Abstract
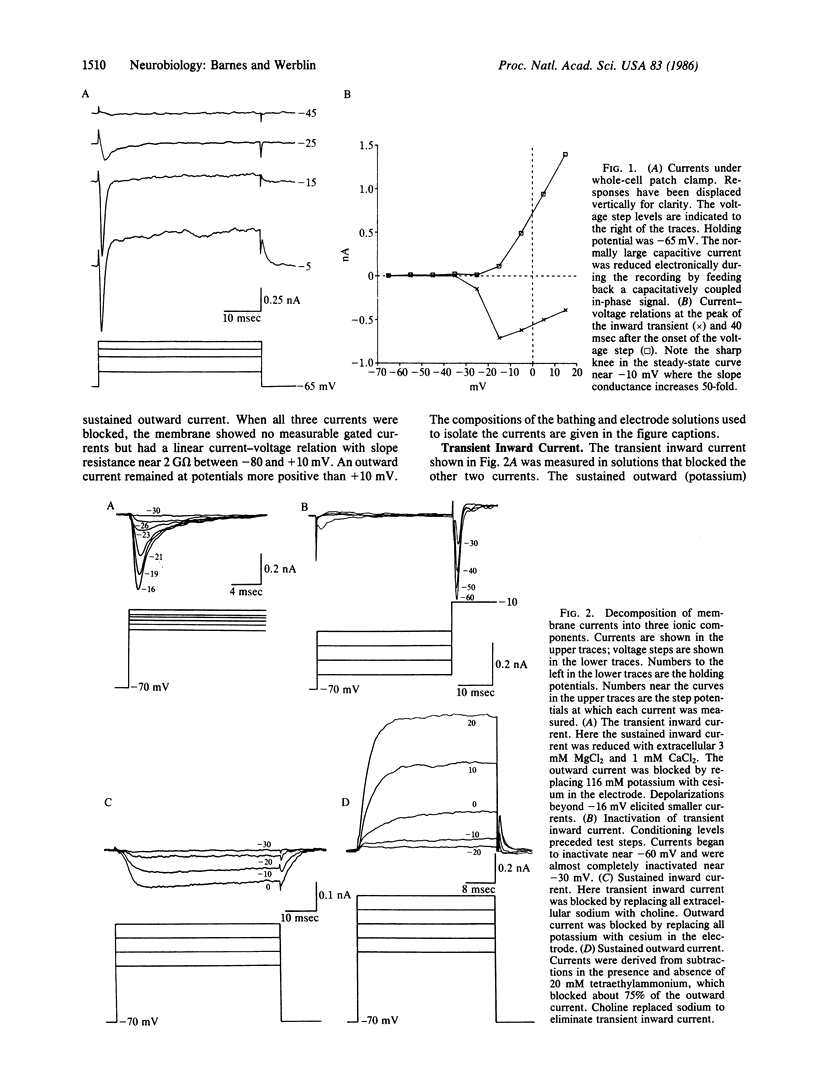
Amacrine cells form the neural networks mediating the second level of lateral interactions in the vertebrate retina. Members of a prominent class of amacrine cells, found in most vertebrates, respond at both the onset and termination of steps of illumination with a single, large transient depolarization. We show here how specific relationships between membrane currents control this single spike activity. Using whole-cell patch clamp on living retinal slices, we studied the membrane currents in amacrine cells. The currents elicited by depolarizing voltage steps could be separated into three main ionic components: a transient inward voltage-gated sodium current, a relatively small sustained inward voltage-gated calcium current, and a calcium-dependent outward current. A specific relationship between the sodium and potassium current alone appears to preclude repetitive spike activity. Potassium current is activated at potentials positive to -20 mV, but the sodium inactivation, between -60 and -20 mV, does not intersect potassium activation. Therefore, a steady depolarizing current step elicits an initial spike but then the membrane cannot be sufficiently hyperpolarized by potassium current to remove sodium inactivation and the cell remains refractory.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Membrane biophysics of calcium currents. Fed Proc. 1981 Jun;40(8):2220–2225. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. Dendritic and somatic spikes in mudpuppy amacrine cells: indentification and TTX sensitivity. Brain Res. 1976 Mar 5;104(1):157–162. doi: 10.1016/0006-8993(76)90657-0. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol. 1977 Jan;264(3):767–785. doi: 10.1113/jphysiol.1977.sp011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]
- Wunk D. F., Werblin F. S. Synaptic inputs to the ganglion cells in the tiger salamander retina. J Gen Physiol. 1979 Mar;73(3):265–286. doi: 10.1085/jgp.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]


