Abstract
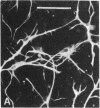
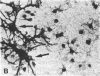
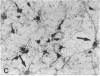
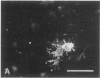
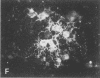
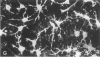
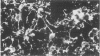
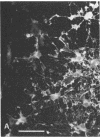
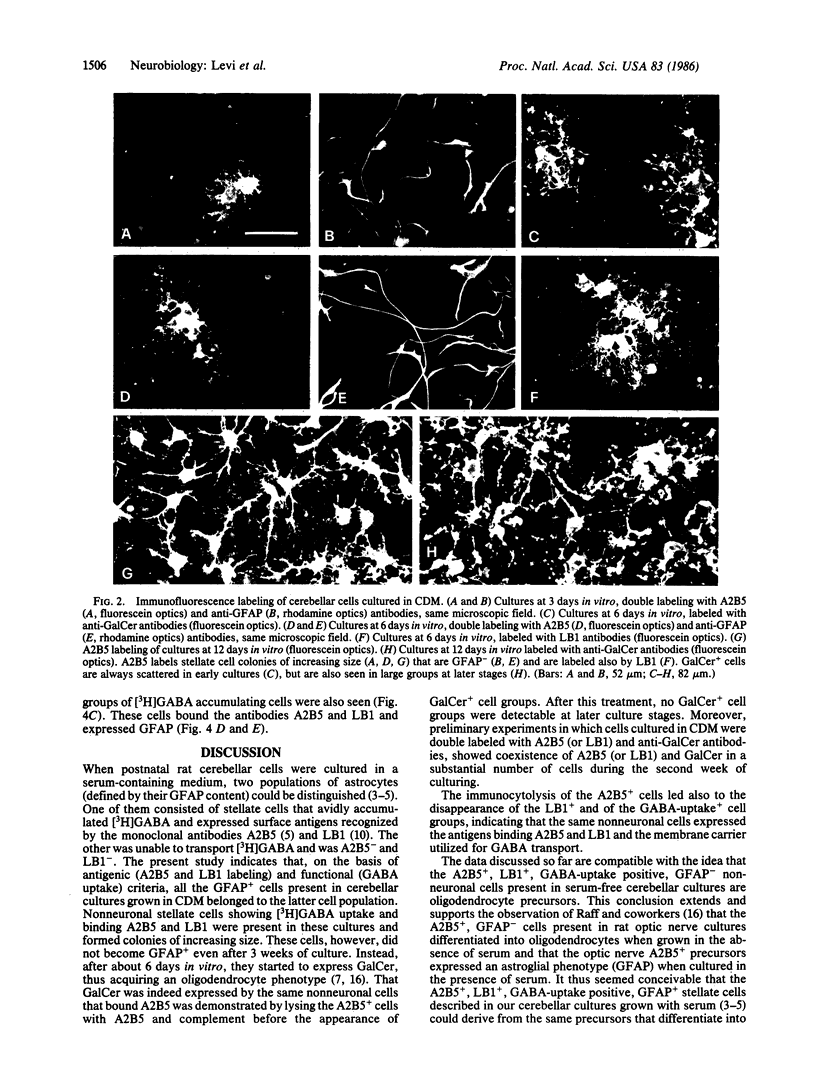
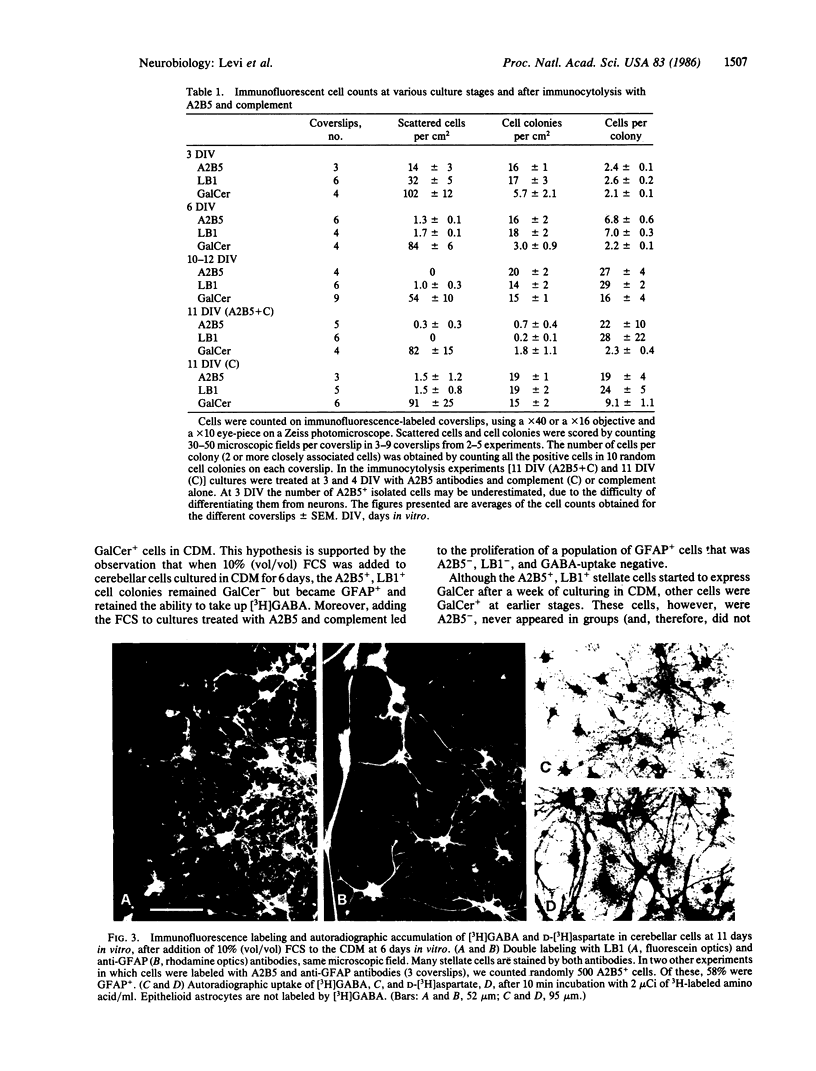
When postnatal rat cerebellar cells were cultured in a chemically defined, serum-free medium, the only type of astrocyte (defined by the expression of the glial fibrillary acidic protein, GFAP) present was unable to accumulate gamma-[3H]aminobutyric acid (GABA), did not express surface antigens recognized by two monoclonal antibodies, A2B5 and LB1, and showed minimal proliferation. In these cultures, nonneuronal A2B5+, LB1+ stellate cells exhibiting "neuron-like" [3H]GABA uptake formed cell colonies of increasing size and were GFAP-. After about one week of culturing, the A2B5+, LB1+, GABA-uptake positive cell groups became galactocerebroside (GalCer) positive. Immunocytolysis of the A2B5+ cells at 3 and 4 days in vitro prevented the appearance of the A2B5+, LB1+, GABA-uptake positive cell colonies, and also of the GalCer+ cell groups. If 10% (vol/vol) fetal calf serum was added to 6-day cultures, the A2B5+, LB1+, GABA-uptake positive cell groups expressed GFAP and not GalCer. If the serum was added to the cultures 2 days after lysing the A2B5+ cells, only A2B5-, LB1-, GABA-uptake negative astrocytes proliferated. It is concluded that the putative fibrous astrocytes previously described in serum-containing cultures (which had a stellate shape and were A2B5+, LB1+, GABA-uptake positive) derive from bipotential precursors that differentiate into oligodendrocytes (GalCer+) in serum-free medium or into astrocytes (GFAP+) in the presence of serum, while the epithelioid A2B5-, LB1-, GABA-uptake negative astrocytes originate from a different precursor not yet identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Williams B. P., Raff M. C. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting, and cell culture. Dev Biol. 1983 Nov;100(1):166–171. doi: 10.1016/0012-1606(83)90207-5. [DOI] [PubMed] [Google Scholar]
- Aloisi F., Ciotti M. T., Levi G. Characterization of GABAergic neurons in cerebellar primary cultures and selective neurotoxic effects of a serum fraction. J Neurosci. 1985 Aug;5(8):2001–2008. doi: 10.1523/JNEUROSCI.05-08-02001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Miller P. E., Navone F., Theurkauf W. E., Vallee R. B. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984 Apr;11(4):817–846. [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone S. R., Levi G., Wilkin G. P., Schneider A., Ciotti M. T. Subpopulations of rat cerebellar astrocytes in primary culture: morphology, cell surface antigens and [3H]GABA transport. Brain Res. 1986 Jan;389(1-2):63–75. doi: 10.1016/0165-3806(86)90173-2. [DOI] [PubMed] [Google Scholar]
- Kingsbury A. E., Gallo V., Woodhams P. L., Balazs R. Survival, morphology and adhesion properties of cerebellar interneurones cultured in chemically defined and serum-supplemented medium. Brain Res. 1985 Jan;349(1-2):17–25. doi: 10.1016/0165-3806(85)90128-2. [DOI] [PubMed] [Google Scholar]
- Lasher R. S. The uptake of (3H)GABA and differentiation of stellate neurons in cultures of dissociated postnatal rat cerebellum. Brain Res. 1974 Apr 5;69(2):235–254. doi: 10.1016/0006-8993(74)90004-3. [DOI] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- Levi G., Wilkin G. P., Ciotti M. T., Johnstone S. Enrichment of differentiated, stellate astrocytes in cerebellar interneuron cultures as studied by GFAP immunofluorescence and autoradiographic uptake patterns with [3H]D-aspartate and [3H]GABA. Brain Res. 1983 Nov;312(2):227–241. doi: 10.1016/0165-3806(83)90139-6. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Raff M. C. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984 Feb;4(2):585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Miller R. H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983 Jun 2;303(5916):390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Herschkowitz N. Uptake of [3H]GABA by oligodendrocytes in dissociated brain cell culture: a combined autoradiographic and immunocytochemical study. Brain Res. 1984 Nov 19;322(1):17–31. doi: 10.1016/0006-8993(84)91176-4. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Schachner M. Expression of Thy-1, H-2, and NS-4 cell surface antigens and tetanus toxin receptors in early postnatal and adult mouse cerebellum. J Neuroimmunol. 1981 Dec;1(4):429–456. doi: 10.1016/0165-5728(81)90022-9. [DOI] [PubMed] [Google Scholar]
- Snodgrass S. R., White W. F., Biales B., Dichter M. Biochemical correlates of GABA function in rat cortical neurons in culture. Brain Res. 1980 May 19;190(1):123–138. doi: 10.1016/0006-8993(80)91164-6. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Levi G., Johnstone S. R., Riddle P. N. Cerebellar astroglial cells in primary culture: expression of different morphological appearances and different ability to take up [3H]D-aspartate and [3H]GABA. Brain Res. 1983 Nov;312(2):265–277. doi: 10.1016/0165-3806(83)90143-8. [DOI] [PubMed] [Google Scholar]