breathprints are unchanged in asthmatic patients. Pre-challenge baseline breathprints at the two visits are shown by plotting Factor 2 (red line) and Factor 3 (blue line); for all factors: p > 0.05. The attached table shows the data.
4. Conclusions
This study shows that an acute decrease in airway caliber per se is not associated with an altered breath molecular profile in asthma as measured by an electronic nose. This suggests that eNose assessment in asthma does not require correction for the degree of airway obstruction. We also demonstrate that inhaled nebulized aerosols can change breathprints irrespective of the change in airway caliber. Finally, breathprints by an eNose do not show significant between-day changes in stable asthma.
These findings imply that the previously observed discrimination of patients with asthma, COPD and controls by breathprint analysis [12,13,15] is due to the differences in underlying inflammation or disease activity rather than the level of airway obstruction. The clinical implication of this study is that monitoring of patients with asthma with repeated eNose assessments can be performed, regardless of acute changes in airway caliber. Exhaled molecular profiles may still vary with the degree of airway obstruction if the latter goes along with changes in airway inflammation. This strengthens the applicability of eNose technology in the future monitoring of diseases with variable airway obstruction and facilitates further studies on the validation of eNose monitoring in patients with asthma and COPD.
Acknowledgments
ZL received a scholarship from the Huygens Scholarship Program (2006-2008) allocated by the Dutch Minister of Education, Culture and Science. This study was funded by the Netherlands Asthma Foundation (grant no 3.2.06.017 and 3.4.08.019).
References and Notes
- 1.National Heart and Lung Institute, National Institutes of Health USA, and the World Health Organization Global Initiative for Asthma. Dec, 2008. Update. Available at: http://www.ginasthma.org/ (accessed on 20 September 2010).
- 2.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Cowan JO, Brassett KP, Herbison GP, Taylor DR. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. New Engl. J. Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 4.Moser B, Bodrogi F, Eibl G, Lechner M, Rieder J, Lirk P. Mass spectrometric profile of exhaled breath--field study by PTR-MS. Respir. Physiol. Neurobiol. 2005;145:295–300. doi: 10.1016/j.resp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Van Berkel JJ, Dallinga JW, Moller GM, Godschalk RW, Moonen E, Wouters EF, Van Schooten FJ. Development of accurate classification method based on the analysis of volatile organic compounds from human exhaled air. J. Chromat. 2008;861:101–107. doi: 10.1016/j.jchromb.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Phillips M, Cataneo RN, Cummin AR, Gagliardi AJ, Gleeson K, Greenberg J, Maxfield RA, Rom WN. Detection of lung cancer with volatile markers in the breath. Chest. 2003;123:2115–2123. doi: 10.1378/chest.123.6.2115. [DOI] [PubMed] [Google Scholar]
- 7.Westhoff M, Litterst P, Freitag L, Urfer W, Bader S, Baumbach JI. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: Results of a pilot study. Thorax. 2009;64:744–748. doi: 10.1136/thx.2008.099465. [DOI] [PubMed] [Google Scholar]
- 8.Lewis NS. Comparisons between mammalian and artificial olfaction based on arrays of carbon black-polymer composite vapor detectors. Acc. Chem. Res. 2004;37:663–672. doi: 10.1021/ar030120m. [DOI] [PubMed] [Google Scholar]
- 9.Röck F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem. Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]
- 10.Hockstein NG, Thaler ER, Lin Y, Lee DD, Hanson CW. Correlation of pneumonia score with electronic nose signature: A prospective study. Ann. Otol. Rhinol. Laryngol. 2005;114:504–508. doi: 10.1177/000348940511400702. [DOI] [PubMed] [Google Scholar]
- 11.Dragonieri S, Annema JT, Schot R, van der Schee MP, Spanevello A, Carratu P, Resta O, Rabe KF, Sterk PJ. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer (Amsterdam, Netherlands) 2009;64:166–170. doi: 10.1016/j.lungcan.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Dragonieri S, Schot R, Mertens BJ, Le Cessie S, Gauw SA, Spanevello A, Resta O, Willard NP, Vink TJ, Rabe KF, Bel EH, Sterk PJ. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clinical Immunol. 2007;120:856–862. doi: 10.1016/j.jaci.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 13.Fens N, Zwinderman AH, van der Schee MP, de Nijs SB, Dijkers E, Roldaan AC, Cheung D, Bel EH, Sterk PJ. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Amer. J. Respir. Crit. Care Med. 2009;180:1076–1082. doi: 10.1164/rccm.200906-0939OC. [DOI] [PubMed] [Google Scholar]
- 14.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnol. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 15.Montuschi P, Santonico M, Mondino C, Pennazza G, Mantini G, Martinelli E, Capuano R, Ciabattoni G, Paolesse R, Di Natale C, Barnes PJ, D'Amico A. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest. 2010;137:790–796. doi: 10.1378/chest.09-1836. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich MJ. Scientists seek to sniff out diseases: electronic “noses” may someday be diagnostic tools. JAMA. 2009;301:585–586. doi: 10.1001/jama.2009.90. [DOI] [PubMed] [Google Scholar]
- 17.Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur. Respir. J. 2009;34:261–275. doi: 10.1183/09031936.00142508. [DOI] [PubMed] [Google Scholar]
- 18.de Gouw HW, Hendriks J, Woltman AM, Twiss IM, Sterk PJ. Exhaled nitric oxide (NO) is reduced shortly after bronchoconstriction to direct and indirect stimuli in asthma. Amer. J. Respir. Crit. Care Med. 1998;158:315–319. doi: 10.1164/ajrccm.158.1.9703005. [DOI] [PubMed] [Google Scholar]
- 19.Ho LP, Wood FT, Robson A, Innes JA, Greening AP. The current single exhalation method of measuring exhales nitric oxide is affected by airway calibre. Eur. Respir. J. 2000;15:1009–1013. doi: 10.1034/j.1399-3003.2000.01506.x. [DOI] [PubMed] [Google Scholar]
- 20.Kharitonov SA, Sapienza MA, Barnes PJ, Chung KF. Prostaglandins E2 and F2alpha reduce exhaled nitric oxide in normal and asthmatic subjects irrespective of airway caliber changes. Amer. J. Respir. Crit. Care Med. 1998;158:1374–1378. doi: 10.1164/ajrccm.158.5.9707076. [DOI] [PubMed] [Google Scholar]
- 21.Boot JD, Tarasevych S, Sterk PJ, Schoemaker RC, Wang L, Amin D, Cohen AF, Diamant Z. Reversal of the late asthmatic response increases exhaled nitric oxide. Respir. Med. 2005;99:1591–1594. doi: 10.1016/j.rmed.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Garnier P, Fajac I, Dessanges JF, Dall'Ava-Santucci J, Lockhart A, Dinh-Xuan AT. Exhaled nitric oxide during acute changes of airways calibre in asthma. Eur. Respir. J. 1996;9:1134–1138. doi: 10.1183/09031936.96.09061134. [DOI] [PubMed] [Google Scholar]
- 23.Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur. Repir. J. 1993;16:53–83. doi: 10.1183/09041950.053s1693. [DOI] [PubMed] [Google Scholar]
- 24.Knobloch H, Turner C, Spooner A, Chambers M. Methodological variation in headspace analysis of liquid samples using electronic nose. Sens. Actuat. B: Chem. 2009;139:353–360. [Google Scholar]
- 25.Tabary O, Escotte S, Couetil JP, Hubert D, Dusser D, Puchelle E, Jacquot J. High susceptibility for cystic fibrosis human airway gland cells to produce IL-8 through the I kappa B kinase alpha pathway in response to extracellular NaCl content. J. Immunol. 2000;164:3377–3384. doi: 10.4049/jimmunol.164.6.3377. [DOI] [PubMed] [Google Scholar]