Abstract
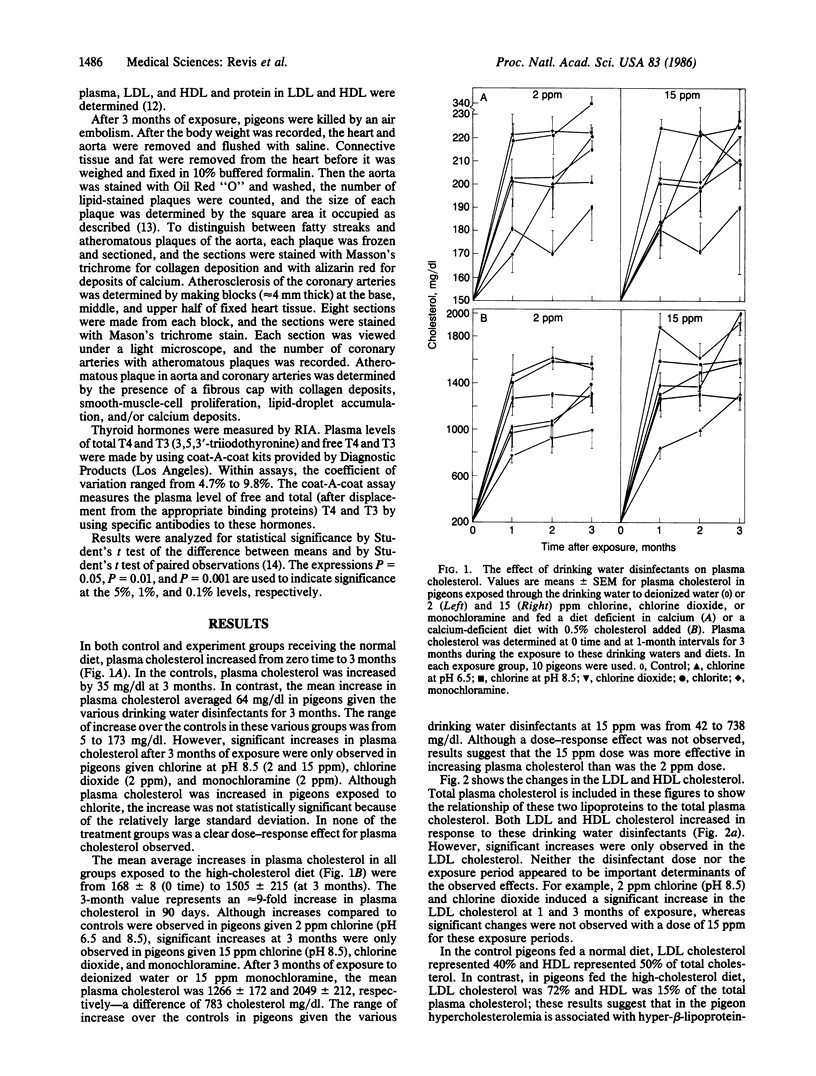
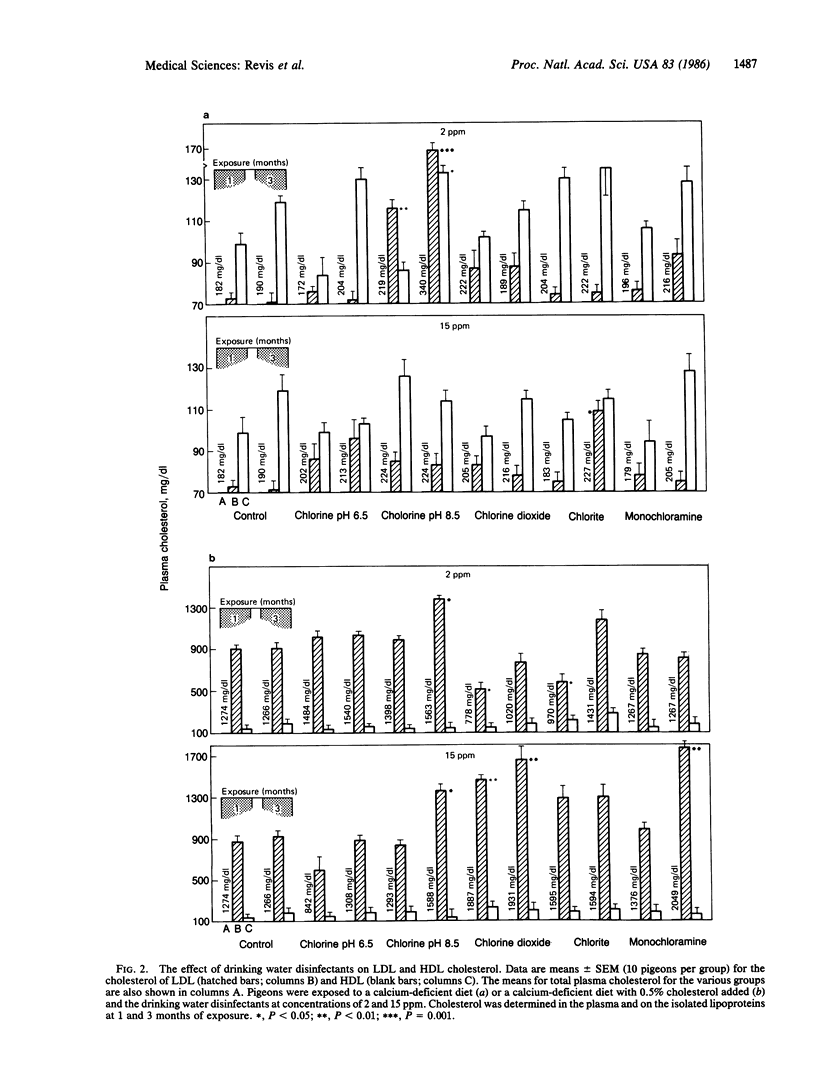
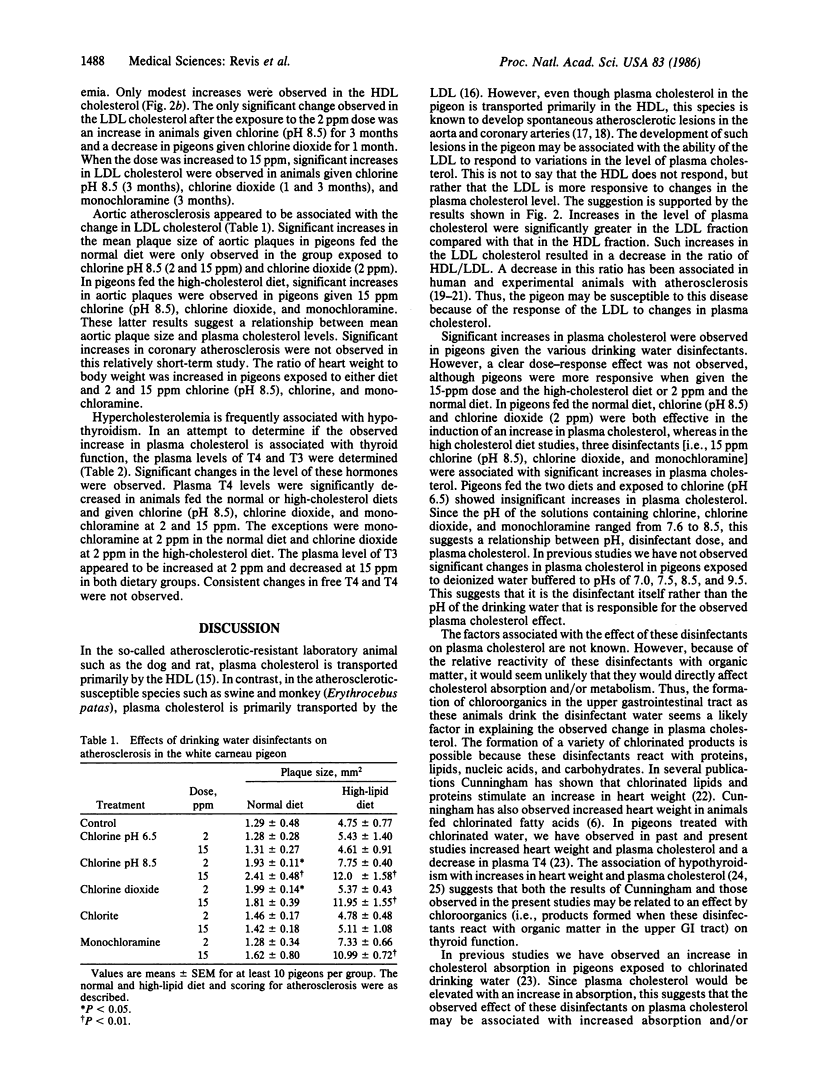
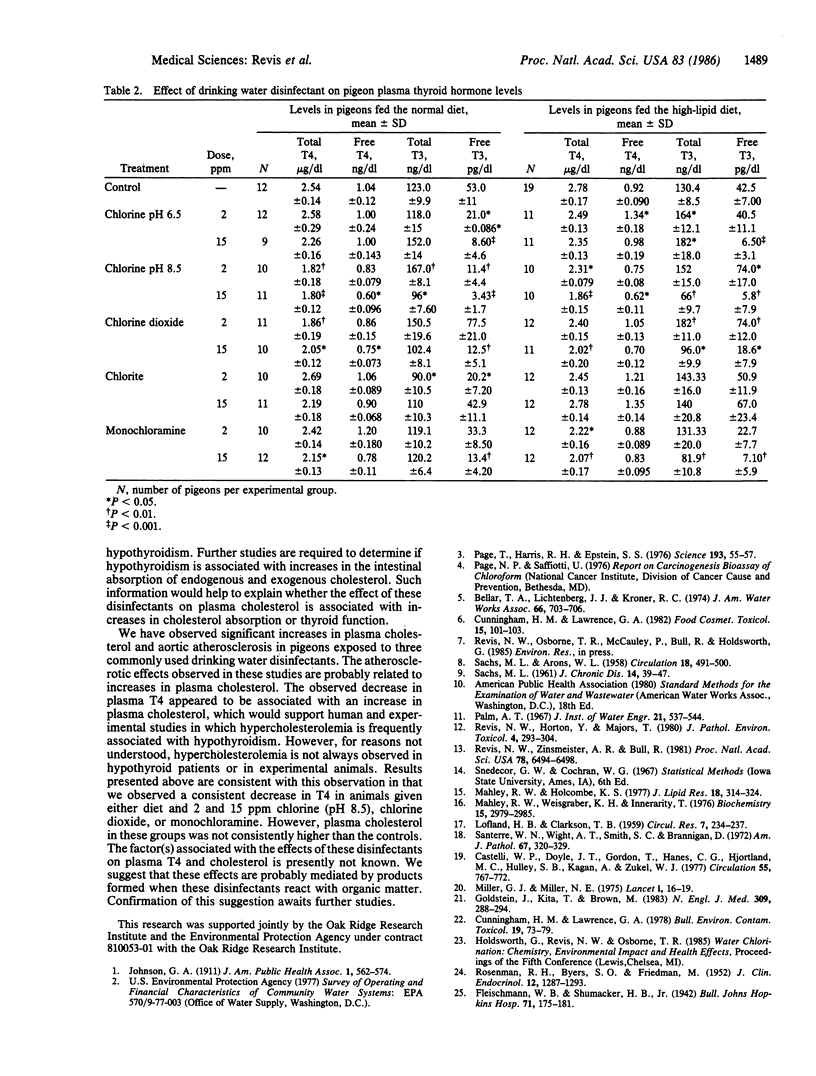
The effects of drinking water containing 2 or 15 ppm chlorine (pH 6.5 and 8.5), chlorine dioxide, and monochloramine on thyroid function and plasma cholesterol were studied because previous investigators have reported cardiovascular abnormalities in experimental animals exposed to chlorinated water. Plasma thyroxine (T4) levels, as compared to controls, were significantly decreased in pigeons fed a normal or high-cholesterol diet and drinking water containing these drinking water disinfectants at a concentration of 15 ppm (the exception was chlorine at pH 6.5) for 3 months. In most of the treatment groups, T4 levels were significantly lower following the exposure to drinking water containing the 2 ppm dose. Increases in plasma cholesterol were frequently observed in the groups with lower T4 levels. This association was most evident in pigeons fed the high-cholesterol diet and exposed to these disinfectants at a dose of 15 ppm. For example, after 3 months of exposure to deionized water or water containing 15 ppm monochloramine, plasma cholesterol was 1266 +/- 172 and 2049 +/- 212 mg/dl, respectively, a difference of 783 mg/dl. The factor(s) associated with the effect of these disinfectants on plasma T4 and cholesterol is not known. We suggest however that these effects are probably mediated by products formed when these disinfectants react with organic matter in the upper gastrointestinal tract.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castelli W. P., Doyle J. T., Gordon T., Hames C. G., Hjortland M. C., Hulley S. B., Kagan A., Zukel W. J. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977 May;55(5):767–772. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- Cunningham H. M., Lawrence G. A. Absorption and metabolism of chlorinated fatty acids and triglycerides in rats. Food Cosmet Toxicol. 1977 Apr;15(2):101–103. doi: 10.1016/s0015-6264(77)80313-1. [DOI] [PubMed] [Google Scholar]
- Cunningham H. M., Lawrence G. A. Effect of chlorinated lipid and protein fractions of cake flour on growth rate and organ weight of rats. Bull Environ Contam Toxicol. 1978 Jan;19(1):73–79. doi: 10.1007/BF01685769. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Kita T., Brown M. S. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983 Aug 4;309(5):288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- LOFLAND H. B., Jr, CLARKSON T. B. A biochemical study of spontaneous atherosclerosis in pigeons. Circ Res. 1959 Mar;7(2):234–237. doi: 10.1161/01.res.7.2.234. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Holcombe K. S. Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res. 1977 May;18(3):314–324. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. Atherogenic hyperlipoproteinemia induced by cholesterol feeding the Patas monkey. Biochemistry. 1976 Jul 13;15(14):2979–2985. doi: 10.1021/bi00659a007. [DOI] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Page T., Harris R. H., Epstein S. S. Drinking water and cancer mortality in Louisiana. Science. 1976 Jul 2;193(4247):55–57. doi: 10.1126/science.935854. [DOI] [PubMed] [Google Scholar]
- ROSENMAN R. H., BYERS S. O., FRIEDMAN M. The mechanism responsible for the altered blood cholesterol content in deranged thyroid states. J Clin Endocrinol Metab. 1952 Oct;12(10):1287–1299. doi: 10.1210/jcem-12-10-1287. [DOI] [PubMed] [Google Scholar]
- Revis N. W., Major T. C., Horton C. Y. The effects of calcium, magnesium, lead, or cadmium on lipoprotein metabolism and atherosclerosis in the pigeon. J Environ Pathol Toxicol. 1980 Sep;4(2-3):293–303. [PubMed] [Google Scholar]
- Revis N. W., Zinsmeister A. R., Bull R. Atherosclerosis and hypertension induction by lead and cadmium ions: an effect prevented by calcium ion. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6494–6498. doi: 10.1073/pnas.78.10.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]



