Abstract
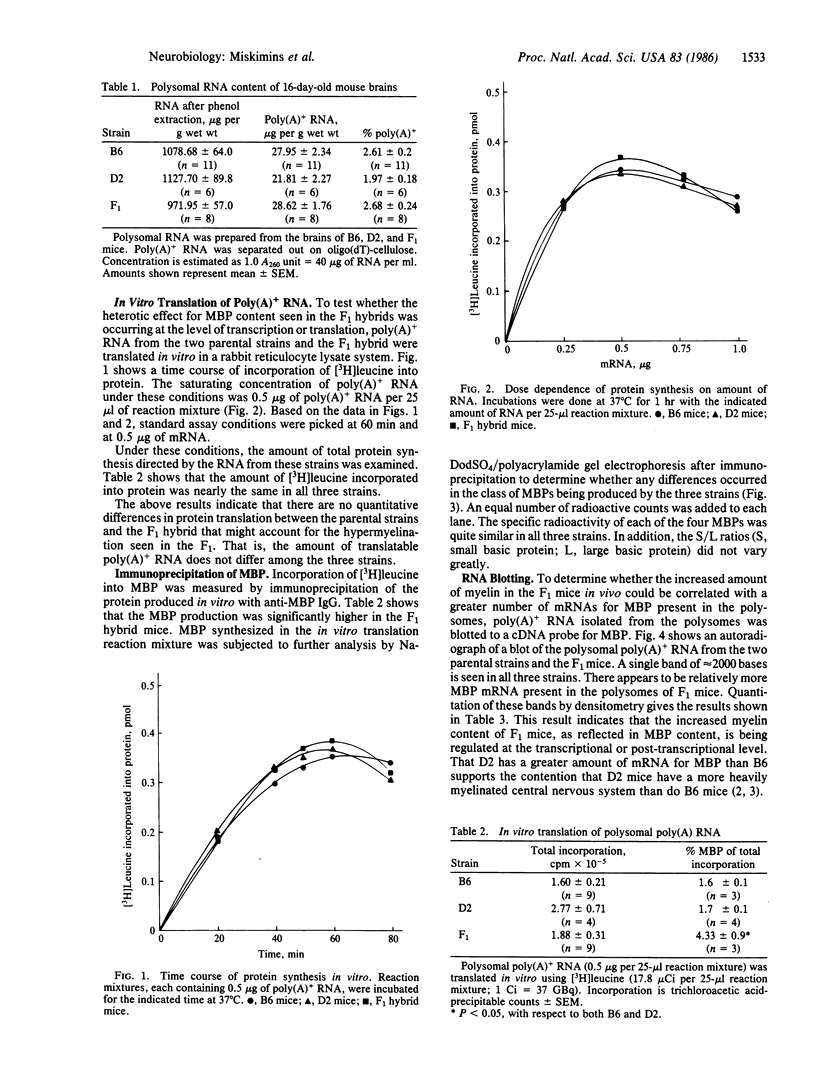
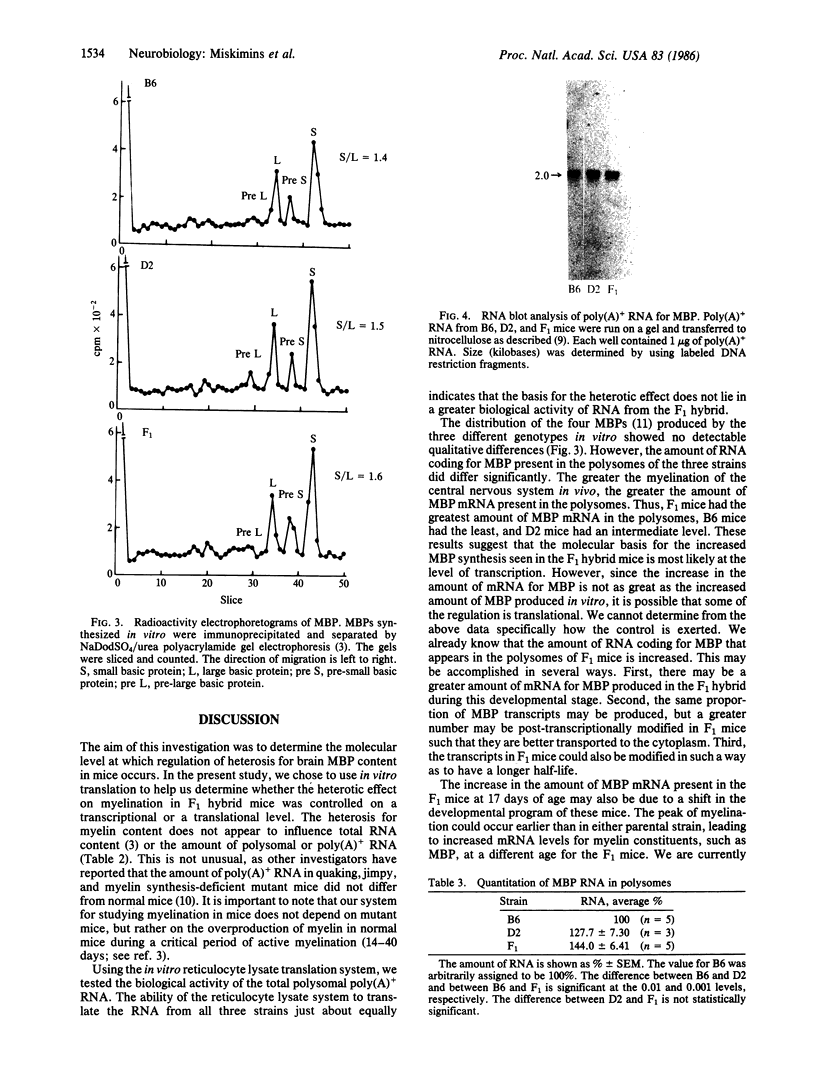
Poly(A)+ mRNA was isolated from the brains of C57BL/6J (B6), DBA/2J (D2), and F1 hybrid mice (B6 X D2) of 16-17 days of age. The yield of polysomal RNA, both poly(A)+ and poly(A)-, from the three strains of mice was comparable. When translated in vitro in a reticulocyte lysate system, the mRNA preparations had the same efficiency with respect to stimulation of amino acid incorporation into protein. However, a significant heterotic effect was seen for the production of myelin basic protein (MBP) by the mRNA from the F1 mice. That is, the fraction of protein synthesized as MBP was greater for the F1 hybrid than for either parental strain. The distribution of the form of MBPs was not different among the three strains. We therefore believe that heterosis for brain MBP content in the F1 hybrid may be regulated at the transcriptional or post-transcriptional level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnow T. B., Carson J. H., Brostoff S. W., Hogan E. L. Myelin basic protein gene expression in quaking, jimpy, and myelin synthesis-deficient mice. Dev Biol. 1984 Nov;106(1):38–44. doi: 10.1016/0012-1606(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Ebato H., Seyfried T. N., Yu R. K. Biochemical study of heterosis for brain myelin content in mice. J Neurochem. 1983 Feb;40(2):440–446. doi: 10.1111/j.1471-4159.1983.tb11302.x. [DOI] [PubMed] [Google Scholar]
- Eylar E. H., Brostoff S., Hashim G., Caccam J., Burnett P. Basic A1 protein of the myelin membrane. The complete amino acid sequence. J Biol Chem. 1971 Sep 25;246(18):5770–5784. [PubMed] [Google Scholar]
- Eylar E. H., Thompson M. Allergic encephalomyelitis: the physico-chemical properities of the basic protein encephalitogen from bovine spinal cord. Arch Biochem Biophys. 1969 Feb;129(2):468–479. doi: 10.1016/0003-9861(69)90204-5. [DOI] [PubMed] [Google Scholar]
- Matthees J., Campagnoni A. T. Cell-free synthesis of the myelin basic proteins in a wheat germ system programmed with brain messenger RNA. J Neurochem. 1980 Oct;35(4):867–872. doi: 10.1111/j.1471-4159.1980.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Seyfried T. N., Yu R. K. Heterosis for brain myelin content in mice. Biochem Genet. 1980 Dec;18(11-12):1229–1237. doi: 10.1007/BF00484350. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Schimke R. T. Synthesis of rat liver albumin in a rabbit reticulocyte cell-free protein-synthesizing system. J Biol Chem. 1973 Nov 25;248(22):7661–7668. [PubMed] [Google Scholar]




