Abstract
hBVR is a Ser/Thr/Tyr kinase/scaffold protein/transcription factor/intracellular transporter of regulators. hBVR is an upstream activator of the insulin/IGF-1/MAPK/PI3K signaling pathway, and of NF-κB. As a reductase, it converts biliverdin to the antioxidant, bilirubin. hBVR gene has 8 exons; exon 1 is not translated. We report the characterization of hBVR promoter and its negative and positive regulation, respectively, by TNF-α and hypoxia. The 5′ end of exon 1 was defined by primer extension analyses; deletion of an inhibitor sequence 350-425 bp upstream of this exon enhanced the promoter activity. One of two NF-κB binding sites in the 836-bp promoter was functional; the P65 subunit of NF-κB and TNF-α acted as inhibitors. On the basis of EMSA and ChIP assays, TNF-α treatment increases binding of NF-κB to its regulatory element. Overexpression of IκB increased hBVR mRNA. Biliverdin, but not bilirubin, was as effective as TNF-α in inhibiting hBVR promoter activity. Only one of 4 hypoxia responsive elements (HREs) bound to HIF-1α and ARNT expressed in HEK293A cells. An abasic site was introduced at the 3′ G of the HRE. This element bound HIF-1 in the gel shift and in in-cell luciferase assays. hBVR was detected in the nucleus at 1, 2, and 4 h after hypoxia (1% O2), at which times its kinase and reductase activities were increased. Because hypoxia positively influences hBVR promoter and phosphorylation and TNF-α activated NF-κB inhibits the promoter, while biliverdin inhibits both NF-κB activity and hBVR promoter, we propose a regulatory mechanism for NF-κB by hypoxia and TNF-α centered on hBVR/biliverdin.—Gibbs, P. E. M., Miralem, T., Maines, M. D. Characterization of the human biliverdin reductase gene structure and regulatory elements: promoter activity is enhanced by hypoxia and suppressed by TNF-α-activated NF-κB.
Keywords: oxidative stress, antioxidants, bile pigments, cytokines, heme oxygenase, HIF-1
Initial description of biliverdin reductase (BVR) defined its sole function as the catalyst for reduction of biliverdin-IXα to bilirubin-IXα and to be exclusive to mammals. The purified enzyme was characterized as a dual cofactor/dual pH-dependent catalyst (1); this activity profile is unique to BVR. The available genomic data indicate a universal distribution of BVR, or BVR-like genes, throughout life forms ranging from cyanobacteria to humans (reviewed in refs. 2, 3). Within the higher life forms, tissue distribution is widespread. Recent investigations have uncovered multiple functions of the protein in the cell. Indeed, no other single protein described to date has a comparable range of diverse cellular activities as those ascribed to the human form of BVR. Accordingly, we were convinced that analysis of its transcriptional regulation is crucial to understanding and manipulating its activities. Prior to this study, examination of hBVR regulation was limited to investigation of its activation by phosphorylation (4, 5).
Biliverdin-IXα is the product of the heme oxygenase (HO) isozymes, the stress-inducible HO-1 and the constitutive HO-2 (6). Induction of HO-1 is generally considered to be cytoprotective, in that it degrades free heme and the heme prosthetic moiety of senescent hemoproteins to biliverdin and CO, releasing the chelated iron; the heme molecule is a potent catalyst for formation of reactive oxygen species (ROS) (7,8,9,10). Biliverdin is biologically active, and its activity is generally inhibitory in nature; inactivation of protein kinases, the transcription factor, NF-κB, and also interference with virus replication have been documented (11,12,13,14). In contrast, increased production of bilirubin is generally associated with positive outcomes for the cell; bilirubin is a chain-breaking intracellular antioxidant and a scavenger of free radicals (15,16,17,18,19,20,21). The reductase function of BVR is therefore considered to be cytoprotective; a concept that is supported by finding that depletion of BVR by treatment of HEK293A cells with si-hRNA augments apoptosis and attenuates response of HO-1 to free radical inducers (22, 23). Indeed, the cytoprotective function of hBVR in the cell has been found to be equivalent to that of GSH (24). Insofar as it uses the same binding cleft for ATP and the NAD(P)H cofactors, the kinase and reductase activities of hBVR are linked (25,26,27).
The uncovered multiple functions of the protein in the cell essentially influence all key components of signal transduction in the stress response. As a dual specificity (Ser/Thr/Tyr) kinase in the insulin/IGF-1 signaling pathway, hBVR is potentially significant in the control of glucose uptake (4, 28). In this signaling pathway hBVR is directly phosphorylated by IRK and phosphorylates IRS-1 (4). hBVR is an activator and intracellular translocater of PKC-βII and PKC-ζ to the membrane and is also a scaffolding/bridge for MEK/ERK1/2/Elk transcriptional activity and nuclear transporter of activator ERK1/2 (5, 29,30). There is also evidence that it is involved in cell differentiation/proliferation and growth (2). AP-1- and AP-2-regulated genes are activated by stress signaling (31,32,33,34,35,36); in this pathway, hBVR has been shown to activate CREB/ATF-1, c-fos, c-jun, and HO-1(22,23, 37). BVR activity is linked to protective effects against inflammation by stimulating production of the prototypical anti-inflammatory cytokine interleukin-10, the regulation of which is dependent in part on the activation of Akt, which has been observed on addition of biliverdin to cells (38). Using si-hBVR and nuclear localization mutant forms of the protein, Tudor et al. (39) have shown that the reductase functions as the nuclear transporter of heme for activation of HO-1 gene expression and potentially for other heme-regulated genes.
We have previously analyzed the gene structure of rat BVR and identified in the sequence upstream of the putative start site the consensus recognition element for the interferon gene regulatory factor INF-1, (the enhancer of cytokine and virus-induced transcriptional activation), and copies of GREs, Pit-1, the regulator of pituitary specific gene expression, and P3A, that is associated with embryonic gene expression and development (40). Because at the time we had considered that BVR is a “housekeeping” gene, its regulation at the transcription level was not examined. On the basis of the following observations, we hypothesized that regulation of hBVR is triggered by stress-inducing stimuli, albeit tightly. Specifically, neuronal expression of BVR decreases with age, particularly in regions that are associated with cognitive functions and linked to expression of HO-2 (17, 41), while BVR transcript levels increased in response to hypoxic heat shock (42). In addition, expression of BVR is increased in human renal carcinoma (43) and in the rat kidney in response to cytokines and LPS exposure (44).
We undertook the present investigation to characterize the human BVR promoter and examine transcriptional regulation of the reductase. Because NF-κB is the downstream effector of PKC-ζ, which is, in turn, activated by hBVR (5) and is considered a primary regulator of the response to extracellular stimuli that cause oxidative stress (45), and because HIF-1 is the transcription factor for hypoxic response, we focused on those factors. The selection of the latter was also consistent with recent reports that suggest a link between BVR expression and hypoxia. Specifically, cellular responses to chronic hypoxia and to hypoxia-reoxygenation in the presence of si-BVR were inhibited (46, 47), while increased levels of BVR at the kidney cell surface activated PI3K under conditions of chronic hypoxia and LPS treatment (38). Together, these observations suggest that hBVR functions as an effector of inflammatory response (38, 47). Moreover, cytokines, including TNF-α, are upstream activators of NF-κB (48, 49). In the light of these observations, we revisited the promoter sequence of the rat BVR gene (40). Aided by the availability of more extensive genomic sequence data (50), we found in the rat promoter region, as in the human, regulatory elements expected to bind NF-κB and HIF-1.
For this study, we cloned the promoter of hBVR from genomic DNA, and used this to drive expression of a luciferase reporter. The complete or partial consensus sequences of numerous regulatory elements are found in the promoter, of which we have characterized functional NF-κB and HIF-1 regulatory elements, and uncovered inhibition of promoter activity by biliverdin, a known inhibitor of NF-κB (12, 13, 51).
MATERIALS AND METHODS
Materials
HEK293A cells were obtained from Invitrogen (Carlsbad, CA, USA). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA, USA). The luciferase vector pGL3-basic and luciferase assay kit were from Promega (Madison, WI, USA). Expression plasmids pcDNA-P65, expressing the P65 subunit of NF-κB, and pcDNA-IκB were a gift from Dr. R. Elyseev (Department of Orthopedics, University of Rochester). TNF-α was a product of Calbiochem (La Jolla, CA, USA). Biliverdin and bilirubin were from Porphyrin Products (Logan, UT, USA).
Plasmids
The 836-bp DNA sequence upstream of the human BLVRA gene was amplified by PCR, using the primers hBVR-836 (CCGCTAGCCAAGCCTTCTCTCTTTGCACTC) and hBVR-flank (CGGCTCGAGATCCCAGCTCCGATTGGC). The amplification product was cloned into the vector pGL3-basic (Promega), between the NheI and XhoI sites. For shorter DNA sequences from this region, the 5′ primers were hBVR-601 (GCCGCTAGCAACAAACCCATTCCCAGGTGG), hBVR-432 (GCCGCTAGCGGCCCGGGGCCACTAAATAC), hBVR-306 (GCCGCTAGCGGCCAGGCACACCGTGAGG), and hBVR-105 (CGCGGTACCGCTAGCCTTCCGTCCACTCCCTTC), while the hBVR-flank 3′ primer was used in all cases. The DNA was cloned between the NheI and XhoI sites of the vector. The promoter constructs were verified by sequencing, using ABI Big-dye 3.1 reagent (Applied Biosystems, Foster City, CA, USA) and capillary electrophoresis. The expression plasmids pEGFP-HIF-1α and pCMV-ARNT1 were gifts from Drs. R. Fleming and G. Johnson-Voll (University of Rochester, Rochester, NY, USA), respectively. The plasmid pcDNA-HA-hBVR contains the open reading frame of hBVR fused downstream of the HA epitope and was generated by PCR using a 5′ primer encoding epitope and hBVR coding sequences.
Construction of plasmids for examining the role of abasic sites in the promoter in intact cells was carried out following a modification of the procedure described by Ozgenc et al.(52). Each strand of a 107-bp sequence (nt −215 to −109) that contains 2 candidate hypoxia responsive element (HRE) elements was assembled from 2 oligonucleotides of the lengths 53 and 58 bp. The shorter, 5′ fragment for each strand is extended to generate a restriction site cohesive end: one strand has that for SpeI, the other that for BclI. Two versions of the shorter oligonucleotides were made: the first containing the wild-type promoter sequence, and the second replacing G with dU at the same sites used in the oligonucleotides tested for HIF-1 binding. The junctions between 5′ and 3′ fragments in each of the two strands are offset by 10 bp, enabling the longer fragment of the complementary strand to function as a scaffold to assemble the complete 111-nt sequence. The oligonucleotides, (excluding the one with the BclI cohesive end), were 5′ phosphorylated with T4 polynucleotide kinase (New England Biolabs, Beverly, MA, USA). Four oligonucleotides were then annealed at a concentration of 10 μM in 100 mM NaCl, and were then ligated by treatment with T4 DNA ligase in the presence of SpeI to favor the formation of 111-nt double-stranded monomers. A 10-fold molar excess of the double-stranded product was then ligated, at high vector concentration, into the NheI site of pGL3-promoter. Both NheI and SpeI were included in the ligation to minimize undesirable products. The efficiency of this reaction was determined by restriction digestion and gel electrophoresis to be >95%. The plasmid, with oligonucleotide ligated to each end, was then digested with BglII, diluted to 2.5 ng/μl, and ligated in the presence of BglII. These conditions favor the formation of circular DNA with a nick in one strand. Gel electrophoresis indicated that the desired product formed at ∼30% efficiency.
Primer elongation and sequencing
Total cellular RNA was purified from HEK293A cells using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions; 12.5 μg of this RNA was annealed to the primers CCACCACGCCAAACTTCCTCT (complementary to coding nt 17–37) or GATTCCGCAAGTCCCTCATCC (complementary to coding nt 68–88), which had been labeled with [γ-32P]-ATP using T4 polynucleotide kinase (New England Biolabs), according to the manufacturer’s instructions. cDNA was synthesized using Superscript reverse transcriptase (Invitrogen), following the manufacturer’s instructions, save for the modification that elongation of the cDNA was started at 42°, and increased stepwise to 50° to counter the GC-rich 5′ sequence of the mRNA (53). The plasmid pcDNA-HA-hBVR was digested with EcoRI and BglII, and was sequenced using the labeled primers, using Sequenase version 2.0. The elongation and sequence products were resolved by electrophoresis on a 0.4-mm buffer gradient sequencing gel and detected by autoradiography.
Cell culture and transfection
HEK293A cells were maintained in DMEM (Invitrogen) containing 10% FBS (Atlanta Biologicals, Lawrenceville, GA, USA). Transfection with plasmid DNA was facilitated by transfectin lipid reagent (Bio-Rad, Hercules, CA, USA). Briefly, the transfectin-DNA mixture in serum-free medium (OptiMEM; Invitrogen) was added to the cells, incubated for 4 h, and the medium was supplemented with 3 vol of DMEM with 10% FBS. pCMV-β-gal plasmid was cotransfected with all luciferase reporter plasmids, as a control for transfection efficiency. Where transfected cells were to be treated with TNF-α, the medium was replaced 12 h after DNA addition with DMEM containing 0.5% FBS or with same medium containing 20 ng/ml TNF-α. After 12 h, the medium was removed; the cells were washed with PBS and then lysed.
Reporter assays
Aliquots of cell lysates were incubated with luciferase substrate (Promega) in a Turner Biosystems microplate instrument (Turner Biosystems San Francisco, CA, USA), and the luminescence was recorded. Further, aliquots were assayed for β-galactosidase activity, using O-nitrophenylthiogalactoside as a substrate. Luciferase activities were then normalized on β-galactosidase to control for variations in transfection efficiency. All assays were performed on samples from independent triplicate wells in each experiment, and all experiments were performed at least three times.
Nuclear extraction and gel mobility-shift assay
In experiments to examine NF-κB binding to hBVR promoter fragments, the cells were starved in medium containing 0.5% FBS overnight, treated with 20 ng/ml TNF-α for 15 min, and used for isolation of nuclear extract, as described in detail elsewhere (13). Double-stranded oligonucleotides containing candidate NF-κB sites from the hBVR promoter (CTTTGTTGGGGAAAATGCCAGTCAACCT−730, AGGCCGGGACCCTCCCTCCCCTTC−102), or a consensus NF-κB binding sequence (5′AGTTGAGGGGACTTTCCCAGGC) were labeled with [γ-32P]-ATP as above. The nuclear extracts were incubated for 10 min at room temperature prior to the addition of labeled oligonucleotide, and continued incubation for 20 min at room temperature. Reaction mixtures contained the same amount of extract protein. To identify NF-κB in the DNA: protein complexes, goat anti-P65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was included in some reactions.
For HIF-1 binding, HIF-1α and ARNT expression plasmids were used to transfect cells; 24 h later, the medium was replaced with OptiMEM, at which time, the cells were transferred to 1% O2 for another 24 h. Cell extracts were prepared in the presence of proteinase (leupeptin, aprotinin, and PMSF) and phosphatase (NaF, NaVO3, and β-glycerophosphate) inhibitors, as detailed elsewhere (54). The extracts were immediately assayed for binding to the double-stranded oligonucleotides GGCAGGCGCACGGACGGTTCT−185 and GCGACCGCACGAGGCCGGG−118 from the hBVR promoter, and to GATCGCCCTACGTGCTGTCTCAGATC, containing a wild-type HRE from the EPO promoter (55). Nonspecific binding was reduced by including 50-fold excess of unlabeled GATCGCCCTAAAAGCTGTCTCAGATC in the reaction. An abasic site was introduced into one strand of the duplex by annealing a dU-containing oligonucleotide to its wild-type complement (giving a C-U pairing) the oligonucleotides used were GGCAGGCGCACGGACGdUTTCT−185 and −118CCCGGCCTCGTGCdUGTCGC, respectively. The duplexes were treated with uracil N-glycosylase (New England Biolabs) for 30 min prior to labeling with 32P, which yields an uniquely located abasic site in the duplex.
The DNA:protein binding complexes were subjected to electrophoresis on a 4% nondenaturing polyacrylamide gel at 4°C and processed for autoradiography. Each experiment was repeated at least three times.
Chromatin immunoprecipitation
Cells (108) were fixed in 1% formaldehyde for 10 min at room temperature, and the reaction was stopped by the addition of 0.125 M glycine. After 5 min at room temperature, the cells were washed with PBS and harvested by centrifugation, followed by the cell pellet being washed by repeated resuspension in PBS. The cells were then suspended in 5 mM Pipes (pH 8.0) and 85 mM KCl, incubated on ice for 20 min, and lysed by 15 strokes of a Dounce homogenizer. Nuclei were collected by centrifugation at 2500 g, resuspended in 50 mM Tris (pH 8.0), 10 mM EDTA, and 1% SDS-containing protease inhibitors, and sonicated. Staph A cells that had been preincubated with salmon sperm DNA and BSA were added to the sheared chromatin for 15 min at 4°C and removed by centrifugation at 10,000 g for 5 min. The supernatant was incubated overnight with the goat anti-P65 antibody (Santa Cruz Biotechnology), followed by addition of rabbit anti-goat IgG, and incubation for a further 1 h. Staph A cells (pretreated as above) were added; the suspension was incubated for 15 min at room temperature and then centrifuged at 10,000 g for 4 min. The pellets were washed twice with 1 ml of 50 mM Tris (pH 8), 2 mM EDTA, and 1 mM PMSF and 4 times with 1 ml of 0.1 M Tris (pH 9), 500 mM LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 1 mM PMSF. Antibody/protein/DNA complexes were eluted twice with 50 mM NaHCO3, 1% SDS. The samples were adjusted to 0.2 M NaCl and incubated at 67°C for 4 h to reverse cross-linking. DNA was purified using the Qiaquick PCR purification kit (Qiagen, Valencia, CA, USA). The ChIP sample was then used for PCR reactions, using primers that flank the NF-κB target sites in the hBVR promoter.
RT-PCR experiments
Cells in 6-well plates were transfected with pcDNA3-P65, pcDNA-IκB or an empty pcDNA3 vector. Four hours after DNA addition, one plate was removed for RNA isolation, and the rest of the cells were allowed to recover in DMEM containing 10% FBS. Cells from the remaining plates were harvested at daily intervals after the last medium change. Cells were lysed with TRIzol reagent, total RNA was purified, and 2.5 μg RNA was used as a template for cDNA synthesis using random hexamer primers. The cDNA was used as a template for qRT PCR, using gene-specific primers and Taq polymerase in an ABI-fast 7500 PCR system. Products were detected by SYBR Green; specificity was determined from melting curves, and in some samples, the reaction products were resolved by gel electrophoresis. Specific signals were normalized on β-actin or on 18S rRNA, and quantified by the ΔΔCT method. Errors in mRNA quantification were calculated on the basis of the se computed in ΔΔCT, and the error was determined by error = mRNA(ΔΔCT) ± mRNA(ΔΔCT ±se). To determine hBVR mRNA stability, cells were treated with 2.5 μg/ml actinomycin D to inhibit transcription, and harvested at intervals thereafter. ΔΔCT was plotted as a function of time and data fitted by linear regression. The mRNA half-life was determined as the reciprocal of the slope of the regression line.
Measurement of hBVR reductase activity
Cells grown to 70% confluence were synchronized with 0.5% FBS, incubated in 1% O2 for 2 h, and lysed while hypoxic in 0.75 M sodium phosphate buffer (pH 7.4) containing 75 mM NaCl, 0.2 mM DTT, 10% glycerol, 1% Nonidet P-40, and protease inhibitors (leupeptin, aprotinin, and PMSF). hBVR activity was measured at pH 6.7 using NADH as the cofactor, as described previously (56). The rate of reduction of biliverdin to bilirubin was determined by the increase in absorbance at 450 nm at 25°C. Specific activity is expressed as nanomoles of bilirubin per minute per milligram of protein.
Confocal microscopy
Cells were transfected with pEGFP-hBVR in chamber slides and incubated in a hypoxic chamber at 1% O2, 5% CO2, in chamber slides at 37°C. The medium was removed, and the cells were fixed with 4% paraformaldehyde, while still in the hypoxic chamber. After further fixation with methanol, the cells were washed, and the nuclei were counterstained with propidium iodide and viewed in an Olympus confocal microscope.
Kinase assays
Cells were transfected with pcDNA-HA-hBVR: after lysis, the HA-hBVR was immunoprecipitated with mouse anti-HA antibodies (Santa Cruz Biotechnology), followed by incubation with protein/G-Sepharose beads. The immunoprecipitated protein was assayed for hBVR kinase activity, as described previously (4), in reactions containing 20 mM MnCl2 and pH 8.0, using myelin basic protein (MBP) as a substrate. Phosphorylated MBP was detected by SDS-PAGE, followed by blotting to nitrocellulose membrane and autoradiography.
In vivo phosphorylation assays
Cells transfected with pcDNA-HA-hBVR were lysed, and HA-hBVR was immunoprecipitated as above. Protein in the immunoprecipitate was dissolved in SDS gel loading buffer, and resolved on 10% polyacrylamide gels. The proteins were blotted to nitrocellulose and probed with a mixture of rabbit antibodies to phosphoserine and phosphothreonine (Zymed Laboratories, San Francisco CA, USA), or with mouse anti-phosphotyrosine antibody (Cell Signaling, Boston, MA, USA). Bound antibody was detected with anti-rabbit IgG secondary antibody and enhanced chemiluminescence. The blot was then stripped and reprobed with rabbit anti-BVR antibody.
Statistical analyses
Experiments were performed ≥3 times. All statistical analyses, including linear regression, were performed using Prism 3.0 software (GraphPad, San Diego, CA, USA). In those experiments in which experimental samples were normalized to a control set as 1.0, a one-sample t test was used to test whether the experimental mean differed from 1. Other pairwise comparisons used a 2-tailed t test.
RESULTS
The human BVR (BLVRA) gene consists of 8 exons, as determined by comparison of cDNA and genomic DNA sequences, and a similar arrangement is observed for the mouse gene. The first exon is entirely untranslated, and a further 21 nt of 5′-untranslated sequence are found in exon 2. There is some uncertainty as to where the hBVR transcript initiates, as sequences in GenBank, including one generated in this laboratory (53) may not derive from truly full-length cDNAs. The reference sequence (NM_000712) exon 1 at 62 nt is the longest found, while two sequences suggest an exon 1 sequence of 55 nt. A. Moreover, no sequence in the expressed sequence tag (EST) database extends further 5′ than the reference sequence. For the purposes of this study, nt 1 of the reference sequence mRNA was tentatively taken as the transcription initiation site.
Primer extension analyses
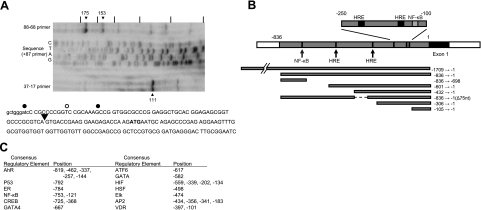
Although the longest of the known exon 1 sequences might be considered as arising from the transcription start site, it is possible that the cDNA from which this sequence is derived was itself a consequence of premature termination in the reverse transcription reaction rather than being a full-length copy. We therefore set out to determine the transcription start site by reverse transcription of hBVR mRNA with gene-specific primers. Two 5′-phosphorylated primers were used for cDNA synthesis, one complementary to coding nt 17–37, the other to nt 68–88. Both of these gave multiple extension products (Fig. 1A). Comparing the mobility of the major cDNA species with that of bands from a sequence reaction driven by the 5′-phosphorylated 68–88 primer allowed measurement of the length of the cDNA products. The major cDNA from the 17–37 primer is 111 nt in length, which suggests a 5′-untranslated sequence of 74 nt and hence that the exon 1 sequence is 53 nt. Two products are observed with the 68–88 primer, of lengths 153 and 175 nt. These correspond to 5′-untranslated sequences of 65 and 87 nt, or 44 and 66 nt for exon 1. The 153-nt product most likely arises from premature termination, implying that at most the transcription initiation site lies 4 bp upstream from the start of the reference mRNA (Fig. 1A).
Figure 1.
Map of the proposed hBVR promoter region. A) In vitro primer extension. Reverse transcription of total HEK293A cell RNA was performed using 32P-labeled hBVR-specific primers (complementary to coding nt 88-68 and 37-17, respectively). The products were resolved on an 8% polyacrylamide gel containing 8 M urea. Size markers were provided by using the 32P-labeled 88-68 primer for dideoxy sequencing of pcDNA-HA-BVR; tick marks are placed at 20-nt intervals. Sequence of nt 1–171 of hBVR mRNA, together with 9 nt of upstream genomic sequence is shown. Black dots indicate cDNA terminations observed for the 88-68 primer; white dot that for the 37-17 primer. B) Map indicates ∼1 kb of genomic sequence immediately 5′ to and including the 62-bp exon 1 sequence of the hBVR mRNA (accession no. NM_000712). White bar at left indicates a region of repetitive DNA elements. Bars below the sequence indicate regions that were amplified and examined for promoter activity. Arrows indicate the location of candidate binding sites for regulatory proteins that are discussed in the text. C) Table shows the locations within the 836 bp of nonrepetitive DNA of candidate regulatory elements, determined from RVista (60) comparisons of the human and mouse genes. Coordinates are the position of the first nucleotide in the element defined by the computer program. AhR, aryl hydrocarbon receptor; ER, estrogen receptor; NF-κB, nuclear factor binding to κ light-chain gene enhancer; CREB, cyclic AMP response element binding protein; ATF6, activating transcription factor 6; GATA4, GATA binding protein 4; GATA, GATA binding proteins; HIF, hypoxia inducible factor; HSF, heat-shock factor; Elk, member of ETS oncogene family; AP-2, activating enhancer binding protein 2; VDR, vitamin D receptor.
Identification of the hBVR promoter
Identification of the human hBVR promoter was further aided by the observation that human BLVRA and its mouse homologue (Blvra) occupy regions that are not syntenic between the species (57)—the mouse gene is flanked by sequences orthologous to human chromosome regions 15q21 and 2q11, whereas the 34-kb mouse sequence itself is syntenic in relation to human 7p13. These authors further demonstrated that there are at most 1182 nt of conserved 5′ flanking and 6612 nt of 3′-flanking sequence surrounding the mouse gene. Therefore, these data constrain the likely region for a conserved promoter sequence. The presence of a block of repetitive sequence elements ∼840 nt 5′ of human exon 1 is considered as a further constraint. The genomic region that includes hBVR exon 1 is shown schematically in Fig. 1B. The 450 nt closest to exon 1, and, indeed, the exon itself and the 5′ end of intron 1, have sequence composition and characteristics that resemble those of a CpG island. Promoters of this type are commonly found in housekeeping genes, although there are exceptions, and they stand as the most frequently observed form of mammalian promoter (58). The human and mouse genomic sequences were aligned using the zPicture program (59), and the resulting alignment was used as input for the program Rvista (60) to yield candidate regulatory sequence elements, as shown in Fig. 1C. These include binding sites for proteins involved in developmental gene regulation, as well as sequences, such as HRE, candidate binding sites for NF-κB, and elements that suggest regulation by steroid hormones or stress.
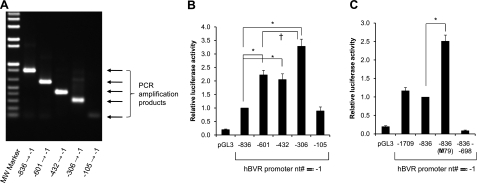
Initially, the 836-nt sequence upstream of exon 1 was amplified by PCR (Fig. 2A) and cloned into a luciferase reporter plasmid. Transfection of cells with this plasmid leads to expression of the reporter gene, and the luciferase activity is found to be increased ∼10-fold compared to the empty vector (Fig. 2B). This suggests that the 836 nucleotide sequence contains sufficient information to direct transcription of the hBVR gene. To determine the location of necessary sequence elements, a series of nested deletions in the putative promoter region was constructed by PCR amplification (Fig. 1B, 2A) and cloned into the reporter vector. Most of these promoters prove to be significantly (P≤0.001) more effective than that in the original construct in driving luciferase expression. The elevated activity is neither due to mutation during PCR amplification, as the constructs were verified by sequencing, nor is it due to variations in DNA quality, since numerous independent preparations of each construct have shown the same pattern of expression. The shortest fragment tested, in hBVR-105, is as active as that in the hBVR-836 construct but less active than most of the other constructs. This indicates that there are positive regulatory elements located between nts −105 and −306. To search for other regulatory elements, a longer genomic sequence, starting 1.7 kb upstream of the transcription initiation site, was cloned into the luciferase reporter. This sequence is likely to extend outside the region of human-mouse homology (57). The construct displays virtually identical activity to that of the 836-bp promoter sequence (Fig. 2C), arguing against the possibility of further regulatory elements in this region. Two deletions were made in the 836-bp promoter construct. The first, a deletion of nt −697 to −1, yields a construct with no reporter activity (Fig. 2C), which implies that all of the essential elements of the promoter lie in the ∼700 bp closest to the initiation site. This is confirmed by a reciprocal construct, which includes nt −696 to −1, which shows promoter activity indistinguishable from that of the −601 construct (data not shown). Furthermore, this suggests the presence of a negative regulatory element in the more distal 140 bp. A second deletion, of 79 bp, between positions −429 and −350, results in a construct that shows ∼2-fold higher activity compared to the 836 bp promoter (Fig. 2C). This indicates that there is a further sequence element in the deleted region that down-regulates the promoter. The presence of such an element is also suggested by the observation that the hBVR-306 fragment is significantly (P<0.01) more active than hBVR-432, or hBVR-601 (Fig. 2B).
Figure 2.
The 5′-flanking region of the hBVR gene is a functional promoter. A) PCR amplification from human genomic DNA of the promoter regions described in Fig. 1B. Products were resolved by agarose gel electrophoresis. B) Genomic fragments in A were cloned upstream of a luciferase reporter gene. HEK293A cells were cotransfected with the resulting plasmids together with pCMV-βgal. At 24 h after DNA addition, the cells were lysed and assayed for luciferase and β-galactosidase. For each sample, luciferase activity was normalized to that of β-galactosidase, and within each experiment, the normalized activity determined for each construct was expressed relative to that of the hBVR-836 construct. *P < 0.01 vs. hBVR-836; †P < 0.01 vs. hBVR-601 or hBVR-432. C) Inhibitor sequence in the hBVR promoter. Luciferase reporter plasmids shown were used to cotransfect cells; determination of luciferase activity was as described in B. *P < 0.001 vs. hBVR-836.
hBVR promoter is inhibited by TNF-α via NF-κB
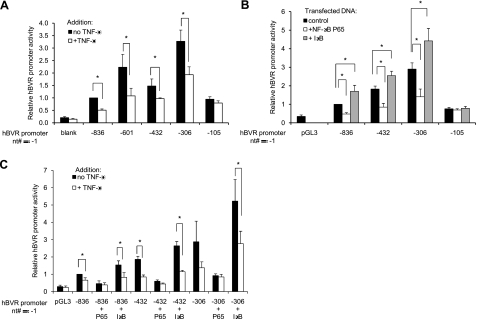
It was noted during computer analysis of the 5′ flanking sequences of hBVR that there are ≥2 candidate NF-κB recognition sites in the human sequence (Fig. 1). These suggested that the promoter might be responsive to TNF-α, which ultimately exerts its effects via NF-κB. Accordingly, luciferase activity was assessed in cells transfected with the reporter plasmids that were subsequently treated with TNF-α for 12 h. To minimize background luciferase expression, the TNF-α treatment was initiated 12 h after the addition of DNA to the cells when very little luciferase protein has accumulated in the cell. As previously mentioned, the shorter promoters are apparently more active in both untreated and treated cells. Although all of the shorter constructs exclude one of the NF-κB recognition sites seen in hBVR-836 (Fig. 1), the addition of TNF-α routinely results in ∼50% reduction in promoter activity in most of the constructs tested (Fig. 3A). These differences are significant (P<0.05). Only the shortest promoter fragment tested, hBVR-105, which retains neither of the putative NF-κB binding sites, fails to respond to TNF-α. We tentatively conclude that the inhibition by TNF-α is mediated via NF-κB binding to a recognition sequence located less than 125 nt from the transcription start site (Fig. 1B).
Figure 3.
TNF-α treatment inhibits hBVR-promoter activity. A) Cells were cotransfected with the indicated plasmids and with pCMV-βgal. At 12 h after the addition of DNA, cells were treated with 20 ng/ml TNF-α for a further 12 h and assayed for luciferase and β-galactosidase activities. Normalized luciferase activity of each experimental sample was expressed relative to that of the untreated hBVR-836 construct. B) Cells were cotransfected with hBVR promoter reporter plasmid, pCMV-β-gal and one of pcDNA-P65, pcDNA-IκB, or the pcDNA3 vector. Cells were lysed 24 h after DNA addition, and lysates were assayed for luciferase and β-galactosidase; data are expressed as in A. C) Cells were transfected as described in B. At 12 h after addition of DNA, cells were treated, as indicated, with 20 ng/ml TNF-α for a further 12 h prior to analysis.
In further experiments characterizing this TNF-α-dependent response of the promoter, the effect of overexpression of NF-κB was examined by cotransfecting cells with the reporter constructs and an expression plasmid encoding either the P65 subunit of NF-κB or IκB. The empty pcDNA3 vector served as the control. NF-κB in untreated cells is largely sequestered in the cytoplasm as a complex with IκB and is thus unavailable to the nucleus. By overexpressing P65, it was likely that the cellular IκB would be titrated, and thus NF-κB would be activated. This would then be expected to result in an inhibition of hBVR promoter activity. Indeed, extracts of cells cotransfected with pcDNA-P65 and one of four different promoter constructs shows significantly (P≤0.001) reduced luciferase activity compared to those cotransfected with the empty pcDNA3 vector (Fig. 3B). Again, the exception is the hBVR-110 construct, which lacks both of the putative NF-κB binding sites. Moreover, treatment of the cells overexpressing NF-κB with TNF-α does not result in further inhibition (Fig. 3C) compared to that seen with NF-κB alone, further arguing that any effect of TNF-α was due to activation of NF-κB. Similarly, overexpression of IκB might be expected to shift the equilibrium between free and complexed NF-κB in resting cells, even further in favor of the complex. Because there is a modest (P<0.01) and reproducible increase in promoter activity in cells overexpressing IκB (Fig. 3B), this evidence indicates that any low level of circulating NF-κB in the resting cell can be further sequestered by the inhibitor. It is notable, too, that TNF-α treatment of these cells reduces activity of the promoter to the same level seen in similarly treated control cells (Fig. 3C). Full-length hBVR was used as bait in a yeast 2-hybrid screen system using a human kidney cDNA library, and ∼17 million yeast colonies were screened for hBVR-interacting proteins. Of the 31 positive clones that were obtained under the highest stringency screening, several were identified as fragments of the IκB kinase. This finding suggests that hBVR itself mediates the activation of NF-κB in the pathway. We also obtained 12 clones in this screen for the Goodpasture antigen binding protein, and we have confirmed that interaction by several independent criteria (61).
NF-κB binds to an hBVR promoter element
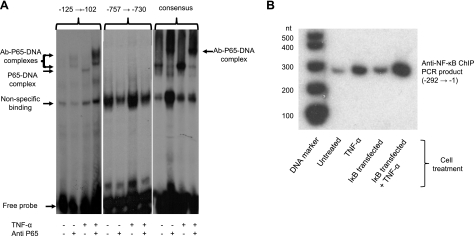
It is apparent from comparison of the deletion series that the proximal NF-κB binding site is necessary for the responses to TNF-α and to overexpression of the NF-κB subunit. However, this does not preclude a role for the more distal site. To address this, a series of gel-shift experiments was performed, using oligonucleotides, including the distal (nt −757 to −730), proximal (nt −125 to −102) and consensus-binding sequences (Fig. 4A). The proximal and consensus oligonucleotides are both bound by protein in the nuclear extracts, and this is enhanced by treatment of the cells with TNF-α (20 ng/ml, 15 min) immediately prior to harvesting. The supershift observed with both of these oligonucleotides in the presence of anti-P65 antibody indicates that binding is due to NF-κB. On the other hand, no specific binding of extract protein to the distal oligonucleotide is observed, even at higher protein concentrations. It is probable, therefore, that only the binding site closest to the site of transcription initiation is involved in regulation of the hBVR gene by TNF-α.
Figure 4.
NF-κB binding to hBVR promoter. A) Electrophoretic mobility shift assay. Cells were starved in medium with 0.5% FBS overnight, then treated with TNF-α, as indicated, for 15 min. Cells were harvested; nuclear extracts were prepared as described in the text, and assayed for protein binding to 3 duplex oligonucleotides: hBVR promoter nt −757 → −730, −125 → −102, (containing the candidate NF-κB binding sites), and an oligonucleotide, including a consensus NF-κB binding sequence. Where indicated, antibody to the NF-κB P65 subunit was included. Products of binding reactions were resolved by electrophoresis on 4% polyacrylamide gels and detected by autoradiography. Two apparent supershift bands are seen with the −125 → −102 oligonucleotide, but only one with consensus. B) NF-κB binds to the proximal target sequence in vivo. Cells were transfected with pcDNA-IκB or treated with TNF-α, as indicated, for 15 min. Cells were fixed with 1% formaldehyde and chromatin was prepared (see text) and immunoprecipitated with antibody to the P65 subunit. DNA in the immunoprecipitate was amplified by PCR using primers for the hBVR promoter region between nt-1 and −290. 32P-labeled PCR products were resolved by PAGE, and detected by autoradiography.
Whether NF-κB binds to the hBVR promoter in vivo was assessed by chromatin immunoprecipitation. There is a low level of binding to the most proximal NF-κB site in untreated cells (Fig. 4B). This is increased by the overexpression of IκB, while binding is enhanced ∼8-fold by treatment of the cells with 20 ng/ml TNF-α for 15 min. Binding of NF-κB to the more distal site is also observed; however, the level of binding is unaltered by TNF-α treatment (data not shown). These observations are consistent with the observed lack of binding of NF-κB to the more distal site in vitro, and also with the enhanced binding to the proximal binding site of nuclear extract proteins prepared from TNF-α-treated cells.
NF-κB regulates hBVR mRNA expression
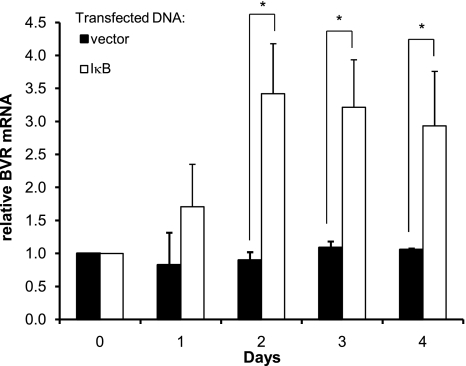
The regulation of endogenous hBVR transcription was addressed by prolonged overexpression of IκB. RNA isolated from the cells at intervals after transfection was used as a template for cDNA synthesis, and the level of hBVR cDNA was measured by qPCR; the data were analyzed using the ΔΔCT method. The specificity of amplification was assessed from melting profiles of the products, and in some cases, the size of amplification products was confirmed by gel electrophoresis. Overexpression of IκB leads to an accumulation of hBVR mRNA over a period of 48 h (Fig. 5); the increases are significant (P<0.01 at d 2 and 3, P<0.05 at d 4). These data are consistent with the observation that overexpression of IκB leads to enhanced promoter activity of reporter constructs. The slow response observed in these experiments are those that would be expected for a gene expressing a stable mRNA. Prolonged treatment of the cells with repeated additions of TNF-α resulted in decreased expression of hBVR mRNA in the short term, but this was not maintained over longer periods (data not shown), and it is possible that the signal is poorly maintained in these cultures. It is also possible that the stability of the hBVR mRNA is a further confounding factor in these experiments, in that the effect of even prolonged reduction in promoter activity might be difficult to detect due to the continued presence of the preexisting mRNA.
Figure 5.
NF-κB regulates endogenous hBVR transcription. Cells were transfected with pcDNA-IκB or empty vector and were harvested at daily intervals. RNA was isolated and used as a template for cDNA synthesis. hBVR cDNA, together with that for β-actin, was amplified by quantitative PCR. Quantification was by the ΔΔCT method.
Biliverdin inhibits activity of the hBVR promoter
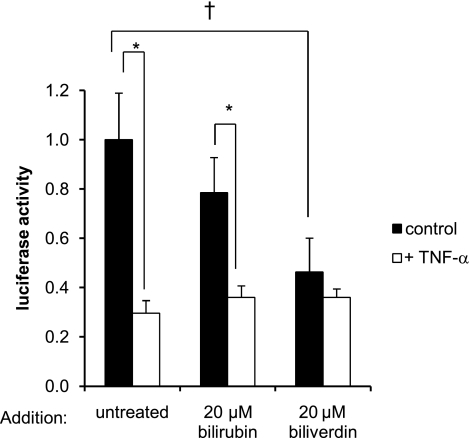
Biliverdin is known to modulate the activity of NF-κB (12–13). Here, we examine the effect of biliverdin on the activity of the hBVR promoter. For these experiments, the hBVR-306 and hBVR-836 constructs were used, and similar results were obtained in both cases. Cells transfected with the luciferase reporters were synchronized in low serum, treated with either 20 μM biliverdin or 20 μM bilirubin for 1 h, and subsequently with TNF-α or vehicle for 12 h. Both biliverdin and bilirubin treatments result in lower reporter expression in the absence of TNF-α (Fig. 6). For biliverdin, this inhibition is significant (P<0.05). The addition of TNF-α to the control or bilirubin-treated cells results in reduced reporter expression (P<0.01), whereas no further inhibition of expression was observed in the cells treated with biliverdin. It is possible that the insignificant inhibition seen with bilirubin might be due to a low availability in the cells, since it is probable that this compound is rapidly excreted after uptake, preventing its accumulation. Both biliverdin and bilirubin associate with the aryl hydrocarbon receptor (AhR) (62), which, in turn, interacts with the aryl hydrocarbon receptor nuclear translocator (ARNT). ARNT is also a subunit of the HIF-1 transcription factor, and we thus explored the role of HIF-1 in regulation of hBVR expression.
Figure 6.
Biliverdin suppresses BVR-promoter activity. Cells in 24-well plates were cotransfected with the hBVR-306 promoter construct and pCMV-βgal. Cells were synchronized in growth medium containing 0.5% FBS, treated first with 20 μM bilirubin or 20 μM biliverdin for 1 h and then with TNF-α for a further 12 h. Luciferase activity was measured and normalized against β-gal activity, as in Fig. 2. †P < 0.05 vs. untreated control; *P < 0.01 vs. no TNF-α treatment.
Hypoxia activates hBVR
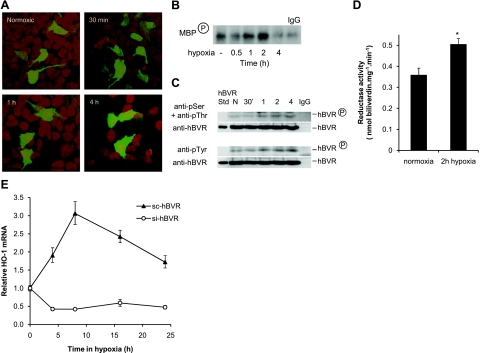
We first addressed whether hypoxia per se affects the activity of hBVR. It is apparent that BVR, under conditions of hypoxia-reperfusion in the kidney, mediates many of the cellular responses in the affected tissue (46,47). We examined by confocal microscopy the subcellular distribution of hBVR during hypoxia. In normoxic cells, hBVR is located primarily in the cytoplasm, with some protein also being seen in the nucleus (Fig. 7A). After transfer to 1% O2, the hBVR gradually accumulates in the nucleus. By 30 to 60 min, a modest increase is seen, whereas at 4 h, the nuclei are seen to have increased levels of hBVR. This parallels an earlier finding that BVR tends to accumulate in the nuclei of kidney cells in rats treated with nephrotoxins such as lipopolysaccharide or bromobenzene (44). Translocation of the protein within the cell is one aspect of altered activity of hBVR, and we therefore examined whether enzyme activity of the protein is also affected by hypoxia. Cells transfected with pcDNA-HA-hBVR were starved overnight in medium with 0.5% FBS and then transferred to 1% O2. Cells were lysed at intervals after transfer, while still in the hypoxic chamber. In one series of experiments, hBVR was immunoprecipitated from the lysate with anti-HA antibody, after which it was assayed for kinase activity (4), using MBP as a substrate. As shown in Fig. 7B, there is an increase in kinase activity during the early stages of hypoxia, peaking at ∼2 h. It was previously demonstrated (25) that the activity of hBVR is dependent on phosphorylation. Accordingly, in a second series of experiments, immunoprecipitates were examined by Western blot analysis, using antibodies against phosphoserine, phosphothreonine, or phosphotyrosine. An increase in phosphorylation signal is seen with either anti-phosphotyrosine or a mixture of anti-phosphoserine and anti-phosphothreonine antibodies (Fig. 7C). Thus, there is a correlation of relocation of hBVR in the cell with increased phosphorylation of the protein and increased kinase activity in response to hypoxia. Moreover, when untransfected cells are exposed to 1% O2, the reductase activity in whole-cell lysates is also observed to be significantly elevated after 2 h (Fig. 7D). Taken together, these data are indicative of increased activity of all functions of hBVR during the early stages of the hypoxic response.
Figure 7.
Activation of the hBVR promoter by HIF-1. A) Confocal microscopy of hypoxic cells. HEK293A cells grown in chamber slides were synchronized by serum starvation for 24 h, transfected with pEGFP-hBVR, and transferred to 1% O2 16 h after DNA addition. Cells were fixed with 4% paraformaldehyde prior to removal from the hypoxic chamber, at the times indicated. B) Protein kinase activity of hBVR is enhanced in hypoxia. Cells were transfected with pcDNA-HA-BVR and transferred after 18 h to 1% O2 for the indicated times. Cells were harvested and lysed, and HA-BVR was immunoprecipitated with anti-HA antibodies. Immunoprecipitate was assayed for hBVR kinase activity, using MBP as substrate and 32P-ATP. Reaction products were resolved by gel electrophoresis, blotted to nitrocellulose, and detected by autoradiography. C) Hypoxia increases hBVR phosphorylation. Lysates prepared from cells transfected and exposed to hypoxia as in B were immunoprecipitated with anti-HA. The immunoprecipitates were resolved by gel electrophoresis and transferred to nitrocellulose. One blot was probed sequentially with a mixture of antibodies to phosphoserine and phosphothreonine, then with anti-hBVR. A second blot was probed first with anti-phosphotyrosine followed by anti-hBVR. D) Hypoxia increases hBVR reductase activity. Cells were grown to 70% confluence, synchronized with 0.5% FBS, and incubated in 1% O2 for 2 h. Cells were lysed while hypoxic, and reductase activity was measured as described in Materials and Methods. *P < 0.01. E) hBVR is required for hypoxic induction of HO-1 expression. HEK293A cells, at ∼80% confluency, were treated with viruses expressing either si-hBVR or a randomized version of the si-sequence (sc-BVR). At 9 h after initiating treatment, cells were again treated with the viruses and transferred to 1% O2 for the times indicated. Further addition of virus was made at 4 and 8 h of hypoxia. RNA was prepared from the cells, and HO-1 mRNA was determined by PCR, as in Fig. 5A.
Relocation of hBVR to the nucleus is consistent with the previous observations that the protein can bind to specific sites within promoters, such as that of HO-1, and activate transcription (22, 37). Moreover, prevention of hBVR nuclear relocation adversely affected induction of HO-1 transcription (39). Since treatment of cells with viruses expressing si-hBVR effectively attenuates induction of HO-1 by arsenite (23), the role of hBVR in induction of HO-1 expression during hypoxia was examined. Hypoxic growth of cells treated with an inactive form of the si-hBVR [sc-hBVR (5)] causes a modest induction of HO-1, as detected by RT-PCR within 8 h of hypoxic treatment, after which the mRNA level begins to decay (Fig. 7E). The HO-1 induction is effectively blocked when si-hBVR was used, indicating the need for hBVR in HO-1 induction.
An HRE is active in regulating hBVR
Four HREs were noted during computer analysis of the promoter sequence (Fig. 1B, C). To test whether these might be functional, the activity of two promoter constructs (BVR-836 and BVR-306) was assessed in the presence of overexpressed HIF-1. Both reporter constructs are stimulated 4- to 5-fold by overexpressing HIF-1 (data not shown). Because there is no difference in the relative responses of the BVR-836 and BVR-306 promoter fragments to HIF-1, it can be argued that the more distal elements, located at approximately nt −530 and −310, are unlikely to be functional. However, elements at nt −190 and −110 could serve as candidate binding sites.
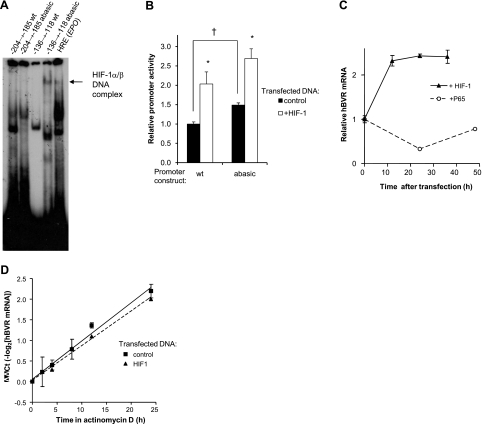
Double-stranded oligonucleotides encompassing two of the candidate HREs, located at nt −204 to −185 and −136 to −118, were labeled with 32P and tested for HIF-1 binding in an electrophoretic mobility shift assay. Cells were transfected with plasmids expressing both subunits of HIF-1 (54) and then incubated in 1% O2 for 24 h. There is no evidence of specific binding to the nt −204 to −185 sequence (Fig. 8A). On the other hand, while minimal binding to the normal nt −136 to −118 fragment is observed, a dramatic increase is seen if an abasic site is included in place of G131 in the complementary strand (Fig. 8A). Because HIF-1 responsive elements are prone to oxidative damage to DNA (63), ultimately leading to formation of an abasic site in one strand, oligonucleotides containing abasic sites were expected to show significantly greater protein binding. Therefore, it is likely that the HRE located closest to the transcription start site is the one responsible for increased activity of the hBVR promoter in response to overexpression of HIF-1. Repeated attempts to observe supershifting with antibodies to either HIF-1α or ARNT1 were unsuccessful (Fig. 8A). This was also true of the control HRE and seems to be a consequence of a problem with the antibodies (although two batches, from different suppliers, were tested).
Figure 8.
Hypoxia activates hBVR transcription. A) Electrophoretic mobility shift assay. HEK293A cells were transfected with HIF-1α and ARNT expression plasmids; at 24 h after transfection, cells were transferred to 1% O2 for 24 h. The cells were lysed in the hypoxic chamber, and the lysates were immediately frozen in dry ice-ethanol. Aliquots of the lysate were assayed for binding to labeled oligonucleotides (located between nts −204 and −185, and −136 and −118), each containing a candidate HIF-binding site. An abasic site was introduced into the latter sequence by replacing G−131 in the complementary strand with dU, and treating the duplex with uracil glycosylase. Resolution and detection of binding products was as in Fig. 5A. Arrow marks a band observed only in hypoxia extracts. B) Abasic sites in the hBVR promoter enhance its activity. A 107-bp segment of hBVR promoter (nt −215 to −109), including the two most start-site proximal HREs, was assembled from 4 oligonucleotides and cloned into the pGL3-promoter plasmid (Materials and Methods). Two versions of the plasmid were built, a wild-type, dG-containing form, and one with dU substitutions in the putative HRE elements. Cells were cotransfected with the assembled construct, together with the pCMV-βgal plasmid and either empty pcDNA3 vector or the pcDNA3-HIFα and pcDNA3-ARNT plasmids. At 18 h after transfection, cells were lysed and assayed for luciferase and β-galactosidase activities. *P<0.01 vs. control; †P<0.001. C) Overexpression of HIF-1 increases hBVR mRNA. Cells were transfected with both pcDNA3-HIFα and pcDNA3-ARNT, and samples were taken at 12-h intervals for RNA isolation. cDNA was prepared, and the hBVR mRNA level was measured by quantitative PCR, normalized on 18S rRNA. Similarly, cells transfected with NF-κB P65 were assayed for hBVR mRNA expression. D) HIF-1 overexpression does not affect hBVR mRNA stability. Cells transfected with pcDNA3-HIFα and pcDNA3-ARNT or with empty vector were treated with 2.5 μg/ml actinomycin D, harvested at the indicated times, and hBVR mRNA was measured by quantitative PCR, as in C. mRNA half-life was calculated as the reciprocal of the slope of the linear regression.
The question of whether an enhanced activity of the promoter would be seen in cells was addressed by constructing plasmids with a synthetic 107-nt sequence, including two of the HRE elements in the same sequence context seen in human genomic DNA, in either the wild-type dG or dU configurations, inserted upstream of a luciferase reporter driven by a minimal promoter. In one plasmid, the wild-type sequence was used, while the other contained a G→U substitution in HRE at the sites used for the gel-shift experiments. These plasmids were cotransfected into cells with either plasmids expressing both subunits of HIF-1 or with empty vector. The cells were harvested 18 h after DNA addition and assayed for expression of luciferase. As shown in Fig. 8B, both constructs display higher expression of luciferase in the presence of overexpressed HIF-1 (P<0.01). In addition, the construct containing dU in each HRE shows higher expression of luciferase (P<0.001) in control cells. This suggests that the hBVR promoter activity can be enhanced by damage to the promoter, for example by ROS. While oxidative damage results in different lesions in the DNA (the dU used in this experiment is somewhat artificial), base excision repair will create an abasic site whether it is oxoguanine or uracil that is excised. It is noted that there is expression of the wild-type promoter sequence in this experiment, in the presence of overexpressed HIF-1.
We further examined whether prolonged overexpression of HIF-1 results in an increased level of mRNA. RNA was harvested from cells at intervals after cotransfection with HIF-1α and ARNT plasmids, and the level of hBVR mRNA was measured by quantitative RT-PCR. The result of a typical experiment is shown in Fig. 8C, where the level of hBVR mRNA is seen to increase in the first 12 h and remains elevated thereafter. The increase in mRNA level is likely due to increased promoter activity. A similar observation, albeit with a more pronounced mRNA induction, has been made in human vascular endothelial cells exposed to hypoxia (A. Jozkowicz and J. Dulak, Jagiellonian University, Krakow, Poland; personal communication). Normal HEK293A cells or those overexpressing HIF-1 were treated with actinomycin D to halt RNA synthesis, and RNA was prepared at intervals after addition. The level of hBVR mRNA was determined by RT-PCR. If, as expected, the decay of hBVR mRNA were to display first-order kinetics, then this would result in a linear relationship between ΔΔCT and time. Moreover, the reciprocal of the slope will yield the mRNA half-life. The result of these experiments is shown in Fig. 8D: the two sets of cells show very similar decay kinetics. The slopes of the two lines (0.093±0.006 for control, 0.084±0.005 for HIF-1) do not differ significantly, indicating that overexpression of HIF-1 does not affect the stability of the hBVR mRNA. The data yield a half-life of the mRNA of 11–12 h, which is indicative of a stable mRNA. Furthermore, these data suggest that the alterations in promoter activity reported here would be expected to require prolonged response times before they were manifested as alterations in either mRNA or protein.
DISCUSSION
The robust expression of hBVR in tissues that are not associated with heme metabolism, such as neurons, coupled with the unique activity profile of the protein, culminated in uncovering the multitude of its functions in signal transduction pathways, such as being an activator/enhancer of kinases and stress-inducible genes, including HO-1, downstream of insulin/IGF-I receptor (2,3). Although substantial information has been gathered about stimuli of hBVR kinase activity, prior to this report, information with regard to hBVR regulation at the promoter level was not available. In this report, we have characterized regulation of hBVR promoter activity by cytokines and hypoxia and offer a potential link between these stimuli and NF-κB.
The presently obtained structural organization of the human BVR promoter was compared with our previously obtained data for the rat gene (40) and sequence database information for the mouse BVR promoter, as well as the gene structures, were compared. At both levels, the organizations differ between humans and rats. Specifically, we found that the rat gene lacks a 5′ untranslated exon and that the composition of the regulatory elements differs as well. In contrast, the mouse and human genes share the same structural features, and in the promoter, the sequences of consensus regulatory elements are more similar. Similarity between human and mouse has also been detected for the HO-1 gene that also share similar structures in both gene and promoter/enhancer regions. In both species, the enhancer elements are located ∼4 and 10 kb upstream of the transcription start site (64,65). Unlike HO-1, in both and human and mouse, the hBVR promoter is located entirely proximal to exon 1. However, while the human BVR promoter region is GC rich and appears to be the typical CpG island commonly found in mammalian promoters, the extent of this GC-enriched sequence is nearly 2-fold greater in the human genome than in that of mouse. Computational analysis of the human BVR promoter revealed the presence of 2 NF-κB and 4 HIF-1 regulatory elements, among others (Fig. 1C).
We used the approach of nested deletions within the human BVR promoter sequences to discern the presence of enhancer and silencer sequences and found the presence of positive and negative regulatory elements. Using a luciferase reporter assay, we found that the shortest element tested, at 105 nt, displayed a low level of promoter activity. The next longest sequence, 306 nt, contained elements that decidedly activated transcription, as this sequence was routinely 3- to 4-fold more active in expression of the reporter gene (Fig. 2B). However, the next promoter fragment (432 nt) was clearly less active than the 306-nt sequence; this indicated the presence between −432 and −306 of a sequence element that inhibited promoter activity. We mapped the most likely inhibitor element to the region between nt −425 and −350, because deletion of this sequence from a longer 836-bp promoter resulted in increased activity (Fig. 2C). A second silencer element was found to be located even more distal to the start site—the −696 and −601 constructs both showed similar activity to the −432 promoter, whereas the −836 nt construct was less active and indeed was the same as the minimal 105-nt promoter (Fig. 2B). Inclusion of further genomic sequence upstream of the −836 species did not result in any further change in activity (Fig. 2C). However, much of the additional sequence consists of long (LINE) and short (SINE) interspersed repetitive sequences found at high copy numbers in the human genome.
The findings presented in this study are interpreted as an indication of hBVR regulation being a balance between the activation by hypoxia and inhibition by NF-κB Furthermore, there is a reciprocal interaction between NF-κB and hBVR regulation; prolonged activation of NF-κB reduced expression of hBVR, while as previously shown, overexpression of hBVR activated NF-κB (13). Because hBVR promoter activity is inhibited by TNF-α, the activator of NF-κB, we predict that during prolonged NF-κB activation, the down-regulated BVR promoter reduces hBVR protein synthesis and a reduced ability to convert the NF-κB inhibitory biliverdin to ineffective bilirubin and hence suppression of NF-κB activity. The enhanced binding of NF-κB to the promoter is detectable within 15 min after TNF-α treatment (Fig. 4B). The noted down-regulation and up-regulation, respectively, in activity of the hBVR promoter on overexpression of P65 or IκB (Fig. 3B), are consistent with this proposed regulatory feedback mechanism. It is curious that NF-κB generally functions as an activator of transcription, whereas in the promoter for the unconventional enzyme hBVR, it is inhibitory.
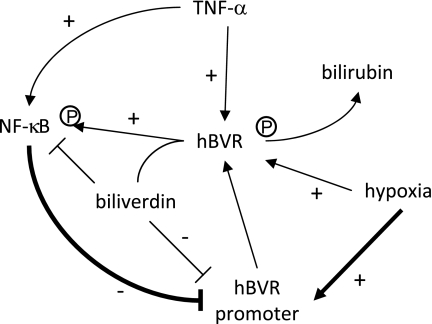
The observed response of the promoter to TNF-α, NF-κB, and hypoxia (Figs. 3 and 8) suggested the presence of a regulatory loop between hypoxia and NF-κB that is linked by hBVR and biliverdin. The depiction of this loop that is supported by past observations and the present findings is schematically presented in Fig. 9. In this scheme, NF-κB is depicted as the inhibitor of BVR gene expression, whereas hypoxia activates the promoter. These two stimuli are linked via hBVR, with biliverdin being an inhibitor of NF-κB and of the hBVR promoter. By the reasoning offered above, in this scheme the cytokine is the ultimate regulator of hBVR and NF-κB interaction. The past observations that biliverdin inhibited NF-κB (12,13), while increased expression of hBVR activated NF-κB (13) are supportive of the proposed scheme. Because in all previous studies, activation of kinases has been observed as the consequence of binding to hBVR (5, 29,30), the yeast 2-hybrid screen approach that predicted an interaction between hBVR and IκB kinase leads us to propose that hBVR binding to IκB kinase causes enhanced phosphorylation of IκB and its release from the nuclear factor. This is supported by the activation of the hBVR promoter by the overexpression of IκB. It follows, if this were the case then it is a plausible mechanism for hBVR activation of the NF-κB pathway. hBVR binding to the P65 subunit of NF-κB (13), which is the regulatory subunit of the complex, represents an additional component of activation.
Figure 9.
Proposed feedback loop for regulation of hBVR by TNF-α/NF-κB and hypoxia. BVR catalyzes conversion of biliverdin to bilirubin, thereby removing the molecule that inhibits (−) the activities of both NK-κB and the hBVR promoter. Activation (+) of the reductase and kinase activities of hBVR is also observed in cells treated with TNF-α or grown under hypoxic conditions. TNF-α treatment activates NF-κB, which in turn inhibits hBVR promoter activity, whereas hypoxia exerts the diametrically opposite effect.
The effects of hypoxia on hBVR and its promoter are diametrically opposite to those of NF-κB. Hypoxia leads to stimulation of hBVR phosphorylation, which, predictably (25), results in activation of hBVR enzyme activities, paralleled by a relocation of hBVR to the nucleus. There is also an activation of hBVR promoter function, leading eventually to increased hBVR mRNA, and hence, increased production of this cytoprotective protein. In contrast to the inhibitory action of NF-κB, the other transcription factor, HIF-1 (HIF-1α/β), binds to a candidate HRE in the hBVR promoter (Fig. 8A), and activates it. Binding in vitro was dependent on the presence of an abasic site in the oligonucleotide, corresponding to the terminal guanosine residue in the HRE (63, 66), but the presence of an abasic site was less critical for promoter activity in the cell, where the abasic site enhanced the activity. An abasic site arises in vivo as a consequence of oxidative damage to guanine bases, and their subsequent removal from the DNA by cellular glycosylases. The difference between the in vitro and in vivo assays suggests that there are additional functional promoter elements and/or activated cellular factors that were missing in the gel-shift assay. The construct used in these experiments retained the sequence context for two HREs seen in the genome. Introduction of an abasic site into the other potential HREs observed in the promoter did not lead to binding of HIF-1. A candidate element for the aryl hydrocarbon receptor (AhR) is located within 10 bp of the HRE (Fig. 1C). Because binding of both HIF-1 and AhR depend on heterodimerization with ARNT for binding to DNA, and because HIF-1 can also bind to the AhR site, cooperative binding of HIF-1 transcription factors to the two adjacent sequences could efficiently activate transcription. Binding and stability of AhR and HIF-1α are also mediated by the coactivator chaperonin HSP-90 (67,68), which might be limiting in the extracts used in vitro.
The observed increased binding of HIF-1 to the abasic site suggests that DNA damage that occurs in the course of exposure to oxidative stress is a means of augmenting BVR expression, resulting in potentiation of induction of HO-1 and the resulting increased production of bilirubin. The promoter of HO-1 has HRE elements (69), but it also has sites within the enhancer regions that respond to c-Fos/c-Jun (32, 64,65), both of which are activated by hBVR (22,23). It is notable that treatment with si-hBVR to reduce the cellular concentration prevents the induction of HO-1 by hypoxia (Fig. 7E). The antioxidant bilirubin is a particularly potent free-radical scavenger. It is as effective as glutathione in protecting from oxidative damage (24). In the course of exposure to oxidative stress, microsomal hemoproteins are denatured. Activation of the BVR/HO-1 axis would present a key factor in the battery of cellular defense mechanisms. Free heme is the most potent catalyst for oxygen radical formation (7).
We also note that hBVR relocates to the nucleus during the early stages of hypoxia (Fig. 7A). Nuclear translocation of hBVR correlates with its regulation of gene expression; specifically, in cells transfected with hBVR carrying a mutation in the NLS, which disables nuclear import, induction of HO-1 by heme was prevented (39). In the nucleus, translocated hBVR binds to chromatin, which is linked to changes in chromatin structure and to regulation of gene expression (39). A similar translocation of BVR into the nucleus was seen in the kidneys of rats on treatment with LPS and the oxidant bromobenzene (44). It is plausible that hBVR accumulation in the hypoxic cell nucleus may also relate in part, to its role as an antioxidant. This concept is in agreement with the reported nuclear localization of HO-1 (70) and under oxidative stress, heme associated with denatured hemoproteins becomes available. Notably, the promoters of HIF-responsive genes are subject to oxidative damage (63, 66); it is not unreasonable to suggest that the elevated antioxidant potential of the nucleus serves to limit oxidative damage and thereby attenuate the activation of the promoters. It is plausible to suggest a regulatory loop between hBVR and HIF-1 activity. The latter activity is dependent on prolyl hydroxylases, which modify two specific residues, P402 and P564(71), and the activity of the hydroxylases is regulated by oxygen tension (72). The presence of hydroxyproline residues in HIF-1α enhances its binding to the von Hippel-Lindau factor (73,74), which ubiquitinates HIF-1α and targets the protein for proteasomal degradation. The mechanistic differences in response of NF-κB and HIF-1 to altered O2 are thus likely to be critical in the different responses of the hBVR promoter.
References
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956–3962. [PubMed] [Google Scholar]
- Maines M. D. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–389. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Maines M. D. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Lerner-Marmarosh N., Shen J., Torno M. D., Kravets A., Hu Z., Maines M. D. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proc Natl Acad Sci U S A. 2005;102:7109–7114. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. Regulation of TNF-α-activated PKC-ζ signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB J. 2007;21:3949–3962. doi: 10.1096/fj.07-8544com. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- Aust S. D., Svingen B. A. The role of iron in enzymatic lipid peroxidation. Pryor W. A., editor. Academic Press; New York: Free Radicals in Biology. 1982;vol. 5:1–28. [Google Scholar]
- Immenschuh S., Schroder H. Heme oxygenase-1 and cardiovascular disease. Histol Histopathol. 2006;21:679–685. doi: 10.14670/HH-21.679. [DOI] [PubMed] [Google Scholar]
- Abraham N. G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Gozzelino R., Jeney V., Soares M. P. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- Tongers J., Fiedler B., Konig D., Kempf T., Klein G., Heineke J., Kraft T., Gambaryan S., Lohmann S. M., Drexler H., Wollert K. C. Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovasc Res. 2004;63:545–552. doi: 10.1016/j.cardiores.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Nakao A., Neto J. S., Kanno S., Stolz D. B., Kimizuka K., Liu F., Bach F. H., Billiar T. R., Choi A. M., Otterbein L. E., Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E., Maines M. D. Biliverdin inhibits activation of NF-κB: reversal of inhibition by human biliverdin reductase. Int J Cancer. 2007;121:2567–2574. doi: 10.1002/ijc.22978. [DOI] [PubMed] [Google Scholar]
- Lehmann E., El-Tantawy W. H., Ocker M., Bartenschlager R., Lohmann V., Hashemolhosseini S., Tiegs G., Sass G. The heme oxygenase 1 product biliverdin interferes with hepatitis C virus replication by increasing antiviral interferon response. Hepatology. 2010;51:398–404. doi: 10.1002/hep.23339. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F. Turning green to gold. Nat Struct Biol. 2001;8:198–200. doi: 10.1038/84915. [DOI] [PubMed] [Google Scholar]
- Baranano D. E., Rao M., Ferris C. D., Snyder S. H. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66:773–779. doi: 10.1124/mol.104.002832. [DOI] [PubMed] [Google Scholar]
- Mancuso C., Bonsignore A., Capone C., Di Stasio E., Pani G. Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid Redox Signal. 2006;8:487–494. doi: 10.1089/ars.2006.8.487. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Morse D., Choi A. M. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzal G. J., Leck M. C., Collinson E., Li C., Stocker R. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J Biol Chem. 2009;284:29251–29259. doi: 10.1074/jbc.M109.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravets A., Hu Z., Miralem T., Torno M. D., Maines M. D. Biliverdin reductase: A novel regulator for induction of activating transcription factor-2 and heme oxygenase-1. J Biol Chem. 2004;279:19916–19923. doi: 10.1074/jbc.M314251200. [DOI] [PubMed] [Google Scholar]
- Miralem T., Hu Z., Torno M. D., Lelli K. M., Maines M. D. Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J Biol Chem. 2005;280:17084–17092. doi: 10.1074/jbc.M413121200. [DOI] [PubMed] [Google Scholar]
- Sedlak T. W., Saleh M., Higginson D. S., Paul B. D., Juluri K. R., Snyder S. H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim M., Brown-Kipphut B. A., Maines M. D. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem. 2001;276:10929–10934. doi: 10.1074/jbc.M010753200. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Park S. Y., Miyatake H., Sun D., Sato M., Yoshida T., Shiro Y. Crystal structure of rat biliverdin reductase. Nat Struct Biol. 2001;8:221–225. doi: 10.1038/84955. [DOI] [PubMed] [Google Scholar]
- Whitby F. G., Phillips J. D., Hill C. P., McCoubrey W., Maines M. D. Crystal structure of a biliverdin IXalpha reductase enzyme-cofactor complex. J Mol Biol. 2002;319:1199–1210. doi: 10.1016/S0022-2836(02)00383-2. [DOI] [PubMed] [Google Scholar]
- Florczyk U. M., Jozkowicz A., Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Miralem T., Lerner-Marmarosh N., Shen J., Gibbs P. E. Human biliverdin reductase, a previously unknown activator of protein kinase C βII. J Biol Chem. 2007;282:8110–8122. doi: 10.1074/jbc.M513427200. [DOI] [PubMed] [Google Scholar]
- Lerner-Marmarosh N., Miralem T., Gibbs P. E., Maines M. D. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc Natl Acad Sci U S A. 2008;105:6870–6875. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992;17:418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- Takeda K., Ishizawa S., Sato M., Yoshida T., Shibahara S. Identification of a cis-acting element that is responsible for cadmium-mediated induction of the human heme oxygenase gene. J Biol Chem. 1994;269:22858–22867. [PubMed] [Google Scholar]
- Lee P. J., Camhi S. L., Chin B. Y., Alam J., Choi A. M. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L175–L182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- Gong P., Stewart D., Hu B., Vinson C., Alam J. Multiple basic-leucine zipper proteins regulate induction of the mouse heme oxygenase-1 gene by arsenite. Arch Biochem Biophys. 2002;405:265–274. doi: 10.1016/s0003-9861(02)00404-6. [DOI] [PubMed] [Google Scholar]
- Lee P. J., Choi A. M. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35:341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- Wright M. M., Kim J., Hock T. D., Leitinger N., Freeman B. A., Agarwal A. Human heme oxygenase-1 induction by nitro-linoleic acid is mediated by cAMP, AP-1 and E-box response element interactions. Biochem J. 2009;422:353–361. doi: 10.1042/BJ20090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z., Salim M., Maines M. D. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J Biol Chem. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- Wegiel B., Baty C. J., Gallo D., Csizmadia E., Scott J. R., Akhavan A., Chin B. Y., Kaczmarek E., Alam J., Bach F. H., Zuckerbraun B. S., Otterbein L. E. Cell surface biliverdin reductase mediates biliverdin-induced anti-inflammatory effects via phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2009;284:21369–21378. doi: 10.1074/jbc.M109.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor C., Lerner-Marmarosh N., Engelborghs Y., Gibbs P. E., Maines M. D. Biliverdin reductase is a transporter of heme into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem J. 2008;413:405–416. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoubrey W. K., Jr, Cooklis M. A., Maines M. D. The structure, organization and differential expression of the rat gene encoding biliverdin reductase. Gene. 1995;160:235–240. doi: 10.1016/0378-1119(95)00112-j. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Maines M. D. Immunohistochemical localization of biliverdin reductase in rat brain: age-related expression of protein and transcript. Brain Res. 1995;672:29–41. doi: 10.1016/0006-8993(94)01290-x. [DOI] [PubMed] [Google Scholar]
- Ewing J. F., Weber C. M., Maines M. D. Biliverdin reductase is heat resistant and coexpressed with constitutive and heat shock forms of heme oxygenase in brain. J Neurochem. 1993;61:1015–1023. doi: 10.1111/j.1471-4159.1993.tb03615.x. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Mayer R. D., Erturk E., Huang T. J., Disantagnese A. The oxidoreductase, biliverdin reductase, is induced in human renal carcinoma—pH and cofactor-specific increase in activity. J Urol. 1999;162:1467–1472. [PubMed] [Google Scholar]
- Maines M. D., Ewing J. F., Huang T. J., Panahian N. Nuclear localization of biliverdin reductase in the rat kidney: response to nephrotoxins that induce heme oxygenase-1. J Pharmacol Exp Ther. 2001;296:1091–1097. [PubMed] [Google Scholar]
- Mercurio F., Manning A. M. NF-κB as a primary regulator of the stress response. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- Pachori A. S., Smith A., McDonald P., Zhang L., Dzau V. J., Melo L. G. Heme-oxygenase-1-induced protection against hypoxia/reoxygenation is dependent on biliverdin reductase and its interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 2007;43:580–592. doi: 10.1016/j.yjmcc.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R., Yao Y., Han M., Zhao X., Liu X. C., Wei J., Luo Y., Zhang J., Zhou J., Wang S., Ma D., Xu G. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol. 2008;19:380–387. doi: 10.1681/ASN.2006111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Pomerantz J. L., Baltimore D. Two pathways to NF-κB. Mol Cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Weinstock G. M., Metzker M. L., Muzny D. M., Sodergren E. J., Scherer S., Scott G., Steffen D., Worley K. C., Burch P. E., Okwuonu G., Hines S., Lewis L., DeRamo C., Delgado O., Dugan-Rocha S., Miner G., Morgan M., Hawes A., Gill R., Celera, Holt R. A., Adams M. D., Amanatides P. G., Baden-Tillson H., Barnstead M., Chin S., Evans C. A., Ferriera S., Fosler C., Glodek A., Gu Z., Jennings D., Kraft C. L., Nguyen T., Pfannkoch C. M., Sitter C., Sutton G. G., Venter J. C., Woodage T., Smith D., Lee H. M., Gustafson E., Cahill P., Kana A., Doucette-Stamm L., Weinstock K., Fechtel K., Weiss R. B., Dunn D. M., Green E. D., Blakesley R. W., Bouffard G. G., De Jong P. J., Osoegawa K., Zhu B., Marra M., Schein J., Bosdet I., Fjell C., Jones S., Krzywinski M., Mathewson C., Siddiqui A., Wye N., McPherson J., Zhao S., Fraser C. M., Shetty J., Shatsman S., Geer K., Chen Y., Abramzon S., Nierman W. C., Havlak P. H., Chen R., Durbin K. J., Egan A., Ren Y., Song X. Z., Li B., Liu Y., Qin X., Cawley S., Cooney A. J., D'Souza L. M., Martin K., Wu J. Q., Gonzalez-Garay M. L., Jackson A. R., Kalafus K. J., McLeod M. P., Milosavljevic A., Virk D., Volkov A., Wheeler D. A., Zhang Z., Bailey J. A., Eichler E. E., Tuzun E., Birney E., Mongin E., Ureta-Vidal A., Woodwark C., Zdobnov E., Bork P., Suyama M., Torrents D., Alexandersson M., Trask B. J., Young J. M., Huang H., Wang H., Xing H., Daniels S., Gietzen D., Schmidt J., Stevens K., Vitt U., Wingrove J., Camara F., Mar Alba M., Abril J. F., Guigo R., Smit A., Dubchak I., Rubin E. M., Couronne O., Poliakov A., Hubner N., Ganten D., Goesele C., Hummel O., Kreitler T., Lee Y. A., Monti J., Schulz H., Zimdahl H., Himmelbauer H., Lehrach H., Jacob H. J., Bromberg S., Gullings-Handley J., Jensen-Seaman M. I., Kwitek A. E., Lazar J., Pasko D., Tonellato P. J., Twigger S., Ponting C. P., Duarte J. M., Rice S., Goodstadt L., Beatson S. A., Emes R. D., Winter E. E., Webber C., Brandt P., Nyakatura G., Adetobi M., Chiaromonte F., Elnitski L., Eswara P., Hardison R. C., Hou M., Kolbe D., Makova K., Miller W., Nekrutenko A., Riemer C., Schwartz S., Taylor J., Yang S., Zhang Y., Lindpaintner K., Andrews T. D., Caccamo M., Clamp M., Clarke L., Curwen V., Durbin R., Eyras E., Searle S. M., Cooper G. M., Batzoglou S., Brudno M., Sidow A., Stone E. A., Payseur B. A., Bourque G., Lopez-Otin C., Puente X. S., Chakrabarti K., Chatterji S., Dewey C., Pachter L., Bray N., Yap V. B., Caspi A., Tesler G., Pevzner P. A., Haussler D., Roskin K. M., Baertsch R., Clawson H., Furey T. S., Hinrichs A. S., Karolchik D., Kent W. J., Rosenbloom K. R., Trumbower H., Weirauch M., Cooper D. N., Stenson P. D., Ma B., Brent M., Arumugam M., Shteynberg D., Copley R. R., Taylor M. S., Riethman H., Mudunuri U., Peterson J., Guyer M., Felsenfeld A., Old S., Mockrin S., Collins F. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Nakao A., Otterbein L., Overhaus M., Sarady J., Tsung A., Kimizuka K., Nalesnik M., Kaizu T., Uchiyama T., Liu F., Murase N., Bauer A., Bach F. H. Biliverdin protects the functional integrity of a transplanted syngeneic small bowel. Gastroenterology. 2004;127:595–606. doi: 10.1053/j.gastro.2004.05.059. [DOI] [PubMed] [Google Scholar]
- Ozgenc A. I., Szekeres E. S., Lawrence C. W. In vivo evidence for a recA-independent recombination process in Escherichia coli that permits completion of replication of DNA containing UV damage in both strands. J Bacteriol. 2005;187:1974–1984. doi: 10.1128/JB.187.6.1974-1984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Polevoda B. V., Huang T. J., McCoubrey W. K., Jr Human biliverdin IXalpha reductase is a zinc-metalloprotein Characterization of purified and Escherichia coli expressed enzymes. Eur J Biochem. 1996;235:372–381. doi: 10.1111/j.1432-1033.1996.00372.x. [DOI] [PubMed] [Google Scholar]
- Michel G., Minet E., Mottet D., Remacle J., Michiels C. Site-directed mutagenesis studies of the hypoxia-inducible factor-1α DNA-binding domain. Biochim Biophys Acta. 2002;1578:73–83. doi: 10.1016/s0167-4781(02)00484-0. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A., Nigisch A., Wegrzyn J., Weigel G., Huk I., Dulak J. Opposite effects of prostaglandin-J2 on VEGF in normoxia and hypoxia: role of HIF-1. Biochem Biophys Res Commun. 2004;314:31–38. doi: 10.1016/j.bbrc.2003.12.059. [DOI] [PubMed] [Google Scholar]
- Huang T. J., Trakshel G. M., Maines M. D. Multiple forms of biliverdin reductase: modification of the pattern of expression in rat liver by bromobenzene. Arch Biochem Biophys. 1989;270:513–520. doi: 10.1016/0003-9861(89)90533-x. [DOI] [PubMed] [Google Scholar]
- Thomas J. W., Green E. D. Comparative sequence analysis of a single-gene conserved segment in mouse and human. Mamm Genome. 2003;14:673–678. doi: 10.1007/s00335-003-2300-1. [DOI] [PubMed] [Google Scholar]
- Sandelin A., Carninci P., Lenhard B., Ponjavic J., Hayashizaki Y., Hume D. A. Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet. 2007;8:424–436. doi: 10.1038/nrg2026. [DOI] [PubMed] [Google Scholar]
- Ovcharenko I., Loots G. G., Hardison R. C., Miller W., Stubbs L. zPicture: dynamic alignment and visualization tool for analyzing conservation profiles. Genome Res. 2004;14:472–477. doi: 10.1101/gr.2129504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots G. G., Ovcharenko I. rVISTA 20: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–W221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralem T., Gibbs P. E., Revert F., Saus J., Maines M. D. Human biliverdin reductase suppresses Goodpasture antigen binding protein (GPBP) kinase activity: the reductase regulates TNF-α- NF-κB-dependent GPBP expression [E-pub ahead of print] J Biol Chem. 2010;285 doi: 10.1074/jbc.M109.032771. doi: 10.1074/jbc.M109.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal C. J., Bend J. R. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- Ziel K. A., Grishko V., Campbell C. C., Breit J. F., Wilson G. L., Gillespie M. N. Oxidants in signal transduction: impact on DNA integrity and gene expression. FASEB J. 2005;19:387–394. doi: 10.1096/fj.04-2805com. [DOI] [PubMed] [Google Scholar]
- Alam J., Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- Alam J., Camhi S., Choi A. M. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem. 1995;270:11977–11984. doi: 10.1074/jbc.270.20.11977. [DOI] [PubMed] [Google Scholar]
- Pastukh V., Ruchko M., Gorodnya O., Wilson G. L., Gillespie M. N. Sequence-specific oxidative base modifications in hypoxia-inducible genes. Free Radic Biol Med. 2007;43:1616–1626. doi: 10.1016/j.freeradbiomed.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Beischlag T. V., Luis Morales J., Hollingshead B. D., Perdew G. H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Li J., Costa M., Gao J., Huang C. JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res. 2010;70:813–823. doi: 10.1158/0008-5472.CAN-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter S. W., Choi A. M. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- Lin Q., Weis S., Yang G., Weng Y. H., Helston R., Rish K., Smith A., Bordner J., Polte T., Gaunitz F., Dennery P. A. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrismann D., Flashman E., Genn D. N., Mathioudakis N., Hewitson K. S., Ratcliffe P. J., Schofield C. J. Studies on the activity of the hypoxia-inducible-factor hydroxylases using an oxygen consumption assay. Biochem J. 2007;401:227–234. doi: 10.1042/BJ20061151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Cockman M. E., Masson N., Mole D. R., Jaakkola P., Chang G. W., Clifford S. C., Maher E. R., Pugh C. W., Ratcliffe P. J., Maxwell P. H. Hypoxia inducible factor-α binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]