Abstract
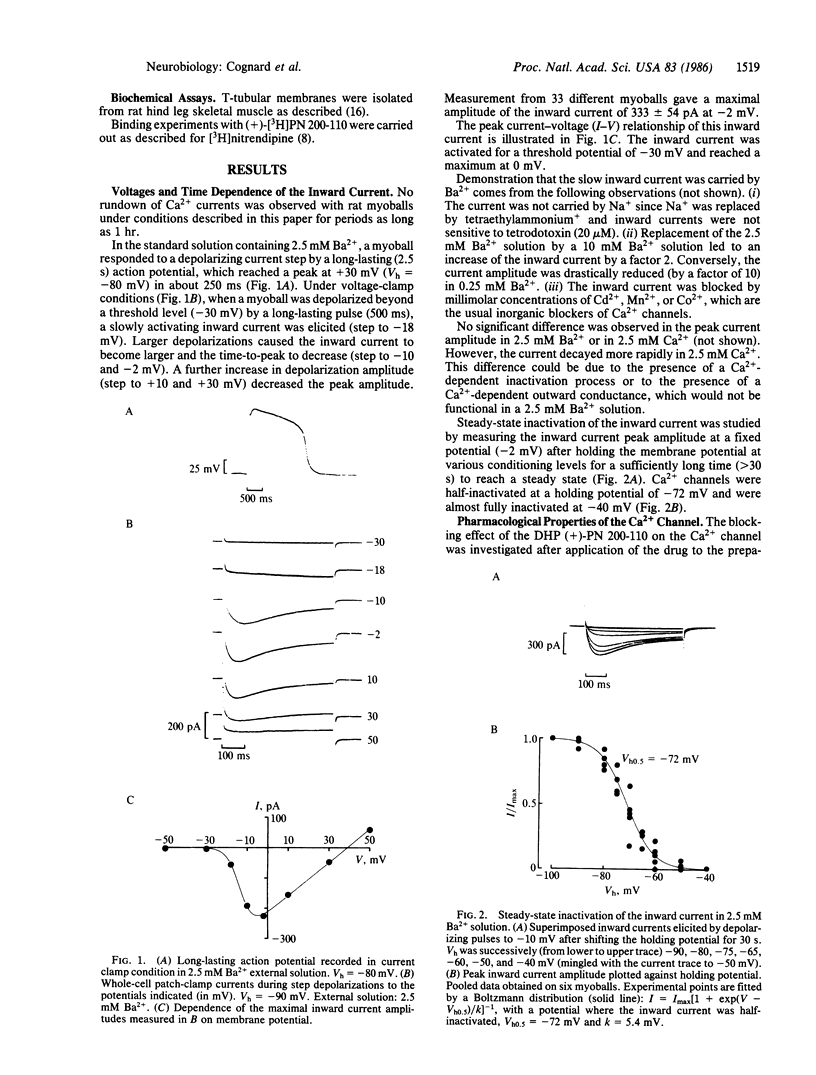
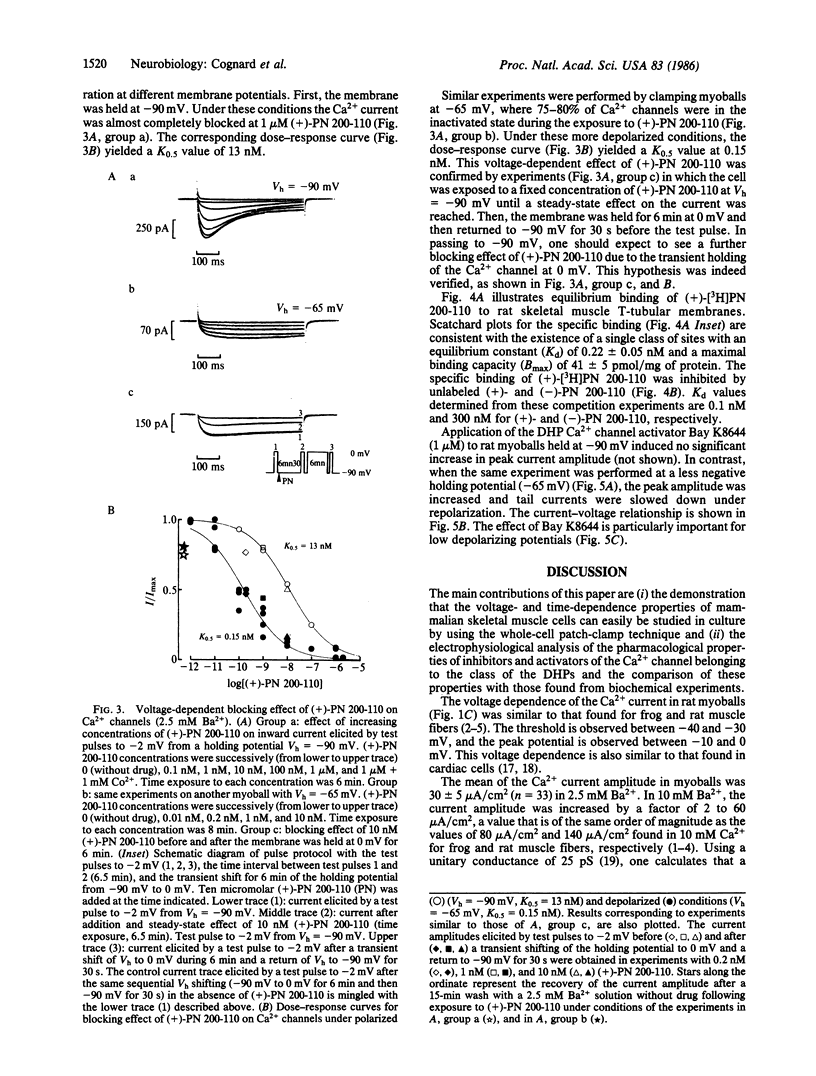
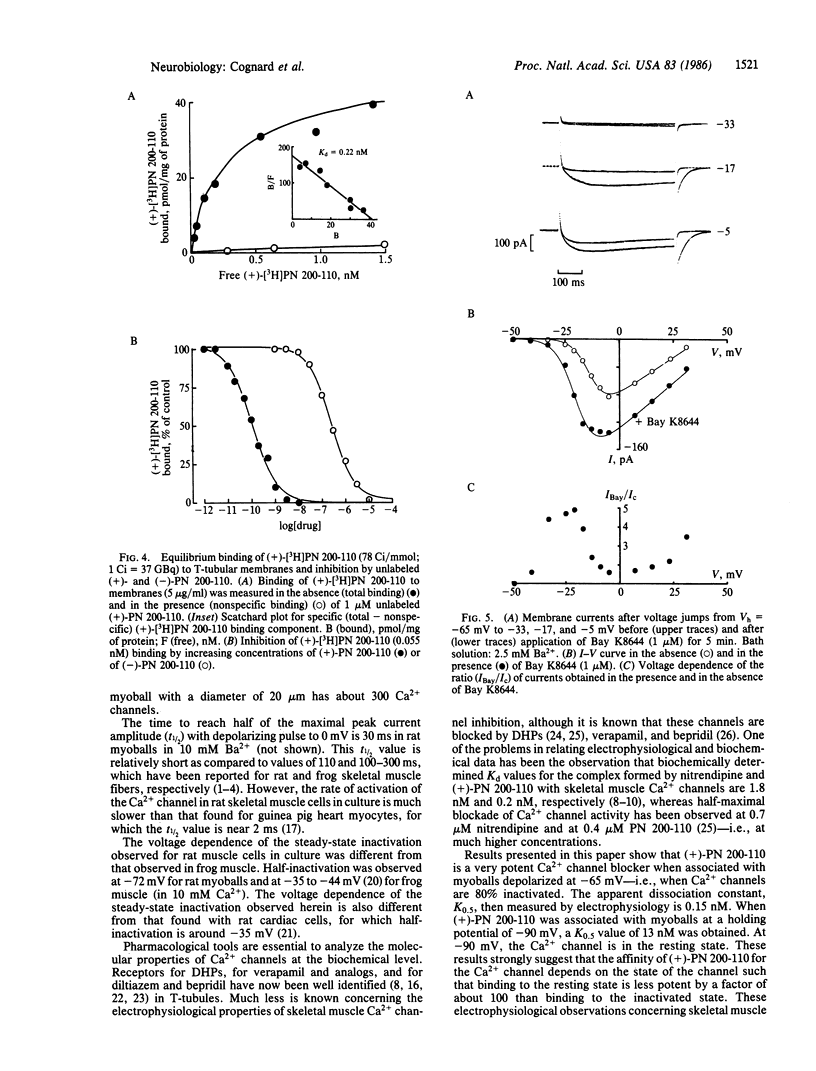
The whole-cell patch-clamp technique has been used to analyze the properties of the dihydropyridine-sensitive Ca2+ channel in rat skeletal muscle cells (myoballs) in culture. The potential dependence of Ca2+-channel activation is similar to that observed in cardiac cells. However, the skeletal muscle Ca2+ channel is activated more slowly (by a factor of about 10). The voltage dependence of Ca2+-channel inactivation indicates a half-maximal inactivation (Vh0.5) at -72 mV as compared to Vh0.5 = -35 mV for cardiac cells. Blockade of the skeletal muscle Ca2+ channel by the dihydropyridine (+)-PN 200-110 is voltage dependent, with a half-maximal effect (K0.5) of 13 nM for an application of the drug to the myoball membrane held at -90 mV and of 0.15 nM for an application at a potential of -65 mV. The 100-fold difference in apparent affinity is interpreted as a preferential association of PN 200-110 with the inactivated form of the Ca2+ channel. The K0.5 value found from electrophysiological experiments for the binding to the inactivated state (K0.5 = 0.15 nM) is nearly identical to the equilibrium dissociation constant found from binding experiments with (+)-[3H]PN 200-110 using transverse-tubular membranes (Kd = 0.22 nM). The dihydropyridine activator Bay K8644 acts by increasing Ca2+ current amplitude and by slowing down deactivation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Barrett E. F., Dribin L. B. Calcium-dependent slow potassium conductance in rat skeletal myotubes. Dev Biol. 1981 Mar;82(2):258–266. doi: 10.1016/0012-1606(81)90450-4. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Fosset M., Lazdunski M. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca2+ channel. Purification and subunit composition. J Biol Chem. 1985 Nov 15;260(26):14255–14263. [PubMed] [Google Scholar]
- Borsotto M., Barhanin J., Norman R. I., Lazdunski M. Purification of the dihydropyridine receptor of the voltage-dependent Ca2+ channel from skeletal muscle transverse tubules using (+) [3H]PN 200-110. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1357–1366. doi: 10.1016/0006-291x(84)91241-5. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Cota G., Nicola Siri L., Stefani E. Calcium channel inactivation in frog (Rana pipiens and Rana moctezuma) skeletal muscle fibres. J Physiol. 1984 Sep;354:99–108. doi: 10.1113/jphysiol.1984.sp015365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Donaldson P. L., Beam K. G. Calcium currents in a fast-twitch skeletal muscle of the rat. J Gen Physiol. 1983 Oct;82(4):449–468. doi: 10.1085/jgp.82.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. Na+ channels with high and low affinity tetrodotoxin binding sites in the mammalian skeletal muscle cell. Difference in functional properties and sequential appearance during rat skeletal myogenesis. J Biol Chem. 1983 Jun 25;258(12):7256–7259. [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. Properties of receptors for the Ca2+-channel blocker verapamil in transverse-tubule membranes of skeletal muscle. Stereospecificity, effect of Ca2+ and other inorganic cations, evidence for two categories of sites and effect of nucleoside triphosphates. Eur J Biochem. 1984 Oct 15;144(2):211–215. doi: 10.1111/j.1432-1033.1984.tb08451.x. [DOI] [PubMed] [Google Scholar]
- Galizzi J. P., Fosset M., Lazdunski M. [3H] verapamil binding sites in skeletal muscle transverse tubule membranes. Biochem Biophys Res Commun. 1984 Jan 13;118(1):239–245. doi: 10.1016/0006-291x(84)91092-1. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Linn T., Rombusch M., Ferry D. R. Temperature-dependent regulation of d-cis-[3H]diltiazem binding to Ca2+ channels by 1,4-dihydropyridine channel agonists and antagonists. FEBS Lett. 1983 Aug 22;160(1-2):226–232. doi: 10.1016/0014-5793(83)80972-7. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Ildefonse M., Jacquemond V., Rougier O., Renaud J. F., Fosset M., Lazdunski M. Excitation contraction coupling in skeletal muscle: evidence for a role of slow Ca2+ channels using Ca2+ channel activators and inhibitors in the dihydropyridine series. Biochem Biophys Res Commun. 1985 Jun 28;129(3):904–909. doi: 10.1016/0006-291x(85)91977-1. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. M., Sperelakis N. Effect of the calcium antagonists bepridil (CERM-1978) and verapamil on Ca++-dependent slow action potentials in frog skeletal muscle. J Pharmacol Exp Ther. 1982 Jul;222(1):80–86. [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Reversal of current through calcium channels in dialysed single heart cells. Nature. 1982 Jun 10;297(5866):498–501. doi: 10.1038/297498a0. [DOI] [PubMed] [Google Scholar]
- Potreau D., Raymond G. Slow inward barium current and contraction on frog single muscle fibres. J Physiol. 1980 Jun;303:91–109. doi: 10.1113/jphysiol.1980.sp013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. F., Méaux J. P., Romey G., Schmid A., Lazdunski M. Activation of the voltage-dependent Ca2+ channel in rat heart cells by dihydropyridine derivatives. Biochem Biophys Res Commun. 1984 Nov 30;125(1):405–412. doi: 10.1016/s0006-291x(84)80382-4. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Sanchez J. A., Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978 Oct;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M. C., Kass R. S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ Res. 1984 Sep;55(3):336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., McCleskey E. W., Almers W. Dihydropyridine receptors in muscle are voltage-dependent but most are not functional calcium channels. 1985 Apr 25-May 1Nature. 314(6013):747–751. doi: 10.1038/314747a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Thomas G., Chung M., Cohen C. J. A dihydropyridine (Bay k 8644) that enhances calcium currents in guinea pig and calf myocardial cells. A new type of positive inotropic agent. Circ Res. 1985 Jan;56(1):87–96. doi: 10.1161/01.res.56.1.87. [DOI] [PubMed] [Google Scholar]



